Abstract
Interleukin-10 (IL-10) is a cytokine that is produced by a variety of immune cells and is known to inhibit T helper cell type 1 (TH1) responses, which are essential to combat tumors. In the present study, we used LSA, a T cell lymphoma cell line that expresses IL-10, to study the role of this cytokine in its tumorigenesis. To this end, LSA cells were modified to overexpress IL-10 or to block its expression via Ret-off-IL-10 vector transfection. Interestingly, blocking IL-10 expression using Ret-off-IL-10 antisense inhibited the growth of LSA in syngeneic C57BL/6 mice. Also, overexpression of IL-10 in LSA cells using Ret-off-IL-10 sense significantly increased LSA tumorigenicity. Additionally, administration of antibodies against IL-10 significantly inhibited LSA tumor growth in vivo. Together, our data stress the importance of tumor-produced IL-10 in regulating the tumorigenicity of this T cell lymphoma, and suggest that antagonizing IL-10 expression in IL-10 secreting lymphomas may have significant potential in their treatment.
Keywords: IL-10 gene expression, lymphoma, tumorigenicity
Introduction
Interleukin-10 (IL-10), originally known as cytokine synthesis inhibitory factor (CSIF) [1], was shown initially to be produced primarily by mouse T helper cell type 2 (TH2) cells, where it inhibited the activation and production of cytokines by TH1 cells [2]. IL-10 has been characterized as an anti-inflammatory cytokine produced by macrophages as well as a wide range of other immune cells [3]. The ability of murine and human IL-10 to inhibit cytokine production by both T cells and lymphokine-activated killer cells was shown to be indirect, and through the inhibition of macrophage functions [4–7]. The macrophage functions adversely affected by IL-10 include monokine synthesis, NO production, and expression of IL-12, class II major histocompatibility complex (MHC) and costimulatory molecules including CD80/CD86 [8–10]. Moreover, IL-10 potently inhibits the production of cytokines including: IL-1α, IL-1β, IL-6, IL-12, IL-18, granulocyte macrophage colony stimulating factor (GM-CSF), G-CSF, M-CSF, tumor necrosis factor (TNF), leukocyte inhibitory factor (LIF) and platelet activating factor (PAF) in activated macrophages as well as its own production by the same cells [11,12]. In addition, IL-10 was shown to inhibit the production of both CC chemokines including monocyte chemoattractant protein-1 (MCP-1), MCP-5, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, MIP-3α, MIP-3β, regulated and normal T cell expressed and secreted (RANTES) and macrophage-derived chemokine (MDC), as well as CXC chemokines including IL-8, interferon-γ-induced protein 10 (IP-10), MIP-2 and keratinocyte-derived chemokine (KC; Gro-α) by activated monocytes [13]. Such chemokines are known to be responsible for the recruitment of monocytes, dendritic cells, neutrophils and T cells at sites of inflammation. Thus, through this effect, IL-10 is thought to potentiate its anti-inflammatory activities. IL-10 not only inhibits production of these effectors, but in addition it enhances expression of their natural antagonists such as interleukin-1 receptor antagonist (IL-1RA) and soluble p55 and p75 tumor necrosis factor receptor (TNFR) [14].
More recently, IL-10 has been implicated in the activities of regulatory T cells as well as T regulatory B cells [15]. The induced T regulatory subpopulation known as Tr1 cells have been shown to regulate immune responses through the secretion of high levels of immunosuppressive cytokines, IL-10 and transforming growth factor-β (TGF-β), thereby suppressing both naive and memory T-cell responses both in vitro and in vivo [16]. The anti-inflammatory effect of IL-10 was well elucidated in the IL-10 deficient (IL-10−/− ) mouse model, for it was shown that such mice developed chronic enterocolitis due to exposure to normal enteric antigens. The inflammatory colitis response was mediated through the exacerbation of CD4+ TH1 cells that were able to induce similar enterocolitis when adoptively transferred to immunocompromised RAG-2−/− mice [17–20].
IL-10 has been shown to play a critical role in suppressing the anti-tumor immunity, thereby facilitating tumor growth and metastasis. Specifically, tumor-associated macrophages (TAMs) produce IL-10 and are also associated with in-tumor immunosuppression, thereby providing a suitable microenvironment for cancer growth [21]. Such studies have suggested that IL-10 produced by TAMs in the tumor microenvironment may suppress immune surveillance against cancer growth. The role of IL-10 in cancer has also been suggested by a number of clinical studies in which high levels of IL-10 were noted in patients with cancer. For instance, high serum IL-10 levels were associated with a worse prognosis in patients with gastric cancer, independently of their tumor stage [22]. In another study, the levels of IL-10 and IL-6 were significantly higher in patients with hepatocellular carcinomas than in healthy subjects [23]. In such patients, it was the high level of IL-10 that correlated with the worst prognosis after tumor resection [23]. While it is not clear whether the high levels of IL-10 seen in patients with cancer are derived from the immune cells of the host or the cancer cells themselves, the fact that some cancer cells produce IL-10 suggests the role of tumor-derived IL-10 in causing suppression of the antitumor immunity and promotion of tumor growth. This may be particularly true for malignancies of T cell origin, which have been shown to express IL-10 constitutively [24].
In IL-10 transgenic mice, it was shown that Lewis lung carcinoma cells (3LL) had more aggressive growth potential that was attributed to the disruption of both T cell and adenomatous polyposis coli (APC) function [25]. Alternatively, the inhibition of IL-10 expression through an IL-10 antisense retrovirus restored the vaccine efficacy of the GM-CSF-producing J558L cells, thereby demonstrating a direct role of IL-10 in paralyzing the GM-CSF-induced antitumor immune response [26]. Furthermore, when a viral construct was injected into the tumors of IL-10 knockout mice, it produced a strong construct-specific interferon-γ (IFN-γ) response. On the other hand, when the same construct was injected into tumors of normal mice, it failed to elicit an anti-construct response. Such studies have demonstrated that tumor-induced IL-10 can block the generation of a tumor-specific TH1 immune response [27].
