Abstract
During the course of pregnancy, dynamic remodeling of the gut microbiota occurs and contributes to maternal metabolic changes through an undefined mechanism. Because short chain fatty acids (SCFAs) are a major product of gut microbiome fermentation, we investigated whether serum SCFA levels during pregnancy are related to key metabolic parameters in mothers and newborns. In this prospective study, 20 pregnant women without gestational diabetes were evaluated at 36–38 weeks of gestation, and their newborns were assessed after parturition. In this cohort, which included normal (n = 10) and obese (n = 10) subjects based on prepregnancy body mass index, serum levels of SCFAs (acetate, propionate, and butyrate), maternal adipokines, maternal glucose, and C-peptide were measured at 36–38 weeks of gestation. Maternal weight gain and newborn anthropometrics were also determined. Data were analyzed using linear regression to test for associations, adjusting for prepregnancy obesity. In this cohort, serum acetate levels were associated with maternal weight gain and maternal adiponectin levels. In addition, serum propionate correlated negatively with maternal leptin levels, newborn length, and body weight. Taken together, this study observed that novel relationships exist among maternal SCFA levels and multiple interrelated maternal/newborn metabolic parameters.
Pregnancy is a dynamic metabolic state that must allocate nutritional resources between mother and fetus. These metabolic changes include alteration in insulin sensitivity and secretion, along with fatty acid mobilization from the adipose depots. Each of these changes occurs dynamically throughout pregnancy to meet the changing nutritional demands of mother and fetus. For example, increased insulin secretion occurs during early pregnancy, initially in the setting of unchanged insulin sensitivity.1 During mid pregnancy, insulin resistance increases, and insulin secretion increases to match this change.1 Along with changes in insulin sensitivity/secretion, maternal adiposity stores increase during early pregnancy, whereas in mid to late pregnancy, adipose depots are diminished.1 Playing a role in these metabolic changes, adipocyte-specific hormones, called “adipokines” (ie, adiponectin and leptin) have been identified for their role in metabolic response during pregnancy because they contribute to the regulation of satiety, adiposity, and insulin resistance.2 More importantly, the metabolic health of the mother has important implications for the health of the infant. For example, it is well established that maternal obesity is a factor in newborn birth weight that, consequently, has lasting metabolic effects throughout the life of the infant.3
Although much is known about metabolism during pregnancy, an intriguing new factor, the gut microbiome, has been identified for its role in contributing to metabolic changes throughout pregnancy.4 In their report, Koren et al4 show that major changes occur throughout pregnancy to the gut microbiota. In particular, they suggest that, during the first trimester, the gut microbiome is most similar to that observed in nonpregnant healthy women; however, the third trimester leads to a large degree of gut microbiota dysbiosis, similar to what occurs in metabolic syndromes such as type 2 diabetes.4 The authors also show these changes contribute to metabolic aberrations, as demonstrated by transfer of human microbiota from either the first-or third-trimester mothers to germ-free mice.4 However, the factor mediating the gut microbiome effect is unclear.4 One possible explanation involves the role of gut bacteria in food fermentation.5 Multiple metabolites are produced during this process, and one of the major products includes short-chain fatty acids (SCFAs).6
Considering the recently described relationship between the gut microbiome and the metabolic response during pregnancy, we sought to explore whether a relationship exists between serum SCFA levels during pregnancy and well-described metabolic factors during pregnancy (ie, prepregnancy obesity, maternal weight gain, glucose and select metabolic hormones such as C-peptide, leptin, and adiponectin). Because maternal health strongly impacts newborn outcomes, we also examined how serum SCFAs are related to newborn anthropometrics. Overall, this is the first study to examine whether a relationship exists between serum SCFAs and well-described metabolic measures in pregnancy and newborn outcomes.
