Abstract
Borrelia burgdorferi binds strongly to the extracellular matrix and cells of the connective tissue, a binding apparently mediated by specific proteins and proteoglycans. We investigated the interactions between B. burgdorferi cells and intact type I collagen using hydrated lattices that reproduce features of in vivo collagen matrices. B. burgdorferi cells of several strains adhered avidly to these acellular matrices by a mechanism that was not mediated by decorin or other proteoglycans. Moreover, following adhesion to these matrices, B. burgdorferi grew and formed microcolonies. The collagen used in these studies was confirmed to lack decorin by immunoblot analysis; B. burgdorferi cells lacking the decorin adhesin bound readily to intact collagen matrices. B. burgdorferi also bound to collagen lattices that incorporated enzymes that degraded glycosaminoglycan chains in any residual proteoglycans. Binding of the bacteria to intact collagen was nonetheless specific, as bacteria did not bind agar and showed only minimal binding to bovine serum albumin, gelatin, pepsinized type I collagen, and intact collagen that had been misassembled under nonphysiological pH and ionic-strength conditions. Proteinase K treatment of B. burgdorferi cells decreased the binding, as did a lack of flagella, suggesting that surface-exposed proteins and motility may be involved in the ability of B. burgdorferi to interact with intact collagen matrices. The high efficiency of binding of B. burgdorferi strains to intact collagen matrices permits replacement of the commonly used isotopic binding assay with visual fluorescent microscopic assays and will facilitate future studies of these interactions.
Borrelia burgdorferi, the etiologic agent of Lyme disease, is transmitted to animals and humans mainly by nymphae of the tick Ixodes scapularis, which during a blood meal deposit a small number of microorganisms into the skin (8, 43, 51). The inoculation of the bacteria by the tick bite results after a few days in a characteristic rash, erythema migrans, which may be accompanied by systemic symptoms, including malaise, fatigue, fever, headache, neck stiffness, arthralgias, or myalgias (30, 51, 58). Adhesion, colonization, and proliferation within the skin and other host organs and tissues by B. burgdorferi necessitates interaction between the spirochete and cells of the connective tissue, including macrophages, dendritic cells, fibroblasts (24, 48), and the associated extracellular matrix (ECM) (24).
B. burgdorferi expresses cell surface proteins that interact specifically with different components of the ECM of the host organism and of mammalian cells in culture (8, 23, 26, 30). These B. burgdorferi surface proteins include the fibronectin receptor encoded by BBK32 (42); proteins that bind directly to glycosaminoglycans (GAGs), such as Bgp (39-41); and membrane lipoproteins, such as DbpA and DbpB, which bind to decorin (4, 16, 20-22). The B. burgdorferi outer membrane protein p66 binds to the beta3 integrin chain of host ECM receptors (9-12). The variable small proteins (Vsp) and variable large proteins (Vlp) of Borrelia turicatae and Borrelia hermsii also bind to GAGs (31). These findings indicate that B. burgdorferi and relapsing fever Borrelia have multiple modes of interaction with the ECM, which may account for the ability of these bacteria to colonize and thrive in many organs (4, 8, 30).
The dermis is the first target and barrier that B. burgdorferi cells encounter in the skin after the tick inoculation. This tissue has an ECM containing several protein and polysaccharide components and is particularly rich in type I collagen. Other bacteria able to colonize and produce lesions in the skin express adhesins by which they bind directly to collagen (29, 32, 35, 46, 49, 53-55). These include the virulence factors CNE of Streptococcus equi (29) and Acm of Enterococcus faecium (35), the surface proteins RspA and RspB of Erysipelothrix rhusiopathiae (46), the S-layer protein CbsA of the nonpathogenic bacterium Lactobacillus crispatus (32, 49), and the adhesin YadA found in Yersinia enterocolitica and Yersinia pseudotuberculosis (53). Treponema also has the ability to bind collagen directly by collagen-binding polypeptides (54, 55).
In contrast to the above, previous studies using isolated collagen have been unable to detect a direct interaction between this ECM component and whole B. burgdorferi cells (4, 20-22). This is despite the fact that B. burgdorferi is mainly an extracellular pathogen that migrates actively from the dermis into the bloodstream and from the blood into connective tissues of organs, such as the heart. In light of this, it has been suggested that the bacterium interacts with and migrates through connective tissues by interacting primarily with the collagen-associated proteoglycan decorin (21, 22, 26, 45, 52, 57), for which well-characterized B. burgdorferi adhesins have been described (4, 16, 20-22). However, this hypothesis is not supported by experiments with decorin knockout mice that showed that decorin binding does not play an essential role in the generation of infection leading to arthritis (5).
Because the structure and organization of type I collagen vary with its mode of extraction and in vitro assembly (2, 15, 17, 18, 56, 57), we decided to investigate the question of B. burgdorferi-collagen interaction using a preparation that reproduces the features of in vivo matrices more authentically than those used previously. We have found that the bacteria bind readily to lattices prepared from intact acetic acid-extracted type I collagen devoid of decorin and other proteoglycans and GAGs that have been assembled under physiological conditions. Moreover, B. burgdorferi readily invades and colonizes such lattices, suggesting an alternative pathway of interaction between the bacterium and the ECM.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. burgdorferi strains used in these experiments included the high-passage noninfectious strain B31-UM transformed with plasmid pCE320, which contains gfpmut1 under the control of the flaB promoter (provided by J. D. Radolf and C. H. Eggers, University of Connecticut Health Center, Farmington, Conn.) (14); the high-passage strain B31 (ATCC 35210; American Type Culture Collection, Manassas, Va.), obtained from Jorge Benach (Department of Molecular Genetics and Microbiology, State University of New York at Stony Brook); the high-passage noninfectious flaB null mutant flaB::Km (flaB mutant MC-1), obtained from Nyles Charon (Department of Microbiology and Immunology, West Virginia University, Morgantown, Va.) (34); the low-passage infectious strain N40, obtained from Linda Bockenstadt (Yale University, New Haven, Conn.); and B314, a high-passage noninfectious derivative of strain B31, which lacks lp54, does not bind to mammalian cells, and does not express the DbpA and DbpB proteins (16, 44), obtained from John Leong and Nikhat Parveen (Department of Molecular Genetics and Microbiology, University of Massachusetts Medical Center, Worcester).
Preparation of intact type I collagen.
