Abstract
Described is an in vitro model of premature senescence in pulmonary adenocarcinoma A549 cells induced by persistent DNA replication stress in response to treatment with the DNA damaging drug mitoxantrone (Mxt). The degree of cellular senescence, based on characteristic changes in cell morphology, is measured by laser scanning cytometry. Specifically, the flattening of cells grown on slides (considered the hallmark of cellular senescence) is measured as the decline in local intensity of DNA-associated DAPI fluorescence (represented by maximal pixels). This change is paralleled by an increase in nuclear area. Thus, the ratio of mean intensity of maximal pixels to nuclear area provides a very sensitive morphometric biomarker for the degree of senescence. This analysis is combined with immunocytochemical detection of senescence markers, such as overexpression of cyclin kinase inhibitors (e.g., p21WAF1) and phosphorylation of ribosomal protein S6 (rpS6), a key marker associated with aging/senescence that is detected using a phospho-specific antibody. These biomarker indices are presented in quantitative terms defined as a senescence index (SI), which is the fraction of the marker in test cultures relative to the same marker in exponentially growing control cultures. This system can be used to evaluate the anti-aging potential of test agents by assessing attenuation of maximal senescence. As an example, the inclusion of berberine, a natural alkaloid with reported anti-aging properties and a long history of use in traditional Chinese medicine, is shown to markedly attenuate the Mxt-induced SI and phosphorylation of rpS6. The multivariate analysis of senescence markers by laser scanning cytometry offers a promising tool to explore the potential anti-aging properties of a variety agents.
Keywords: cell cycle, nuclear size, laser scanning cytometry, ribosomal protein S6, mTOR signaling, berberine
INTRODUCTION
Cellular senescence can be categorized into two types. The first is replicative senescence, which is seen as the loss of replicative capacity of normal diploid cells after a certain number of cell divisions, known as Hayflick’s limit (Hayflick, 1965). Replicative senescence is a consequence of the progressive erosion of telomere ends with each division, until the cell reaches a state of telomere dysfunction (Harley et al., 1990). The second category is premature senescence, which is independent of telomere shortening (reviewed in Kuilman et al., 2010). Among the factors inducing premature senescence are persistent DNA replication stress (Sherr and DePinho, 2000; Parrinello et al., 2003), activation of oncogenes (Serrano et al., 1997), and loss of tumor suppressor genes (Chen et al., 2005). In certain malignancies such as acute leukemias, the predominant mode of cell death during chemo- or radiotherapy is apoptosis. In solid tumors, however, the mechanism of growth inhibition often relies on impaired cell reproductive capacity, referred to as drugor radiation-induced senescence, which can also be defined as premature senescence (Gerwitz et al., 2008; Litwiniec et al., 2010). In addition, there is a growing body of evidence that induction of premature senescence in vivo plays a role as a barrier in tumor development by preventing tumorigenesis of induced pluripotent stem cells (iPSCs; Banito and Gil, 2010).
Regardless of the type of senescence or mechanism of induction, there are common biomarkers that can be used to identify senescent cells. The most typical are morphological changes (Cristofallo and Pignolo, 1993). Senescent cells are characterized by increased size, marked flattening when grown on a solid surface, enlarged and irregularly shaped nuclei, numerous cytoplasmic granularities, and low saturation density at the plateau phase of growth. In addition, the cytoplasm contains increased numbers of microfilaments, enlarged lysosomal bodies, and prominent Golgi apparatus (Funayama and Ishikawa, 2007; Cho and Hwang, 2010). Some types of senescent cells demonstrate abnormalities in nuclear chromatin, defined as senescence-associated heterochromatic foci (SAHF), which are abundant in histone H3 modified at lysine 9 (K9M H3) and its binding partner heterochromatin protein 1 (HP1; Narita and Lowe, 2004; Li et al., 2007; Zhang and Adams, 2007).
Several molecular biomarkers are also expressed in senescent cells. The suppression of proliferation is associated with upregulation of inhibitors of cyclin-dependent kinases (CKIs), including p21WAF1, p16INK4A, and p27KIP1, and with activation of tumor suppressor p53 signaling pathways (Korotchkina et al., 2009; Qian and Chen, 2010; Shen and Maki, 2010). Persistent expression of markers of DNA damage response such as γH2AX and activation of ATM, a consequence of oxidative DNA damage by endogenous oxidants (Huang et al., 2006; Tanaka et al., 2006; Bernaś et al., 2013), is another attribute of senescent cells (Mah et al., 2010; Halicka et al., 2012). Although these markers are neither unique nor highly specific to senescent cells, the characteristic features of cellular morphology combined with the induction of senescence-associated β-galactosidase (SA-β-gal) activity are considered to be the most specific biomarkers of senescent cells (Dimri et al., 1995; Itahana et al., 2007).
