Abstract
Fondaparinux is an antithrombin-dependent factor Xa inhibitor that is used for thromboprophylaxis of patients undergoing hip fracture surgery, hip or knee replacement, or abdominal surgery. It is cleared by the kidney and should be used with caution in patients with renal impairment and avoided in patients with severe renal insufficiency. Recently, several studies have demonstrated that a lower dose of fondaparinux in patients with moderate renal impairment appears to be safe and effective. The purpose of this study was to obtain pharmacokinetic and clinical data on the use of prophylactic fondaparinux inpatients with renal insufficiency undergoing major abdominal surgery for cancer (n=8) or orthopedic surgery (n=1). Anti-factor Xa levels were obtained, and a published population pharmacokinetic model for fondaparinux was fit to the data. The data were analyzed using NONMEM software. Fondaparinux did not appear to accumulate in these patients, even when the drug was administered for up to twelve days. Pharmacokinetic analysis revealed thatthe apparent clearance in this population, who were primarily undergoing cancer surgery, was similar to prior studies in orthopedic surgery patients. In contrast, lower estimates were obtained for volume of distribution and absorption rate constant parameters.None of the patients sustained a hemorrhagic complication attributable to fondaparinux. One patient developed hypoxia in the setting of transient atrial fibrillation and clinical suspicion for pulmonary embolism, but this was not confirmed radiographically. These results support the use of 1.5 mg of fondaparinux every 24 hours for thromboprophylaxis in patients with renal insufficiency undergoing high-risk surgical procedures.
Keywords: thromboprophylaxis, renal failure, fondaparinux
Fondaparinux is a synthetic, antithrombin-dependent, factor Xa-specific inhibitor that is approved in the U.S. for the prevention of deep vein thrombosis in patients undergoing hip fracture surgery, hip or knee replacement surgery, or abdominal surgery. Numerous studies have demonstrated this drug's safety and efficacy in these clinical settings [1-3], and the 2012 Guidelines from the American College of Chest Physicians includes fondaparinux as a thromboprophylactic alternative for patients undergoing orthopedic [4] as well as for general and abdomino-pelvic surgical procedures [5].
Fondaparinux is mainly excreted by the kidneys, with a terminal plasma half-life of 17 to 21 hours, depending on patient age [6]. Following total hip arthroplasty, anti-factor Xa levels were shown to gradually increase from median values of 0.0 to 0.24 mg/L over a two-week period during which patients were treated with 2.5 mg/day of fondaparinux, and this accumulation was more likely in patients with impaired renal function [7]. Consequently, it is generally recommended that fondaparinux be used with caution in patients with renal impairment and avoided altogether in patients with severe renal insufficiency (creatinine clearance < 30 mL/min).
Several studies have explored the use of a lower dose of fondaparinux (1.5 mg daily) in orthopedic patients with moderate renal impairment (20 to 50 mL/min) [8, 9]. In the PROPICE trial, a real-world, prospective, multi-center, cohort study, 442 patients with renal impairment undergoing total hip or knee replacement, or hip fracture surgery, received 1.5 mg/day of fondaparinux for 16 ± 12.5 days [9]. Major bleeding events occurred in 20 patients (4.5%), and clinically relevant bleeding occurred in 2 patients (0.5%). Three symptomatic distal venous thrombotic events occurred (0.5%), two while on therapy and one after fondaparinux had been stopped. More recently, the FONDAIR study investigated the safety and efficacy of fondaparinux at a dose of 1.5 mg daily in 206 acutely ill medical patients with renal impairment (creatinine clearance of 20 to 50 mL/min) [10]. One patient sustained a major bleeding event (0.49%), eight had clinically-relevant non-major bleeding (3.88%), and three developed symptomatic venous thromboembolism (1.46%) [10].
Relatively limited data are available that provide anti-factor Xa data in patients with renal insufficiency treated with fondaparinux. Recently, Delavenne and colleagues reported pharmacokinetic data from the PROPICE study, which investigated patients with renal impairment undergoing major orthopedic surgery [11]. They demonstrated that fondaparinux exposure was lower in patients with renal impairment treated with 1.5 mg daily compared to patients with normal renal function treated with 2.5 mg daily (p<0.01) [11]. We extended these data by investigating a small cohort of patients with renal insufficiency undergoing major abdomino-pelvic or orthopedic surgery.