Despite such indications, there is no direct evidence that the IL-10 produced by T cell lymphomas may direct the growth pattern of the tumor in the host. To investigate this, in the present study, we used a murine T cell lymphoma line that constitutively produced IL-10, and genetically modified the cell line to either overexpress IL-10 or down-regulate IL-10 expression, and examined their ability to grow in the syngeneic host. These studies demonstrated the importance of tumor-derived IL-10 in supporting and enhancing the tumorigenicity of the T cell lymphoma.
Materials and methods
Mice
Female, adult, C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Cell lines
The LSA tumor cell line is a radiation leukemia virus (Rad LV) induced T cell lymphoma syngeneic to C57BL/6 mice [28]. It is an immunogenic tumor that has been well characterized by us in previous studies to trigger a strong TH1 as well as cytotoxic T cell response [29,30]. AutoD1T is a spontaneously transformed CD4+ T cell clone that was shown to be dependent on the autocrine stimulation of IL-2 for its growth. AutoD1T is only tumorigenic in immunodeficient mice. It was used as a control because it did not express mRNA for IL-10. All cells were maintained in complete RPMI-1640 supplemented with 5% fetal calf serum (FCS).
Antibodies
Affinity purified normal rat serum was purchased from Jackson Immunoresearch Laboratories (West Grove, PA). The anti-IL-10 rat immunoglobulin G (IgG) hybridoma cell line (SXC1) was originally provided by Dr. Tim Mosmann, and was grown ex vivo. Supernatants were collected and concentrated, and then anti-IL-10 monoclonal antibodies (mAbs) were affinity purified over a protein A column (HiTrap™ Protein A HP; GE Life Sciences) before in vivo use.
Cell proliferation
The proliferation of the cells in culture was studied by enumerating viable cells using a trypan-blue dye exclusion assay. The LSA and AutoD1T cell lines were cultured in 96-well tissue-culture plates at a concentration of 5 × 104 cells/well in 0.2 mL complete RPMI-1640 medium supplemented with 10% FCS. To these cultures, 10 ng/mL of recombinant IL-10 (rIL-10; R&D Systems) was added and cells were incubated at 37 ° C with 5% CO2 for 48 h before counting the viable cells. Each set was done in triplet wells, and the experiment was repeated twice. Data from one representative experiment are shown.
Reverse transcription-polymerase chain reaction
Reverse transcription-polymerase chain reaction (RT-PCR) was conducted to detect the expression of mRNA for the following genes: IL-4, IL-10 and β-actin. Briefly, cells were grown for 12, 24, 36 and 48 h, total cytoplasmic RNA was extracted from 107 cells, and 1.5 μg from each sample was reverse transcribed. Then the resulting cDNA was amplified using specific primers for each gene (IL-4 and -10 primers provided by Clontech) with sequences as follows: for IL-4, 5′-CCA GCT AGT TGT CAT CCT GCT CTT CTT TCT CG and 3′-CAG TGA TGT GGA CTT GGA CTC ATT CAT GGT GC; IL-10, 5′-ATG CAG GAC TTT AAG GGT TAC TTG GGT T and 3′-ATT TCG GAG AGA GGT ACA AAC GAG GTT T; β-actin, 5′-TAT CCT GAC CCT GAA GTA CCC CAT T and 3′-AGC ACA GCT TCT CTT TGA TGT CAC G. PCR products were visualized in 1.5% agarose gels following staining with ethidium bromide. The demonstration of a single 464, 455 and 384 bp band was considered indicative of β-actin, IL-10 and IL-4 genes, respectively.
Enzyme linked immunosorbent assay
The secreted IL-10 from cell lines was assessed using the DuoSet enzyme linked immunosorbent assay (ELISA) from R&D Systems (Minneapolis, MN; Cat. # DY417). The ELISA was set up according to the manufacturer's manual. Briefly, 96 flat-bottom ELISA plates were coated with capture antibody and subsequently blocked with blocking buffer having 1% bovine serum albumin (BSA) and 5% sucrose. Supernatants were collected from ex vivo grown cell lines, centrifuged at 300 × g for 10 min, and filtered through a 0.45 μm syringe filter. Several dilutions of supernatants as well as a positive control were added in triplicate before adding the detecting antibody. The plates were read in a microplate reader at 450 and 570 nm as reference wavelengths.
Establishment of IL-10 sense and antisense transfectant cell lines
The IL-10 gene was obtained from clone IL-10-F116 (American Type Culture Collection; ATCC), and cloned into pRetro-off vector (Clontech; Cat. # 6157-1), a vector that allows the inducible expression of the target gene. In pRetro-off, the vector is turned off in the presence of doxycycline (Dox) hydrochloride (Sigma; # D9891). Then, plasmid vectors were transformed into Escherichi coli DH5α (ATCC), and clones were selected to have IL-10 inserted in either the sense or antisense direction using PCR and restriction digestion. Figure 1 depicts the pRetro-off vector with IL-10 gene insert. The Ret-off -IL-10 sense and Ret-off -IL-10 antisense, as well as Ret-off, were transfected into the packaging cell line, Phoenix Ectopic (provided by Dr. G. Nolan and ATCC) following protocol description in the Clontech manual (PT30001-1). Briefly, the packaging Phoenix cell line was plated in the presence of 5 μg/mL of chloroquine, then 15 μg of each purified plasmid vector DNA was transfected using calcium phosphate, CalPhos Maximizer (Clontech; Cat. # 8021-1, -2). Supernatants from different transfectant packaging Phoenix cells containing retroviral particles were used to infect the LSA cell line in the presence of 8 mg/mL of polybrene. The resulting cell lines were selected in media containing 1.5 mg/mL puromycin for 1 week and subsequently cloned. The transfection and orientation were confirmed by PCR of the genomic DNA using vector specif c primers (data not shown). The resulting clones were called LSA Ret, LSA Ret-IL-10 sense and LSA Ret-IL-10 antisense. These clones were able to grow ex vivo to a similar extent.
Figure 1.