The laser scanning cytometer (LSC) is a microscope slide−based image cytometer that offers analytical capabilities to probe individual cells, including rapid multicolor measurement of fluorescence intensity and detection of changes in morphology (Bedner et al., 1999; Darzynkiewicz et al., 1999; Henriksen et al., 2011). These capabilities have been used to develop a quantitative biomarker for the degree (depth) of cellular senescence by measuring cellular flattening and nuclear enlargement (Zhao et al., 2010; Zhao and Darzynkiewicz, 2013). Specifically, after DNA staining with the fluorochrome 4,6-diamidino-2-phenylindole (DAPI), a simple LSC assay of nuclear area combined with the mean intensity of maximal pixels of DAPI fluorescence per nucleus provides a measure of the change in nuclear/cellular geometry that is considered to be one of the most characteristic features of cellular senescence (Zhao et al., 2010; McKenna et al., 2012). In addition, markers associated with cellular senescence and detected immunocytochemically can be measured concurrently with morphometric changes. This protocol is presently used to assess the ability of a potential anti-aging (gero-suppressive) agent to attenuate induction of cellular senescence by persistent DNA replication stress.
QUANTITATIVE ANALYSIS OF CHANGES IN CELL MORPHOLOGY ASSOCIATED WITH INDUCED PREMATURE SENESCENCE
The following protocol was developed using human pulmonary adenocarcinoma A549 cells that are induced to senescence by a low concentration of mitoxantrone (Mxt), a DNA topoisomerase II inhibitor that binds to DNA (Kapuscinski and Darzynkiewicz, 1985, 1986) and perturbs the normal changes in DNA topology (over- and underwinding) associated with replication and transcription (D’Arpa et al., 1990; Pommier et al., 2010). Such treatment causes persistent DNA replication stress. Berberine (BRB), a naturally occurring alkaloid with a long history of use in traditional Chinese and Ayurvedic medicine, is used as an example of a potential anti-aging agent (Lee et al., 2006; Halicka et al., 2012; Zhao et al., 2013). A549 cultures are treated with Mxt with or without BRB, and the depth of cellular senescence is assessed and compared between BRB-treated and untreated cultures.
In addition to analyzing senescence-associated nuclear morphological changes, this protocol uses immunocytochemical detection of CKI p21WAF1, and can be used to detect other CKIs considered to be markers of senescent cells (p27KIP1 or p16INK4a; Demidenko et al., 2009; Korotchkina et al., 2009; Qian and Chen, 2010; Shen and Mak, 2010). The protocol also measures ribosomal protein S6 (rpS6) phosphorylated at Ser235/236 (rpS6P) using a phospho-specific antibody. Phosphorylation of rpS6, the downstream effector of the mTOR signaling pathway, is considered the key factor responsible for aging and induction of premature cellular senescence (Blagosklonny, 2008; Zoncu et al., 2011; Magnuson et al., 2012). Attenuation of phosphorylation of rpS6 is therefore consistent with the expectation that an agent has anti-aging properties.
This methodology can also be used to measure senescence-associated changes in other cell types (e.g., WI-38), as well as changes in other biomarkers, such as induction of SA-β-gal or γH2AX and activation of ATM (Zhao et al., 2010, 2013; Halicka et al., 2012; McKenna et al., 2012; Zhao and Darzynkiewicz, 2013). Activation of SA-β-gal is measured using the Chemicon Cellular Senescence Assay Kit (Millipore) and the capability of LSC to measure both light absorption and intensity of fluorescence (Bedner et al., 1999; Darzynkiewicz et al., 1999; Henriksen et al., 2011; Zhao et al., 2013).
Other types of fluorescence microscope or imaging system may also be used, provided that the capability for fluorescence analysis (hardware and software) allows one to assess nuclear area as well as maximal pixel intensity of DNA-associated nuclear fluorescence (e.g., using DAPI or another DNA-specific fluorochrome).