Materials and Methods
Study design
This was a prospective, open-label, single-center, pharmacokinetic (PK) study (ClinicalTrials.gov identifier NCT01121770). The protocol was approved by the Duke University Medical Center Institutional Review Board, and informed consent was obtained from all patients prior to participation in the study.
Patients
The inclusion criteria for this study were: (1) inpatients ≥18 years of age with an estimated creatinine clearance between 20 to 50 mL/min; (2) anticipated hospitalization including either abdominal surgery or an elective orthopedic procedure; (3) need for prophylactic anticoagulant therapy during the hospitalization; and (4) ability to give informed consent. The estimated creatinine clearance was calculated using the Cockcroft-Gault equation [12, 13]. Exclusion criteria included: (1) anticipated use of aspirin, clopidogrel, or non-steroidal inflammatory agents during the time when participating in the study; (2) weight less than 50 kg; (3) clinical indication for therapeutic anticoagulation; (4) currently pregnant or breast-feeding; (5) reported hypersensitivity to fondaparinux; (6) history of thrombocytopenia with a positive in vitro test for anti-platelet antibodies in the presence of fondaparinux; (7) bacterial endocarditis; or (8) brain malignancy. Patients were also excluded if there were any concerns expressed by the primary team that the patient might be at an increased risk for hemorrhage. Eligibility assessments were made in the outpatient orthopedic and general surgery clinics prior to admission for surgery. This visit included the creatinine measurement for calculation of the creatinine clearance.
Study Drug
Fondaparinux was provided by the manufacturer in individual vials (1.5 mg in 0.3 mL). Study drug was stored in the Duke Investigational Pharmacy Service and dispensed for individual patient use when needed.
Protocol
Prophylactic anticoagulation was initiated in the post-operative setting when the bleeding risk was assessed as being minimal by the surgical and anesthesiology services, as per standard clinical practice. Fondaparinux was administered at a dose of 1.5 mg subcutaneously every 24 hours, or at an adjusted dosing interval determined from the anti-factor Xa level, for the duration of the patient's hospital stay. Study drug was discontinued for major bleeding events and thrombotic events. Continuation of the study drug for minor bleeding events was determined by the primary physician for the patient and the principal investigator. At the time of discharge from the hospital, continued thromboprophylactic therapy in the outpatient setting was permitted at the discretion of the patient's primary provider.
Outcome measures
The primary objective of the study was to characterize fondaparinux pharmacokinetics (PK) in patients with severe renal dysfunction. Blood PK samples were collected at the following time points following the third consecutive dose: 2 to 6 hours after the dose; approximately 12 ± 4 hours after the dose; and 1 to 4 hours prior to the next scheduled dose. PK samples were subsequently collected daily for as long as the patient was in the hospital.
The secondary objective was to investigate the safety and efficacy of fondaparinux by monitoring for major and minor hemorrhagic events and thrombotic complications by daily assessment during study participation. Major bleeding events were defined as any bleeding associated with severe hemodynamic instability that required transfusion support; bleeding in a critical site (e.g., retroperitoneal, intracranial); bleeding resulting in a 2 gm/dL drop in hemoglobin or higher; or bleeding resulting in death. Minor bleeding included any bleeding or increased bruising not considered major. Thrombotic events included new arterial or venous thrombosis of any extremity, stroke, myocardial infarction, or pulmonary embolism.
Anti-factor Xa activity was determined by an amidolytic assay using the HemosIL™ Heparin kit (Instrumentation Laboratories, Co., Bedford MA) on an ACL TOP coagulation analyzer. Concentration curves were constructed using a pool of platelet-poor plasma (Precision BioLogic, Dartmouth, Nova Scotia, Canada) spiked with increasing concentrations of fondaparinux. Anti-factor Xa activity was linearly correlated to the logarithm of the absorbance over the therapeutic range of the agent. Using this assay, the lower end for reproducible detection of the drug was 0.1 mcg/mL, and the therapeutic range for fondaparinux was between 0.20 to 0.70 mcg/mL (10% CV for results in the Coagulation Laboratory).