METHODS
Subjects
The subjects included in this study were selected from a cohort reported previously.7 Each of the selected women delivered at The Prentice Women’s Hospital of Northwestern Memorial Hospital. Prepregnancy body mass index (BMI) was based on self-reported height and weight, and was confirmed by chart review. From this cohort, 10 obese subjects (prepregnancy BMI, >30 kg/m2) and 10 normal-weight subjects (prepregnancy BMI, 18– 25 kg/m2), were matched by maternal age and gestation length. Inclusion criteria for this study included singleton-only pregnancy, term pregnancies, and normal blood pressure. Other eligibility criteria were serum glucose level less than 130 mg/dL during their routine 50-g oral glucose challenge test for gestational diabetes screening. Participants provided self-reported race and ethnicity, which was then categorized as African-American/black, white, Hispanic, or Asian. All subjects provided written informed consent, and the study was approved by the Northwestern University Institutional Review Board for conduct of research on human subjects.
Biochemical measures
During the course of the study, maternal blood was collected between 36 weeks and 38 weeks of gestation. All blood was stored at −70°C until assayed. Plasma glucose was measured with Synchron CX Delta Systems instrumentation (Beckman Coulter, Inc., Brea, Calif) using an oxygen rate method with a glucose oxygen electrode, and had an interassay coefficient of variation of 2.0%–2.3%. Maternal triglycerides were measured with the Triglycerides GPO reagent using the Beckman Coulter Unicel DXC800 analyzer (Beckman Coulter). Concentrations of C-peptide, leptin, and adiponectin were assayed using radioimmunoassay kits from Millipore Corporation (Billerica, Mass). All biochemical assays were performed in duplicate.
Short-chain fatty acids
Serum acetate, propionate, and butyrate were measured in samples by gas chromatography. Briefly, an 800-µL serum aliquot was filtered, and the protein-free filtrate was stored at −20°C before vacuum distillation. The distillation was performed using a 225-µL protein-free sample with a 25-µL internal standard solution added. Next, gas chromatography was performed and SCFA concentration was determined.8
Neonatal anthropometrics
Infant length was obtained with the baby positioned on a hard-surface measuring board. The length measurement was performed in duplicate and recorded to the nearest 0.1 cm, and the results were averaged. Air displacement plethysmography with the Pea Pod Infant Body Composition System (Cosmed, Rome, Italy) was used to measure infant weight and volume, as described previously.7,9 Using pressure-volume equations, body composition, including fat mass and fat-free mass, was calculated to provide percentage body fat.
Statistical analysis
Values are reported as the mean ± standard error of the mean. P values were calculated using Student’s t test (2 tailed) with a significance level of P < 0.05 using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, Calif). Multivariable linear regression models were used to evaluate the associations between maternal SCFA levels, and maternal and neonatal characteristics. For this study, subjects were selected such that 50% (n = 10) were obese (prepregnancy BMI, ≥30 kg/m2) and 50% (n = 10) were nonobese. We accounted for this in our regression analyses by adjusting for prepregnancy obesity status as a dichotomous variable. SAS 9.4 (Cary, NC) was used to conduct the regression analyses.
RESULTS
Baseline characteristics
Twenty subjects were examined in this study (Table I) and were classified based on their prepregnancy BMI. Of these 20 subjects, 10 were obese (BMI, >30) and 10 were of normal weight (BMI, 20–25). Participant characteristics are displayed in Table I. Comparing glucose levels, the fasting glucose levels were significantly greater in the obese vs normal-weight group; however, the 1-hour glucose levels during the glucose challenge were not statistically different (Table I). Blood triglyceride levels during pregnancy in obese subjects trended higher (P = 0.07) than in the normal-weight group.
Table I.