Materials
Human pulmonary adenocarcinoma A549 cells (non-small cell lung carcinoma; ATCC)
Supplemented Ham’s F12K medium (see recipe)
Mitoxantrone (Mxt; Sigma-Aldrich)
Berberine (BRB; Sigma-Aldrich)
Phosphate-buffered saline (PBS), pH 7.4
1% (v/v) formaldehyde (methanol-free, ultrapure, Polysciences) in PBS, ice cold
70% (v/v) ethanol, ice cold
0.1% (v/v) Triton X-100 (Sigma-Aldrich) in PBS
1% (w/v) bovine serum albumin (BSA; Sigma-Aldrich) in PBS
Primary antibody: anti-p21WAF1, anti-p16INK4a, anti-p27KIP1 (all Santa Cruz Biotechnology), and/or anti-rpS6P (Epitomics)
Secondary antibody: appropriate species-specific antibody labeled with AlexaFluor 488, 633, or 647 (Invitrogen/Life Technologies)
DAPI staining solution (see recipe)
Antifade mounting medium (Invitrogen/Life Technologies)
Single- or multi-chambered incubation slides with chamber caps (Nunc Lab-Tek II)
Coplin jars
Moisturized chamber
Coverslips
Laser scanning microscope (LSC), preferentially new model of iCys Research Imaging Cytometer with iGeneration software (initially manufactured by CompuCyte, currently by Thorlabs)
Induce cell senescence
-
1
Prepare cultures of A549 cells on single- or multi-chambered incubation slides using supplemented Ham’s F12K medium. Seed cells at ~25% to 30% confluency and grow in a 37°C incubator with 5% CO2 in air and 100% humidity.
Other cell types that grow on slides can be used, such as human diploid WI-38 fibroblasts (ATCC) in MEM Eagle-Earle (Gibco/Invitrogen).
-
2
For exponentially growing control cells, culture in medium alone and terminate (harvest) after 24 hr.
-
3
For test cells, culture with medium alone for 4 hr to allow attachment, then add 2 nM Mxt alone or with varying concentrations of BRB (e.g., 0, 5, 10, 30, and 60 µM). Terminate cultures after 3, 4, and/or 5 days.
Cells with Mxt alone provide a measure of maximal senescence, while cells with Mxt plus BRB allow measurement of the possible attenuation of senescence. Other senescence-inducing drugs can be used instead of Mxt, for example, 0.5 or 1.0 µM histone deacetylase inhibitor Trichostatin A (TSA; Sigma-Aldrich) or 1 or 2 µg/ml DNA-crosslinking agent mitomycin C (McKenna et al., 2012). Other presumed gero-suppressive agents can be tested at the desired concentrations.
Harvest, fix, and stain cells
-
4
Remove culture medium from chamber and replace with enough PBS to fill the chamber.
-
5
Remove PBS and fill chamber with ice-cold 1% formaldehyde in PBS. Close chamber caps and keep slides on ice for 15 min.
CAUTION: Formaldehyde is a strong carcinogen. Care should be exercised in handling this reagent. Methanol-free formaldehyde is used instead of paraformaldehyde (a solid-state substance), as the latter is even more hazardous because it must be heated to hydrolyze it to obtain formaldehyde.
-
6
Remove formaldehyde and replace with ice-cold 70% ethanol. Close chamber caps and transfer slides to a freezer at −20°C for storage, if needed.
Slides can be stored in 70% ethanol at −20°C for several days.
-
7
Carefully remove the chamber walls from the slides as described by the manufacturer, then incubate the slides in PBS in Coplin jars for 5 min at room temperature. Repeat incubation in fresh PBS for another 5 min.
-
8
Remove slides and place them horizontally in a moisturized chamber.
-
9
Carefully layer a small volume (~100 µl) of 0.1% Triton X-100 in PBS over the cells and incubate 15 min at room temperature.
-
10
Remove Triton/PBS by touching the edge of the slide to a paper towel. Apply ~100 µl of 1% BSA in PBS and incubate another 30 min at room temperature.
-
11
Replace BSA solution with 100 µl BSA solution containing a 1:100 dilution of the desired primary antibody (p21, p16, p27, or rpS6P). Incubate 1.5 hr at room temperature or overnight at 4°C.
See Critical Parameters and Troubleshooting for a discussion of pilot antibody dilution experiments.
Primary and/or secondary antibodies from suppliers other than those listed above can be used. When ordering, ensure that antibodies are recommended for immunocytochemistry and/or flow cytometry, not only western blotting or immunoprecipitation. Multiple primary antibodies can be used, as long as there is no cross-reactivity with secondary antibodies.
-
12
Rinse cells by dipping slides in a Coplin jar filled with 1% BSA in PBS.
-
13
Apply 100 µl BSA solution containing a 1:100 dilution of the appropriate AlexaFluor-tagged secondary antibody. Incubate 45 min at room temperature.
-
14
Rinse slides with PBS.
-
15
Apply 100 µl DAPI staining solution and incubate 15 min at room temperature.
-
16
Rinse slides with PBS.
-
17
Apply a drop of antifade mounting medium and mount under a coverslip.
