Abstract
Objective:
To explore the effect of sequential treatment with glucocorticoid and tumor necrosis factor-alpha inhibitors in patients with Takayasu arteritis (TA).
Materials and Methods:
In five patients with TA, the effects of the sequential treatment with prednisone for 5-7 months and then with adalimumab (ADA) + methotrexate (MTX) or infliximab + MTX, or with ADA only, for 12 months on both clinical and laboratory findings were evaluated.
Results:
All treatments improved both symptoms and laboratory parameters without the development of side-effects.
Conclusions:
It was hypothesized that MMP-9 and neutrophil gelatinase-associated lipocalin could be markers of the response to the treatments.
Keywords: Adalimumab, infliximab, methotrexate, matrix metalloproteinases-9, neutrophil gelatinase-associated lipocalin, takayasu arteritis
INTRODUCTION
Takayasu arteritis (TA) is an idiopathic chronic inflammatory granulomatous disease affecting aorta and its main branches.[1,2] The pathogenesis of TA includes vessel injury due to inflammatory mediators, e.g., Tumor necrosis factor-α (TNF-α), released by T cells, natural killers, γ/δ T cells and macrophages;[3,4,5] However, an imbalance between matrix metalloproteinases (MMPs) and their tissue inhibitors of metalloproteinases (TIMPs) may also be involved.[6] In particular, it has been reported that MMPs 1, 3 and 9 were significantly higher in subjects with active disease.[7,8]
Previously, we documented an association between MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL), a protein belonging to the lipocalin family. NGAL was able to modulate the activity of MMP-9 through the activation of the NGAL/MMP-9 complex.[9,10] NGAL plasma concentration has been associated with vascular diseases.[11]
The clinical manifestation of TA is related to both sites of affected vessel and severity of inflammation and the acute stage is represented by fever, weight loss, and elevated C-reactive protein (CRP) levels.[12] Unfortunately, due to the delay in diagnosing, patients experience claudication, absence of pulses, hypertension, myocardial infarction and cerebrovascular diseases.[13]
The treatment include anti-inflammatory (glucocorticoids) and immunosuppressive drugs (cyclophosphamide, methotrexate [MTX] and azathioprine);[14,15] in unresponsive patients, or in patients experiencing adverse drug reactions (ADRs), a treatment with TNF-α inhibitors + immunosuppressive may be used.[16,17,18]
In this study, we reported in 5 patients with acute TA the effects of a sequential treatment with prednisone for 5 or 7 months and then with TNF-α inhibitors for 12 months on both clinical and laboratory parameters.
MATERIALS AND METHODS
In an open label, parallel groups, double clinical centers study, conducted during the period between January 2009 and December 2012 we enrolled patients with clinical, laboratory (erythrocyte sedimentation rate [ESR] >30 mm/h and CRP; >5 mg/L) and radiological findings of acute TA. Angiography and echo-color Doppler were used to evaluate the presence and the site of vessel lesions. The disease activity was determined using the Birmingham Vascular Activity Score.[19]
This study was approved by the Institutional Review Board - Independent Ethics Committee of Interuniversity Center of Phlebolymphology - International Research and Educational Program in Clinical and Experimental Biotechnology - Headquarters at University Magna Graecia of Catanzaro and before the beginning of the study, all participants were informed about the aim, procedures, risks and benefits of the study and the they provided a written informed consent. At the time of admission and during the follow-up, a blood sample was taken from each patient in order to evaluate the plasma levels of ESR, CRP, MMP-9 and NGAL. The levels of MMPs and NGAL were evaluated through Elisa test in agreement with our previous papers.[9,20,21]
The enrolled patients received a sequential treatment with prednisone for 5 or 7 months and then with adalimumab (ADA) + MTX or infliximab (IFX) + MTX for 12 months. Follow-up was performed every month and the development of ADRs were evaluated in agreement with our previous study.[22,23,24,25,26,27,28,29,30,31]
This study may be considered to be exploratory; therefore we did not determine a power calculation.