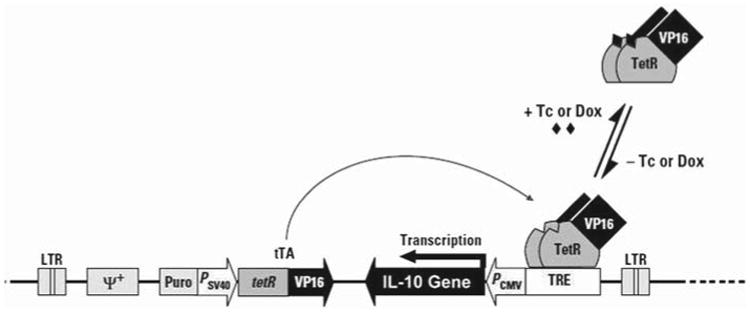
The pRetro-off vector with the IL-10 gene insert. The tetracycline (Tc)-controlled transactivator (tTA) is expressed from the integrated retroviral constructs tetR and VP16. In this vector, tTA binds the tet-responsive element (TRE), activating transcription only in the absence of Tc or its derivative doxycycline (Dox). PCMV is the promoter for cytomegalovirus. In addition, the vector includes genes for puromycin-resistance and ψ+, the packing signal for retrovirus particle formation. IL-10 was inserted in either the sense or antisense direction to produce Ret-off-IL-10 sense and Ret-off-IL-10 antisense, respectively. (Figure modified from Clontech retroviral Tet vector user manual PT30001-1, page 6.)
In vivo tumorigenesis of LSA
To determine the ability of various LSA transfectants to induce tumors in vivo, we injected 5 × 106 cells i.p. into groups of five C57BL/6 mice and studied the survival pattern. Moribund mice were immediately euthanized. The mice were monitored for survival daily, and once dead, mice were examined for tumor growth by opening the intraperitoneal cavity and studying the metastasis. In some experiments, to study the role of IL-10, we injected mice with 1 × 106 LSA cells followed by anti-IL-10 Abs (1.5 mg/mouse) or normal rat serum (control) every other day for 16 days. The survival of tumor-bearing mice was studied as described above.
Statistical analysis
Statistical analyses were carried out between experimental and control groups using Microsoft Excel and Student's t-test, and a probability p <0.05 was considered to be statistically significant. For tumor-bearing mouse survival, Kaplan–Meier survival curve analysis was conducted (MedCalc®) and statistically significant difference was assessed using the log-rank test.
Results
Expression of IL-10 by LSA and AutoD1T lymphoma cell lines
To investigate whether the immunosuppressive cytokine IL-10 was involved in the regulation of tumor growth in vivo, we first examined the expression of IL-10 mRNA using RT-PCR on LSA and AutoD1T lymphoma cell lines. Cells were grown over periods of 12, 24, 36 or 48 h and harvested, and the total RNA from such cells was subjected to RT-PCR as described above in “Materials and methods.” Results, depicted in Figure 2, showed that LSA tumor cells expressed the mRNA for IL-10 constitutively, while the AutoD1T cell line did not. The LSA tumor cells secreted IL-10, as demonstrated in Figure 3. In these experiments, LSA obtained from tumor-bearing C57BL/6 mice were washed three times and subsequently grown ex vivo for 48 h, and culture supernatants were collected, filtered and assessed for the presence of IL-10 using capture ELISA. The data showed that LSA from tumor-bearing mice secreted significant levels of IL-10 (Figure 3). Interestingly, when the LSA cell line was maintained ex vivo for several passages, it lost expression of IL-10 and became less tumorigenic in the normal syngeneic host (data not shown).
Figure 2.
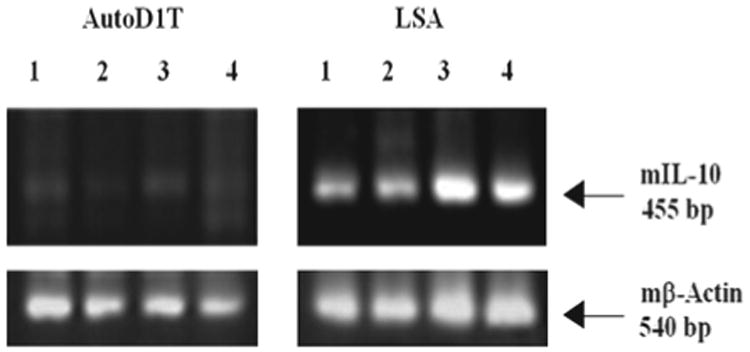
Expression of IL-10 gene in T cell lymphoma cell lines. LSA and AutoD1T cell lines were grown for 12, 24, 36 or 48 h in vitro and RT-PCR was performed to detect both mIL-10 and mβ-actin as an internal control. Lanes 1–4 are PCR products of 12, 24, 36 or 48 h of culture, respectively.
Figure 3.
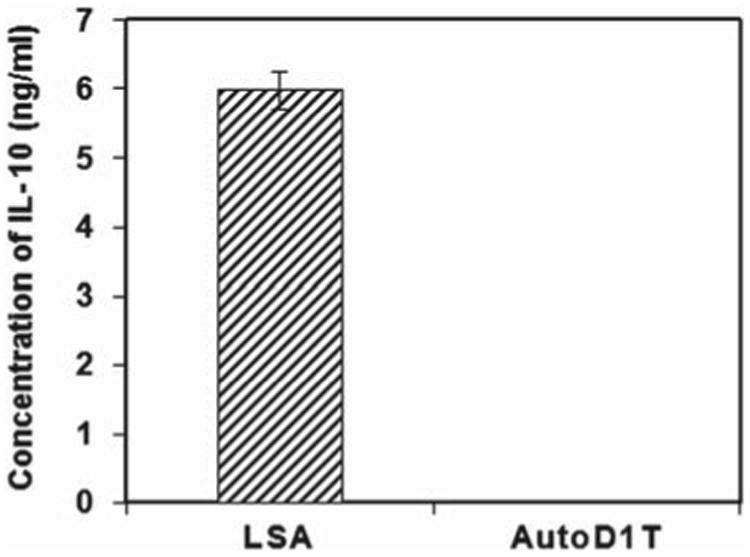
Secretion of mIL-10 by in vivo grown LSA. LSA tumor cells were collected from LSA-bearing C57BL/6 mice. These cells were grown ex vivo for 48 h. Supernatants were collected and assessed for IL-10 content using ELISA (R&D Systems). Concentrations were calculated by comparing to IL-10 standard and mean of concentrations (ng/mL) ± SE was plotted. AutoD1T tumor cell line was used as a negative control.