Additional assays performed at baseline included a serum pregnancy test, platelet count, prothrombin time (PT), and an activated partial thromboplastin time (aPTT). Tests performed weekly included a hemoglobin, platelet count, and serum creatinine.
Statistical and PK Analyses
Descriptive statistical analyses of the patient cohort were performed with Microsoft Excel (Redmond, WA). Figures were generated using the lattice package in the software R (version 2.15.2) [14]. A population PK analysis utilizing sparse pharmacokinetic data collected in the study subjects was performed in the software NONlinear Mixed Effects Modeling (NONMEM, Version 7.2, Icon Development Solutions, Ellicott City, Maryland). A published population pharmacokinetic model for fondaparinux was fit to the data utilizing reported final parameter estimates as initial values in this analysis [11]. The structural component was a two-compartment body model with first order absorption. In the published model, creatinine clearance (CrCl) was found to be significantly related to the apparent clearance (CL/F) and scaled using the CrClas described below. In this allometric function, CL/Fi represents the individual estimate for CL/F for the ith subject; CL/Fstd represents the typical population clearance estimate in a subject with the median CrCl in the sample; CrCli is the creatinine clearance value for the ith subject, which is divided by median creatinine clearance for the population; and θCL is an empirical allometric exponent. Inter-individual variability, which was estimated for the first order absorption rate constant (KA) and CL/F, was described using an exponential function where ηi is a rando n with mean zero and variance ω2.
In these analyses a combined proportional and additive error model was used to describe the intra-individual variability. Population parameter estimates and empirical Bayes estimates (EBE) (i.e., individual PK parameters) for this sample, after applying the published model, are reported. For values less than 0.1 mcg/mL, half the limit of quantification (i.e., 0.05 mcg/mL) was used [15].
Results
Patients
Nine patients with renal insufficiency were enrolled into the study between September 2010 and February 2012. Baseline characteristics of the study participants are summarized in Table 1. All of the patients underwent surgical procedures for a urologic malignancy (six with bladder cancer, two with renal cancer) except for one participant, who underwent a total knee arthroplasty. The average hospital length-of-stay for the study participants was 8.2 days (range, 5 to 13 days), and, with one exception, all participants underwent their surgical procedure on the day of admission to the hospital (one patient underwent surgery on Day 2). Estimated blood loss during surgery for the orthopedic patient was 300 cc, and for the urologic patients ranged from 300 to 3300 cc (mean, 1419 cc). Two of the urologic patients also underwent a splenectomy in addition to the planned procedure, one due to a splenic laceration sustained during dissection of dense splenic adhesions of a large renal mass and one due to tumor invasion of the spleen.
Table 1.
Demographics of study patients.
| Characteristic | Participants |
|---|---|
| Age (years) | 76 (52 to 86)* |
| Male | 6 |
| Race | 7 Caucasian, 2 African-American |
| Weight, mean (kg) | 69 (53 to 97) |
| Creatinine at enrollment (mg/dL) | 1.3 (1.1 to 1.7) |
| Calculated creatinine clearance at enrollment (mL/min)† | 44.4 (29.5 to 54.6) |
| Maximal postoperative creatinine (mg/dL) | 1.5 (1.0 to 4.5) |
Data are median (range) except where ranges are not indicated.
Calculated by Cockcroft-Gault formula.
Drug Administration
Fondaparinux dosing was initiated within 24 hours of the surgical procedure in 5 patients. Initiation of fondaparinux was delayed in 4 patients for 3 to 7 days, related to hemorrhagic concerns after the surgical procedure. Thromboprophylaxis continued for an average of 5 days (range, 2 to 12 days). Sequential doses were administered within 24 hours of the prior dose except for one dose that was delayed for ∼10 hours, due to clerical error in timing. Patient #8 was converted from thromboprophylaxis with fondaparinux to therapeutic anticoagulation with enoxaparin after only two days of prophylactic therapy when a clinical diagnosis of pulmonary embolism was made (described further below). Two patients discontinued fondaparinux the day before discharge; all other patients continued fondaparinux until the day of discharge.
