Abstract
Objective
To characterize the impact of S-adenosylmethionine (SAMe) on homocysteine, and potential risk of adverse cardiovascular effects, by examining plasma levels of SAMe, S-adenosylhomocysteine (SAH), total homocysteine (tHCY), methionine (MET), and 5-methyltetrahydrofolate (5-MHTF) in 35 of 73 patients from a 6-week double-blind randomized trial of SAMe augmentation in serotonin-reuptake inhibitor partial responders with DSM-IV major depressive disorder (MDD), published in 2010.
Method
Subjects were randomized from 6/4/2004-8/8/2008 to adjunctive placebo or SAMe 800-1600 mg/day for 6 weeks. Primary outcome measures included changes in one-carbon cycle intermediates within each treatment arm (by paired t-test) and between treatment arms (by independent samples t-test). Univariate analysis of variance and Fisher’s Protected Least Significant Difference were carried out to compare post-treatment levels of each one-carbon cycle intermediate. Secondary outcome measures included associations between clinical improvement and change in plasma intermediates, examined by linear regression (for change in HAM-D) and logistic regression (for response or remission).
Results
We found significant differences in pre-treatment plasma levels of tHCY (p=0.03) between the SAMe and placebo arms. Following 6 weeks of treatment, plasma SAMe (p=0.002) and SAH (p<0.0001) levels increased significantly in the SAMe arm; no intermediates in the placebo group changed significantly. Post-treatment plasma SAMe (p=0.0035), SAH (P<0.0001), and tHCY (p=0.0016) differed significantly between the SAMe and placebo groups. No significant associations were found between plasma intermediate levels and clinical improvement, response, or remission.
Conclusion
Despite concerns about the impact that SAMe therapy may have on homocysteine, and risk of adverse cardiovascular effects, the lack of significant increase in tHCY after treatment suggests that no toxic effects from SAMe should be expected. Our findings, however, have some significant limitations and should be interpreted with caution.
Keywords: SAMe, S-adenosylmethionine, bioavailability, depression, homocysteine, methionine, methyltetrahydrofolate
Introduction
S-adenosylmethionine (SAMe) is a natural antidepressant widely used throughout Europe for decades1. Since the introduction of SAMe as a dietary supplement to the US market in the late 1990s, its popularity has increased2. The literature overall has supported SAMe’s efficacy and safety for acute treatment of major depressive disorder (MDD)1, despite the fact that some early studies were limited by the rapid degradation of an unstable SAMe formulation, which dampened treatment efficacy2. Most commercially available SAMe preparations nowadays are stable and have longer shelf-lives, though relatively few recent investigations have examined the stability and bioavailability of these newer compounds in the context of treatment studies.
Mechanisms of antidepressant action of SAMe may include methyl donation in neurotransmitter synthesis2, antioxidative effects (radical scavenging, glutathione precursor), anti-inflammatory effects, and neuroprotective effects3,4. Given that depression is increasingly considered as an inflammatory disorder with activation of immune, oxidative and nitrosative stress (IO&NS) pathways which may cause decreased neurogenesis and enhanced neurodegeneration, and that attenuation of the IO&NS pathways is considered part of the working mechanisms of various antidepressants, such as SSRIs5,6, tricyclic antidepressants7, mirtazapine8, and riluzole9, SAMe’s putative antidepressant effect appears perfectly viable.
While SAMe appears to be safe and well-tolerated, its proposed activity and metabolism which involves methylation, transulfuration, and aminopropylation1,10, has raised some concerns about safety, particularly with regard to its role in the one-carbon cycle. SAMe is a ubiquitous molecule found in every cell of the body, the synthesis of which is dependent on the dietary intake of folate and vitamin B1210. The folate form 5-methyltetrahydrofolate (5-MTHF) transfers a methyl group to homocysteine (HCY) to form methionine (MET), in a reaction that requires vitamin B12. Methionine is in turn converted to SAMe by the enzyme methionine adenosyl transferase (MAT). The primary role of SAMe is as a universal methyl group donor that participates in a wide range of methylation reactions10. The byproduct of all methylation reactions is S-adenosyl homocysteine (SAH), which is in turn converted to homocysteine (HCY). HCY may then enter the transulfuration pathway and synthesis of cystathionine or undergo re-methylation to methionine (Figure 1).
Figure 1. Interconversions between SAMe and other one-carbon cycle intermediates.