Descriptive characteristics and short-chain fatty acids levels during pregnancy
| Variables | Total (n = 20) | Obese (n = 10) | Normal (n = 10) | P value* |
|---|---|---|---|---|
| Baseline measures, mean (SEM) | ||||
| Age, y | 31.9 (1.0) | 32.1 (0.9) | 31.7 (2.0) | 0.81 |
| Gestational age, wk | 39.5 (0.2) | 39.7 (.3) | 39.4 (0.2) | 0.34 |
| Weight gain, kg | 14.8 (1.2) | 14.2 (1.6) | 15.4 (1.9) | 0.68 |
| Prepregnancy BMI, kg/m2 | 28.3 (1.6) | 34.9 (0.9) | 21.6 (0.5) | 0.00001 |
| Fasting glucose, mg/dL | 82 (1.7) | 85.7 (2.3) | 78.2 (1.8) | 0.03 |
| 1-Hour glucose, mg/dL† | 98.2 (3.6) | 104.8 (4.5) | 91.6 (4.9) | 0.11 |
| Triglyceride, mg/dL | 186.4 (10.8) | 205 (17) | 168 (11) | 0.07 |
| Hormones, mean (SEM) | ||||
| Leptin, ng/mL | 41.2 (4.5) | 58.2 (3.2) | 24.3 (3.3) | 0.00001 |
| Adiponectin, µg/mL | 10.6 (1.0) | 10.4 (1.5) | 10.8 (1.3) | 0.81 |
| C-peptide, ng/mL | 3.1 (0.4) | 4.3 (0.6) | 2.0 (0.1) | 0.003 |
| Short-chain fatty acids, mean (SEM) | ||||
| Acetate, µM | 26.3 (1.9) | 25.6 (3.0) | 26.9 (0.4) | 0.70 |
| Propionate, µM | 1.9 (0.1) | 1.8 (0.1) | 2.0 (0.1) | 0.18 |
| Butyrate, µM | 0.7 (0.1) | 0.5 (0.06) | 0.9 (0.2) | 0.21 |
Abbreviations: BMI, body mass index; SEM, standard error of the mean.
P value, as calculated by Student’s t test, compares the measures between obese and normal groups.
Value is glucose level 1 hour after oral glucose challenge.
Comparison of maternal hormone levels and SCFA levels
A comparison of maternal levels of C-peptide, leptin, and adiponectin showed that leptin and C-peptide levels were twice as high in obese subjects vs normal subjects during pregnancy; however, adiponectin levels were not different between groups (Table I). In both groups, serum acetate levels were greater with lower levels of propionate, followed by butyrate (Table I)—a pattern that has been observed by multiple other studies.10 However, there was no significant difference in serum acetate, propionate, and butyrate levels between groups.
Association of serum SCFAs with prepregnancy obesity, maternal weight gain, and maternal hormone levels
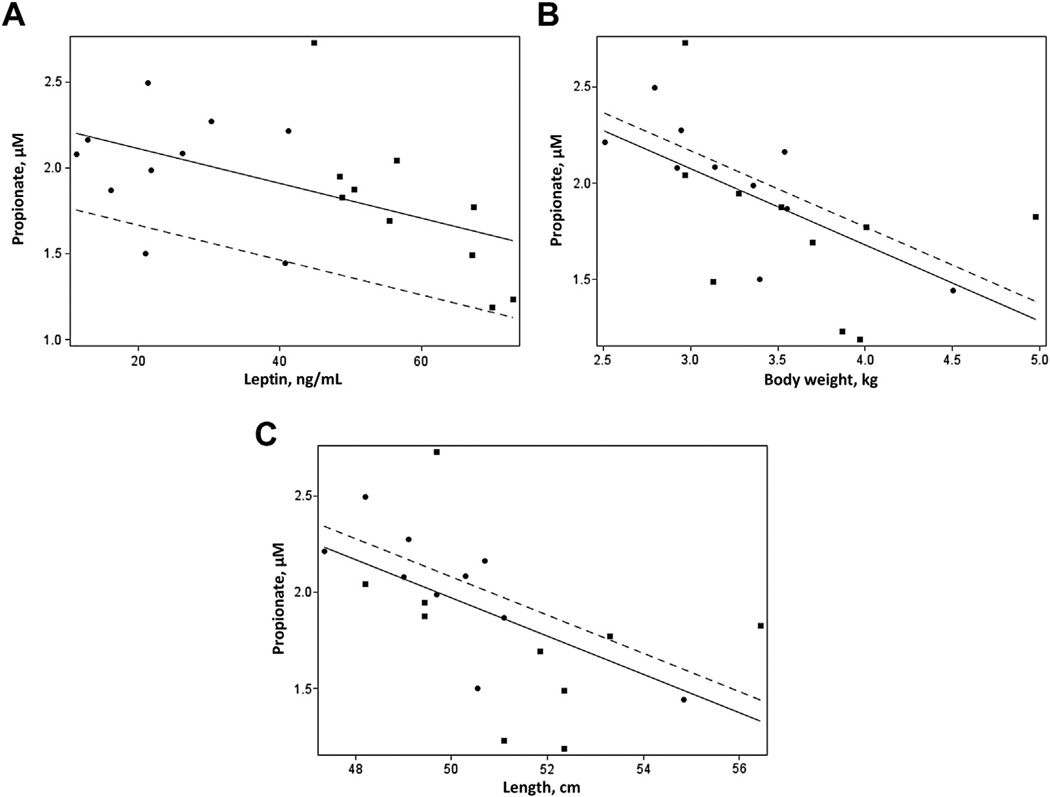
To ascertain whether maternal SCFAs are related to indicators of obesity and metabolism, we next analyzed these data adjusting for maternal prepregnancy obesity (Table II). Of the 3 SCFAs measured, only serum acetate was associated positively with maternal adiponectin levels. Based on the known association of adiponectin and weight gain in obesity, it was not surprising that serum acetate was also associated with weight gain during pregnancy. Interestingly, serum propionate correlated negatively with maternal leptin (Table II, Fig 1, A). No associations were observed with serum butyrate and these variables.
Table II.
Associations between maternal short-chain fatty acids, and maternal and neonatal parameters
| Measures | Acetate, µM |
Propionate, µM |
Butyrate, µM |
