Sir,
Cannabis continues to be most common illicit drug of abuse with around 119-224 million users worldwide. However, it remains one of the least studied illicit drugs.
Buprenorphine is an approved medicine for short and long term management of opioid dependence.[1] Studies have reported a high prevalence of cannabis use among individuals enrolled in treatment for opioid dependence (ranging from 39-66%).[2] Studies exploring impact of cannabis use on treatment outcome for opioid dependence have shown varied results. While some have reported an adverse impact on outcome,[2] others have failed to demonstrate such association.[3]
Concurrent use of cannabis along with opioid substitution therapy (OST) can reflect inadequate control of opioid withdrawals. In fact, successful prescribed use of cannabis as an adjunct to or substitute for opiates in the treatment of chronic pain has been reported from some settings. Continued cannabis use, while on OST, can have potentially adverse effects on treatment outcome. These individuals continue to be exposed to the risks associated with cannabis use. Also they can experience interaction of cannabis metabolites and OST agents. Additionally, focus on opioids as primary drug of abuse can lead to limited focus on ongoing cannabis use among these individuals.
Hence, it is important to assess the individuals on OST for cannabis use. Urinalysis based screening for psychoactive substances remains a widely accepted and practiced approach in substance use disorder treatment centers.[4] This study aimed at exploring the presence of cannabis among urine samples of individuals on OST with buprenorphine or buprenorphine-naloxone combination. No such literature is available from South Asian region.
The study was conducted at a tertiary care substance use disorder treatment centre in North India. The study involved a retrospective review of the laboratory records of individuals on OST from the centre. Urinalysis records of individuals were retrieved and analyzed for the purpose of the study. A total of 100 urinalysis conducted over a 6 month period were included in this study.
A modified hydrolysis method followed by thin layer chromatography (TLC) was used for the detection of abused drugs in urine. The urine samples were collected and processed as per methods described earlier.[4] Cassette test (Alfa scientific Designs, Inc. Poway, CA, USA) was used to detect presence of cannabis. This devise is a qualitative immunoassay intended to be used to detect Δ9- THC-9- carboxylic acid (THC), a major metabolite of cannabis, in human urine at a cut off level of 50 ng/ml. Data were analyzed using SPSS ver 11.0. Student's t test and Chi square (Fisher's exact) test was performed for in between group differences. The level of statistical significance was kept at P < 0.05.
The study was approved by the Institute Ethics Committee.
A total of 100 urinalyses samples from individuals on OST with buprenorphine (n = 25) and buprenorphine-naloxone combination (n = 75) were included in the study. The mean age of the individuals was 37.75 (SD ± 11.05) years. All were males. The mean age did not differ among those on buprenorphine (35.04, SD ± 8.37 years) and buprenorphine-naloxone combination (38.65, SD ± 11.72 years) (t 1.42, P = 0.15).
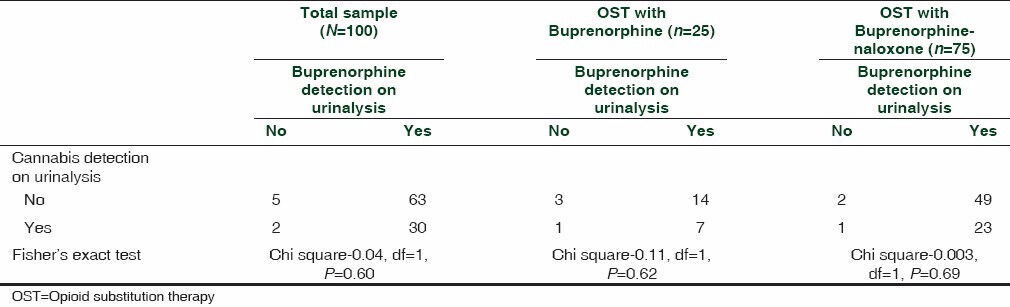
Buprenorphine was detected in 93% of the urine samples. Cannabis was detected in 32% of the samples. Cannabis detection rate was comparable between the samples tested positive and negative for buprenorphine (chi square - 0.041, df = 1, P = 0.60). Subgroup analysis among those on buprenorphine (chi square -0.11, df = 1, P = 0.62) and buprenorphine-naloxone combination (chi square -0.003, df = 1, P = 0.69) was also comparable for samples tested positive or negative for buprenorphine [Table 1].
Table 1.
Cannabis detection and buprenorphine detection among those prescribed OST
Concerns of neglect of cannabis use among recipients of OST have been expressed for long.[5] Cannabis use along with OST can have potentially deleterious effects. Reports have associated chronic use of cannabis and buprenorphine with brain hemorrhage and cerebral vasospasm.[6] DuPont and Saylor[5] reported that use of cannabis among those on methadone maintenance is associated with an adverse outcome. Wasserman et al.[7] reported that marijuana use was associated with a greater risk of lapse to heroin use among those on methadone maintenance.
Around 32% of the samples tested positive for cannabis in this study. Studies among those seeking treatment for opioid dependence have reported a high rate of cannabis use. Budney et al.,[3] reported that 66% of 107 individuals enrolled in treatment for opioid dependence were current marijuana users and 94% continued to use during treatment. Blandthorn et al.,[8] reported rate of cannabis use to be 39% among 98 pregnant women (20 on buprenorphine).