RESULTS
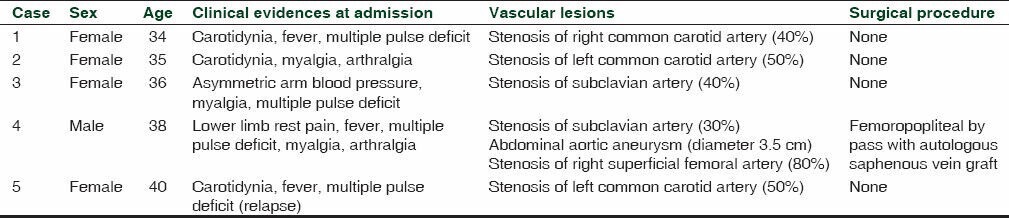
During the study period, 5 new patients, of whom 4 were females and 1 male (median age 36), with acute TA were enrolled [Table 1] and signed the informed consent. Clinical evaluation documented that patients lamented fatigue, myalgia and arthralgia with a pain score of 8, measured through the visual analog scale.
Table 1.
Demographic characteristic of each patient enrolled in this study
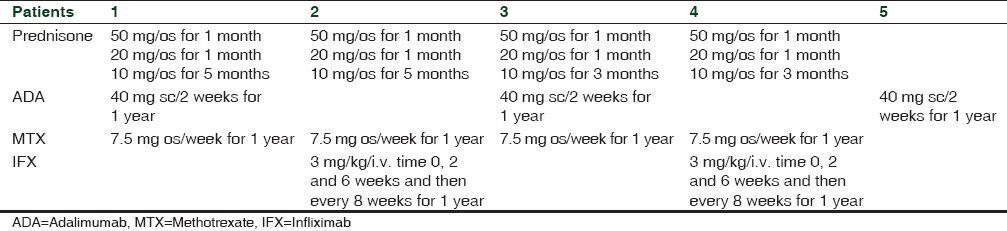
Patients were treated with Prednisone for 5 or 7 months and then with ADA + MTX for 1 year or with IFX + MTX for 1 year [Table 2].
Table 2.
Protocol treatment used in this study
In these patients, laboratory findings revealed an improvement of inflammatory index (ESR and CRP) after 2 months from the beginning of prednisone treatment. Moreover, a complete normalization of inflammatory index was recorded 4 months after the beginning of prednisone and these values maintained steady during treatment with ADA or IFX without difference between groups [Table 3].
Table 3.
Laboratory findings of ESR (mm/h) and CRP (mg/L) in enrolled patients
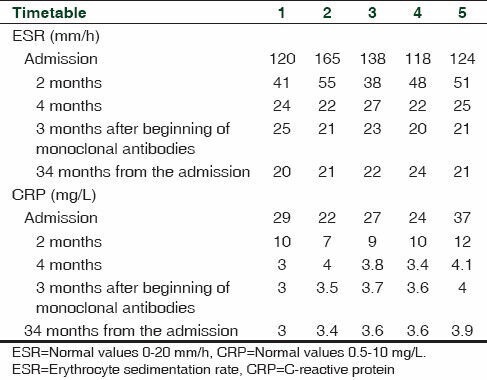
A patient (patient 5) with relapse, enrolled in one of our previously published study [Table 1],[14] who developed ADRs to corticosteroid and intolerance to MTX received a treatment with ADA only, with a good control of laboratory index [Table 3]. All patients also showed an improvement in their clinical conditions (VAS from 8 to 1 at the end of the study). No relapses and no ADRs occurred during the study and during the follow-up of 34 months.
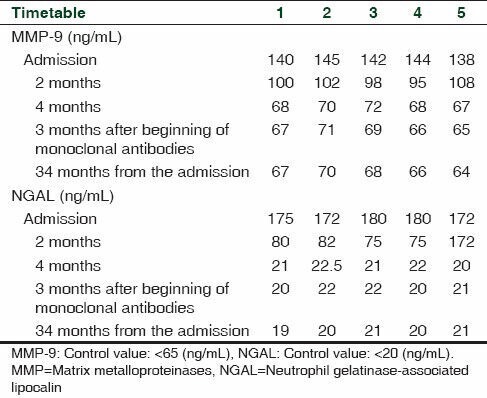
In all patients (Case 1-5) ELISA findings revealed higher levels of both plasma MMP-9 (mean 140 ± 4 ng/mL) and NGAL (mean 176 ± 5 ng/mL) with respect to control patients (5 healthy volunteers: 4 F, 1 M, median age 34; MMP-9: 65 ng/mL; NGAL: 20 ng/mL). In enrolled patients (Cases 1-4) the prednisone treatment induced a time-related decrease in both plasma MMP-9 and NGAL levels [Table 4], that have been maintained in normal values during the treatment with monoclonal antibodies [Table 4].