Anti-factor Xa Levels
All patients had two or more anti-factor Xa levels checked (mean, 6.7 measurements per patient; range, 2 to 17 measurements), for a total of 59 anti-factor Xa measurements. Most of the measured trough levels (obtained 1 to 4 hours before a scheduled dose) were minimally elevated or below the lower cut-off limit of the assay (Table 2). Peak levels (obtained 2 to 6 hours after a dose) ranged from 0.1 to 0.45 mcg/mL (Table 2). The highest anti-factor Xa level obtained was 0.64 mcg/mL for Patient #5, who had a baseline creatinine clearance of 33.6 mg/mL, obtained ∼12 hours after the third dose. Three patients received fondaparinux for five or more days, but there was no evidence for significant drug accumulation, with a mean peak level of 0.22 mcg/mL (range, 0.1-0.36 mcg/mL; n=5) and two trough levels of 0.1 mcg/mL, all obtained after day four of treatment with fondaparinux.
Table 2. Anti-factor Xa levels.
| Timepoints* | Measurements (N) | Median Anti-factor Xa level, mcg/mL | Range, mcg/mL |
|---|---|---|---|
| 2-6 hrs after dosing (peak) | 10 | 0.25 | <0.1-0.45 |
| 12 ± 4 hrs after dosing (mid-point) | 16 | 0.16 | 0.1-0.64 |
| 1-4 hrs before subsequent dose (trough) | 7 | 0.10 | <0.1-0.22 |
Twenty-four anti-factor Xa results fell outside these timepoint ranges.
Statistical/PK Analysis
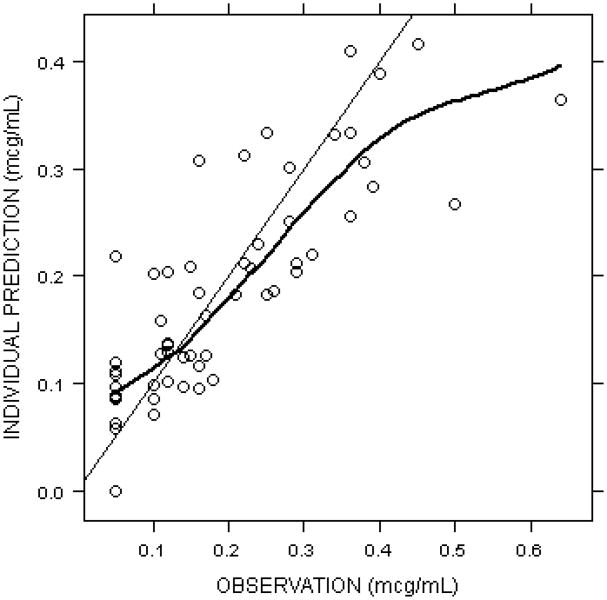
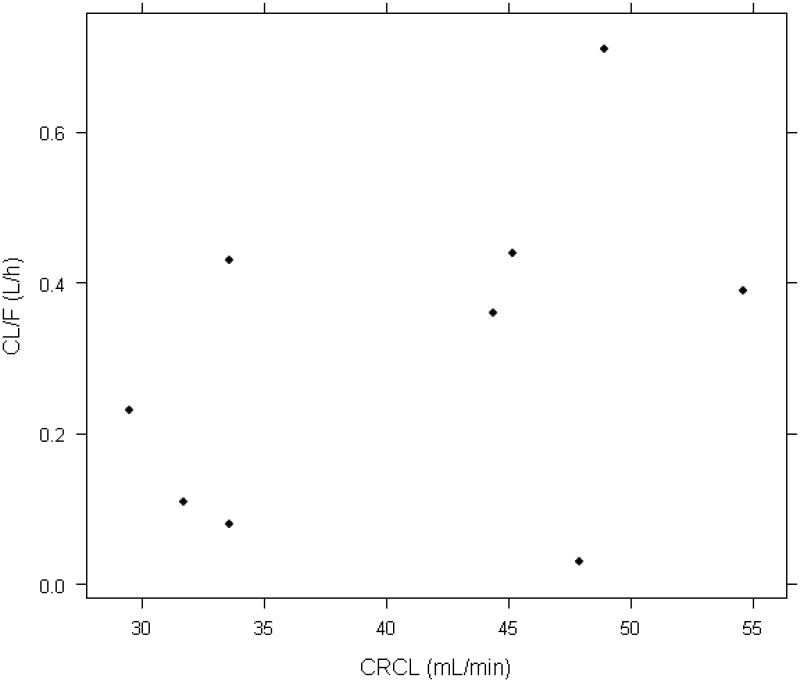
Observed versus individual model predictions for all collected data is shown in Figure 1. In the current sample, population estimates for CLstd/F and Q/F were 0.24 L/h and 0.55 L/h, respectively; whereas, VC and VP were estimated as 3.79 and 5.13 L, respectively. The population estimate for the allometric exponent, θCL, was 1.04. Inter-individual variability could be estimated for KA (31%) and CL/F (104%). Intra-individual variability estimated using a combined proportional and additive relationship was 34% and 0.03 mcg/mL, respectively. For individual subjects, the median and range for structural model parameters where inter-individual variability was incorporated are estimated as 0.34 h-1 (0.26-0.41, KA) and 0.38 L/h (0.03-0.71, CL/F). The relationship between creatinine clearance and the individual estimate for CL/F is depicted in Figure 2.