Effect of anti-IL-10 antibodies on survival of LSA-bearing C57BL/6 mice
To determine whether the secretion of IL-10 contributes to the tumorigenicity of LSA in the normal syngeneic host, we attempted to block IL-10 using anti-IL-10 mAbs in LSA tumor-bearing mice. In vivo tumors were introduced by injecting 1 × 106 LSA cells i.p. into C57BL/6 mice. The mice subsequently received 1.5 mg of anti-IL-10 mAbs or control Abs (control) i.p. once every other day for 16 days. The data illustrated in Figure 4, Kaplan–Meier survival curve analysis, show that there were significant survival advantages (p = 0.0089, log-rank) for blocking IL-10 activity in the anti-IL-10 mAb treated group. While these studies indicated that IL-10 played a critical role in tumor growth in vivo, it was not clear whether the effect of anti-IL-10 mAbs was directed against host- or tumor-derived IL-10 or both.
Figure 4.
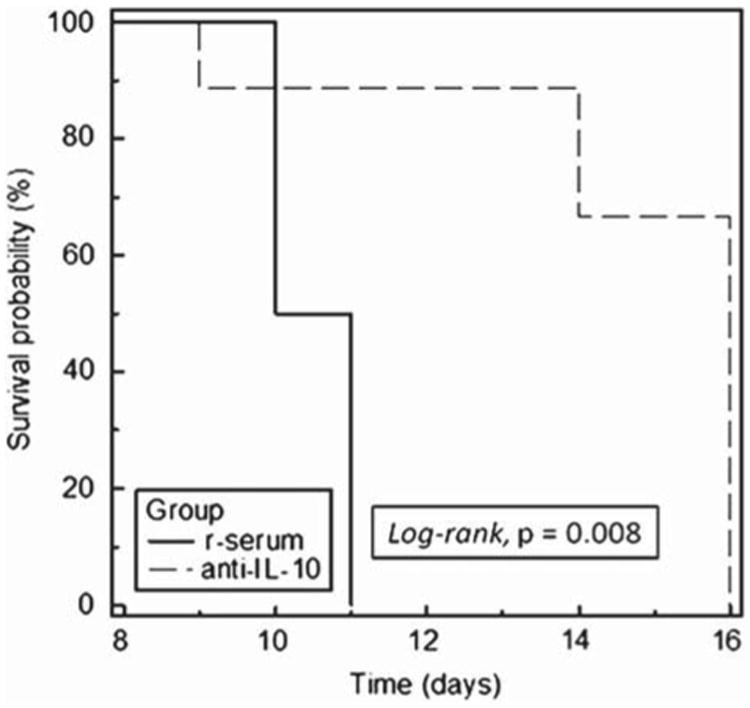
Effect of anti-IL-10 mAbs on survival of LSA-bearing C57BL/6 mice. Two groups of C57BL/6 mice were injected (i.p.) with 106 LSA followed by 1.5 mg of mAbs against IL-10 (anti-IL-10) or normal rat serum (r-serum) on alternate days. Survival of the tumor bearing mice was monitored and Kaplan–Meier survival curve analysis was plotted accordingly.
Dox-regulated expression of IL-10 mRNA in tumor cell lines
To further address specifically the role of IL-10 produced by the LSA tumor cells, we attempted to genetically down- or up-regulate the expression of IL-10 in LSA cells. This was accomplished using the Ret-off vector as described in “Materials and methods.” In the LSA Ret-IL-10 sense cells, the presence of IL-10 gene in the sense direction in front of the cytomegalovirus (CMV) promoter, and in the absence of Dox, would lead to the production of extra copies of IL-10 mRNA, thereby increasing IL-10 production in these cells. Alternatively, in the LSA Ret-IL-10 antisense cells, and in the absence of Dox, the transfected vector would produce IL-10 antisense mRNA. Such copies of IL-10 antisense mRNA are expected to hybridize to IL-10 sense mRNA, forming double-stranded RNA (dsRNA), which is known to be a potent suppressor of gene expression [31]. To examine whether regulated expression of IL-10 was established, the different transfectant cell lines were incubated for 24 h in the presence or absence of doxycycline. The cells were washed and mRNA was extracted for RT-PCR using primer pairs from Clontech for IL-10 as described in “Materials and methods.” The data depicted in Figure 5 demonstrate that when vector expression was induced in the absence of Dox, the LSA Ret-IL-10 sense transfected cells up-regulated IL-10 expression. Alternatively, the expression of IL-10 was markedly down-regulated in the Ret-IL-10 antisense transfected LSA cells. The presence of the vector alone did not change IL-10 expression in LSA cells.
Figure 5.
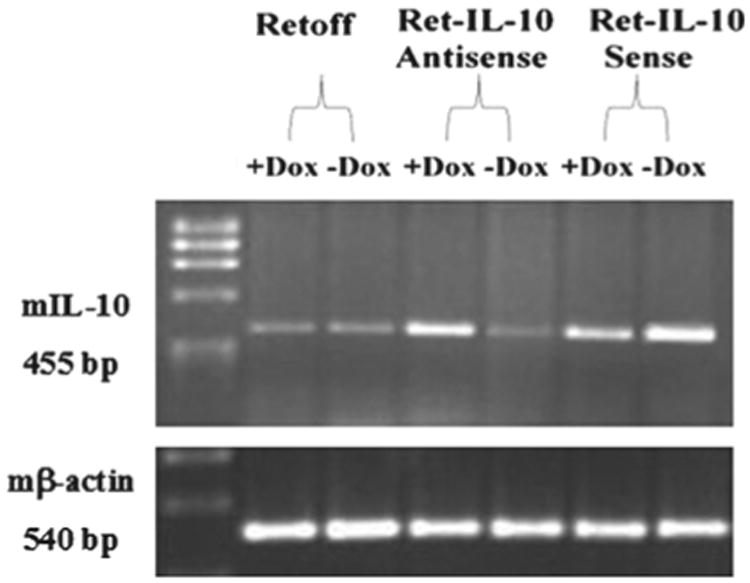
Expression of IL-10 in LSA transfected clones. Clones transfected with the Ret-off vector, Ret-IL-10 antisense and Ret-IL-10 sense were grown for 24 h in the presence or absence of Dox. mRNA was extracted and RT-PCR was conducted for expression of IL-10 gene and mβ-actin as a control, as described in “Materials and methods.” Gel images depict PCR products from the different clones for IL-10 gene and β-actin.