Slides may be stored up to 1 week at 4°C. To preserve specimens for longer periods of time (up to 1 month) or to transport them, seal coverslips with nail polish or melted paraffin.
Measure fluorescence and acquire and analyze data
-
18
Measure fluorescence intensity on an LSC using the following excitation and emission parameters:
Excitation:DAPI fluorescence 405 nm diode laser AlexaFluor 488 488 nm diode laser AlexaFluor 633 or 647 633 nm He Ne laser Photomultipliers for fluorescence:DAPI blue AlexaFluor 488 green AlexaFluor 633 or 647 far red See Critical Parameters and Troubleshooting for a discussion other markers of senescence that can be measured.
-
19
While measuring DAPI fluorescence, record:
mean intensity of maximal pixels per nucleus (maximal pixels)
nuclear area (i.e., number of pixels over the whole nucleus)
integral of intensity of DAPI fluorescence (i.e., the sum of intensities of all pixels over the nucleus).
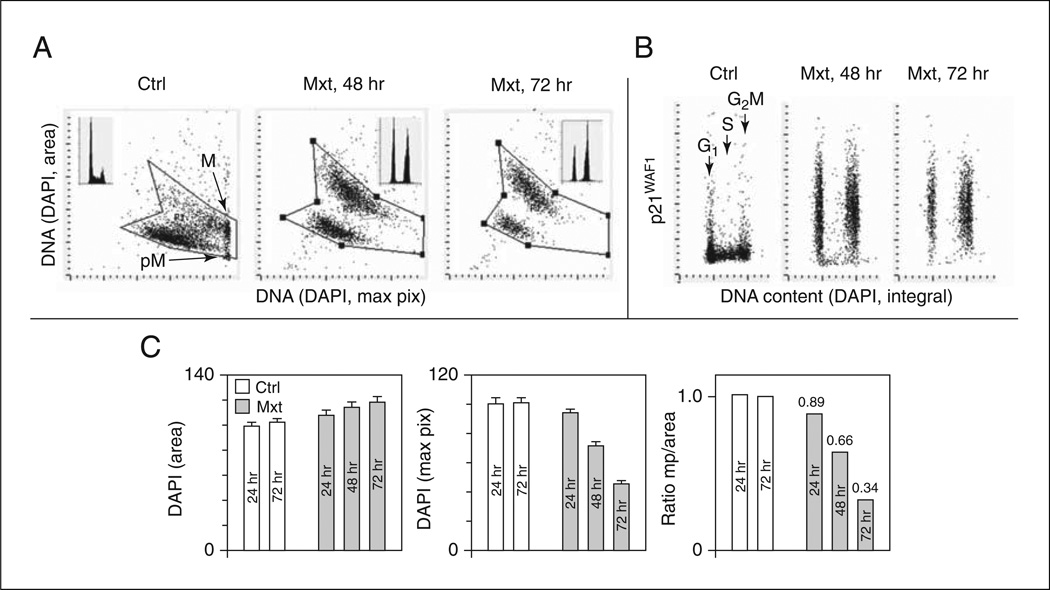
The integral of DAPI fluorescence intensity per nucleus provides information on cellular DNA content and thereby on the cell cycle phase (G1, S, G2 M) distribution of the cell population (Fig. 9.47.1A; DNA content frequency histograms; Darzynkiewicz et al., 2010).
-
20
Calculate the ratio of changes in these parameters in cells subjected to Mxt treatment (inducing senescence) compared to exponentially growing control cells to obtain the senescence index (SI).
The SI represents a fraction (or fold increase) of the measured parameter in cells subjected only to DNA replication stress to induce senescence (e.g., using Mxt or mitomycin C).
-
21
Calculate the change in SI (ΔSI) in cells treated with Mxt plus BRB with respect to cells exposed to Mxt alone.