Tail tendons of young adult rats (from carcasses of terminated controls in other protocols) were extracted with 0.5 M guanidine hydrochloride (GuHCl) (Sigma Chemical Co., St. Louis, Mo.) in 0.5 M Tris-HCl, pH 7.2, for 24 h at 4°C to remove GAGs and proteoglycans (3). The GuHCl-treated tendons were then extracted with 3% acetic acid and centrifuged for 3 h at 14,000 rpm (Sorvall SS-34 rotor) at 4°C. The supernatant, containing acid-soluble collagen, was then centrifuged at 4°C and 35,000 rpm in a Beckman ultracentrifuge (Ti-45 rotor) to sediment any remaining bacteria and small particulates, and the solubilized collagen was dialyzed (12,000- to 14,000-MW cutoff) for 48 h with three changes of 1/10-strength Ham's F-12 medium (F-12) without bicarbonate (Flow Laboratories, Inc., McLean, Va.) (36). The dialyzed collagen was stored at 4°C until it was used.
Preparation of native, pepsinized, and misassembled collagen lattices and other substrata.
Native collagen lattices were prepared as described previously (36). Briefly, dialyzed intact collagen was adjusted with 1/10-strength F-12 without bicarbonate to a concentration of 8.1 mg/ml. An aliquot of this solution (0.7 ml) was mixed on ice with 0.1 ml of 9.3× F-12, 0.1 ml of distilled water, and 0.1 ml of sodium bicarbonate (11.76 mg/ml) with rapid stirring; 1 ml of 1× complete F-12 was then added to this solution with continued stirring (36). The final concentration of collagen was 2.33 mg/ml. Misassembled intact collagen was prepared by leaving out the 9.3× F-12 and adding an additional 0.1 ml of sodium bicarbonate. The misassembled gels were washed several times with phosphate-buffered saline (PBS) before being used to correct their pH. Glycosidase-treated collagen lattices were prepared in the same manner, but instead of 0.1 ml of water we added 0.1 ml of a solution containing chondroitinase ABC (5 mU/ml from Proteus vulgaris; Seikagaku Corporation, Tokyo, Japan) and heparinase I and heparitinase III (1 U/ml each; Sigma). Prolonged (3-h) digestions with these enzymes were also used to determine that the digestion that we obtained at 1 h was complete, as there was no change in the pattern of bands obtained at these two times. For lattices containing pepsinized collagen, commercial rat tail tendon collagen which had undergone pepsin treatment to remove telopeptides (BD Biosciences, Bedford, Mich.) was gelled by neutralization with NaOH according to the manufacturer's instructions to a final protein concentration of 2.33 mg/ml. For Borrelia adhesion, invasion, and growth assays, slide flasks (Nunc, Ruskilde, Denmark) were coated either with the described collagen lattices or with 1% agar (DIFCO Laboratories, Detroit, Mich.), 5% gelatin (DIFCO), or 1% bovine serum albumin (BSA) (Sigma).
Glycosidase treatment and decorin detection.
Acid-soluble intact collagen with and without GuHCl treatment, pepsinized collagen, and rat skin dermis, untreated or treated with GAG-cleaving glycosidases (30), were separated by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS-7.5% PAGE) (27). To remove GAG chains from decorin and other potential collagen-associated proteoglycans, we digested collagen or tissue samples with chondroitinase ABC (5 mU/ml), heparinase I (1 U/ml), and heparitinase III (1 U/ml) in 0.4 M Tris-HCl, pH 8.0-0.4 M sodium acetate-4 mM CaCl2-0.1% BSA for different intervals from 10 min to 3 h at 37°C. Chondroitinase ABC cleaves dermatan sulfate present on the decorin proteoglycan known to mediate binding between B. burgdorferi cells and collagen, as well as chondroitin-4-sulfate and chondroitin-6-sulfate potentially present on other proteoglycans (30). Heparinase I and heparitinase III cleave GAG chains of heparan sulfate proteoglycans. Proteins were detected by Coomassie blue staining of SDS-PAGE gels or were transferred to 0.45 μm-pore-size nitrocellulose membranes (Bio-Rad, Hercules, Calif.) by electroblotting for 1.5 h (Semi-Phor Hoefer Scientific Instruments, San Francisco, Calif.) for silver staining (25) or immunodetection using goat polyclonal anti-human decorin (Decorin Ab-1; Oncogene Research Products, San Diego, Calif.), alkaline phosphatase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, Pa.), and BCIP (5-bromo-4-chloro-3-indolyl phosphate p-toluidine) and p-nitroblue tetrazolium chloride (Sigma).
Adherence assays.
Strains of B. burgdorferi were grown to logarithmic phase in Barbour-Stoenner-Kelly H (BSK-H) medium (Sigma) supplemented with 6% rabbit serum (Sigma) and centrifuged at 4,000 rpm for 20 min in a Hermle centrifuge (Denville Scientific, Metuchen, Ill.) using the 22.08 VO1 rotor. The supernatant was discarded, and the pellet was washed once in PBS (pH 7.4; Sigma) and recentrifuged. The concentration of cells inoculated into each well was measured by counting bacteria in 10 dark fields using a 40× objective; the number of Borrelia cells was calculated by multiplying the mean by 106 (47). The cells were resuspended in 1 ml of PBS to a concentration of 3.0 × 107, inoculated in each slide flask, and incubated for 1 h at 34°C in an incubator (Thelco, Chicago, Ill.) over different matrices. After gentle washing with PBS (three times for 1 min each time), the attached spirochetes were visualized by acridine orange staining or by their own fluorescence (strain B31-UM) using a fluorescence microscope (Olympus BX 60). The attachment of different strains to the matrices was measured by counting the number of attached B. burgdorferi cells per field in 10 fields using a 40× objective. Averages were obtained for three different flasks per matrix and are reported as percentages of cells from the original inoculum that bound to the matrices.
Protease treatment of whole B. burgdorferi cells.
