Abstract
Alveolar macrophages (AM) represent important effector cells in the innate immune response to the AIDS-related pathogen Pneumocystis, but the early AM host defense signaling events are poorly defined. Using AM from healthy individuals, we showed in the present study that Pneumocystis organisms stimulate AM NF-κB p50 and p65 nuclear translocation in a time-dependent and multiplicity-of-infection-dependent manner as determined by electrophoretic mobility shift assay and immunofluorescence microscopy and that NF-κB nuclear translocation is associated with I-κB phosphorylation. Importantly, competitive inhibition of mannose receptor and targeted short interfering RNA-mediated gene suppression of mannose receptor mRNA and protein is associated with complete elimination of NF-κB nuclear translocation in response to Pneumocystis. Furthermore, human immunodeficiency virus (HIV) infection of AM (as a model human disease state of reduced AM mannose receptor expression and function) inhibits Pneumocystis-mediated NF-κB nuclear translocation and is associated with reduced I-κB phosphorylation and reduced interleukin-8 (IL-8) release. In contrast, NF-κB nuclear translocation and IL-8 release in response to lipopolysaccharide are intact in AM from both healthy and HIV-infected individuals, indicating that the observed impairment is not a global disturbance of the NF-κB pathway. Thus, in addition to phagocytic and endocytic effector functions, the present study identifies mannose receptors as pattern recognition receptors capable of NF-κB activation in response to infectious non-self challenge. AM mannose receptor-mediated NF-κB activation may represent an important mechanism of the host cell response to Pneumocystis, and altered NF-κB activation in the context of HIV infection may impair a critical innate immune signaling response and may contribute to pathogenesis of opportunistic lung infections.
Life-threatening Pneumocystis pneumonia frequently complicates human immunodeficiency virus (HIV) infection, although the underlying mechanisms contributing to disease pathogenesis remain incompletely understood. Alveolar macrophages (AM) represent the predominant host defense cell in the alveolar airspace, and experimental data support the concept that AM contribute to an effective host response to Pneumocystis (2, 21, 22, 30, 31, 41, 44, 51, 52). AM from healthy individuals bind and phagocytose Pneumocystis (16, 25), and recognition likely occurs via ligation of pathogen-associated molecular patterns on the surfaces of the organisms (such as Pneumocystis glycoprotein A) (39) by macrophage pattern recognition receptors of innate immunity such as mannose receptors (16). Macrophage receptor ligation by unopsonized Pneumocystis results in a respiratory burst (26) and release of interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) (24), although the specific intracellular signaling pathways activated by Pneumocystis have not been completely investigated (17). In contrast to those from healthy individuals, macrophages from HIV-infected subjects at high clinical risk for Pneumocystis pneumonia demonstrate select defects in Pneumocystis binding and phagocytosis (25) and a reduced respiratory burst in response to Pneumocystis (26). Furthermore, in vitro HIV infection of macrophages attenuates TNF-α release in response to Pneumocystis (24), suggesting that innate receptor-mediated signal transduction pathways may be altered by HIV infection.
Nuclear factor κB (NF-κB) is a central mediator of gene transcription for a variety of critical cellular responses (19) and represents an important signal transduction pathway of the innate immune response to infectious challenge (46). NF-κB is complexed with inhibitory I-κB proteins in the cytoplasm. Following appropriate stimulation, phosphorylation of I-κB by I-κB kinase (IKK) allows dissociation and nuclear translocation of NF-κB. In AM from healthy individuals, NF-κB is activated following lipopolysaccharide (LPS) stimulation (4, 33), and this response appears to be intact in AM from asymptomatic HIV-infected persons (33). A recent investigation demonstrated that a β-glucan component of Pneumocystis can activate NF-κB in murine RAW 264.7 macrophages, although the receptor mediating NF-κB signaling was not identified (28). However, human AM NF-κB activation in response to unopsonized Pneumocystis organisms has not been previously examined, the receptor mediating NF-κB activation has not been identified, and the influence of HIV infection on this particular pathway has not been investigated. To explore the mechanisms for Pneumocystis pneumonia pathogenesis, this study examined the NF-κB response of human AM to Pneumocystis organisms in vitro and investigated the influence of HIV infection on Pneumocystis-mediated NF-κB signaling.
MATERIALS AND METHODS
Study subjects.