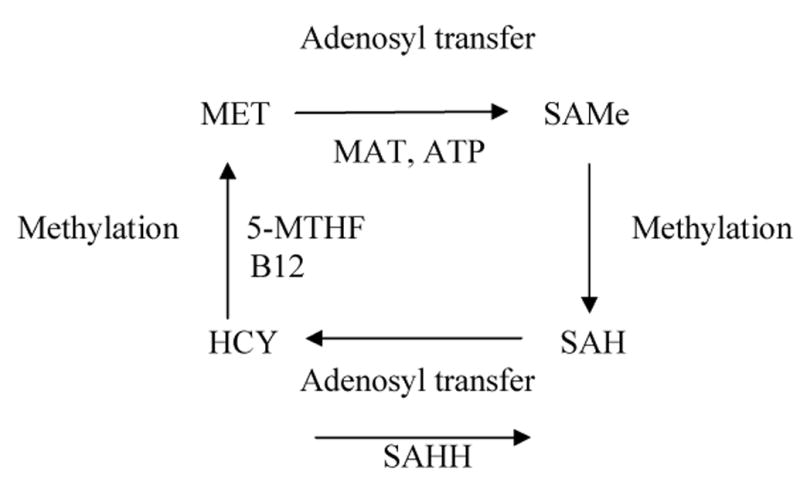
Abbreviations:
ATP = Adenosine triphosphate
B12 = Vitamin B12
HCY = Homocysteine
MET = Methionine
MAT = Methionine adenosine transferase
5-MTHF = 5-Methyltetrahydrofolate
SAH = S-adenosylhomocysteine
SAMe = S-adenosylmethionine
SAHH = S-adenosylhomocysteine hydrolase
Methionine levels are closely related to homocysteine metabolism10. High intake of methionine can increase plasma tHCY levels substantially as a result of increased metabolism through the methylation cycle. Methionine loading can be used to stress the methylation and transulfuration pathways and thereby detect mild disturbances in enzyme activity related to these pathways. Under these conditions a high oral dose of methionine is administered and increase and decline in plasma tHCY is monitored hourly over a 4-hour period11. By analogy, some investigators have expressed concern that the administration of supraphysiologic doses of SAMe could result in increased levels of plasma tHCY12. Increased plasma tHCY has been shown to be a risk factor for cardiovascular and cerebrovascular disease, a condition to which depressed populations may be especially vulnerable13.
Relatively few clinical trials have examined the impact of SAMe on total plasma homocysteine in depressed populations14, though our group found a modest decrease in homocysteine in subjects taking SSRIs or SNRIs in combination with SAMe15. Nonetheless, it is generally advised that physicians monitor homocysteine levels in certain patients receiving SAMe, particularly if they have a personal or family history of heart disease, or who have normally elevated levels of homocysteine13. Continued investigation into the effect of SAMe on homocysteine and other one-carbon-cycle intermediates could better clarify safety concerns associated with use of SAMe in depressed populations.
We recently completed a double blind, randomized controlled clinical trial of SAMe augmentation in antidepressant partial responders (Study NCT00093847). Our results suggested efficacy and safety16. In this report, we present our analysis of plasma levels of SAMe before and after treatment, in order to assess the absorption of this particular SAMe preparation. We also examined whether there were any significant changes in the levels of other one-carbon cycle components, specifically S-adenosyl homocysteine, total homocysteine, methionine, and 5-MTHF. It was hoped that the findings would provide insight on the overall biochemical impact of SAMe administration, and to clarify potential safety concerns for SAMe users. Likewise, we were interested in learning whether there were any associations between plasma levels of SAMe and other one-carbon cycle intermediates and clinical improvement. Based on the findings from Goren et al14 we predicted that patients who received SAMe would experience significant changes in blood levels of SAMe, and speculated that greater changes in SAMe blood levels would be associated with a more robust clinical improvement.
Method
Methods for the parent clinical trial are reported in Papakostas et al16. Briefly, 73 serotonin-reuptake inhibitor (including SSRIs and SNRIs) non-responders with MDD who signed a consent form approved by our institutional review board (IRB) were enrolled from 6/4/2004-8/8/2008 in a 6-week, double-blind, randomized trial of adjunctive oral SAMe, at a starting dose of 800mg daily and final target dose of 1600mg daily (divided on a twice daily regimen). The 400 mg tableted form of SAMe was provided by Pharmavite LLC, Northridge, CA. Patients continued on their antidepressant at a stable dose throughout the 6-week trial. Other concomitant psychotropic drugs such as benzodiazepines, non-SRI antidepressants, mood stabilizers, and antipsychotics were not allowed. Patients with unstable medical illness, including cardiovascular disease, were excluded. The primary outcome measure for the study was response rates according to the 17-item Hamilton Depression Rating Scale (HAM-D-17)17.