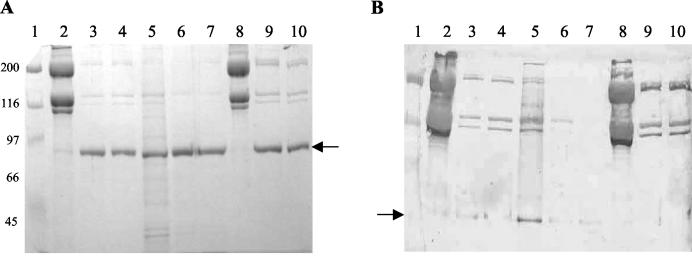
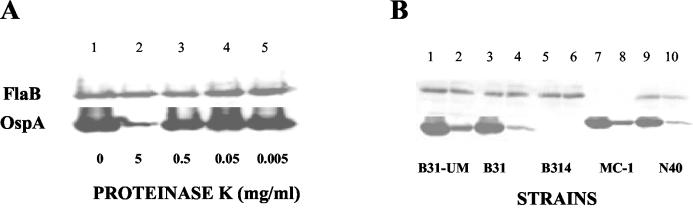
Whole B. burgdorferi cells were treated with proteinase K (Sigma) (37) by incubating 108 washed cells in PBS containing 5 mM MgCl2 (PBS-Mg)-proteinase K (final concentrations, 0 to 5 mg per ml) for 40 min at 24°C. PBS-Mg alone was added to negative control tubes. After incubation, the reaction was terminated by adding 10 μl of phenylmethylsulfonyl fluoride (50 mg/ml in isopropanol; Sigma), and the cells were washed twice with PBS-Mg, pH 7.5 (37), and resuspended in PBS for adhesion assays. The spirochetes were actively motile before and after the protease treatments. For protein determination, the pellet was resuspended in gel sample buffer (28) and boiled for 5 min. Proteins were resolved by electrophoresis and detected by silver staining on nitrocellulose membranes (25) (see Fig. 2C) or were transferred to a nitrocellulose membrane (0.45 μm pore size) for immunodetection. Primary antibodies included monoclonal anti-B. burgdorferi outer surface protein A (OspA) MAb H5332 (1) and polyclonal anti-B. burgdorferi FlaB (provided by J. D. Radolf). The blot was developed using an ECF Western blotting kit (Amersham Biosciences, Little Chalfont, United Kingdom), and signal detection was performed using a Molecular Dynamics (Sunnyvale, Calif.) Storm System 860 PhosphorImager.
FIG. 2.
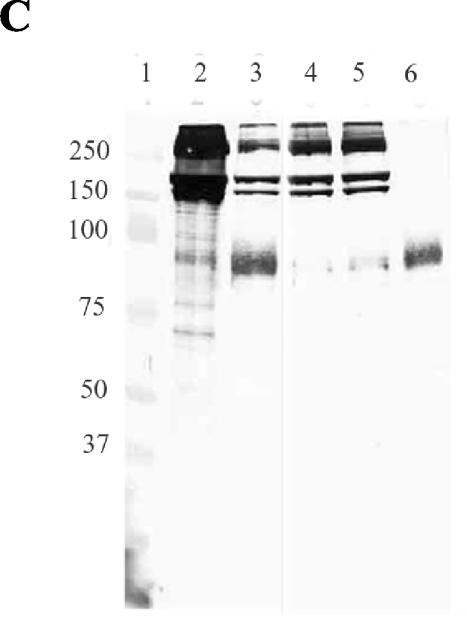
Decorin and GAGs in collagen and tissue samples. (A and B) Type I collagen samples and proteins extracted from rat dermis preparations were separated by electrophoresis in SDS-PAGE (7.5% polyacrylamide) gels. (A) Proteins were detected by Coomassie staining. Molecular mass markers (lane 1; in kilodaltons) are on the left. Lanes 2 and 8, intact collagen; lane 5, rat skin dermis; lanes 3, 4, 9, and 10, intact collagen after chondroitinase treatment (5 mU); lanes 6 and 7, rat skin dermis after chondroitinase treatment. The arrow indicates the position of chondroitinase (∼80 kDa). (B) Immunoblot using anti-decorin antibody. The lanes are as in panel A. Several immunoreactive bands between 90 and 110 kDa appear in most of the samples, but they were also present when the primary antibody was omitted (not shown). Bands that migrated at the expected position of the decorin core protein (∼45 to 47 kDa) (arrow) appeared in the undigested skin sample (lane 5), in the digested skin sample (lanes 6 and 7), and in trace amounts in the digested intact collagen prior to extraction with GuHCl (lanes 3 and 4), but not after extraction (lanes 9 and 10). (C) Type I collagen preparations were separated by gradient electrophoresis in SDS-PAGE (10 to 20% polyacrylamide) gels. Proteins were detected by silver staining after transfer onto nitrocellulose membranes (25). The molecular mass markers (lane 1) are indicated on the left. Lane 2, intact collagen after extraction with GuHCl as described in Materials and Methods; lane 3, intact collagen after enzymatic digestion with chondroitinase ABC (5 mU); lanes 4 and 5, intact collagen after assembly in the presence of chondroitinase ABC (5 mU), heparinase I (1 U), and heparitinase III (1 U); lane 6, mixture of the three enzymes alone.
Determination of viability of B. burgdorferi cells.
The viability of the Borrelia cells after different treatments and under different conditions was confirmed by observation of motility and measured in strain MC-1 by the LIVE/DEAD Bac Light Viability kit (Molecular Probes, Eugene, Oreg.).
Statistical analysis.
The range and mean (±3 standard deviations) of binding to each matrix for each B. burgdorferi strain was determined by one-way analysis of variance. The binding of different B. burgdorferi strains to the same matrices and of the same strains to different matrices were analyzed by nonparametric methods and analyzed by the Tukey-Kramer multiple-comparisons test. We considered a P value of <0.01 significant.
RESULTS
B. burgdorferi binds directly to type I collagen lattices.
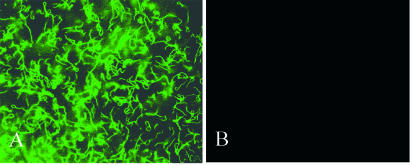
Previous studies demonstrated indirect binding of B. burgdorferi to type I collagen mediated by the proteoglycan decorin (4, 16, 20, 21) but were unable to demonstrate direct binding of the bacteria to collagen (4, 20-22). Because the earlier studies used commercially prepared, pepsin-treated type I collagen, it remained possible that matrices assembled from intact collagen (referred to as “native” lattices here) would provide an alternative binding substratum for B. burgdorferi. To test this possibility, we prepared acetic acid-soluble collagen from rat tail tendons (the same source used for the commercial preparation) but did not include a pepsin treatment step (36). Hydrated lattices containing 2.33 mg of nonpepsinized type I collagen/ml were assembled on slide flasks. Similar slide flasks were coated with 1% agar. Whereas B. burgdorferi strain B31-UM failed to attach to agar, a high percentage of the initial inoculum bound to native type I collagen lattices (Fig. 1). Binding of B. burgdorferi to hydrated lattices prepared using commercial pepsinized collagen was substantially less under the same conditions (see below). Before any relevant quantitative comparisons could be made, however, it was necessary to deal with the potential presence of decorin and other proteoglycans in the various collagen preparations.