Prospectively recruited healthy and asymptomatic HIV-seropositive individuals were without evidence for active pulmonary disease and had normal spirometry. Healthy individuals were without known risk factors for HIV infection and were confirmed to be HIV seronegative by enzyme-linked immunosorbent assay (ELISA), which was performed according to the instructions of the manufacturer (Abbott Diagnostics, North Chicago, Ill.). Demographic characteristics for all participants were recorded on standardized forms and included age, gender, smoking status, HIV risk factor, medical history, and prescribed antiretroviral medications.
Bronchoscopy.
Pulmonary immune cells were obtained by bronchoalveolar lavage (BAL) using standard technique as previously described (25). All procedures were performed on consenting adults following protocols approved by the Beth Israel Deaconess Medical Center institutional review board. The cells were separated from the pooled BAL fluid as previously described (25) and counted on a hemacytometer with light microscopy.
Human AM.
For flow cytometry measurements, BAL cell suspensions were used directly. For other determinations, AM were isolated by adherence to plastic-bottom tissue culture plates (3 × 106 cells/well in six-well plates) for electrophoretic mobility shift assay (EMSA) and Western blotting or to 13-mm-diameter round glass coverslips (2.5 × 105 cells/coverslip in 24-well plates) for NF-κB nuclear translocation as previously described (25). Isolation of AM from all healthy and HIV-seropositive persons yielded cells which were ≥98% viable as determined by trypan blue dye exclusion and demonstrated >95% positive nonspecific esterase staining.
Rat AM.
For selected experiments to validate the response of human AM to rat-derived Pneumocystis, rat AM were obtained from euthanatized adult female CD rats (225 to 250 g) by tracheo-BAL with a Teflon 18-gauge catheter (Critikon, Tampa Bay, Fla.). Lungs were lavaged with 8-ml aliquots of sterile phosphate-buffered saline (PBS) at room temperature (RT) for a total of eight washes, the lavaged fluid was pooled and centrifuged at 300 × g for 10 min, and the cell pellet was resuspended in RPMI 1640 medium with penicillin and streptomycin. Cell count and viability were determined by hemacytometer counting and trypan blue exclusion (Sigma Chemical Co., St. Louis, Mo.). AM were isolated by adherence in six-well plates (3 × 106 cells/well for 3 h at 37°C in 5% CO2) and washed, and the medium was replaced with fresh complete RPMI 1640 medium with 10% fetal bovine serum.
AM innate immune receptor stimulation.
To examine the NF-κB signaling pathway in response to AM innate immune receptor ligation, LPS (Sigma), zymosan (Sigma), and Pneumocystis organisms were used. LPS is a recognized inducer of NF-κB in AM (4) and served as a positive control. Unopsonized zymosan and Pneumocystis interact with macrophage innate immune receptors (16, 23).
Since sustainable cultivation of Pneumocystis is not possible and Pneumocystis derived from human disease (P. jiriveci [49]) is rarely available, P. carinii organisms were obtained from male Lewis rats (University of Cincinnati Lab Animal Medicine Facility, Cincinnati, Ohio) that were chronically immunosuppressed by weekly subcutaneous injections of Depo-Medrol (Pharmacia & Upjohn, Kalamazoo, Mich.) at a dose of 4 mg/kg/0.2 ml (7, 10). The infection is established by intratracheal inoculation of 106 P. carinii nuclei/0.2 ml of PBS into rats that receive 2 weeks of immunosuppressive pretreatment (11). Rats were sacrificed 8 weeks after inoculation, and organisms were purified from host lung tissue and cryopreserved as previously described (5, 7). Organisms were cocultured on an adherent A549 (ATCC CCL 185) feeder cell monolayer for 24 h to disperse the clumps (8). Isolated P. carinii organisms were quantified by enumeration of nuclei stained with a modified Giemsa stain, and the viability was verified by an ATP bioluminescence assay (5). These mixed-life-cycle preparations yielded approximately 90% trophozoite and 10% cyst forms and were characterized by contour-clamped homogeneous electric field analysis as karyotype form 1 (9). The organism preparations were then cryopreserved as previously described (7). Pneumocystis viability was >85%, and cryopreservation did not significantly affect organism viability. Prior to use, cryovials containing the organisms were rapidly thawed in a 37°C water bath, and the organisms were isolated by centrifugation, washed extensively with PBS, and then added to cultures at specified concentrations. P. carinii preparations were relatively free of contaminating rat-derived proteins (27), and preparations were endotoxin free (<1.0 endoxotin U/ml) as determined by E-toxate Limulus polyphemus assay (Sigma).