Cannabis use has been reported to be associated with an increased likelihood of relapse at 6-month follow-up.[7] Aharonovich et al.,[1] found that one-third of 250 patients from inpatient psychiatric/substance abuse setting used cannabis following discharge. Post-discharge cannabis use significantly increased the hazard of first use of any substance and strongly reduced the likelihood of stable remission from use of any substance. Postdischarge cannabis use affected first use of alcohol, stable remission, and subsequent relapse of alcohol use as well as first use of cocaine and stable remission. However, it was unrelated to heroin use outcomes in this study.
Interestingly use of cannabis among those seeking treatment for opioid dependence has not always been found to be associated with an adverse outcome. Budney et al.,[3] failed to find any significant adverse relation between cannabis use and treatment outcome in this study. Although, cannabis users were significantly more likely to report financial difficulties, be involved in drug dealing and engage in sharing of needles.
Cannabis use among those using opioids has been reported to be a potential deterrent to high risk use of opioids. Daily use of cannabis has been interpreted as a form of self-regulation and potentially deterrent to transition from noninjecting heroin use to injecting heroin use.
Cannabis has been hypothesized to have an inverted U-shaped relation with retention in naltrexone based treatment for opioid dependence. Opioid-dependent individuals on naltrexone using cannabis intermittently have shown better retention as compared to individuals with either heavy cannabis use or no cannabis use.
Cannabis stimulates appetite and has antiemetic, antispasmodic, and analgesic effects. Intermittent administration of exogenous cannabinoids can attenuate sympathetic nervous activation and has indirect dopaminergic agonist effect at the brain reward system.[9] Inadequate dose of buprenorphine/buprenorphine-naloxone combination to individuals maintained on OST can be experienced as uncomfortable state. Also partial agonist nature of buprenorphine means that the desired (euphoric) effect experienced by those on OST is likely to be less than that experienced while using pure agonists such as heroin. Hence, these individuals inspite of being on OST are likely to use cannabis. Infact, more days of cannabis use has been found to be correlated with higher maximum daily dose of buprenorphine-naloxone. Also, it has been recommended to make cannabis available within therapeutic settings with an aim to reduce problematic use of pharmaceutical opiates and other potentially harmful substances. Such an approach has been hypothesized to reduce opioid-related morbidity.
High prevalence of cannabis use and multiple possible interactions between its use and medications for opioid dependence make it important to address cannabis use among those receiving OST. There is limited literature exploring this issue, especially from South Asian countries. The rates of detection of cannabis were comparable among those receiving buprenorphine and buprenorphine-naloxone combination in this study.
The current study offers insights in to a rather underexplored area. In this study, subjects who were on buprenorphine or buprenorphine-naloxone combination were examined. Use of buprenorphine-naloxone combination is being seen as a significant development in office-based management of opioid dependence. Use of urinalysis is a well-accepted objective measure of drug use and monitoring of therapy in long term management of opioid dependence.[4]
There are certain limitations of this study. It was based on retrospective review of the available laboratory records. Many variable such as dose of medication, baseline cannabis use characteristics and subjective reports of cannabis use while on OST were not explored. Cannabis can be detected in urine for many weeks after a period of regular use. Change in cannabis use pattern after initiation of OST remained unexplored in this study.
The findings of this study suggest that a significant proportion of individuals on OST tested positive for cannabis. It is therefore recommended to use urinalysis during OST to monitor ongoing use of cannabis. Detection of cannabis on urinalysis should be followed by exploration of possible causes for the same as its implications on outcome.
ACKNOWLEDGEMENTS
The technical assistance given by Mr. Nizamuddin Saifi NDDTC laboratory is highly acknowledged. This work is supported by National Drug Dependence Treatment Centre, AIIMS, India.
REFERENCES
- 1.Balhara YP. Time to include buprenorphine-naloxone combination in the WHO Model List of Essential Medicines. J Opioid Manag. 2013;9:237. [PubMed] [Google Scholar]
- 2.Roux P, Carrieri PM, Cohen J, Ravaux I, Spire B, Gossop M, et al. Non-medical use of opioids among HIV-infected opioid dependent individuals on opioid maintenance treatment: The need for a more comprehensive approach. Harm Reduct J. 2011;8:31. doi: 10.1186/1477-7517-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budney AJ, Bickel WK, Amass L. Marijuana use and treatment outcome among opioid-dependent patients. Addiction. 1998;93:493–503. doi: 10.1046/j.1360-0443.1998.9344935.x. [DOI] [PubMed] [Google Scholar]
- 4.Balhara YP, Jain R. Urinalysis-based comparative evaluation of pattern of use of dextropropoxyphene and buprenorphine among opioid-dependent subjects. J Opioid Manag. 2012;8:45–9. [PubMed] [Google Scholar]
- 5.DuPont RL, Saylor KE. Marijuana and benzodiazepines in patients receiving methadone treatment. JAMA. 1989;261:3409. [PubMed] [Google Scholar]
- 6.Renard D, Gaillard N. Brain haemorrhage and cerebral vasospasm associated with chronic use of cannabis and buprenorphine. Cerebrovasc Dis. 2008;25:282–3. doi: 10.1159/000119638. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman DA, Weinstein MG, Havassy BE, Hall SM. Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend. 1998;52:183–92. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 8.Blandthorn J, Forster DA, Love V. Neonatal and maternal outcomes following maternal use of buprenorphine or methadone during pregnancy: Findings of a retrospective audit. Women Birth. 2011;24:32–9. doi: 10.1016/j.wombi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Jain R, Balhara YP. Neurobiology of cannabis addiction. Indian J Physiol Pharmacol. 2008;52:217–32. [PubMed] [Google Scholar]