|---|---|---|---|
| Maternal characteristics | |||
| Weight gain, kg | −0.35* | 0.00 | −0.01 |
| Maternal hormones | |||
| Adiponectin, µg/mL | 1.13* | 0.01 | 0.00 |
| Leptin, ng/mL | −0.37 | −0.02* | −0.01 |
| C-peptide, ng/mL | −2.22 | −0.08 | −0.04 |
| Neonatal characteristics | |||
| Length, cm | −0.42 | −0.09* | −0.08 |
| Body weight, kg | 0.82 | −0.37* | −0.26 |
| Fat mass, % | 7.07 | −0.83 | −0.43 |
| Body fat, % | 0.44 | −0.03 | −0.01 |
Values shown are β coefficients in models adjusting for maternal prepregnancy obesity. These values represent the change in outcome per 1 U change in maternal characteristics, maternal hormones, and neonatal characteristics.
P values that reached statistical significance are indicated (P < 0.05).
Fig. 1.
(A–C) Scatterplots showing the association between serum propionate levels and maternal leptin levels (A), neonatal body weight (B), and neonatal length (C). The nonobese subjects are represented by circles and dashed lines; the obese subjects are represented by squares and solid lines.
Association of maternal serum SCFAs with newborn anthropometrics
Next in this cohort, we examined how serum SCFA levels in the mother were related to newborn anthropometrics (Table II). Maternal acetate and butyrate levels were not associated with any of these measures. However, serum propionate levels showed a negative association with neonatal length and body weight (Fig 1, B and C).
DISCUSSION
Our data showed associations between serum SCFAs and measures of maternal weight gain, maternal hormones, and neonatal parameters. In particular, serum acetate and propionate were associated with multiple interrelated variables: gestational weight gain, maternal adiponectin levels, maternal leptin levels, neonatal length, and body weight.
Considering that the origin of SCFAs is primarily the gut flora during fermentation, our data raise the question: are SCFAs one of the factors connecting the gut microbiome to metabolic changes during pregnancy? If this is true, bacteria that produce particular SCFAs preferentially may be beneficial to pregnancy. For example, in this study, propionate was associated negatively with maternal leptin and measures of infant size (ie, length and body weight). Therefore, greater propionate levels may be associated with lower maternal leptin levels and smaller newborn size, and thus provide a beneficial or protective effect for the mother during pregnancy and possibly for the newborn. This possibility has precedent; treatment with propionate has been observed to be beneficial against the development of obesity.11 Moreover, how SCFAs might be helpful has been speculated by other groups, including the role of SCFAs as appetite suppressants11,12 and as regulators of hormones such as incretins11 or leptin.13 Systematic studies are needed to determine more concretely any possible role of SCFAs in metabolic responses during pregnancy.