Participants in the study were asked to provide a blood sample to be analyzed for SAMe, SAH, tHCY, MET, and 5-MHTF, prior to randomization and at the completion of the study. Blood samples were processed and analyzed as follows:
Plasma was separated from blood within 30 minutes and stored at -80oC until time of analysis. Plasma levels of SAM, and SAH were measured by a modification of the stable-isotope dilution liquid chromatography-electrospray injection tandem mass spectrometry (LC-ESI-MS/MS) previously described18. Total plasma HCY and methionine were determined by LC-ESI-MS/MS as previously described19. 5-MTHF in plasma was determined by LC-ESI-MS/MS by diluting sample 5:1 (v/v) with a solution of dithiothriotol, ascorbic acid and 13C55-MTHF (Shircks, Switzerland). Sample was deproteinized with acetonitrile and supernatant was extracted and analyzed by LC-ESI-MS/MS on a 4000 QTRAP as previously described with some modification20.
Comparisons in mean levels of one-carbon cycle intermediates between the treatment and placebo group for pre- and post-treatment samples were carried out using the 2-sample t-test. To further characterize the impact of SAMe on one-carbon cycle components, we carried out a univariate analysis of variance for each post-treatment one-carbon intermediate as the dependent variable(s), assigned treatment, baseline levels of the intermediate as fixed factors, and the interaction between the two was examined as well. Fisher’s Protected Least Significant Difference (PLSD) was used to compare post-treatment levels of each one-carbon cycle intermediate, after adjusting for respective pre-treatment levels.
To examine whether clinical improvement was associated with change in plasma levels of the one-carbon intermediates in patients receiving SAMe, we carried out multiple linear regression using the final HAM-D-17 score as the dependent variable, and the baseline HAM-D-17 score, and pre-and post-treatment levels of the one-carbon intermediates as independent variables. Logistic regression was performed to examine any association between response or remission (dependent variables) and pre-and post-treatment levels of the one-carbon intermediates (independent variables).
All calculations were performed with Statview software (Cary, North Carolina). All significance levels were set at p<0.05.
Results
Data for both pre- and post-treatment levels of the one-carbon cycle intermediates were available for 35 of the original 73 patients from the parent study. Some patients had declined to have the additional blood draws, or were lost to follow-up, thus having no post-treatment data available. Some blood samples were damaged during storage and could not be analyzed. The 35 patients consisted of 20 from the SAMe arm (mean age 55±13 years, 50% female) and 15 from the Placebo arm (mean age 47±11, 87% female). The differences in age were not significant (p=0.06). In the SAMe arm, 80% of patients were on SSRIs and 20% were on SNRIs; in the placebo arm, 80% of patients were on SSRIs and 20% were on SNRIs. Full distribution of antidepressants is reported in Papakostas et al16.
Given that the intermediates are interdependent, Bonferroni correction for multiple comparisons was not used in the analysis. Prior to randomization, there were no significant differences in plasma levels of SAMe, SAH, MET, and 5-MHTF between patients who were randomized to SAMe or to placebo, but SAMe patients had significantly greater tHCY levels compared to placebo patients (p=0.03) (Table 1).
Table 1.
Pre-and Post-Treatment Plasma Levels of SAMe and other One-Carbon-Cycle Intermediates
| Pre-Treatment | Post-Treatment | Changes in treatment arms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAMe (n=20) | Placebo (n=15) | Significance* (SAMe vs PBO) | SAMe (n=20) | Placebo (n=15) | Signif** (SAMe vs PBO) | Signif+ (SAMe arm) | Signif++ (PBO arm) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| SAMe (nmol/L) | 82.9 | 22.44 | 71.24 | 12.44 | t=1.81, p=0.08 | 503.77 | 535.76 | 81.27 | 31.52 | p=0.0035 | t=-3.5, p=0.002 | t=-1.1, p=0.29 |
| SAH (nmol/L) | 35.7 | 6.74 | 30.87 | 10.56 | t=1.65, p=0.11 | 56.1 | 18.25 | 30.14 | 8.25 | p<0.0001 | t=-4.9, p<0.0001 | t=0.2, p=0.81 |
| tHCY (umol/L) | 9.78 | 2.27 | 8.04 | 2.31 | t=2.22, p=0.03 | 10.57 | 3.64 | 7.97 | 1.9 | p=0.0016 | t=-1.4, p=0.19 | t=0.18, p=0.86 |
| MET (umol/L) | 13.28 | 10.07 | 11.13 | 7.65 | t=0.69, p=0.49 | 12.34 | 6.35 | 11.07 | 5.73 | p=0.51 | t=0.43, p=0.68 | t=0.04, p=0.97 |
| 5-MTHF (nmol/L) | 34.59 | 18.19 | 26.53 | 10.06 | t=1.54, p=0.13 | 50.68 | 83.04 | 30.28 | 13.79 | p=0.19 | t=-1.0, p=0.32 | t=-0.92, p=0.37 |
Based on two-sample t-test for SAMe versus Placebo.