FIG. 1.
B. burgdorferi binds type I collagen. Shown are fluorescence microscopy photographs of B. burgdorferi B31-UM binding to intact collagen before treatment with GuHCl (A) and 1% agar (B). Borrelia cells (108 per ml) were inoculated onto each matrix, incubated, and washed as described in Materials and Methods.
Assay for decorin in collagen preparations.
Because earlier work had demonstrated that certain B. burgdorferi surface proteins bind to decorin and other proteoglycans present in native collagenous matrices and some collagen extracts (4, 20, 21, 30, 39, 41), it was important to test whether the observed bacterium-collagen interactions were mediated by proteoglycans. Assays of both commercial collagen and initial preparations of intact collagen indicated trace amounts of high-molecular-weight material reactive with an anti-human decorin antibody (Fig. 2A and B). Intact collagen was subsequently prepared from rat tail tendons extracted with 0.5 M GuHCl prior to acetic acid solubilization. The presence of decorin and other proteoglycans in the various collagen preparations was assayed by SDS-PAGE on samples before and after combined treatment with chondroitinase ABC, heparinase I, and heparitinase III to remove the GAG chains of proteoglycans. A band of ∼45 to 47 kDa, the expected molecular mass of the decorin core protein (45, 50), was reactive with an anti-decorin antibody that appeared on immunoblots in enzyme-treated samples of the crude rat tail tendon and rat skin collagen preparations but not in the GuHCl-extracted collagen samples (Fig. 2B). Decorin was therefore undetectable in the purified intact collagen preparations used in subsequent assays.
Binding of B. burgdorferi to proteoglycan-depleted type I collagen lattices.
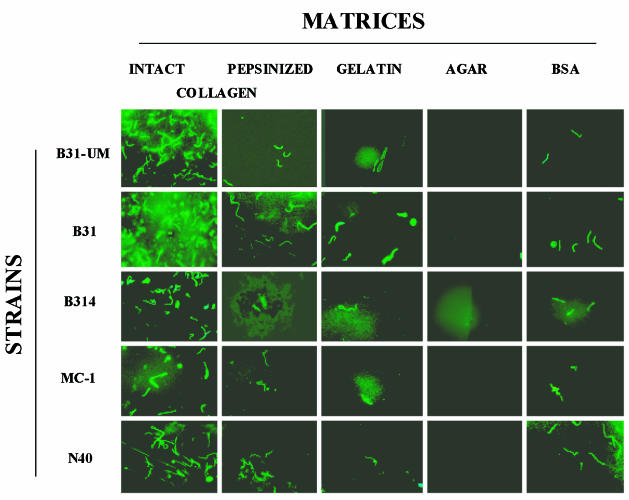
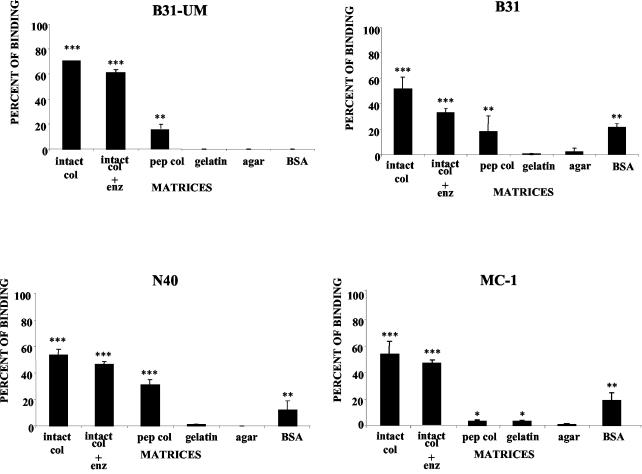
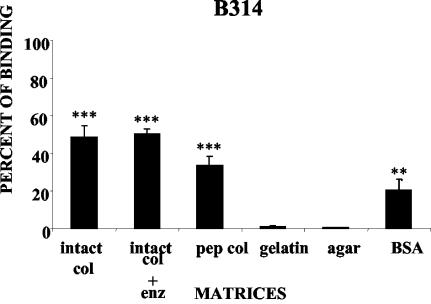
Binding assays were performed with several strains of B. burgdorferi using lattices prepared from GuHCl-extracted intact collagen and from pepsinized collagen, both at a final concentration of 2.33 mg/ml, as described above (Fig. 1). Binding assays were also performed using as substrata misassembled intact collagen (see Materials and Methods) at the same protein concentration, gelatin (5%), BSA (1%), and agar (1%). While all B. burgdorferi strains used in this study clearly adhered to the type I collagen lattices (Fig. 3 and 4), for each strain the relative magnitude of cell binding depended on the characteristics of the matrix (Fig. 4). In particular, in all cases, the number of bacteria bound to native lattices constructed from proteoglycan-depleted intact type I collagen was significantly greater than that bound to pepsinized type I collagen (Fig. 3 and 4) or to BSA (12 to 20% of the cells in the original inoculum) and gelatin, as indicated above. Less than 10% of the cells in the original inoculum were able to bind to collagen lattices fabricated with misassembled intact collagen (not shown). The differences in the binding of each B. burgdorferi strain to different matrices and of the same strain to different matrices were significant in many cases (Fig. 3 and 4). For example, binding of all B. burgdorferi strains to the intact collagen matrix was significantly higher than their binding to pepsinized collagen, gelatin, BSA, and agar matrices (P < 0.001). However, there was no significant difference between the levels of binding of B. burgdorferi strains MC-1, N40, and B314 to intact and enzymatically treated collagen matrices (P > 0.05).
FIG. 3.
Strains of B. burgdorferi display different capacities to bind to different matrices. B. burgdorferi strains B31-UM, B31, B314, MC-1, and N40 were added to the different matrices prepared as described in Materials and Methods. The inocula were all 3 × 107 per ml. The results are shown in graphic form in Fig. 4.
FIG. 4.