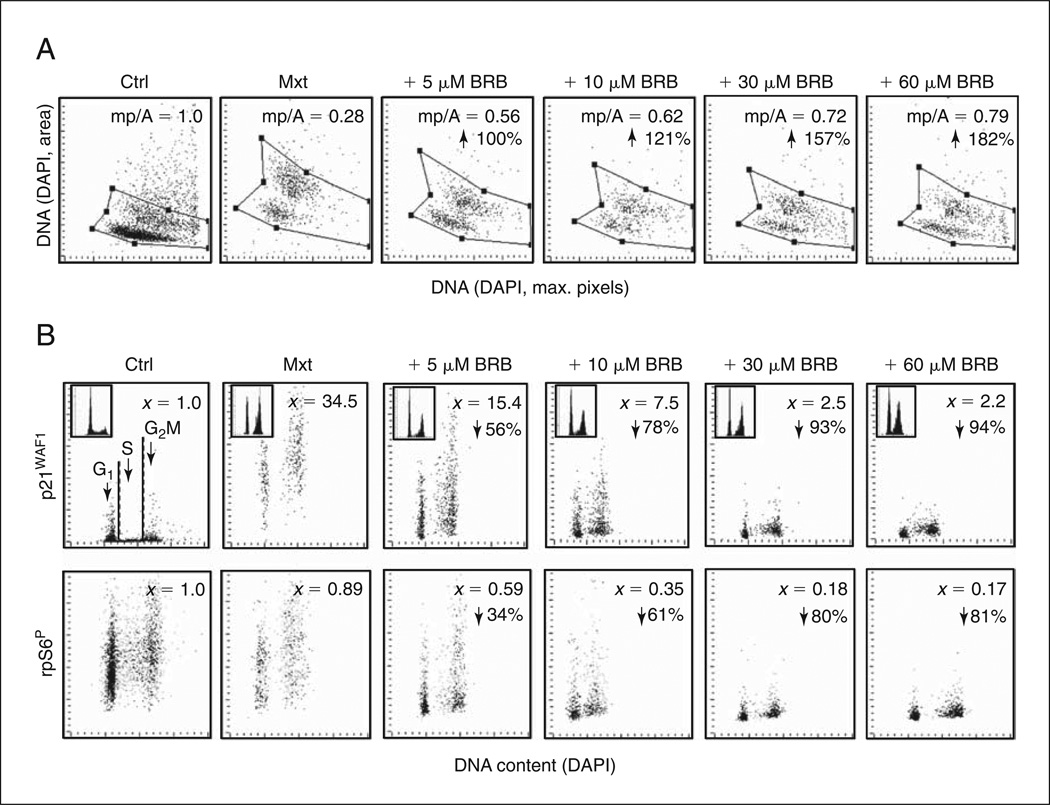
ΔSI reveals the ability of the potential anti-aging agent (e.g., BRB) to reduce the degree (depth) of cell senescence. Examples of analysis of SI and ΔSI are provided in Figure 9.47.2.
Figure 9.47.1.
Induction of premature cellular senescence in A549 cells measured by laser scanning cytometry (LSC; iCys Research Imaging Cytometer; Zhao et al., 2010). A549 cells were untreated (Ctrl) or treated with 2 nM Mxt for 48 or 72 hr. (A) Cell morphometric features revealed by nuclear DNA (DAPI) fluorescence reporting on the bivariate distributions (scatter plots) of nuclear area versus mean intensity of maximal pixels of fluorescence per nucleus, respectively. In Ctrl cells, intensity of the mean value of maximal pixels is highest in mitotic (M) and immediately post-mitotic (pM) G1 cells; nuclei of these cells also have a small area of DAPI fluorescence, consistent with their high degree of chromatin condensation (Bedner et al., 1999; Darzynkiewicz et al., 1999; Henriksen et al., 2011). In senescing cells, nuclear area increases while maximal pixel intensity decreases (Zhao et al., 2010, 2013; McKenna et al., 2012). These morphometric changes reflect enlargement of the projected nuclear area and decreased intensity of DAPI local staining per unit area due to a flattened cellular appearance, the hallmark of cellular senescence (Dimri et al., 1995; Itahana et al., 2007). The insets show DNA content frequency histograms of cells from the respective cultures. Persistent DNA damage by Mxt (replication stress) leads to a decrease in the frequency of S phase cells and cell arrest in G2. (B) Bivariate distributions of DNA content versus p21WAF1, another marker of cellular senescence (Dimri et al., 1995; Itahana et al., 2007), report expression of this CKI with respect to cell cycle phase. In the Mxt-treated cells, note that the increase in p21 expression is concurrent with the reduction in frequency of S-phase cells. (C) Mean values (± SD) of DAPI (nuclear) area, DAPI mean maximal pixels, and the ratio of maximal pixels to nuclear area (mp/area) in control and Mxt-treated cells. Numbers above bars in the ratio graph indicate the senescence index (SI) of Mxt-treated cells (ratio of mp/area in Mxt-treated cells to mp/area ratio in Ctrl cells).
Figure 9.47.2.