Based on Fisher’s Protected Least Significant Difference (PLSD after adjusting for pre-treatment levels) for SAMe versus Placebo.
Paired t-test for pre and post treatment levels of intermediates for SAMe arm.
Paired t-test for pre and post treatment levels of intermediates for Placebo arm.
Outcomes analysis focused on both the within-treatment (SAMe group before and after treatment, and placebo group before and after treatment) change in the one-carbon cycle intermediates, as well as the between-treatment change (for SAMe versus placebo groups). Following 6 weeks of treatment, plasma SAMe (p=0.002) and SAH (p<0.0001) levels increased significantly in subjects randomized to the SAMe arm, but there were no significant changes in levels of tHCY, MET, and 5-MTHF (Table 1). In the placebo arm, there were no significant changes in any of the one-carbon cycle intermediates (Table 1).
To compare outcomes in patients receiving SAMe with those receiving placebo, we used Fisher’s Protected Least Significant Difference (PLSD) after adjusting for baseline plasma levels of each intermediate. We found significant differences in plasma levels of SAMe (p=0.0035), SAH (P<0.0001), and tHCY (p=0.0016) between the two treatment arms. Total plasma methionine (MET), and 5-MTHF levels were not significantly different between SAMe and placebo groups following treatment (Table 1).
When adjusting for baseline plasma levels of each intermediate, univariate ANOVA found a significant effect for baseline tHCY on post-treatment tHCY (p<0.0001), and a significant effect of baseline MET on post-treatment MET (p=0.008) (Table 2). Regarding post-treatment MTHF, significant effects were found for assigned treatment (p=0.03), pre-treatment MTHF (p=0.01), and the interaction between assigned treatment and pre-treatment MTHF (p=0.02) (Table 2).
Table 2.
Univariate ANOVA Examining Impact of Baseline Levels of One-Carbon Cycle Intermediates on Post-Treatment Levels
| Intermediate | df | F | p | Power |
|---|---|---|---|---|
| SAMe post | ||||
| Treatment | 1 | 0.06 | 0.81 | 0.06 |
| SAMe Pre | 1 | 0.47 | 0.5 | 0.1 |
| Treatment X SAMe pre | 1 | 0.58 | 0.45 | 0.11 |
| SAH post | ||||
| Treatment | 1 | 0.57 | 0.46 | 0.11 |
| SAH Pre | 1 | 0.8 | 0.38 | 0.13 |
| Treatment X SAH pre | 1 | 0.13 | 0.72 | 0.06 |
| tHCY post | ||||
| Treatment | 1 | 1.58 | 0.22 | 0.22 |
| tHCY Pre | 1 | 25.39 | <0.0001 | 1 |
| Treatment X tHCY pre | 1 | 2.75 | 0.11 | 0.35 |
| MET post | ||||
| Treatment | 1 | 1.09 | 0.3 | 0.17 |
| MET Pre | 1 | 8.11 | 0.008 | 0.8 |
| Treatment X MET pre | 1 | 1.07 | 0.31 | 0.16 |
| 5-MTHF post | ||||
| Treatment | 1 | 5.03 | 0.03 | 0.58 |
| 5-MTHF Pre | 1 | 7.29 | 0.01 | 0.75 |
| Treatment X 5-MTHF pre | 1 | 5.7 | 0.02 | 0.64 |
Baseline HAM-D-17 scores were 18.95±2.61 for the SAMe group, and 19.80±2.43 for the placebo group (p=0.33). In subjects receiving SAMe (n=20), multiple regression showed no significant association between change in HAM-D-17 score and plasma levels of any of the one-carbon cycle intermediates following treatment. Logistic regression analysis showed no significant association between response or remission and plasma levels of any of the one-carbon intermediates.
Discussion
The enteric-coated SAMe formulation used in this study was absorbed, as indicated by an approximate 6-fold increase in plasma levels of SAMe compared to pre-treatment values, representing a level much greater than our (per author TB’s lab) observed normal reference range of 22–131 nmols/L in 62 subjects aged 56–82 years. This also suggests good compliance with study treatment, which renders the positive findings of the parent study16 even more convincing.
The metabolic links between SAMe and homocysteine have raised the question about the possibility that SAMe could result in increased plasma tHCY levels, a risk factor for occlusive vascular disorders. Thus far, no evidence has been found to suggest that HCY levels rise in patients receiving oral SAMe.