Table 4.
Elisa values of MMP-9 (ng/mL) and NGAL (ng/mL) in enrolled patients
DISCUSSION
In this study, we evaluated the effects of a different drug treatment of TA in patients with acute TA symptoms.
Till date the etiology of TA disease is enigmatic since several mechanisms such as post-infective, autoimmune, ethnic susceptibility and genetic predisposition have been postulated.[32] Oxidative stress (i.e., reactive oxygen species) and reactive nitrosative species represent a cardinal feature of inflammatory process and are involved in vascular abnormalities influencing extracellular matrix remodeling through the activation of MMPs.[8] MMPs regulate extracellular structural proteins and consequent tissue remodeling and seem to be involved in various vascular disease and related complications.[11,33] NGAL is a protein belonging to the lipocalin family, is expressed by activated neutrophils and is able to modulate the activity of MMP-9 through the activation of the NGAL/MMP-9 complex. In vessels MMPs influence the migration, proliferation and apoptosis of vascular smooth muscle, endothelial cells and inflammatory cells. NGAL plasma concentrations have been associated with vascular diseases.[34,35,36,37] In agreement with these data, in our study we enrolled 5 patients with fever myalgia, claudication intermittens; angiography documented the presence of stenosis in upper diaphragmatic vessels. Previously it has been showed that MMP-1, 3 and 9 were significantly higher in subjects with active disease when compared to those study subjects who were in remission.[33,34,35]
The limitation of this study is related to the very low number of patients enrolled that is related with the very low frequency of this disease.
CONCLUSION
Elisa test revealed higher levels of both plasma MMP-9 and NGAL in Takayasu patients respect to control patients. Prednisone administered for 5 months showed the same effects on both clinical and laboratory findings of a longer treatment (7 months). Moreover, we showed that both ADA and IFX administered on-label with MTX improved the inflammatory indexes inducing a significant decrease in plasma levels of CRP, ESR, MMP-9 and NGAL. The patient treated on-label with ADA in monotherapy documented a good control of symptoms and of laboratory findings. No side effects appeared during the treatments. The limitation of this study is very low number of patients due to very low frequency of this disease. In conclusion, monoclonal antibodies are able to improve the symptoms of Takayasu without the development of side effects and we suggest that treatment with prednisone for 5 months leads to a good clinical outcome, although further studies are needed to validate these data. Finally, the evaluation of MMP-9 and NGAL plasma levels could be useful during the follow-up of this disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ishihara T, Haraguchi G, Tezuka D, Kamiishi T, Inagaki H, Isobe M. Diagnosis and assessment of Takayasu arteritis by multiple biomarkers. Circ J. 2013;77:477–83. doi: 10.1253/circj.cj-12-0131. [DOI] [PubMed] [Google Scholar]
- 2.Kallappa Parameshwarappa S, Mandjiny N, Kavumkal Rajagopalan B, Radhakrishnan N, Samavedam S, Unnikrishnan M. Intact giant abdominal aortic aneurysm due to Takayasu arteritis. Ann Vasc Surg. 2013;27:671.e11–4. doi: 10.1016/j.avsg.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Seko Y. Takayasu arteritis: Insights into immunopathology. Jpn Heart J. 2000;41:15–26. doi: 10.1536/jhj.41.15. [DOI] [PubMed] [Google Scholar]
- 4.Seko Y, Sugishita K, Sato O, Takagi A, Tada Y, Matsuo H, et al. Expression of costimulatory molecules (4-1BBL and Fas) and major histocompatibility class I chain-related A in aortic tissue with Takayasu's arteritis. J Vasc Res. 2004;41:84–90. doi: 10.1159/000076437. [DOI] [PubMed] [Google Scholar]