Percentages of B. burgdorferi strains binding to different matrices. B. burgdorferi strains B31-UM, B31, B314, MC-1, and N40 were added to the different matrices prepared as described in Materials and Methods. enz: chondroitinase ABC, heparinase I, and heparitinase III. The percentages of bound cells were determined by nonparametric methods and analyzed by the Tukey-Kramer test. The binding to each matrix was compared to binding to 1% agar, which served as a negative control. The error bars indicate standard deviations. Asterisks indicate significant differences with agar: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Previous work demonstrated that binding of B. burgdorferi to decorin required both the core protein and GAG moieties (21, 30). Although the GuHCl-extracted collagen contained no detectable decorin (Fig. 2B), we wanted to exclude the possibility that the binding of bacteria to type I collagen lattices was mediated by GAG chains of any residual proteoglycans. Extracted collagen separated by SDS-PAGE gradient gel electrophoresis exhibited high-molecular-weight heterodisperse silver-staining molecular species on nitrocellulose transfers (Fig. 2C, lane 2). When this preparation was digested with chondroitinase ABC, the staining pattern resolved into bands corresponding to collagen alone (Fig. 2C, lane 3), indicating extensive digestion of GAGs under these conditions. A similar pattern of protein bands was obtained from collagen lattices prepared by adding a mixture of chondroitinase ABC, heparinase I, and heparitinase III to the collagen mixtures during assembly (Fig. 2C, lanes 4 and 5). We found that the digestion of GAGs by glycosidases had no significant effect on the binding of most B. burgdorferi strains to type I collagen (Fig. 4).
Influence of growth phase and contact time on B. burgdorferi binding to type I collagen.
Experiments were performed to assess whether the binding of B. burgdorferi to collagen is dependent on the phase of growth of Borrelia cells or if the time of contact influences the capacity to bind to collagen. We found that B. burgdorferi strains B31-UM and N40 collected at early, mid-, and late logarithmic growth phases did not exhibit variations in their abilities to bind to type I collagen lattices. The number of B. burgdorferi cells binding to collagen increased with the time of contact; maximum binding was obtained after 1 h of incubation (data not shown).
Effect of proteinase treatment on B. burgdorferi binding to type I collagen.
To investigate whether the observed binding of B. burgdorferi to collagen depends on identifiable structures on the surfaces of B. burgdorferi cells, we treated whole B. burgdorferi cells with proteinase K (37). We made no a priori assumption about the nature of the surface component potentially involved and thus used a broad range of enzyme concentrations, up to 5 mg per ml (Fig. 5A). After treatment, strains B31, B314, B31-UM, N40, and MC-1 were analyzed by immunoblotting with a monoclonal antibody against OspA (H5332) and a polyclonal anti-FlaB antibody. Treatment with proteinase K, even at the highest enzyme concentrations, partially digested OspA without affecting FlaB (Fig. 5B). After this treatment, binding to the collagen matrix was significantly reduced, to ∼10% of control values. This suggests that cell surface proteins are involved in the attachment of B. burgdorferi cells to collagen.
FIG. 5.
Effects of proteinase K treatment of different strains of B. burgdorferi. (A) At increasing concentrations of proteinase K, there was increased digestion of OspA, while the amount of FlaB remained unchanged. (B) Total cell extracts from B. burgdorferi strains B31-UM, B31, B314, MC-1, and N40 (lanes 1, 3, 5, 7, and 9; digested with 5 mg of proteinase K/ml, lanes 2, 4, 6, 8, and 10) were separated by electrophoresis on SDS-12% polyacrylamide gels. Proteins were detected by immunoblotting with antibodies.
Role of motility in mediating binding of B. burgdorferi to type I collagen.
Because proteinase K treatment spared FlaB, it was possible that the continued collagen binding of the treated bacteria was mediated by motility-dependent invasion of the matrix. This possibility is also consistent with the fact that cells of the nonmotile strain MC-1, which lacks flagella, showed reduced binding to the matrix (Fig. 3 and 4). Examination at successive levels of focus of collagen lattices on which motile Borrelia cells were seeded demonstrated that the bacteria invaded the matrix to a depth of ∼0.085 mm within the first 60 min.
Growth of B. burgdorferi in type I collagen.
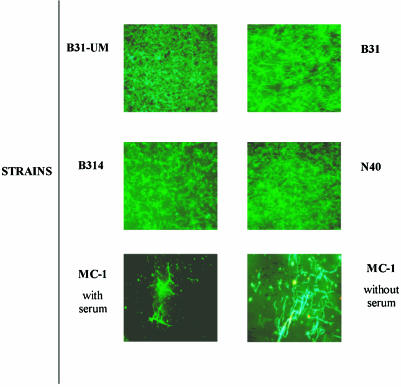
To determine whether, once attached, B. burgdorferi cells could grow on or within a type I collagen matrix, we grew the five strains of B. burgdorferi in BSK-H medium with or without rabbit serum (to rule out the presence of proteases) to a concentration of 107 cells/ml, inoculated them onto type I collagen, and observed the lattices at 3, 7, 10, and 14 days. Four of the five strains studied were actively motile; these invaded the matrix and formed microcolonies. While the nonmotile strain MC-1 cells grew less well than cells of the other strains, staining with the LIVE/DEAD Bac Light Viability kit indicated that they also were alive (Fig. 6).
FIG. 6.
Microcolonies of different strains of B. burgdorferi on matrices of intact collagen. B. burgdorferi strains were cultured on type I intact collagen matrices for as long as 14 days in the presence of BSK-H medium with and without rabbit serum. Fluorescence microscopy photographs of the five strains are shown. After 14 days, all the Borrelia strains (except MC-1) were actively motile, and MC-1 was alive, as demonstrated by staining with the LIVE/DEAD Bac Light Viability kit. Strain MC-1 grew better in medium lacking rabbit serum.
DISCUSSION
In the mammalian hosts of B. burgdorferi the bacteria colonize and proliferate within the connective tissue of their target organs, which include the skin, heart, joints, and central nervous system (7, 41, 58). The insertion of B. burgdorferi into the skin by the bite of Ixodes ticks places the bacteria in the viscoelastic ECM of the dermis (24), where their ability to bind to proteins and proteoglycans may aid their dissemination.