Figure 1.
Observed versus individual model predicted fondaparinux concentrations.Anti-factor Xa levels vs. time are shown for all collected data points.For values less than 0.1 mcg/mL, half the limit of quantification (i.e., 0.05 mcg/mL) is plotted.
Figure 2.
Relationship between creatinine clearance and the individual estimate for apparent clearance (CL/F).
Clinical Events
One patient developed hypoxia in the early postoperative setting, within 24 hours of the first dose of fondaparinux. This patient had a prior history of deep vein thrombosis and had an inferior vena cava filter placed prior to surgery. He also had a transient run of asymptomatic atrial fibrillation early in his postoperative course and subsequently developed increased oxygen requirements. A CT angiogram was not performed due to renal insufficiency (creatinine of 2.2), and a ventilation-perfusion scan was not performed because of an abnormal chest X-ray. An ultrasound revealed two small echogenic eccentric foci within the right internal jugular vein, consistent with non-occlusive thrombus vs. sequelae of prior thrombus. No other evidence of thrombus was identified in the patient, but he was converted to therapeutic anticoagulation given his history and the clinical concern for possible pulmonary embolism.
None of the patients developed clinically overt hemorrhagic complications. A single patient experienced a drop in the hemoglobin level by more than 2 gm/dL while receiving prophylactic fondaparinux, and this patient also received one unit of packed red blood cells on Hospital Day 4. The fondaparinux level in this patient at the time when the hemoglobin dropped was <0.1 mcg/mL, and it was not discontinued until later in the hospital course. A similar drop in the hemoglobin level occurred in two additional patients, but in both of these individuals, the drop occurred after the surgery but prior to initiating the fondaparinux.
Discussion
Renal insufficiency is a common condition found in patients undergoing surgical procedures, especially in the older patient population. Patients with chronic kidney disease undergoing orthopedic surgery are at an increased risk for postoperative morbidity [16], including thrombotic complications [17]. Similarly, patients with renal insufficiency are at an increased risk for cardiovascular events following urologic cancer surgery [18]. Consistent with these reports, one of the patients reported in this study developed worsening hypoxia in the early postoperative setting that was concerning for a possible venous thromboembolic event.
We investigated the use of fondaparinux as a thromboprophylactic agent in patients with moderate renal insufficiency undergoing abdominopelvic surgery for treatment of cancer or orthopedic surgery. Several studies have suggested that a reduced dose of fondaparinux, 1.5 mg/day subcutaneously, is effective and safe in patients with renal insufficiency. This dose has been looked at in patients with renal insufficiency undergoing orthopedic surgery [9] but it has not been previously described in patients undergoing cancer surgery.
Peak anti-factor Xa levels ranged from below the lower limit of detection to 0.45 mcg/mL (Table 2). These data complement existing datasets by providing a range of anti-factor Xa levels that can be expected at the time points studied in patients with renal insufficiency, if there is a clinical indication to check the level. It is possible that an individual patient who has higher levels than those observed in this study might have an increased risk for hemorrhagic complications, but this would need to be confirmed prospectively.