- 5.Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu's arteritis: A 2011 update. Autoimmun Rev. 2011;11:61–7. doi: 10.1016/j.autrev.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28:2108–14. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 7.Parakh R, Yadav A. Takayasu's arteritis: An Indian perspective. Eur J Vasc Endovasc Surg. 2007;33:578–82. doi: 10.1016/j.ejvs.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan N, Dhawan V, Mahmood S, Malik S, Jain S. Extracellular matrix remodeling in Takayasu's arteritis: Role of matrix metalloproteinases and adventitial inflammation. Arch Med Res. 2012;43:406–10. doi: 10.1016/j.arcmed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 9.de Franciscis S, Mastroroberto P, Gallelli L, Buffone G, Montemurro R, Serra R. Increased plasma levels of metalloproteinase-9 and neutrophil gelatinase-associated lipocalin in a rare case of multiple artery aneurysm. Ann Vasc Surg. 2013;27:1185.e5–7. doi: 10.1016/j.avsg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Serra R, Buffone G, Falcone D, Molinari V, Scaramuzzino M, Gallelli L, et al. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. 2013;21:395–401. doi: 10.1111/wrr.12035. [DOI] [PubMed] [Google Scholar]
- 11.Ramos-Mozo P, Madrigal-Matute J, Vega de Ceniga M, Blanco-Colio LM, Meilhac O, Feldman L, et al. Increased plasma levels of NGAL, a marker of neutrophil activation, in patients with abdominal aortic aneurysm. Atherosclerosis. 2012;220:552–6. doi: 10.1016/j.atherosclerosis.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Nooshin D, Neda P, Shahdokht S, Ali J. Ten-year Investigation of Clinical, Laboratory and Radiologic Manifestations and Complications in Patients with Takayasu's arteritis in Three University Hospitals. Malays J Med Sci. 2013;20:44–50. [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and pathological studies of aortitis syndrome. Committee report. Jpn Heart J. 1968;9:76–87. doi: 10.1536/ihj.9.76. [DOI] [PubMed] [Google Scholar]
- 14.de Franciscis S, Serra R, Luongo A, Sabino G, Puzziello A. The management of Takayasu's arteritis: Personal experience. Ann Vasc Surg. 2007;21:754–60. doi: 10.1016/j.avsg.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Borg FA, Dasgupta B. Treatment and outcomes of large vessel arteritis. Best Pract Res Clin Rheumatol. 2009;23:325–37. doi: 10.1016/j.berh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Comarmond C, Plaisier E, Dahan K, Mirault T, Emmerich J, Amoura Z, et al. Anti TNF-α in refractory Takayasu's arteritis: Cases series and review of the literature. Autoimmun Rev. 2012;11:678–84. doi: 10.1016/j.autrev.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Mahlmann A, Pfluecke C, Ouda A, Simonis G, Weiss N, Kappert U. Combined immunosuppressive therapy including a TNF-alpha blocker induces remission in a difficult to treat patient with Takayasu arteriitis and coronary involvement. Vasa. 2012;41:451–7. doi: 10.1024/0301-1526/a000236. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt J, Kermani TA, Bacani AK, Crowson CS, Matteson EL, Warrington KJ. Tumor necrosis factor inhibitors in patients with Takayasu arteritis: Experience from a referral center with long-term followup. Arthritis Care Res (Hoboken) 2012;64:1079–83. doi: 10.1002/acr.21636. [DOI] [PubMed] [Google Scholar]
- 19.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–8. [PubMed] [Google Scholar]
- 20.Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extra cellullar matrix in patients with chronic venous leg ulcers. Acta Phlebol. 2013;14:99–107. [Google Scholar]
- 21.de Franciscis S, Gallelli L, Battaglia L, Molinari V, Montemurro R, Stillitano DM, et al. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J. 2013 doi: 10.1111/iwj.12085. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallelli L, Colosimo M, Pirritano D, Ferraro M, De Fazio S, Marigliano NM, et al. Retrospective evaluation of adverse drug reactions induced by nonsteroidal anti-inflammatory drugs. Clin Drug Investig. 2007;27:115–22. doi: 10.2165/00044011-200727020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Gallelli L, Colosimo M, Tolotta GA, Falcone D, Luberto L, Curto LS, et al. Prospective randomized double-blind trial of racecadotril compared with loperamide in elderly people with gastroenteritis living in nursing homes. Eur J Clin Pharmacol. 2010;66:137–44. doi: 10.1007/s00228-009-0751-3. [DOI] [PubMed] [Google Scholar]