Attenuation of Mxt-induced senescence in A549 cells by BRB as measured by cell morphometric features and expression of p21WAF1 and rpS6P. Exponentially growing cells were untreated (Ctrl) or treated with 2 nm Mxt in the absence or presence of 5 to 60 µM BRB for 5 days. (A) Bivariate distributions of nuclear area (DNA-DAPI) versus mean intensity of maximal pixels of DAPI fluorescence per cell. The ratio of maximal pixels to nuclear area (mp/A) is expressed as SI (i.e., relative to the same ratio in Ctrl cells). Note that SI is progressively reduced (in a dose-dependent manner) in BRB-treated cells relative to cells treated with Mxt alone. Arrows are percent increase in mp/A in BRB-treated cultures (i.e., percent reduction of SI) with respect to Mxt alone. (B) Bivariate distributions of p21WAF1 and rpS6P versus cellular DNA content. x is fold increase in mean expression for all cells with respect to Ctrl cells; arrows are percent reduction of expression in BRB-treated cultures compared to Mxt alone. Since aging and induction of premature senescence is considered to be driven by constitutive mTOR signaling, which converges on activation of rpS6 (Blagosklonny, 2008; Zoncu et al., 2011; Magnuson et al., 2012), potential anti-aging agents are expected to reduce the level of rpS6 phosphorylation.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see Selected Suppliers of Reagents and Equipment.
DAPI staining solution
Stock solution: Dissolve 1 mg 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich) in 1 ml deionized water (2.66 mM). Store up to several months at 4°C in the dark.
Working solution: Dilute 5 µl stock solution in 2 ml PBS (final 2.5 µg/ml). Prepare fresh before use.
Supplemented Ham’s F12K medium
Ham’s F12K tissue culture medium (Gibco/Invitrogen)
2 mM l-glutamine
10% bovine serum
100 U/ml penicillin
100 µg/ml streptomycin
Store up to 1 year at 4°C
COMMENTARY
Background Information
The anti-aging properties of potential gero-suppressive agents are being investigated in vivo by measuring their effects on longevity in both invertebrate and vertebrate organisms. Some of the compounds, such as rapamycin and metformin, have already been shown to significantly prolong life of several animals including mice (reviewed in Darzynkiewicz et al., 2014). These investigations, especially those involving vertebrates, provide the most relevant evidence for anti-aging properties, but are time consuming and expensive. To date, there have been no cytometric methods for investigating gero-suppression.
Using the advantages of imaging cytometry provided by the iCys laser-scanning, slide-based cytometer, this quantitative cytometric approach can be used to assess the degree (depth) of cellular senescence based on changes in cellular morphology. This assessment can be combined with other biomarkers of senescence (Zhao et al., 2010; McKenna et al., 2012; Zhao and Darzynkiewicz, 2013). This approach has been used to test the effectiveness of several reported gero-suppressive agents, including metformin, rapamycin, berberine, vitamin D3, resveratrol, 2-deoxyglucose, and acetylsalicylic acid (Halicka et al., 2012; Darzynkiewicz et al., 2014). In these studies, however, cells were grown in the presence of the indicated agent and evaluated for its effects on the level of constitutive signaling along the mTOR pathway (phosphorylation of mTOR, 4EBP1, and rpS6) as well as on DNA damage signaling (ATM activation, phosphorylation of H2AX). All seven compounds were, to varying degrees, found to attenuate both mTOR as well as DNA damage signaling. Testing the ability of potential gero-suppressive agents to suppress the induction of cellular senescence in the model of persistent DNA replication stress caused by Mxt, this methodology is presented here in protocol format. The results from this protocol (presented in Fig. 9.47.2) indicate that BRB attenuates induction of cellular senescence in a concentration-dependent manner.
Critical Parameters and Troubleshooting
Serial dilution test for immunocytochemical detection
For optimal immunocytochemical detection, it is advised to test various dilutions of the primary and secondary antibodies in pilot titration experiments. In addition to the concentration recommended by the supplier, 2- and 4-fold lower and higher concentrations should be tested. The optimal concentration is the one that gives the highest signal-to-noise ratio (ratio of highest mean fluorescence intensity in positively stained cells to mean fluorescence intensity of negative control cells). The negative control for assessing signal-to-noise ratio should be a negative isotype control antibody used exactly as the antigen-specific antibody and followed by the secondary Ab.
Using additional markers for cell senescence
In addition to measuring DAPI fluorescence concurrently with expression of p21WAF1, p16INK4a, p27KIP1, or rpS6P, other markers relevant to cell senescence can be analyzed. Useful markers of DNA damage response include expression of γH2AX and/or activation of Ataxia telangiectasia mutated (ATM) protein kinase, which can be detected by phospho-specific antibodies and measured by LSC (Tanaka et al., 2007; Zhao et al., 2007). Replication stress, the primary inducer of cellular senescence, activates the DNA damage response, which is detected by γH2AX and activation of ATM (Zhao et al., 2010; McKenna et al., 2012).