Our subjects who received SAMe had a statistically significant increase in plasma levels of SAH, and this was significantly greater than in placebo patients, suggesting that SAMe was metabolized through methylation-dependent pathways leading to increased levels of SAH, which were somewhat higher than our observed reference range of 8-43 nmol/L in 62 subjects aged 56-82 years. Patients in the SAMe arm had higher pre-treatment levels of tHCY, SAMe, and SAH than patients randomized to placebo, but only total HCY was significantly greater, and this difference became more strongly significant at the end of the treatment period. The significant difference in tHCY at baseline may represent an idiosyncrasy of a small sample. In any case, the change in tHCY in SAMe patients after 6 weeks was modest and not significant. There were no significant pre- or post-treatment differences between SAMe and placebo patients in total methionine or 5-MTHF, the latter of which remained in the normal range of 8-75nmol/L21.
One might expect that an elevated level of SAH would result in a robust elevation in tHCY and/or MET, yet this was not the case in our sample. Homocysteine remained within our observed normal range of 2-14 umol/L and below 15uM, which is generally considered the upper limit of safety. MET remained below the normal mean level of 35 uM22. There are at least two possible explanations for this observation: 1) tHCY may initially elevate as a result of conversion of SAH via SAH Hydrolase, but could in turn be rapidly converted to MET via methionine synthase and in turn converted back to SAMe via MAT. 2) tHCY could be rapidly converted to cystathione via transulfuration, thus leaving the rest of the cycle unaffected.
Our findings suggest that humans may have a capacity to self-regulate the one-carbon cycle so as to prevent excessive levels of potentially harmful intermediates from building up. If this is so, then SAMe administration would therefore be very safe from the standpoint of pathological homocysteine elevation. While SAH is generally considered toxic23 it is not clear what impact it may have on mood per se. One particular concern might be about subjects who have elevated plasma tHCY before treatment due to folate and or B12 deficiency. Such individuals may be unable to metabolize HCY and this may be exacerbated after SAMe treatment. Since we did not examine B12 levels in our sample, it is unclear why the SAMe arm had a baseline level of tHCY significantly greater than the placebo arm. However, neither treatment arm experienced a significant change in tHCY levels by the conclusion of the study, suggesting that the SAMe group was not at a metabolic disadvantage compared to the placebo group. Likewise, our examination of pre-treatment levels of the intermediates as predictors of response did not suggest that they impacted clinical improvement differently between the two treatment arms. These issues merit further investigation.
Although we found no significant association between clinical improvement and changes in plasma levels of SAMe or the other intermediates, we did not control the timing of blood collection after SAMe dosing. The absorption of SAMe can vary between individuals and may peak from 2-5 hours, and the decay curve can also vary24.The interval of time between the last SAMe dose and blood collection is therefore a factor that determines blood SAMe concentration. In addition, we do not know how plasma levels of SAMe and related intermediates correlate with those found at the level of the brain, where it presumably exerts its psychotropic effects. This makes it difficult to extrapolate between plasma levels and treatment response. It should be noted, however, that not all antidepressants have a linear dose-response curve25, and this may reflect a limited association between plasma levels and efficacy. This finding should therefore not cast doubt on the antidepressant efficacy of SAMe. Future investigations would benefit from collection of blood samples for analyses of SAMe and its intermediates at several interim time points between randomization and completion of the study in order to evaluate the course or any fluctuations in plasma levels.
Even without this limitation, the sample may have been too small to detect any changes, or there may be a ceiling effect, given that patients received high doses (1600mg/day) of SAMe, which could result in saturation of the relevant pathways and receptors. Given that there is no consensus on a maximum dose of SAMe, and that our clinical practice experience has shown that many patients require doses in the range of 2000-3000 mg/day, further dose-finding studies may be called for, in which plasma levels of SAMe and related intermediates could be examined for associations with dose and response.
Our study is limited by the relatively small patient sample, and by the short term (6 weeks) of the trial, which does not allow us to determine whether longer-term treatment would have resulted in different levels of the one-carbon intermediates. As discussed in the Results section, we obtained pre- and post-treatment blood samples for only 35 of the 55 subjects who completed the study. We compared demographic and clinical characteristics in the 35 sampled patients against the 38 nonsampled patients, and found no significant differences in age, gender, age of depressive onset, number of lifetime depressive episodes, duration of current depressive episode, and baseline severity of depression (P>0.05 for all comparisons; data not shown), suggesting that the sampled patients are a reasonable representation of the parent study sample.
We do not know whether patients were taking B-vitamin supplements such as vitamin B12 or folate, and we did not measure plasma B12 in any of our patients; supplementation could conceivably have had an impact on the efficacy findings of the main outcome paper, since these vitamins may facilitate the metabolism of homocysteine in some cases of hyperhomocysteinemia12, and the addition of folate and vitamin B12 supplements for patients taking SAMe has been recommended12.