Tumorigenicity of IL-10 gene modulated LSA clones
Following the confirmation of regulated expression of the IL-10 gene, the various transfected LSA clones were tested for their ability to induce tumors in vivo. Groups of C57BL/6 mice were injected i.p. with 5 × 106 cells of each of LSA Ret-off, LSA Ret-IL-10 sense or LSA Ret-IL-10 antisense and monitored over a period of 25 days. Results, as shown in Figure 6, demonstrated that 100% of mice with LSA Ret-IL-10 antisense survived, and did not show any symptoms of tumor growth when the experiment was terminated. On the other hand, Kaplan–Meier survival curve analysis in the group injected with LSA Ret-IL-10 sense was significantly less than that for the control group injected with LSA Ret-off (p <0.0001, log-rank). These data clearly demonstrate that the abrogation of IL-10 expression had rendered the LSA cell line non-tumorigenic, while overexpressing the IL-10 gene resulted in increased tumorigenicity and a significant reduction in mean survival time.
Figure 6.
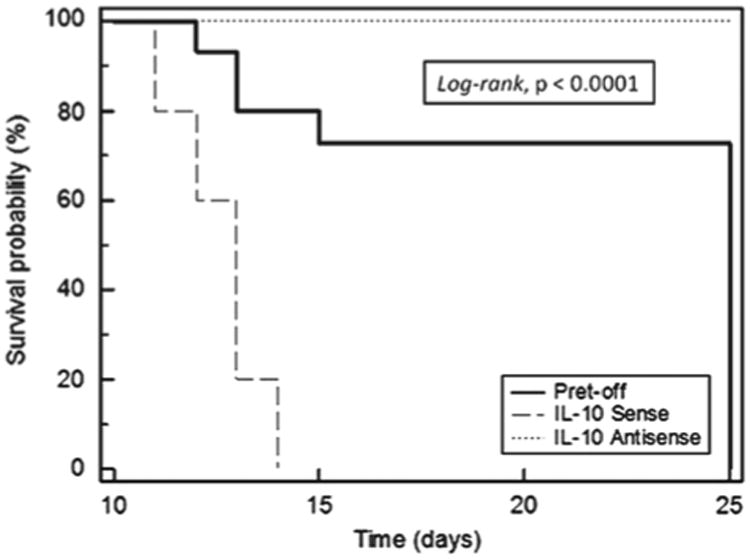
Survival of C57BL/6 bearing IL-10 modified LSA clones. Groups of C57BL/6 mice were injected with 106 LSA tumor cells from the following: clone transfected with Ret, Ret-IL-10 sense and Ret-IL-10 antisense. Survival of the tumor bearing mice was monitored and Kaplan–Meier survival curve analysis was plotted accordingly.
Effect of IL-10 on IL-4 gene expression and proliferation of tumor cell lines
To examine the effect of IL-10 on the growth of the LSA cell line ex vivo, 10 ng/mL of exogenous rIL-10 was added to the LSA cell line. Also, the AutoD1T cell line was used as a control, since it has been shown to be exclusively dependent on IL-2 autocrine stimulation [32]. Cells were grown for 48 h and counted for viable cells using trypan-blue exclusion. Data illustrated in Figure 7(A) show that the addition of rIL-10 significantly reduced the growth of both AutoD1T and LSA cell lines, with p-values 0.0007 and 0.04, respectively. The inhibitory effect on the ex vivo cell proliferation can be attributed to the effect of exogenous rIL-10 on the autocrine growth factor, namely IL-2, that maintains the growth of both cell lines. The negative effect of IL-10 on IL-2 expression has been well established [33]. Because the LSA cell line has been shown previously to be dependent on both IL-2 and IL-4 autocrine stimulation [34], we addressed the effect of IL-10 expression or lack of it on the expression of IL-4 in different IL-10 transfectant LSA cell lines. To this end, Ret-IL-10 sense, Ret-IL-10 antisense and Ret-modified LSA were grown for 24 h in the presence or absence of Dox, and mRNA was assessed for IL-4 expression. Figure 7(B) shows that when IL-10 vector expression was induced in the absence of Dox, LSA cells up-regulated IL-4 mRNA expression. Alternatively, when LSA cell line expression of IL-10 was down-regulated through the induction of IL-10 antisense, LSA cells expressed lower levels of IL-4 mRNA.
Figure 7.