Fluorochromes other than DAPI that can be used to stain DNA include Hoechst 33342, DyeCycle Violet, DRAQ5, and propidium iodide (PI). The use of PI requires pretreatment of cells with RNase A (Darzynkiewicz et al., 2010).
Among the parameters measured by LSC to characterize depth of cell senescence are: (1) mean intensity of maximal pixels of DNA-associated (DAPI) fluorescence per nucleus; (2) area of nucleus based on the image of DAPI fluorescence, which equals the number of DAPI pixels; (3) ratio of mean intensity of maximal pixels to nuclear area, and/or (4) mean fluorescence intensity of an antibody against a marker protein (e.g., p21WAF1, p16INK4a, p27KIP1, rpS6P, or γH2AX) integrated over the nucleus. Cell density on the slide is also recorded, providing information about the saturation density of cells at confluence (Zhao et al., 2010; McKenna et al., 2012; Zhao and Darzynkiewicz, 2013).
Anticipated Results
Figures 9.47.1 and 9.47.2 illustrate the expected results and explain analyses of raw data. To assess depth of cellular senescence and make comparisons between treatments, the data are expressed as the senescence index (SI), which represents the ratio of a parameter (e.g., nuclear area/maximal pixels, expression of markers) in cells treated with a senescence inducer (e.g., Mxt) to untreated, exponentially growing cells (Ctrl). The attenuation of senescence by the gero-suppressive agent is represented as a percent change (expected to be a decrease) of the SI in cells treated with inducer plus test agent (Mxt plus BRB) with respect to the SI of cells treated with the inducer alone.
Time Considerations
Preparation of cultures and incubation in the presence of the inducing and gero-suppressive agents may take 3 to 6 days. After 15 min fixation in formaldehyde, the cells can be stored in 70% ethanol for several days. Immunocytochemical and DAPI labeling take ~3 hr (or ~20 hr if cells are treated with primary antibody overnight). Analysis of slides by LSC takes ~15 min per slide, depending on cell density.
Acknowledgements
This work was supported by the National Cancer Institute (RO1 28 704) and the Robert A. Welke Cancer Research Foundation.
Literature Cited
- Banito A, Gil J. Induced pluripotent stem cells and senescence: Learning the biology to improve the technology. EMBO Rep. 2010;11:353–359. doi: 10.1038/embor.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedner E, Li X, Gorczyca W, Melamed MR, Darzynkiewicz Z. Analysis of apoptosis by laser scanning cytometry. Cytometry. 1999;35:181–195. doi: 10.1002/(sici)1097-0320(19990301)35:3<181::aid-cyto1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Bernaś T, Berniak K, Rybak P, Zarębski M, Zhao H, Darzynkiewicz Z, Dobrucki JW. Analysis of spatial correlations between patterns of DNA damage response and DNA replication in nuclei of cells subjected to replication stress or oxidative damage. Cytometry A. 2013;83:825–932. doi: 10.1002/cyto.a.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin H-K, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Hwang ES. Fluorescence-based detection and quantification of features of cellular senescence. Methods Cell Biol. 2010;103:149–188. doi: 10.1016/B978-0-12-385493-3.00007-3. [DOI] [PubMed] [Google Scholar]
- Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol. Rev. 1993;78:617–625. doi: 10.1152/physrev.1993.73.3.617. [DOI] [PubMed] [Google Scholar]
- D’Arpa P, Beardmore C, Liu LF. Involvement of nucleic acid synthesis in cell killing mechanisms of topoisomerase poisons. Cancer Res. 1990;50:6919–6924. [PubMed] [Google Scholar]
- Darzynkiewicz Z, Bedner E, Gorczyca W, Melamed MR. Laser scanning cytometry. A new instrumentation with many applications. Exp. Cell Res. 1999;249:1–12. doi: 10.1006/excr.1999.4477. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Halicka HD, Zhao H. Analysis of cellular DNA by flow and laser scanning cytometry. Adv. Exp. Med. Biol. 2010;676:137–147. doi: 10.1007/978-1-4419-6199-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Zhao H, Halicka HD, Li J, Lee Y-S, Hsieh T-C, Wu J. In search of anti-aging modalities: Evaluation of mTOR- and ROS/DNA damage-signaling by cytometry. Cytometry A. 2014;85A:386–389. doi: 10.1002/cyto.a.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc. Natl. Acad. Sci. U.S.A. 2009;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Rockelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci U.S.A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama R, Ishikawa F. Cellular senescence and chromatin structure. Chromosoma. 2007;116:431–440. doi: 10.1007/s00412-007-0115-7. [DOI] [PubMed] [Google Scholar]