Another limitation in our study is that patients were allowed only SSRIs and SNRIs, so the findings may not be generalizable to patients who are taking other antidepressants. Finally, our study did not have a comparison arm of non-depressed patients, who could in theory have had different degrees of SAMe-related change in the one-carbon intermediates compared to their depressed counterparts. Given what we have learned from the myriad studies that have identified putative biomarkers of depression26,27, it is reasonable to suggest that one-carbon cycle-related differences may exist between depressed individuals and healthy controls.
Our results support Alpert et al’s12 recommendation that, at least in the short-term, it should not be necessary to monitor homocysteine levels in patients taking SAMe, unless they have a significant personal history or family history of cardiovascular illness, or who are already known to have significantly elevated homocysteine levels12. In the absence of more data about the impact of elevated levels of SAH, it is not clear whether monitoring SAH in individuals receiving SAMe would be desirable. Further investigation is necessary to better clarify the role of SAH in the treatment of depression, and its relationship with SAMe.
Clinical Points.
Clinicians are concerned about the risk of homocysteine elevation and potential cardiac risk from administration of S-adenosyl methionine (SAMe)
In depressed patients who received SAMe augmentation, we found no significant elevation of plasma levels of total homocysteine
SAMe appears to be safe to administer to patients with depression
Acknowledgments
The parent study was funded by a National Institute of Mental Health grant 5-K23 MH-069629 (GP) and NIH/NCCAM grant AT002311-01 (TB)
SAMe and matching placebo pills were provided free of cost by Pharmavite LLC (Mission Hills, CA).
Financial Disclosures
Dr. Mischoulon has received research support for other clinical trials from Amarin (Laxdale), Bristol-Myers Squibb, Cederroth, Lichtwer Pharma GmbH, Nordic Naturals, Ganeden, Swiss Medica, and Fisher-Wallace; he has received consulting and writing honoraria from Pamlab; he has received speaking honoraria from Bristol-Myers Squibb, Nordic Naturals, Pfizer, Pamlab, and Virbac as well as from Reed Medical Education (a company working as a logistics collaborator for the Massachusetts General Hospital Psychiatry Academy); he has received royalty income from Back Bay Scientific for PMS Escape, and from Lippincott Williams & Wilkins for the book, “Natural Medications for Psychiatric Disorders: Considering the Alternatives” (Editors: David Mischoulon and Jerrold F Rosenbaum).
Dr. Alpert has received research support from: Abbott Laboratories, Alkermes, Lichtwer Pharma GmbH, Lorex Pharmaceuticals; Aspect Medical Systems, Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Cyberonics, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., and Wyeth-Ayerst Laboratories. He has participated on advisory boards for or consulted to: Eli Lilly & Company, Pamlab LLC, and Pharmavite LLC. He has participated on speakers’ honoraria from: Eli Lilly & Company, Xian-Janssen, Organon, MGH Psychiatry Academy/Reed Medical Education, MGH Psychiatry Academy/Primedia, and the American Psychiatric Association and has received editorial fees from Belvoir Publishing.
Dr. Arning reports no relevant conflicts of interest.
Dr Bottiglieri reports having been the Chairman of the Advisory Board for Methylation Sciences Inc; holding stock options in Methylation Sciences Inc; and having received grant/research funding from PamLab LLC, distributor of B vitamins as a medical food.
Dr. Fava has received research support from Abbott Laboratories, Alkermes, Aspect Medical Systems, AstraZeneca, Bio Research, BrainCells, Bristol-Myers Squibb, Cephalon, Clinical Trial Solutions, Eli Lilly, Forest Pharmaceuticals, Ganeden Biotech, GlaxoSmithKline, J and J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, NARSAD, the National Center for Complementary and Alternative Medicine, the National Institute on Drug and Alcohol Abuse, the National Institute of Mental Health, Novartis, Organon Inc., Pamlab, Pfizer, Pharmavite, Roche, Sanofi-Aventis, Shire, Solvay Pharmaceuticals, Synthelabo, and Wyeth-Ayerst Laboratories; he has served on the advisory boards of or as a consultant to Abbott Laboratories, Amarin, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer AG, Best Practice Project Management, BioMarin Pharmaceuticals, Biovail Pharmaceuticals, BrainCells, Bristol-Myers Squibb, Cephalon, Clinical Trials Solutions, CNS Response, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eli Lilly, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, GlaxoSmithKline, Grunenthal GmbH Janssen Pharmaceutica, Jazz Pharmaceuticals, J and J Pharmaceuticals, Knoll Pharmaceuticals, Labopharm, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, Methylation Sciences, Neuronetics, Novartis, Nutrition 21, Organon, Pamlab, Pfizer, PharmaStar, Pharmavite, Precision Human Biolaboratory, PsychoGenics, Roche, Sanofi-Aventis, Sepracor, Schering-Plough, Solvay Pharmaceuticals, Somaxon, Somerset Pharmaceuticals, Synthelabo, Takeda, Tetragenex, TransForm Pharmaceuticals, Transcept Pharmaceuticals, Vanda Pharmaceuticals, and Wyeth-Ayerst Laboratories; he has received speaker and publishing fees from Advanced Meeting Partners, the American Psychiatric Association, AstraZeneca, Belvoir, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, Imedex, Novartis, Organon, Pfizer, PharmaStar, Massachusetts General Hospital Psychiatry Academy/Primedia, Massachusetts General Hospital Psychiatry Academy/Reed-Elsevier, UBC Pharma, and Wyeth-Ayerst Laboratories; he is a shareholder with Compellis; he has patent applications for sequential parallel comparison of design and for a combination of azapirones and bupropion in major depressive disorder; and he receives copyright royalties for the following Massachusetts General Hospital assessment tools: the Cognitive and Physical Functioning Questionnaire, the Sexual Functioning Inventory, the Antidepressant Treatment Response Questionnaire, the Discontinuation-Emergent Sign and Symptom scale, and SAFER.