In vitro HIV-1 infection of AM from healthy individuals.
To assess the direct effect of HIV-1 infection on macrophage innate immune receptor surface expression and receptor-mediated signal transduction, AM from healthy individuals were infected in vitro as previously described (25, 43), utilizing a monocytotropic (R5) isolate (HIV Bal). Briefly, AM in ultra-low-binding plates (Corning Inc.) (3 × 106 cells/well of six-well plates) for flow cytometry or adherent AM (3 × 106 cells/well of six-well plates for EMSA and Western blotting and 1 × 105 cells/well for P. carinii binding and NF-κB nuclear translocation) were incubated with the HIV-1 isolate (>105 pg of HIV p24/ml) for 2 h at 37°C, washed five times to remove free virus, and maintained in complete culture medium for 2 weeks. Uninfected AM were also maintained as controls. The culture medium was changed every 3 to 4 days, and productive HIV-1 infection was verified by the measurement of HIV p24 antigen in the culture supernatants by ELISA (Dupont, Boston, Mass.).
siRNA gene silencing of mannose receptor.
To determine the specific contribution of AM mannose receptors to Pneumocystis-mediated NF-κB signaling, AM were isolated by adherence in six-well tissue culture plates (3 × 106 cells/well) and cultured for 48 to 72 h prior to gene silencing. Specific short interfering RNAs (siRNAs) were transfected into AM by using TransMessenger transfection reagent (Qiagen, Valencia, Calif.) according to the manufacturer's protocol. Briefly, 2 to 4 μg of appropriate siRNA was mixed with 4 μl of Enhancer R (specific RNA-condensing reagent) and 100 μl of buffer EC-R (RNA-condensing buffer), incubated at RT for 5 min, and spun down, and the supernatant was isolated. Eight microliters of TransMessenger transfection reagent was then added to the supernatant, and the mixture was vortexed and incubated for 10 min at RT. The entire siRNA reaction mixture was then added to a well of AM containing 900 μl of RPMI 1640 medium without serum or antibiotics and incubated for 3 to 4 h at 37°C with 5% CO2. The cells were then washed with sterile PBS; complete RPMI 1640 medium with 10% fetal bovine serum, 1% penicillin, streptomycin, and amphotericin was added; and the cells were incubated for 60 h at 37°C in 5% CO2. The following oligonucleotides (Qiagen) were used: (i) for human mannose receptor siRNA1 sequences (annealed double-stranded siRNA), DNA target sequence AAGGGATCGGGTTTATGGAGC from bp 389 to 410, 5′-GGGAUCGGGUUUAUGGAGC-3′, and 3′-GCUCCAUAAACCCGAUCCC-5′; (ii) for human mannose receptor siRNA2 sequences (annealed double-stranded siRNA), DNA target sequence AAATCCGCTTTAACGTGGCAC from bp 702 to 723 bp, 5′-AUCCGCUUUAACGUGGCAC-3′, and 3′-UAGGCGAAAUUGCACCGUG-5′; (iii) for human mannose receptor siRNA3 sequences (annealed double-stranded siRNA), DNA target sequence AAGTGGTACGCAGATTGCACG from bp 528 to 549, 5′-GUGGUACGCAGAUUGCACG-3′, and 3′-CGUGCAAUCUGCGUACCAC-5′; (iv) for laminarin A/C siRNA sequences (annealed double-stranded siRNA), (CUGGACUUCCAGAAGAACA)d(TT) and R(UGUUCUUCUGGAAGUCCAG)d(TT); (v) for single-stranded mannose receptor sequences, DNA target sequence AAGTGGTACGCAGATTGCACG, r(GUGGUACGCAGAUUGCACG)d(TT) (sense), and r(CGUGCAAUCUGCGUACCAC)d(TT) (antisense); and (vi) for control mannose receptor siRNA (nonsilencing) and transfection efficiency control siRNA for mannose receptor siRNA transfection, DNA target sequence AATTCTCCGAACGTGTCACGT and siRNA duplex sequences 5′-rhodamine(TAMRA)-r(UUCUCCGAACGUGUCACGU)d(TT) (sense)-r(ACGUGACACGUUCGGAGAA)d(TT) (antisense) and 5′-fluorescein-r(UUCUCCGAACGUGUCACGU)d(TT) (sense)-r(ACGUGACACGUUCGGAGAA)d(TT) (antisense).