The time-dependent expansion of the distinctive skin lesion of Lyme disease, erythema migrans (51), could be the result of active motility of B. burgdorferi mediated by flagella, transport by currents of fluids in the connective tissue, or transport by capillary flow, all forces capable of moving the bacteria from the center of the lesion toward its periphery (7). Several reports have demonstrated that B. burgdorferi is able to bind type I collagen indirectly via small proteoglycans and other molecules present in connective tissue, such as decorin, GAGs, and fibronectin (4, 20, 21, 23, 30, 42). The same reports, however, provided evidence that B. burgdorferi does not bind directly to collagen (4, 5, 20, 21, 23, 30, 42). Since these studies were performed with collagen that had been treated with pepsin prior to commercial distribution, a treatment found to perturb its fibrillar structure (15, 18), the failure to demonstrate binding of the bacteria may have been a function of the properties of the collagen used.
The studies presented here, using intact type I collagen lattices prepared and assembled under conditions that favor native matrix properties and cell interactions (15), demonstrated direct binding of five strains of B. burgdorferi, B31, B314, B31-UM, N40, and MC-1, to such lattices despite the absence of decorin. Our use of a visual assay permitted us to detect some binding of these bacteria to pepsinized collagen similar to that used by previous investigators, although always to a much smaller extent than to the intact collagen (Fig. 3 and 4). In these cases, however, we cannot be certain that residual decorin was not responsible for the binding, since unlike our intact collagen, the commercial collagen we used was not preextracted with GuHCl.
Our results strongly suggest that the binding of B. burgdorferi to intact type I collagen is highly specific. Nonetheless, these results also indicate that there are differences in the extents of binding of each B. burgdorferi strain to different matrices. The factors responsible for these differences remain to be determined. The fact that infectious and noninfectious strains of Borrelia adhered to native collagen matrices suggests that plasmid-borne genes are probably not critically involved in this process, as lack of infectiousness has been related to plasmid loss (38).
It is possible that more extensive binding of the bacteria to matrices constructed from intact rather than commercial collagen can be attributed to the lack in the latter of pepsin-sensitive N and C telopeptides, which could potentially play a role in the interactions between B. burgdorferi cells and collagen. However, it is well known that type I collagen assembled from telopeptide-lacking molecules has a perturbed fibrillar structure (18), and the decrease in binding of bacteria to intact collagen assembled under nonphysiological conditions suggests that organizational properties of the fibrillar matrix, rather than specific cell binding properties of the telopeptides, were responsible for the binding affinity of Borrelia for intact collagen. Another factor that may have contributed to the lower level of binding to commercial collagen is the possible presence of endotoxin and collagen peptide fragments in these preparations (17, 18, 56).
Under the conditions used in this study, the binding of B. burgdorferi to collagen was not mediated by decorin or other proteoglycans or GAGs. We confirmed the lack of decorin in our collagen preparation after mild GuHCl extraction immunologically (Fig. 2B). Treating the collagen with GAG-degrading enzymes, moreover, had no effect on the capacity of Borrelia to bind to it (Fig. 3 and 4). Finally, strain B314, which does not express the decorin adhesin (16, 44), bound to intact type I collagen as efficiently as other strains (Fig. 3, 4, and 6). The fact that B. burgdorferi cells can bind to type I collagen in the absence of intact proteoglycans suggests that this interaction may be mediated by molecules different from previously characterized microbial surface component recognizing adhesive matrix molecules (MSCRAMMs) present on the bacterial surface. This strongly suggests the existence of alternative pathways of interaction between B. burgdorferi and the ECM (8, 16, 35, 40). We therefore investigated the susceptibility of this phenomenon to digestion of the surface of B. burgdorferi with proteinase K (37). We found that the capacity to bind to collagen was partially inhibited by this treatment, suggesting that there are indeed surface proteins that mediate collagen binding. The presence of putative adhesins could then affect the attachment rate. After digestion with proteinase K (5 mg/ml), 10% of the original still binds to the collagen matrices, suggesting that the majority of proteins involved in attaching B. burgdorferi cells to collagen are on the cell surface. However, this residual binding also suggests that some of these proteins may be arranged on the membrane-like protein P66, which is partially protected from the effect of proteases (6).
The notion that burrowing is involved in the B. burgdorferi-collagen interaction gains support from our observation that strain MC-1, which lacks flagella, binds less well than the strains expressing flagella and displaying normal motility (7, 33, 34), especially on prolonged incubation. This suggests that either the flagellum or the motility mediated by it is in some way associated with the phenomenon we have described. Since the flagellum is not exposed on the surface of the Borrelia cell, it is likely that motile cells burrow into the matrices and thereby increase their contact and adherence to collagen. Previous work has shown that the locomotion of B. burgdorferi increases in direct proportion to the viscoelasticity of the surrounding medium (24), and we indeed observed that the adhesion of the motile B. burgdorferi cells was accompanied by their moving deep into the matrices to a distance of several cell lengths.
Binding of B. burgdorferi to collagen occurred within 5 min. By increasing the time of exposure, we were able to increase the number of adhering bacteria, but after 1 h the differences were not significant, similar to the behavior of B. burgdorferi-binding decorin described by Guo et al. (21). Furthermore, Borrelia cells remained bound to the matrices for as long as 14 days in the presence of nutrient medium BSK-H with and without serum, indicating that Borrelia has the ability to grow within these matrices and to form microcolonies, despite the potential collagenolytic activity of B. burgdorferi (19) (Fig. 6). Clearly, under these experimental conditions, the growth of B. burgdorferi must be independent of the activation of any host-derived proteases (58). The enhanced growth potential of strain MC-1, lacking flagella, in media lacking serum was an unexpected finding that warrants further study.
While each B. burgdorferi strain used in these experiments, with the exception of the nonmotile strain MC-1, exhibited the same ability to bind to intact type I collagen, strains B314 and N40 bound more avidly to commercial type I collagen and BSA than the other strains. Interestingly, the abilities of strain B314, which lacks decorin binding adhesins, and the infectious strain N40 to bind to the different kinds of lattices are almost identical (Fig. 3 and 4). Recently, it has been found that Streptococcus pyogenes associated with rheumatic fever can aggregate collagen type IV on its surface in a process mediated by the M protein and the capsule (13), suggesting that bacteria, possibly including Borrelia, express factors other than MSCRAMMs which enable them to bind directly to collagen. Interestingly, this direct binding to collagen appears to increase the ability of S. pyogenes to infect the skin and to trigger the production of antibodies against collagen that may mediate arthritis (5, 13). Since B. burgdorferi, like S. pyogenes, is a skin-infecting and arthritogenic bacterium, it will be of interest to determine whether the binding of collagen by B. burgdorferi that we have detected plays a similar role.