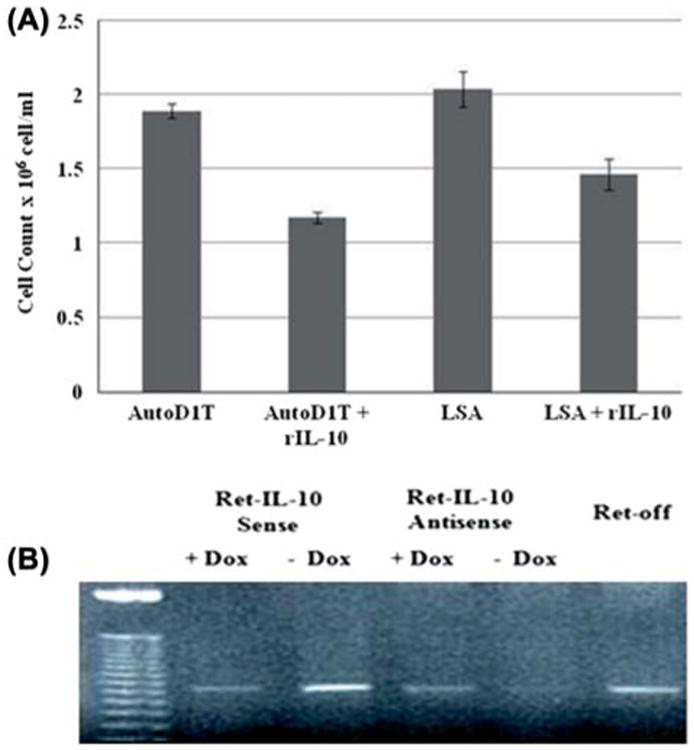
Effect of exogenous rIL-10 on ex vivo growth of LSA. (A) Proliferation was assessed using trypan-blue exclusion assay. AutoD1T (negative for IL-10 and IL-2 dependent) and LSA tumor cells were grown ex vivo in the presence or absence of 10 ng/mL rIL-10. Cultures were carried out in triplicate and mean ± SE was plotted. (B) Effect of IL-10 expression on IL-4 levels in ex vivo LSA Ret-off modified cell lines. Clones transfected with the Ret vector, Ret-IL-10 antisense and Ret-IL-10 sense were grown for 24 h in the presence or absence of Dox. RT-PCR was performed to detect expression of IL-4 gene (384 bp). Gel image depicts PCR products from different clones along with (50 bp) ladder following staining with ethidium bromide.
Discussion
Although the relationship between IL-10 and cancer growth has been well studied, the precise role of IL-10 produced by the tumor in promoting its growth in vivo has not been clearly addressed. While most studies have focused on the ability of tumor cells to evade the action of host immune surveillance by inducing suppressor cells such as TAMs or T regulatory cells (Tregs) that produce IL-10, whether the IL-10 produced by the tumor can have an effect on the growth and metastasis is not clear. While antibodies against IL-10 are useful in addressing the overall role of IL-10 in tumor promotion, whether the effect of this treatment results from neutralization of IL-10 produced by the host-derived immune cells or the tumor cells is not clear. To overcome this problem and to specifically address the role of tumor-derived IL-10, we decided to up- or down-regulate IL-10 expression in the same tumor cell line using a molecular transfection approach. We were surprised to note that IL-10 produced by the tumor cells plays a critical role in tumorigenesis inasmuch as the IL-10 deficient clone failed to induce tumors in vivo. Also, the tumor cell clone expressing higher levels of IL-10 caused increased tumor growth and early death in mice when compared to control LSA tumor cells. Together, these data suggest that IL-10 produced by the tumor cells may predict the extent of tumorigenesis in vivo and the survival of the host.
Several studies have focused on the tumor microenvironment and suggested that cytokines such as TGF-β and IL-10 produced at this site may cause local immunosuppression, thereby leading to immune evasion by the tumor cells [24]. IL-10 is produced by a wide range of immune cells including T cells, B cells, monocytes and macrophages. Of these cells, the Tregs and TAMs found in the tumor microenvironment may play a critical role. IL-10 mediates its effect by inhibiting proinflammatory cytokines such as IL-2, TNF-α, IFN-γ and GM-CSF produced by TH1 cells [24]. In this context, our previous studies demonstrated that the LSA tumor triggers a TH1 response, which, however, fails to eradicate the tumor by virtue of the fact that the LSA tumor also induces suppressor T cells. Thus, adoptive transfer of LSA tumor-specific CD4+ T cells into nude mice could protect the mice from LSA tumor challenge. Also, we demonstrated earlier that cytotoxic T lymphocyte (CTL) clones generated against the LSA cell line can confer significant protection once adoptively transferred to LSA-bearing syngeneic mice [35]. Therefore, it is likely that LSA tumor-derived IL-10 may suppress the tumor specific TH1 cells, thereby facilitating its growth. IL-10 also plays an important role in inhibiting the expression of co-stimulatory molecules including CD80, CD86 and MHC class II, and the maturation of monocyte-derived dendritic cells (DCs) and their capacity to initiate TH1 responses [24].
IL-10 can also induce type 1 regulatory T (Tr1) cells that are known to suppress the immune response, and we have noted previously that LSA tumor cells induce large numbers of such suppressor T cells in vivo, whose elimination leads to complete tumor rejection [36].
IL-10 has also been shown to down-regulate the expression of MHC class I expression. For example, pretreatment of melanoma cell lines with IL-10 made them less sensitive to tumor-specific CTLs and, furthermore, this effect directly correlated with the ability of IL-10 to down-regulate MHC class I expression [37]. Such findings have been used to propose that secretion of IL-10 by tumors may help evade the cytolytic killing by CTLs [37–40]. However, down-regulation of MHC on tumor cells should facilitate their killing by natural killer (NK) cells. Such findings were indeed reported by Salazar-Onfray et al. [38], who noted that IL-10 transfection of mouse T cell lymphoma RMA cell line (C57BL/6 syngeneic) resulted in rendering RMA cells CTL resistant, yet more NK cell sensitive. However, in such instances, the tumors may use additional mechanisms to escape the killing by NK cells. For example, we have noted that the LSA tumor expresses TGF- β (data not shown) and IL-4 constitutively [34], where TGF- β has been shown to inhibit the CD16-mediated NK cell IFN- γ production and antibody-dependent cell-mediated cytotoxicity (ADCC) [41]. Also, IL-4 was shown to block and reduce the expression of NK cell receptors such as Ly49E and NKG2 [42], and thereby suppress NK cell responses. In this study, we have shown that overexpression of IL-10 resulted in an increase in the IL-4 mRNA expression, while silencing of IL-10 gene expression resulted in the depletion of IL-4 mRNA expression. Such detrimental effects of TGF- β and IL-4 on NK cells may lessen the sensitivity of IL-10 secreting LSA lymphoma cells to killing by the NK cells.