Dr. Papakostas has served as a consultant for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Evotec AG, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab, Pfizer, Pierre Fabre Laboratories, Shire Pharmaceuticals, and Wyeth; has received honoraria from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Evotec AG, GlaxoSmithKline, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Lundbeck, Otsuka Pharmaceuticals, Pamlab, Pfizer, Pierre Fabre Laboratories, Shire Pharmaceuticals, Titan Pharmaceuticals, and Wyeth; he has received research support from Bristol-Myers Squibb, Forest Pharmaceuticals, the National Institute of Mental Health, Pamlab, Pfizer, and Ridge Diagnostics (formerly known as Precision Human Biolaboratories); and he has served on the speaker’s bureau for Bristol-Myers Squibb and Pfizer.
Footnotes
ClinicalTrials.gov registry number: NCT00093847
Parent study grant number: 5-K23 MH-069629
Previous Presentation
None
References
- 1.Papakostas GI. Evidence for S-adenosyl-L-methionine (SAM-e) for the treatment of major depressive disorder. J Clin Psychiatry. 2002;70(Suppl 5):18–22. doi: 10.4088/JCP.8157su1c.04. [DOI] [PubMed] [Google Scholar]
- 2.Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76:1158S–1161S. doi: 10.1093/ajcn/76/5.1158S. [DOI] [PubMed] [Google Scholar]
- 3.Lieber CS. S-adenosyl-L-methionine: its role in the treatment of liver disorders. Am J Clin Nutr. 2002;76:1183S–7S. doi: 10.1093/ajcn/76/5.1183S. [DOI] [PubMed] [Google Scholar]
- 4.Suchy J, Lee S, Ahmed A, Shea TB. Dietary supplementation with S-adenosyl methionine delays the onset of motor neuron pathology in a murine model of amyotrophic lateral sclerosis. Neuromolecular Med. 2010;12:86–97. doi: 10.1007/s12017-009-8089-7. [DOI] [PubMed] [Google Scholar]
- 5.Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:676–92. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson's disease. J Immunol. 2010;185:1230–1237. doi: 10.4049/jimmunol.1000208. [DOI] [PubMed] [Google Scholar]
- 7.Vismari L, Alves GJ, Palermo-Neto J. Amitriptyline and acute inflammation: a study using intravital microscopy and the carrageenan-induced paw edema model. Pharmacology. 2010;86:231–239. doi: 10.1159/000317064. [DOI] [PubMed] [Google Scholar]
- 8.Tulner DM, Smith OR, Schins A, de Jonge P, Quere M, Delanghe JR, Crijns HJ, den Boer JA, Korf J, Honig A. Antidepressive effect of mirtazapine in post-myocardial infarction depression is associated with soluble TNF-R1 increase: data from the MIND-IT. Neuropsychobiology. 2011;63:169–176. doi: 10.1159/000321624. [DOI] [PubMed] [Google Scholar]
- 9.Leonard BE. The concept of depression as a dysfunction of the immune system. Curr Immunol Rev. 2010;6:205–212. doi: 10.2174/157339510791823835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottiglieri T. S-Adenosyl-L-methionine (SAMe): from the bench to the bedside--molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–1157S. doi: 10.1093/ajcn/76/5.1151S. [DOI] [PubMed] [Google Scholar]
- 11.Still RA, McDowell IF. Clinical implications of plasma homocysteine measurement in cardiovascular disease. J Clin Pathol. 1998;51:183–188. doi: 10.1136/jcp.51.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alpert JE, Papakostas GI, Mischoulon D. Natural Medications for Psychiatric Disorders: Considering the Alternatives, 2nd edition. Lippincott Williams & Wilkins; Philadelphia: 2008. One-carbon metabolism and the treatment of depression: roles of s-adenosyl-l-methionine and folate. In: Mischoulon D, Rosenbaum J (eds) pp. 68–83. [Google Scholar]