In summary, we have demonstrated that B. burgdorferi strains bind matrices of type I collagen independently of the presence of decorin and other proteoglycans and of the decorin binding protein on the surfaces of B. burgdorferi cells. We have developed a simple assay to detect the interactions of B. burgdorferi with collagen matrices and generated preliminary evidence that surface receptors, in conjunction with flagellum-mediated motility, may be involved in this interaction. The interactions of B. burgdorferi cells with collagen are a potentially important factor in the ability of the bacterium to target, disseminate within, and colonize host tissues. We anticipate that further studies with this system will expand the characterization of the molecular mechanisms involved in these processes and help identify potential therapeutic modalities.
Acknowledgments
This work was supported by Public Health Service grant AI 48856 to F. C. Cabello.
We thank John Leong and Nikhat Parveen for the B314 strain, Julia Bugrysheva and Junghee J. Shin for their help with immunoblots, and Henry Godfrey for discussions. Julia Bugrysheva also provided B. burgdorferi strain B31F transformed with plasmid pCE320.
Editor: D. L. Burns
REFERENCES
- 1.Barbour, A. G., S. L. Tessier, and W. J. Todd. 1983. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect. Immun. 41:795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birk, D. E., and T. F. Linsenmayer. 1994. Collagen fibril assembly, deposition and organization into tissue-specific matrices, p. 91-128. In P. D. Yurchenco, D. E. Birk, and R. P. Mechan (ed.), Extracellular matrix assembly and structure. Academic Press, New York, N.Y.
- 3.Brightman, A. O., B. P. Rajwa, J. E. Sturgis, M. E. McCallister, J. P. Robinson, and S. L. Voytik-Harbin. 2000. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers 54:222-234. [DOI] [PubMed] [Google Scholar]
- 4.Brown, E. L., P. B. Guo, P. O'Neal, and M. Hook. 1999. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J. Biol. Chem. 274:26272-26278. [DOI] [PubMed] [Google Scholar]
- 5.Brown, E. L., R. M. Wooten, B. J. Johnson, R. V. Iozzo, A. Smith, M. C. Dolan, P. B. Guo, J. J. Weis, and M. Hook. 2001. Resistance to Lyme disease in decorin-deficient mice. J. Clin. Investig. 107:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunikis, J., and A. G. Barbour. 1999. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect. Immun. 67:2874-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charon, N. W., and S. F. Goldstein. 2002. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu. Rev. Genet. 36:47-73. [DOI] [PubMed] [Google Scholar]
- 8.Coburn, J. 2001. Adhesion mechanisms of the Lyme disease spirochete, Borrelia burgdorferi. Curr. Drug Targets Infect. Disord. 1:171-179. [DOI] [PubMed] [Google Scholar]
- 9.Coburn, J., W. Chege, L. Magoun, S. C. Bodary, and J. M. Leong. 1999. Characterization of a candidate Borrelia burgdorferi beta3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 34:926-940. [DOI] [PubMed] [Google Scholar]
- 10.Coburn, J., L. Magoun, S. C. Bodary, and J. M. Leong. 1998. Integrins α(v)β3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect. Immun. 66:1946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cugini, C., M. Medrano, T. G. Schwan, and J. Coburn. 2003. Regulation of expression of the Borrelia burgdorferi β(3)-chain integrin ligand, P66, in ticks and in culture. Infect. Immun. 71:1001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defoe, G., and J. Coburn. 2001. Delineation of Borrelia burgdorferi p66 sequences required for integrin α(IIb)β(3) recognition. Infect. Immun. 69:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinkla, K., M. Rohde, W. T. Jansen, E. L. Kaplan, G. S. Chhawal, and S. R. Talay. 2003. Rheumatic fever-associated Streptococcus pyogenes isolates aggregate collagen. J. Clin. Investig. 111:1905-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 15.Elsdale, T., and J. Bard. 1972. Collagen substrata for studies on cell behavior. J. Cell Biol. 54:626-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer, J. R., N. Parveen, L. Magoun, and J. M. Leong. 2003. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 100:7307-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forgacs, G., S. A. Newman, B. Hinner, C. W. Maier, and E. Sackmann. 2003. Assembly of collagen matrices as a phase transition revealed by structural and rheologic studies. Biophys. J. 84:1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelman, R. A., D. C. Poppke, and K. A. Piez. 1979. Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J. Biol. Chem. 254:11741-11745. [PubMed] [Google Scholar]
- 19.Grab, D. J., R. Kennedy, and M. T. Philipp. 1996. Borrelia burgdorferi possesses a collagenolytic activity. FEMS Microbiol. Lett. 144:39-45. [DOI] [PubMed] [Google Scholar]
- 20.Guo, P. B., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Hook. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 21.Guo, P. B., S. J. Norris, L. C. Rosenberg, and M. Hook. 1995. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect. Immun. 69:3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson, M. S., D. R. Cassatt, P. B. Guo, N. K. Patel, M. P. McCarthy, D. W. Dorward, and M. Hook. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 66:2143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Hook. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 24.Kimsey, R. B., and A. Spielman. 1990. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J. Infect. Dis. 162:1205-1208. [DOI] [PubMed] [Google Scholar]
- 25.Kovarik, A., K. Hlubinova, A. Vrbenska, and J. Prachar. 1987. An improved colloidal silver staining method of protein blots on nitrocellulose membranes. Folia Biol. (Prague) 33:253-257. [PubMed] [Google Scholar]
- 26.Kresse, H., and E. Schonherr. 2001. Proteoglycans of the extracellular matrix and growth control. J. Cell. Physiol. 189:266-274. [DOI] [PubMed] [Google Scholar]
- 27.Kuwaba, K., M. Kobayashi, Y. Nomura, S. Irie, and Y. Koyama. 2001. Size control of decorin dermatan sulfate during remodeling of collagen fibrils in healing skin. J. Dermatol. Sci. 29:185-194. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lannergard, J., L. Frykberg, and B. Guss. 2003. CNE, a collagen-binding protein of Streptococcus equi. FEMS Microbiol. Lett. 222:69-74. [DOI] [PubMed] [Google Scholar]