In addition to the demonstration that immune cells of the host may produce IL-10 during tumor growth, there are also a number of studies that have demonstrated that tumors can also produce IL-10. For example, in breast cancer, the expression of IL-10 was proven to be elevated through immunohistochemistry [43]. In addition, it was shown that patients with leukemias including acute myeloid leukemia (AML) and adult T-cell leukemia/lymphoma (ATLL) exhibited high levels of IL-10 in their serum [44,45]. Elevated IL-10 levels strongly correlated with poor survival of patients with metastatic melanoma [46]. It was also found that mRNA expression of IL-10 was associated with Foxp3 mRNA expression, where both were shown to be significantly up-regulated in PgR-negative or HER2-positive tumors [47]. Also, in one study, it was proposed that IL-10 may increase the metastatic potential of tumors inasmuch as IL-10 was more frequently expressed in metastatic tumors than in primary tumors [48]. The present study supports this concept by experimentally altering the expression levels of IL-10 in the same tumor and demonstrating correlation with in vivo tumor growth.
In earlier studies, we demonstrated that LSA tumor cells constitutively expressed IL-2 and IL-4 and that administration of anti-IL-2 and anti-IL-4 mAbs resulted in the inhibition of LSA growth in vivo [34]. This study suggested that LSA tumor cells use IL-2 and IL-4 as autocrine growth factors to survive and grow. The present study demonstrated that the in vivo tumorigenicity of LSA in the immunocompetent host was additionally dependent on the production of IL-10. Together these studies demonstrate that some T cell lymphomas not only need the autocrine growth factors to survive but also need immunosuppressive cytokines such as IL-10 to evade the immune surveillance of the host. While the majority of studies have indicated that IL-10 produced during tumor growth can cause immunosuppression in the host, it should be noted that in some instances, IL-10 production may also promote anti-tumor immunity. For example, transfected TSA mammary murine tumor cells syngeneic to BALB/c mice that produced high levels of IL-10 were found to be more rapidly eliminated than controls. This was attributed to increased lytic activity mediated by CTLs and NK cells [49]. Such findings can be explained by the fact that the effect of IL-10 may depend on the levels of IL-10 produced [25]. Also, production of high doses of IL-10 may have an inhibitory effect on suppressor mechanisms of the host. For example, Chinese hamster ovary (CHO) cells transfected with mouse IL-10 were rapidly rejected in severe combined immunodeficient (SCID) mice [50]. The authors suggested that in mice injected with wild-type CHO cells there was active infiltration of macrophages, which may have suppressed the immune response, while in CHO cells expressing IL-10 there was a lack of such macrophage infiltration, thereby facilitating tumor rejection potentially by NK cells. It is noteworthy that we have shown previously that LSA tumor cells produce FasL, which can also help this tumor to escape the killing by tumor-specific T cells by inducing apoptosis in T cells when they come into contact with the tumor cells [51,52]. Together, such studies suggest that tumors may use multiple pathways to overcome the immune surveillance mechanisms. A combination of such mechanisms, including IL-10 production, may ultimately facilitate tumorigenesis in vivo and escape from immune surveillance from the host.
Acknowledgments
This work was partly supported by grants from NIH: ES09098, HL058641and AI01392.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Vieira P, de Waal-Malefyt R, Dang MN, et al. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci USA. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse helper T cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 4.deWaal Malefyt R, Haanen J, Spits H, et al. IL-10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II MHC expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen presenting cell to inhibit cytokine production by TH1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 6.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–3139. [PubMed] [Google Scholar]
- 7.Hsu DH, Moore KW, Spits H. Differential effects of interleukin-4 and -10 on interleukin-2-induced interferon-g synthesis and lymphokine-activated killer activity. Int Immunol. 1992;4:563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- 8.Oswald IP, Gazzinelli RT, Sher A, et al. IL-10 synergizes with IL-4 and TGF-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 9.Ralph P, Nakoinz I, Sampson-Johannes A, et al. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992;148:808–814. [PubMed] [Google Scholar]
- 10.Murphy EE, Terres G, Macatonia SE, et al. B7 and interleukin-12 cooperate for proliferation and IFN γ production by mouse Th1 clones that are unresponsive to B7 costimulation. J Exp Med. 1994;80:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Andrea A, Aste AM, Valiante NM, et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber MF, Williams CC, Gerrard TL. Macrophage-colony-stimulating factor expression by anti-CD45 stimulated human monocytes is transcriptionally up-regulated by IL-1 beta and inhibited by IL-4 and IL-10. J Immunol. 1994;152:1354–1361. [PubMed] [Google Scholar]
- 13.Kopydlowski KM, Salkowski CA, Cody MJ, et al. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–1544. [PubMed] [Google Scholar]
- 14.Hart PH, Hunt EK, Bonder CS, et al. Regulation of surface and soluble TNF receptor expression on human monocytes and synovial fluid macrophages by IL-4 and IL-10. J Immunol. 1996;157:3672–3680. [PubMed] [Google Scholar]
- 15.Gray D, Gray M. What are regulatory B cells? Eur J Immunol. 2010;40:2677–2679. doi: 10.1002/eji.201040961. [DOI] [PubMed] [Google Scholar]
- 16.Roncarolo MG, Gregori S, Battaglia M, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 17.Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 18.Berg DJ, Davidson N, Kühn R, et al. Enterocolitis and colon cancer in interleukin- 10-deficient mice are associated with aberrant cytokine production and CD4+TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennick DM, Fort MM. Lessons from genetically engineered animal models XII. IL-10-deficient (IL-10-/-) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:829–833. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- 20.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 21.Eruslanov E, Kaliberov S, Daurkin I, et al. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J Immunol. 2009;182:7548–7557. doi: 10.4049/jimmunol.0802358. [DOI] [PubMed] [Google Scholar]