Double-stranded siRNA1, siRNA2, and siRNA3 specifically targeted different domains of the mannose receptor. Double-stranded laminarin siRNA and single-stranded mannose receptor siRNA were used as controls to examine specificity of gene silencing. The nonsilencing rhodamine-labeled siRNA was used to determine transfection efficiency.
Immunofluorescence microscopy detection of NF-κB p65 nuclear translocation.
To demonstrate AM NF-κB nuclear translocation, isolated AM on glass coverslips in 24-well culture plates (2.5 × 105 cells/coverslip) were incubated with LPS or Pneumocystis at 37°C in 5% CO2 for 1 h, rinsed twice with PBS, fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, Pa.) in PBS for 10 min at RT, and permeabilized with Tween 20 for 10 min at RT. The AM were treated with 1% bovine serum albumin (BSA) in PBS for 1 h to reduce nonspecific staining and then incubated overnight with unconjugated rat anti-human NF-κB p65 polyclonal antibody (1:100 dilution) (Santa Cruz Biotechnology, Inc.), washed, and incubated with Cy3-conjugated goat anti-rabbit immunoglobulin G secondary antibody (1:100) (Sigma) for 1 h at 37°C. The cells were counterstained with the nucleic acid stain DAPI (4′,6′-diamidino-2-phenylindole) (1:500) (Molecular Probes, Eugene, Oreg.) for 30 min at RT. After being washed five times with PBS, the coverslips were mounted with Mowiol 4-88 (Hoechst Celanese, Somerville, N.J.), and images were captured with a fluorescence microscope equipped with a digital camera (Nikon Eclipse E800; Diagnostic Instruments, Sterling Heights, Mich.). Controls included cells stained with primary or secondary antibody only.
Mannose receptor mRNA detection by reverse transcriptase-PCR (RT-PCR).
Reverse transcription reactions were performed with a first-strand cDNA synthesis kit and a PCR core kit (Roche, Indianapolis, Ind.) according to the manufacturer's instructions. The primers 5′-TGGCAACTGGGCTCTAATCT-3′ and 5′-ATACTTGTGAGGTCACCGCC-3′ were used for PCR. The PCR conditions for the first-strand cDNA synthesis were 35 cycles of 96°C for 1 min, 57°C for 1 min, and 42°C for 60 min. The PCR conditions for amplification of specific DNA fragments were 30 cycles of 94°C for 30 s, 65°C for 1 min, and 72°C for 3 min. PCR products were resolved on a 1.2% agarose gel.
Quantitative analysis of mannose receptor mRNA by real-time RT-PCR.
Total RNA was isolated from AM by using RNAzol according to the instructions of the manufacturer (Invitrogen, Carlsbad, Calif.). Amplification of the human mannose receptor was performed with the following PCR cycle sequence: 30 min at 50°C (stage 1), 15 min at 95°C (stage 2), and 15 s at 94°C followed by 60 s at 60°C (stage 3) (repeated for 40 cycles). Detection was performed with an ABI Prism 7700 sequence detector (ABI, Foster City, Calif.). The primers used for quantitative real-time PCR were 5′-CGCTACTAGGCAATGCCAATG-3′ (forward) and 5′-CGTGCAATCTGCTACCACT-3′ (reverse). The TaqMan probe sequence is AGCAACCTGTGCATTCCCGTTCAAGTT. Data were normalized to human 18S rRNA (internal control) labeled with VIC/TAMRA (ABI). Results of the real-time PCR data were represented as comparative Ct values (defined as the threshold cycle of PCR at which amplified product is first detected). Data are expressed as the fold induction compared to unstimulated cells.
EMSA.