- 13.Frasure-Smith N, Lespérance F, Habra M, Talajic M, Khairy P, Dorian P, et al. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–140. doi: 10.1161/CIRCULATIONAHA.109.851675. 133p following 140. [DOI] [PubMed] [Google Scholar]
- 14.Gören JL, Stoll AL, Damico KE, Sarmiento IA, Cohen BM. Bioavailability and lack of toxicity of S-adenosyl-L-methionine (SAMe) in humans. Pharmacotherapy. 200424:1501–1507. doi: 10.1592/phco.24.16.1501.50943. [DOI] [PubMed] [Google Scholar]
- 15.Alpert JE, Papakostas G, Mischoulon D, Worthington JJ, 3rd, Petersen T, Mahal Y, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 200424:661–664. doi: 10.1097/01.jcp.0000145339.45794.cd. [DOI] [PubMed] [Google Scholar]
- 16.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl Methionine (SAMe) Augmentation of Serotonin Reuptake Inhibitors (SRIs) for SRI- Non-responders with Major Depressive Disorder: A Double-blind, Randomized Clinical Trial. American J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Struys EA, Jansen EE, de Meer K, Jakobs C. Determination of S-adenosylmethionine and S-adenosylhomocysteine in plasma and cerebrospinal fluid by stable-isotope dilution tandem mass spectrometry. Clin Chem. 2000;46:1650–1656. [PubMed] [Google Scholar]
- 19.Ducros V, Belva-Besnet H, Casetta B, Favier A. A robust liquid chromatography tandem mass spectrometry method for total plasma homocysteine determination in clinical practice. Clin Chem Lab Med. 2006;44:987–989. doi: 10.1515/CCLM.2006.178. [DOI] [PubMed] [Google Scholar]
- 20.Nelson BC, Pfeiffer CM, Margolis SA, Nelson CP. Solid-phase extraction-electrospray ionization mass spectrometry for the quantification of folate in human plasma or serum. Anal Biochem. 2004;325:41–51. doi: 10.1016/j.ab.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Loehrer FM, Angst CP, Haefeli WE, Jordan PP, Ritz R, Fowler B. Low whole-blood S-adenosylmethionine and correlation between 5- methyltetrahydrofolate and homocysteine in coronary artery disease. Arterioscler Thromb Vasc Biol. 1996;16:727–733. doi: 10.1161/01.atv.16.6.727. [DOI] [PubMed] [Google Scholar]
- 22.Stegink LD, Filer LJ, Jr, Baker GL. Plasma methionine levels in normal adult subjects after oral loading with L-methionine and N-acetyl-L-methionine. J Nutr. 1980;110:42–49. doi: 10.1093/jn/110.1.42. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy BP, Bottiglieri T, Arning E, Ziegler MG, Hansen LA, Masliah E. Elevated S-adenosylhomocysteine in Alzheimer brain: influence on methyltransferases and cognitive function. J Neural Transm. 2004;111:547–567. doi: 10.1007/s00702-003-0096-5. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, He Y, Du YX, Tang LL, Wang GJ, Fawcett JP. Pharmacokinetic properties of S-adenosylmethionine after oral and intravenous administration of its tosylate disulfate salt: a multiple-dose, open-label, parallel-group study in healthy Chinese volunteers. Clin Ther. 2009;31:311–320. doi: 10.1016/j.clinthera.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Solvason HB, DeBattista C. Antidepressant Dosing for the Acute Treatment of Unipolar Depression. Primary Psychiatry. 2009;16:30–36. [Google Scholar]
- 26.Leuchter AF, Cook IA, Hamilton SP, Narr KL, Toga A, Hunter AM, Faull K, Whitelegge J, Andrews AM, Loo J, Way B, Nelson SF, Horvath S, Lebowitz BD. Biomarkers to predict antidepressant response. Curr Psychiatry Rep. 2010;12:553–562. doi: 10.1007/s11920-010-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartova L, Berger A, Pezawas L. Is there a personalized medicine for mood disorders? Eur Arch Psychiatry Clin Neurosci. 2010;260(Suppl 2):S121–126. doi: 10.1007/s00406-010-0152-8. [DOI] [PubMed] [Google Scholar]