- 30.Leong, J. M., H. Wang, L. Magoun, J. A. Field, P. E. Morrissey, D. Robbins, J. B. Tatro, J. Corburn, and N. Parveen. 1998. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect. Immun. 66:994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magoun, L., W. R. Zuckert, D. Robbins, N. Parveen, K. R. Alugupalli, T. G. Schwan, A. G. Barbour, and J. M. Leong. 2000. Variable small protein (Vsp)-dependent and Vsp-independent pathways for glycosaminoglycan recognition by relapsing fever spirochaetes. Mol. Microbiol. 36:886-897. [DOI] [PubMed] [Google Scholar]
- 32.Martinez, B., J. Sillanpaa, E. Smit, T. K. Korhonen, and P. H. Pouwels. 2000. Expression of cbsA encoding the collagen-binding S-protein of Lactobacillus crispatus JCM5810 in Lactobacillus casei ATCC 393T. J. Bacteriol. 182:6857-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morisaki, H., S. Nagai, H. Ohshima, E. Ikemoto, and K. Kogure. 1999. The effect of motility and cell-surface polymers on bacterial attachment. Microbiology 145:2797-2802. [DOI] [PubMed] [Google Scholar]
- 34.Motaleb, M. A., L. Corum, J. L. Bono, A. F. Elias, P. Rosa, D. S. Samuels, and N. W. Charon. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA 97:10899-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nallapareddy, S. R., G. M. Weinstock, and B. E. Murray. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733-1743. [DOI] [PubMed] [Google Scholar]
- 36.Newman, S. A., D. A. Frenz, J. J. Tomasek, and D. D. Rabuzzi. 1985. Matrix-driven translocation of cells and nonliving particles. Science 228:885-889. [DOI] [PubMed] [Google Scholar]
- 37.Norris, S. J., C. J. Carter, J. K. Howell, and A. G. Barbour. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 60:4662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris, S. J., J. K. Howell, S. A. Garza, M. S. Ferdows, and A. G. Barbour. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parveen, N., M. J. Caimano, J. D. Radolf, and J. M. Leong. 2003. Adaptation of the Lyme disease spirochete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol. Microbiol. 47:1433-1444. [DOI] [PubMed] [Google Scholar]
- 40.Parveen, N., and J. M. Leong. 2000. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:1220-1234. [DOI] [PubMed] [Google Scholar]
- 41.Parveen, N., D. Robbins, and J. M. Leong. 1999. Strain variation in glycosaminoglycan recognition influences cell-type-specific binding by Lyme disease spirochetes. Infect. Immun. 67:1743-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Probert, W. S., J. H. Kim, M. Hook, and B. J. Johnson. 2001. Mapping the ligand-binding region of Borrelia burgdorferi fibronectin-binding protein BBK32. Infect. Immun. 69:4129-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahn, D. W. 1998. Natural history of Lyme disease, p. 35-48. In D. W. Rahn and J. Evans (ed.), Lyme disease. American College of Physicians, Philadelphia, Pa.
- 44.Sadziene, A., B. Wilske, M. S. Ferdows, and A. G. Barbour. 1993. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect. Immun. 61:2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schonherr, E., H. Hausser, L. Beavan, and H. Kresse. 1995. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J. Biol. Chem. 270:8877-8883. [DOI] [PubMed] [Google Scholar]
- 46.Shimoji, Y., Y. Ogawa, M. Osaki, H. Kabeya, S. Maruyama, T. Mikami, and T. Sekizaki. 2003. Adhesive surface proteins of Erysipelothrix rhusiopathiae bind to polystyrene, fibronectin, and type I and IV collagens. J. Bacteriol. 185:2739-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sicklinger, M., R. Wienecke, and U. Neubert. 2003. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J. Clin. Microbiol. 41:1791-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silberer, M., F. Koszik, G. Stingl, and E. Aberer. 2000. Downregulation of class II molecules on epidermal Langerhans cells in Lyme borreliosis. Br. J. Dermatol. 143:786-794. [DOI] [PubMed] [Google Scholar]
- 49.Sillanpaa, J., B. Martinez, J. Antikainen, T. Toba, N. Kalkkinen, S. Tankka, K. Lounatmaa, J. Keranen, M. Hook, B. Westerlund-Wikstrom, P. H. Pouwels, and T. K. Korhonen. 2000. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 182:6440-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stander, M., U. Naumann, L. Dumitrescu, M. Heneka, P. Loschmann, E. Gulbins, J. Dichgans, and M. Weller. 1998. Decorin gene transfer-mediated suppression of TGF-beta synthesis abrogates experimental malignant glioma growth in vivo. Gene Ther. 5:1187-1194. [DOI] [PubMed] [Google Scholar]
- 51.Steere, A. C., G. McHugh, C. Suarez, J. Hoitt, N. Damle, and V. K. Sikand. 2003. Prospective study of coinfection in patients with erythema migrans. Clin. Infect. Dis. 36:1078-1081. [DOI] [PubMed] [Google Scholar]
- 52.Svensson, L., D. Heinegard, and A. Oldberg. 1995. Decorin-binding sites for collagen type I are mainly located in leucine-rich repeats 4-5. J. Biol. Chem. 270:20712-20716. [DOI] [PubMed] [Google Scholar]
- 53.Tahir, Y. E., P. Kuusela, and M. Skurnik. 2000. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol. Microbiol. 37:192-206. [DOI] [PubMed] [Google Scholar]
- 54.Umemoto, T., M. Li, and I. Namikawa. 1997. Adherence of human oral spirochetes by collagen-binding proteins. Microbiol. Immunol. 41:917-923. [DOI] [PubMed] [Google Scholar]
- 55.Umemoto, T., and I. Namikawa. 1994. Binding of host-associated treponeme proteins to collagens and laminin: a possible mechanism of spirochetal adherence to host tissues. Microbiol. Immunol. 38:655-663. [DOI] [PubMed] [Google Scholar]
- 56.Vogel, K. G. 1994. Glycosaminoglycans and proteoglycans, p. 243-279. In P. D. Yurchenco, D. E. Birk, and R. P. Mechan (ed.), Extracellular matrix assembly and structure. Academic Press, New York, N.Y.
- 57.Vogel, K. G., M. Paulsson, and D. Heinegard. 1984. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J. 223:587-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao, Z., H. Chang, R. P. Trevino, K. Whren, J. Bhawan, and M. S. Klempner. 2003. Selective up-regulation of matrix metalloproteinase-9 expression in human erythema migrans skin lesions of acute Lyme disease. J. Infect. Dis. 188:1098-1104. [DOI] [PubMed] [Google Scholar]