- 24.Gallelli L, Ferreri G, Colosimo M, Pirritano D, Flocco MA, Pelaia G, et al. Retrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro, Italy. Pharmacol Res. 2003;47:493–9. doi: 10.1016/s1043-6618(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 25.Gallelli L, Nardi M, Prantera T, Barbera S, Raffaele M, Arminio D, et al. Retrospective analysis of adverse drug reactions induced by gemcitabine treatment in patients with non-small cell lung cancer. Pharmacol Res. 2004;49:259–63. doi: 10.1016/j.phrs.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Gallelli L, Ferreri G, Colosimo M, Pirritano D, Guadagnino L, Pelaia G, et al. Adverse drug reactions to antibiotics observed in two pulmonology divisions of catanzaro, Italy: A six-year retrospective study. Pharmacol Res. 2002;46:395–400. doi: 10.1016/s1043661802002104. [DOI] [PubMed] [Google Scholar]
- 27.Gareri P, Gallelli L, Ferreri Ibbadu G, Lacava R, Russo E, De Sarro G. Melaena following Use of the Cholinesterase Inhibitor Rivastigmine. Clin Drug Investig. 2005;25:215–7. doi: 10.2165/00044011-200525030-00008. [DOI] [PubMed] [Google Scholar]
- 28.Scicchitano F, Giofrè C, Palleria C, Mazzitello C, Ciriaco M, Gallelli L, et al. Pharmacovigilance and drug safety 2011 in Calabria (Italy): Adverse events analysis. J Res Med Sci. 2012;17:872–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Gallelli L, Guadagnino V, Caroleo B, Marigliano N, De Sarro GB, Izzi A. Bilateral Skin Ulceration after Injection of Pegylated Interferon-alpha-2b in a Patient with Chronic Hepatitis C. Clin Drug Investig. 2003;23:615–9. doi: 10.2165/00044011-200323090-00009. [DOI] [PubMed] [Google Scholar]
- 30.Rende P, Paletta L, Gallelli G, Raffaele G, Natale V, Brissa N, et al. Retrospective evaluation of adverse drug reactions induced by antihypertensive treatment. J Pharmacol Pharmacother. 2013;4(Suppl 1):S47–50. doi: 10.4103/0976-500X.120954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallelli L, Palleria C, De Vuono A, Mumoli L, Vasapollo P, Piro B, et al. Safety and efficacy of generic drugs with respect to brand formulation. J Pharmacol Pharmacother. 2013;4(Suppl 1):S110–4. doi: 10.4103/0976-500X.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isobe M. Takayasu arteritis revisited: Current diagnosis and treatment. Int J Cardiol. 2013;168:3–10. doi: 10.1016/j.ijcard.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Serra R, Buffone G, Costanzo G, Montemurro R, Scarcello E, Stillitano DM, et al. Altered metalloproteinase-9 expression as the least common denominator between varicocele, inguinal hernia and chronic venous disorders. Ann Vasc Surg. 2013 doi: 10.1016/j.avsg.2013.07.026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Amato B, Coretti G, Compagna R, Amato M, Buffone G, Gigliotti D, et al. Role of matrix metalloproteinases in non-healing venous ulcers. Int Wound J. 2013 doi: 10.1111/iwj.12181. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, et al. Extracellular matrix assessment of infected chronic leg ulcers: Role of metalloproteinases and inflammatory cytokines. Int Wound J. 2014 doi: 10.1111/iwj.12225. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serra R, Grande R, Butrico L, Buffone G, Caliò FG, Squillace A, et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J. 2014 doi: 10.1111/iwj.12240. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busceti MT, Grande R, Amato B, Gasbarro V, Buffone G, Amato M, et al. Pulmonary Embolism, Metalloproteinases, and Neutrophil Gelatinase Associated Lipocalin. Acta Phlebol. 2013;14:115–21. [Google Scholar]