Our study has multiple limitations. First, we explored associations only between variables, not causality. In addition, a small cohort of subjects was investigated. Subsequent larger studies are needed to investigate these data further. Future studies should include dietary assessments of the subjects because diet is an important factor in gut-derived SCFA production. Also, it is important to mention that other sources of SCFA production may occur—in particular, from cellular fatty acid oxidation6—that may confound the interpretation of SCFAs as solely from the gut microbiome. Moreover, the relative contribution to SCFAs in circulation from gut-derived or other sources of SCFA production14 is not well studied.
To further this area of investigation, future studies with human subjects are needed. However, it is not appropriate at this time to modify the gut microbiome in humans during pregnancy because of the lack of insight into its effect on the fetus. Thus, the use of rodent models will offer the opportunity to explore these observational data. Studies can explore these questions using germ-free mice (ie, mice that have not been colonized with bacteria) or antibiotics to knock down the gut microbiome in the animals. In addition, in these studies, it will be important to consider that alternatives sources of serumSCFAs may exist in the body. Although this study provides new evidence for the association between SCFAs and pregnancy, future studies are necessary to validate, expand, and explore further the significance of these findings.
AT A GLANCE COMMENTARY.
Background
The gut microbiome was recently identified as a novel factor involved in metabolic changes during pregnancy.
Translational Significance
As a result of this finding, our study investigated this relationship by studying one of the key factors the gut microbiome generates—short-chain fatty acids—and how they may be related to metabolic parameters in mothers and newborns.
ACKNOWLEDGMENTS
BTL is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Career Development (grant no. 1IK2BX001587-01). JJ is supported by grant no. K12 HD055884 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Abbreviations
- BMI
body mass index
- SCFA
short-chain fatty acid
Footnotes
Conflicts of Interest: The authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
REFERENCES
- 1.Ramos MP, Crespo-Solans MD, del Campo S, Cacho J, Herrera E. Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am J Physiol. 2003;285:E318–E328. doi: 10.1152/ajpendo.00456.2002. [DOI] [PubMed] [Google Scholar]
- 2.Zavalza-Gomez AB, Anaya-Prado R, Rincon-Sanchez AR, Mora-Martinez JM. Adipokines and insulin resistance during pregnancy. Diabetes Res Clin Pract. 2008;80:8–15. doi: 10.1016/j.diabres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65:65–69. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- 4.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL., Jr Short chain fatty acids and their receptors: new metabolic targets. Transl Research. 2013;161:131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Josefson JL, Feinglass J, Rademaker AW, et al. Maternal obesity and vitamin D sufficiency are associated with cord blood vitamin D insufficiency. J Clin Endocrinol Metab. 2013;98:114–119. doi: 10.1210/jc.2012-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Layden BT, Yalamanchi SK, Wolever TM, Dunaif A, Lowe WL., Jr Negative association of acetate with visceral adipose tissue and insulin levels. Diabetes Metab Syndr Obes. 2012;5:49–55. doi: 10.2147/DMSO.S29244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body omposition in infants. Pediatr Res. 2003;53:486–492. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 10.Vogt JA, Pencharz PB, Wolever TM. L-Rhamnose increases serum propionate in humans. Am J Clin Nutr. 2004;80:89–94. doi: 10.1093/ajcn/80.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Arora T, Sharma R, Frost G. Propionate: Anti-obesity and satiety enhancing factor? Appetite. 2011;56:511–515. doi: 10.1016/j.appet.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Lin HV, Frassetto A, Kowalik EJ, Jr, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaibi MS, Stocker CJ, O’Dowd J, et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010;584:2381–2386. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Sakakibara I, Fujino T, Ishii M, et al. Fasting-induced hypothermia and reduced energy production in mice lacking acetyl-CoA synthetase 2. Cell Metab. 2009;9:191–202. doi: 10.1016/j.cmet.2008.12.008. [DOI] [PubMed] [Google Scholar]