We applied a published population pharmacokinetic model developed in a large sample of patients with renal impairment undergoing orthopedic surgery [11] to this small sample of high-risk operative patients. When structural population PK parameter estimates are compared, the apparent clearance in this and the prior population was similar, whereas lower estimates were obtained for volume of distribution and absorption rate constant parameters. Greater imprecision in parameter estimates was observed, likely due in part to a small sample size and sparse sampling. Inter-individual variability estimate for KA was lower (31% vs. 90%), whereas variability in CL/F was higher (104% vs. 33%) in the current sample. Although the range of CrCl values observed in this sample was narrower, the majority of the patients had a urologic malignancy, which may have contributed to the greater inter-individual variability in CL/F.
We did not observe increased bleeding with fondaparinux at 1.5 mg/day in the patients treated in this small study. Although one patient did receive a transfusion because of a dropping hematocrit, none of the patients developed a clinically overt hemorrhagic event. In addition, the anti-factor Xa levels obtained immediately prior to a subsequent dose were either below the lower limit of detection, or only minimally elevated (Table 2).
One patient developed worsening hypoxia that was concerning for pulmonary embolism, although this could not be directly proven. The risk for postoperative venous thromboembolism is particularly high in patients undergoing abdominal or pelvic surgery for cancer [5], and this patient also had a prior history of deep vein thrombosis. Since he had been on warfarin prior to the surgery, he was simply restarted on therapeutic anticoagulation. It is notable that there are no large phase III randomized trials of thromboembolism prophylaxis in urological patients (most studies combine this patient population with patients undergoing various abdominal and pelvic procedures)[5]. This patient population has a high rate of renal insufficiency, however, making drug dosing difficult.
One limitation of our study is the small number of study participants, primarily due to difficulties encountered in enrolling a sufficient number of patients who stayed in the hospital for an appropriate length of time for the study. Orthopedic surgery patients were frequently discharged within a few days of surgery, even those with renal insufficiency. Our study was not designed to be conducted in the outpatient setting, restricting our population of eligible patients to individuals who were expected to be in the hospital for at least three or four days following surgery. It is possible that fondaparinux at this dose could be used in the outpatient setting for postoperative thromboprophylaxis in patients with renal insufficiency, but our study did not address this approach to therapy.
In conclusion, we demonstrated that 1.5 mg fondaparinux administered subcutaneously every 24 hours in high-risk surgical patients with renal impairment was associated with trough anti-factor Xa levels ranging from < 0.1 mcg/mL to 0.22 mcg/mL, even after as many as12 doses of study drug (Table 2). We theorize that this dose of fondaparinux is likely to be safe given the resulting plasma concentrations of drug are comparable to those seen in patients with better renal function. These patients are clinically complex and at risk for hemorrhagic as well as thrombotic events. Determining safe strategies for thromboprophylaxis is essential in this post-operative population of patients with renal insufficiency.
Acknowledgments
This study was funded, in part, by an unrestricted research grant from GlaxoSmithKline (TLO). DG is funded by NICHD under the Best Pharmaceuticals for Children Act through a T32 grant (GM086330). MCW receives support from the National Institutes of Health (1K23HD064814), from the nonprofit Thrasher Research Fund (www.thrasherresearch.org), and from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). TLO was supported by a research grant from the Centers of Disease Control and Prevention during the course of this study (DD-000014). We thank Pat Jordan for recruiting and enrolling patients into this study.
Role of the Funding Source: Study materials (drug) and/or additional financial support were provided by GlaxoSmithKline. Financial supporters had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: TLO has received research support from Eisai, Pfizer, Daiichi Sankyo, and Instrumentation Laboratory, Inc, and has received consultation fees from Instrumentation Laboratory, Inc.