- 22.Ikeguchi M, Hatada T, Yamamoto M, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. 2009;12:95–100. doi: 10.1007/s10120-009-0509-8. [DOI] [PubMed] [Google Scholar]
- 23.Chau GY, Wu CW, Lui WY, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552–528. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18:171–182. doi: 10.1016/j.cytogfr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Stolina M, Lin Y, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–5028. [PubMed] [Google Scholar]
- 26.Qin Z, Noffz G, Mohaupt M, et al. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony-stimulating factor gene-modified tumor cells. J Immunol. 1997;159:770–776. [PubMed] [Google Scholar]
- 27.Halak BK, Maguire HC, Jr, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–917. [PubMed] [Google Scholar]
- 28.Silini G, Maruyama Y. Studies of the LSA ascites lymphoma of C57BL mice. I. Transplantation characteristics and radiosensitivity of cells during serial passage. J Natl Cancer Inst. 1965;35:841–849. [PubMed] [Google Scholar]
- 29.Nagarkatti M, Toney DM, Nagarkatti PS. Immunomodulation by various nitrosoureas and its effect on the survival of the murine host bearing a syngeneic tumor. Cancer Res. 1989;49:6587–6592. [PubMed] [Google Scholar]
- 30.Nagarkatti M, Clary SR, Nagarkatti PS. Characterization of tumor-infiltrating CD4+ T cells as Th1 cells based on lymphokine secretion and functional properties. J Immunol. 1990;144:4898–4905. [PubMed] [Google Scholar]
- 31.Weiss B, Davidkova G, Zhou LW. Antisense RNA gene therapy for studying and modulating biological processes. Cell Mol Life Sci. 1999;55:334–358. doi: 10.1007/s000180050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagarkatti M, Hassuneh M, Seth A, et al. Constitutive activation of the interleukin-2 gene in the induction of spontaneous in vitro transformation and tumorigenicity of T cells. Proc Natl Acad Sci USA. 1994;91:7638–7642. doi: 10.1073/pnas.91.16.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 34.Hassuneh MR, Nagarkatti PS, Nagarkatti M. Evidence for the participation of IL-2 and IL-4 in the regulation of autonomous growth and tumorigenesis of transformed cells of lymphoid origin. Blood. 1997;89:610–620. [PubMed] [Google Scholar]
- 35.Hammond-McKibben DM, Seth A, Nagarkatti PS, et al. Characterization of factors regulating successful immunotherapy using a tumor-specific cytotoxic T lymphocyte clone:role of interleukin-2, cycling pattern of lytic activity and adhesion molecules. Int J Cancer. 1995;60:828–836. doi: 10.1002/ijc.2910600618. [DOI] [PubMed] [Google Scholar]
- 36.Nagarkatti M, Kaplan AM. The role of suppressor T cells in BCNU-mediated rejection of a syngeneic tumor. J Immunol. 1985;135:1510–1517. [PubMed] [Google Scholar]
- 37.Matsuda M, Salazar F, Petersson M, et al. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar-Onfray F, Petersson M, Franksson L, et al. IL-10 converts mouse lymphoma cells to a CTL-resistant, NK- sensitive phenotype with low but peptide-inducible MHC class I expression. J Immunol. 1995;154:6291–6298. [PubMed] [Google Scholar]
- 39.Rohrer JW, Coggin JH. CD8 T cell clones inhibit antitumor T cell function by secreting IL-10. J Immunol. 1995;155:5719–5727. [PubMed] [Google Scholar]
- 40.Wang L, Goillot E, Tepper RI. IL-10 inhibits alloreactive cytotoxic T lymphocyte generation in vivo. Cell Immunol. 1994;159:152–169. doi: 10.1006/cimm.1994.1304. [DOI] [PubMed] [Google Scholar]
- 41.Trotta R, Col JD, Yu J, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181:3784–3792. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gays F, Martin K, Kenefeck R, et al. Multiple cytokines regulate the NK gene complex-encoded receptor repertoire of mature NK cells and T cells. J Immunol. 2005;175:2938–2947. doi: 10.4049/jimmunol.175.5.2938. [DOI] [PubMed] [Google Scholar]
- 43.Llanes-Fernández L, Arango-Prado MC, Alcocer-González JM, et al. Association between the expression of IL-10 and T cell activation proteins loss in early breast cancer patients. J Cancer Res Clin Oncol. 2009;135:255–264. doi: 10.1007/s00432-008-0446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inagaki A, Ishida T, Ishii T, et al. Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: high interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int J Cancer. 2006;118:3054–3061. doi: 10.1002/ijc.21688. [DOI] [PubMed] [Google Scholar]
- 45.Tsimberidou AM, Estey E, Wen S, et al. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer. 2008;113:1605–1613. doi: 10.1002/cncr.23785. [DOI] [PubMed] [Google Scholar]
- 46.Nemunaitis J, Fong T, Shabe P, et al. Comparison of serum interleukin-10 (IL-10) levels between normal volunteers and patients with advanced melanoma. Cancer Invest. 2001;19:239–247. doi: 10.1081/cnv-100102550. [DOI] [PubMed] [Google Scholar]
- 47.Ohara M, Yamaguchi Y, Matsuura K, et al. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol Immunother. 2009;58:441–447. doi: 10.1007/s00262-008-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dummer W, Bastian BC, Ernst N, et al. Interleukin-10 production in malignant melanoma: preferential detection of IL-10-secreting tumor cells in metastatic lesions. Int J Cancer. 1996;66:607–610. doi: 10.1002/(SICI)1097-0215(19960529)66:5<607::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Giovarelli M, Musiani P, Modesti A, et al. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol. 1995;155:3112–3123. [PubMed] [Google Scholar]
- 50.Richter G, Krüger-Krasagakes S, Hein G, et al. Interleukin 10 transfected into Chinese hamster ovary cells prevents tumor growth and macrophage infiltration. Cancer Res. 1993;53:4134–4137. [PubMed] [Google Scholar]
- 51.Zeytun A, Hassuneh M, Nagarkatti M, et al. Fas-Fas ligand-based interactions between tumor cells and tumor-specific cytotoxic T lymphocytes: a lethal two way street. Blood. 1997;90:1952–1959. [PubMed] [Google Scholar]
- 52.Zeytun A, Nagarkatti M, Nagarkatti PS. Growth of FasL-bearing tumor cells in syngeneic murine host induces apoptosis and toxicity in Fas(+) organs. Blood. 2000;95:2111–2117. [PubMed] [Google Scholar]