The EMSA studies used a nonradioactive (biotin label) gel shift assay to investigate protein-DNA interactions. Nuclear extracts of the AM were prepared by using the NE-PER kit (Pierce, Rockford, Ill.) according to the manufacturer's protocol. The double-stranded 5′-biotin-labeled NF-κB oligonucleotide probe (consensus sequence 5′-AGTTGAGGGGACTTTCCCAGGC-3′; Panomics, Inc., Redwood City, Calif.) was incubated with the nuclear extract in 1× binding buffer-5 mM MgCl2-50 μl of poly(dI · dC)-0.05% NP-40 for 20 min. Following addition of 5 μl of sample buffer, the mixture was then resolved by gel electrophoresis (5% polyacrylamide) at 100 V for 1.5 h. Gels were transferred to a nylon membrane at 380 mA for 1 h, followed by UV cross-linking with 254-nm bulbs, and the bands were detected by using a light shift chemiluminescent EMSA kit (Pierce). To validate the gel shift assay, selected experiments used the I-κB phosphorylation inhibitor Bay 11-7082 (10 μM) (Biomol Research Laboratories, Inc., Plymouth Meeting, Pa.) (36). Quantitative analysis was performed by densitometry (Molecular Dynamics)
EMSA with supershift assay.
The procedures for the supershift experiments were conducted as described above, with the exception that 2 μg of anti-p50, -p65, -p52, -RelB, and -c-Rel (Geneka, Montreal, Canada) antibodies or normal control serum was incubated with the nuclear cell extract for 30 min on ice before the addition of the biotin-end-labeled oligonucleotide probe. The samples were then treated as described above.
Western blotting.
AM cell cytoplasmic protein extracts were prepared with the NE-PER kit (Pierce) according to the manufacturer's protocol. Western blotting was performed by utilizing a standard protocol (12). Briefly, the cytoplasmic extracts were diluted (2:1) in Laemmli sample buffer, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to an Immobilon polyvinylidene difluoride membrane (Bio-Rad, Hercules, Calif.). The membrane was washed with Tris-buffered saline-Tween 20 (TTBS), blocked in a solution of Tris-buffered saline containing 5% nonfat dry milk, and then washed twice. The membrane was then incubated with anti-I-κBα protein and anti-phosphorylated I-κBα protein antibodies (Santa Cruz) overnight at 4°C, washed three times with TTBS, and incubated with the secondary antibody conjugate solution (1:3,000) for 2 h. Samples were washed three times with TTBS, and then the signal was detected with the Immuno-Star chemiluminescent protein detection system (Bio-Rad). Protein concentration were determined by the Bio-Rad protein assay with bovine serum albumin as a standard.
Flow cytometry determination of surface receptor expression.
Human AM mannose receptor and CD14 receptor surface expression was analyzed on specimens of BAL cell suspensions with an Epics XL flow cytometer (Beckman-Coulter, Miami, Fla.) with a laser power of 5.76 mW. The instrument was calibrated before each measurement with standardized fluorescent particles (Immunocheck; AMAC, Inc., Westbrook, Maine). AM cell size and cell granularity were expressed by forward and right-angle (side) light scatter, respectively. Fluorescent signals of the cells were measured simultaneously with three photomultiplier tubes and optical filters and expressed as the mean of the log fluorescence intensity of the cell population within the gate. AM were labeled with primary phycoerythrin (PE)-conjugated antibodies to human mannose receptor and CD14 receptor (Beckman-Coulter) for 1 h at 4°C in the dark, washed twice with balanced salt solution with calcium and magnesium in 0.1% bovine serum albumin, fixed with 250 μl of OptiLyse C buffer (Beckman-Coulter) diluted with PBS, and analyzed by flow cytometry. AM were first identified by characteristic forward and side scatter parameters on unstained AM, and the population was confirmed by staining with PE-conjugated primary anti-human HLA-DR (Beckman-Coulter). The results were recorded as mean relative fluorescence units (RFU) and the percentage of the population staining positive. Negative control cells were stained with isotype control primary conjugated antibodies. Samples were prepared and analyzed in duplicate, and a minimum of 5,000 cells were counted for each sample.
Flow cytometry detection of intracellular HIV-1 p24 core antigen.