- Gerwitz DA, Holt SE, Elmore LW. Accelerated senescence: An emerging role in tumor cell response to chemotherapy and radiation. Biochem. Pharmacol. 2008;76:947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Halicka HD, Zhao H, Li J, Lee Y-S, Hsieh T-C, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive DNA damage- and mTOR-signaling. Aging. 2012;4:952–965. doi: 10.18632/aging.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Henriksen M, Miller B, Newmark J, Al-Kofahi Y, Holden E. Laser scanning cytometry and its applications: A pioneering technology in the field of quantitative imaging. Methods Cell Biol. 2011;102:161–205. doi: 10.1016/B978-0-12-374912-3.00007-9. [DOI] [PubMed] [Google Scholar]
- Huang X, Tanaka T, Kurose A, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation on Ser-139 in cells untreated by genotoxic agents is cell-cycle phase specific and attenuated by scavenging reactive oxygen species. Int. J. Oncol. 2006;29:495–501. [PubMed] [Google Scholar]
- Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: The senescence-associated β-galactosidase assay. Methods Mol. Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J, Darzynkiewicz Z. Interactions of antitumor agents ametantrone and mitoxantrone (Novantrone) with double-stranded DNA. Biochem. Pharmacol. 1985;34:4203–4213. doi: 10.1016/0006-2952(85)90275-8. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J, Darzynkiewicz Z. Relationship between the pharmacological activity of antitumor drugs ametantrone and mitoxantrone (Novantrone) and their ability to condense nucleic acids. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6302–6306. doi: 10.1073/pnas.83.17.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotchkina LG, Demidenko ZN, Gudkov AV, Blagosklonny MV. Cellular quiescence caused by Mdm2 inhibitor nutlin-3A. Cell Cycle. 2009;8:3777–3781. doi: 10.4161/cc.8.22.10121. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi W, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- Li Q, Ma L, Zhang Z-Y, Tong T-J. SAHF: A new biomarker of cellular senescence. Progr. Biochem. Biophys. 2007;11:1123–1128. [Google Scholar]
- Litwiniec A, Grzanka A, Helmin-Basa A, Gackowska L, Grzanka D. Features of senescence and cell death induced by doxorubicin in A549 cells: Organization and level of selected cytoskeletal proteins. J. Canc. Res. Clin. Oncol. 2010;136:717–736. doi: 10.1007/s00432-009-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signaling networks. Biochem. J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Mah LJ, El-Osta A, Karagiannis TC. γH2AX as a molecular marker of aging and disease. Epigenetics. 2010;5:129–136. doi: 10.4161/epi.5.2.11080. [DOI] [PubMed] [Google Scholar]
- McKenna E, Traganos F, Zhao H, Darzynkiewicz Z. Persistent DNA damage caused by low levels of mitomycin Cinduces irreversible senescence of A549 cells. Cell Cycle. 2012;12:3132–3140. doi: 10.4161/cc.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Lowe SW. Executing cell senescence. Cell Cycle. 2004;3:244–246. [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Chen X. Tumor suppression by p53: Making cells senescent. Histol. Histopathol. 2010;25:515–526. doi: 10.14670/hh-25.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shen H, Maki CG. Persistent p21 expression after Nutlin-3a removal is associated with senescence-like arrest in 4N cells. J. Biol. Chem. 2010;285:23105–23114. doi: 10.1074/jbc.M110.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: Mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle. 2006;5:1940–1945. doi: 10.4161/cc.5.17.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kajstura M, Halicka HD, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation are strongly amplified during mitogenic stimulation of lymphocytes. Cell Prolif. 2007;40:1–13. doi: 10.1111/j.1365-2184.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Adams PD. Heterochromatin and its relationship to cell senescence. Cell Cycle. 2007;6:784–789. doi: 10.4161/cc.6.7.4079. [DOI] [PubMed] [Google Scholar]
- Zhao H, Darzynkiewicz Z. Biomarkers of cell senescence assessed by imaging cytometry. Methods Mol. Biol. 2013;965:83–92. doi: 10.1007/978-1-62703-239-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Tanaka T, Halicka HD, Traganos F, Zarebski M, Dobrucki J, Darzynkiewicz Z. Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports the aging-related processes. Cytometry A. 2007;71:905–914. doi: 10.1002/cyto.a.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Halicka HD, Traganos F, Jorgensen E, Darzynkiewicz Z. New biomarkers probing depth of cell senescence assessed by laser scanning cytometry. Cytometry A. 2010;77:99–107. doi: 10.1002/cyto.a.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Halicka HD, Li J, Darzynkiewicz Z. Berberine suppresses gero-conversion from cell cycle arrest to senescence. Aging. 2013;6:623–636. doi: 10.18632/aging.100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]