References
- 1.Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005;92(10):1212–20. doi: 10.1002/bjs.5154. [DOI] [PubMed] [Google Scholar]
- 2.Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002;359(9319):1721–6. doi: 10.1016/S0140-6736(02)08648-8. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson BI, Bauer KA, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345(18):1298–304. doi: 10.1056/NEJMoa011100. [DOI] [PubMed] [Google Scholar]
- 4.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW., Jr . Chest. 9th. 2 Suppl. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis; pp. e278S–325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Chest. 9th. 2 Suppl. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines; 2012. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis; pp. e227S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donat F, Duret JP, Santoni A, Cariou R, Necciari J, Magnani H, de Greef R. The pharmacokinetics of fondaparinux sodium in healthy volunteers. Clin Pharmacokinet. 2002;41(Suppl 2):1–9. doi: 10.2165/00003088-200241002-00001. [DOI] [PubMed] [Google Scholar]
- 7.Yukizawa Y, Inaba Y, Watanabe S, Yajima S, Kobayashi N, Ishida T, Iwamoto N, Hyonmin C, Nakamura M, Saito T. Plasma accumulation of fondaparinux 2.5 mg in patients after total hip arthroplasty. J Thromb Thrombolysis. 2012;34(4):526–32. doi: 10.1007/s11239-012-0773-z. [DOI] [PubMed] [Google Scholar]
- 8.Turpie AG, Lensing AW, Fuji T, Boyle DA. Pharmacokinetic and clinical data supporting the use of fondaparinux 1.5 mg once daily in the prevention of venous thromboembolism in renally impaired patients. Blood Coagul Fibrinolysis. 2009;20(2):114–21. doi: 10.1097/MBC.0b013e328323da86. [DOI] [PubMed] [Google Scholar]
- 9.Mismetti P, Samama CM, Rosencher N, Vielpeau C, Nguyen P, Deygas B, Presles E, Laporte S. Venous thromboembolism prevention with fondaparinux 1.5 mg in renally impaired patients undergoing major orthopaedic surgery. A real-world, prospective, multicentre, cohort study. Thromb Haemost. 2012;107(6):1151–60. doi: 10.1160/TH11-09-0640. [DOI] [PubMed] [Google Scholar]
- 10.Ageno W, Riva N, Noris P, Di Nisio M, La Regina M, Arioli D, Ria L, Monzani V, Cuppini S, Lupia E, Pierfranceschi MG, Dentali F. Safety and efficacy of low dose fondaparinux (1.5 mg) for the prevention of venous thromboembolism in acutely ill medical patients with renal impairment. The FONDAIR study. J Thromb Haemost. 2012 doi: 10.1111/j.1538-7836.2012.04908.x. [DOI] [PubMed] [Google Scholar]
- 11.Delavenne X, Zufferey P, Nguyen P, Rosencher N, Samama CM, Bazzoli C, Mismetti P, Laporte S. Pharmacokinetics of fondaparinux 1.5 mg once daily in a real-world cohort of patients with renal impairment undergoing major orthopaedic surgery. Eur J Clin Pharmacol. 2012;68(10):1403–10. doi: 10.1007/s00228-012-1263-0. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Dowling TC, Wang ES, Ferrucci L, Sorkin JD. Glomerular Filtration Rate Equations Overestimate Creatinine Clearance in Older Individuals Enrolled in the Baltimore Longitudinal Study on Aging: Impact on Renal Drug Dosing. Pharmacotherapy. 2013 doi: 10.1002/phar.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkar D. Lattice: Multivariate Data Visualization with R. New York: Springer; 2008. [Google Scholar]
- 15.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 16.Ackland GL, Moran N, Cone S, Grocott MP, Mythen MG. Chronic kidney disease and postoperative morbidity after elective orthopedic surgery. Anesth Analg. 2011;112(6):1375–81. doi: 10.1213/ANE.0b013e3181ee8456. [DOI] [PubMed] [Google Scholar]
- 17.Shorr AF, Eriksson BI, Jaffer AK, Smith J. Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–20. doi: 10.1111/j.1538-7836.2012.04803.x. [DOI] [PubMed] [Google Scholar]
- 18.Takeshita H, Yokoyama M, Fujii Y, Chiba K, Ishioka J, Noro A, Kihara K. Impact of renal function on cardiovascular events in patients undergoing radical nephrectomy for renal cancer. Int J Urol. 2012;19(8):722–8. doi: 10.1111/j.1442-2042.2012.03015.x. [DOI] [PubMed] [Google Scholar]