BAL cell suspensions were used for flow cytometry detection for intracellular staining for HIV-1 p24 antigen by monoclonal antibody KC57 conjugated with fluorescein isothiocyanate (Beckman-Coulter) according to the manufacturer's protocol. Briefly, 0.5 × 106 to 1.0 × 106 cells per condition were incubated in 1 ml of a mixture of 20 μg of lysolecithin per ml and 1% paraformaldehyde for 2 min at RT and centrifuged at 500 × g for 5 min at 4°C, and the cell pellet was incubated in 2 ml of cold (−20°C) absolute methanol on ice for 15 min and washed twice with 1 ml of cold 0.1% NP-40. The cell pellet was incubated with 5 μl of monoclonal antibody or isotype control in PBS for 15 min at RT and washed twice with 1 ml of PBS, with final resuspension in PBS. Specimens were analyzed by flow cytometry. AM were first identified by characteristic forward and side scatter parameters on unstained cells. The percentage of positive-staining cells compared to the control was recorded. Positive-staining cells were recorded as mean RFU. Samples were prepared and analyzed in duplicate, and a minimum of 5,000 cells were counted for each sample.
IL-8 detection by ELISA.
Human and rat AM were incubated with P. carinii or LPS for 24 h at 37°C with 5% CO2. Culture supernatants were harvested and centrifuged to remove cellular debris, and aliquots were stored at −80°C until assayed. Specific immunoreactivity for human IL-8 (R&D Systems, Minneapolis, Minn.) or rat CINC-1 (analogue to human IL-8; Assay Designs, Inc., Ann Arbor, Mich.) in culture supernatants was measured by ELISA according to the manufacturer's protocol. Samples were assayed in duplicate on a Biotek plate reader and quantitated with a standard curve. The lower limit of detectability for IL-8 is 10 pg/ml, and that for CINC-1 is 2 pg/ml.
Statistical analysis.
Experiments were performed in duplicate or triplicate and were repeated with AM from at least three different individuals (or animals), except as noted. Data were analyzed with an Apple G3 Power PC computer with StatView (SAS Institute, Inc., Cary, N.C.) and INSTAT2 (Graph Pad Software, San Diego, Calif.) statistical software. Nonparametric data were analyzed by analysis of variance. A P value of <0.05 was considered statistically significant.
RESULTS
Study subjects.
Bronchoscopy was performed on 34 subjects, including 24 healthy individuals (mean age, 35 ± 15 years; including 7 females and 13 smokers) and 10 asymptomatic HIV-infected subjects (mean age, 32 ± 12 years; including1 female and 7 smokers). None of the individuals had evidence of active respiratory disease. For the HIV-infected subjects, peripheral blood CD4 lymphocytes counts were 230 to 595 cells/mm3, HIV risk factors included intravenous drug abuse and homosexual exposures, all were prescribed highly active antiretroviral therapy, all had an undetectable serum viral load (<200 HIV-1 RNA copies/ml), and none had a prior opportunistic pneumonia.
NF-κB nuclear translocation in AM from healthy individuals.
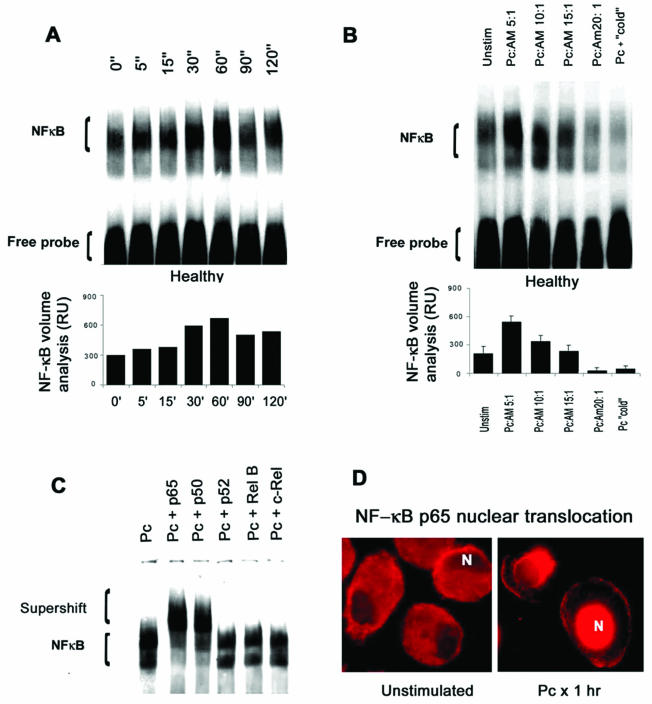
To determine the optimal time point for AM NF-κB nuclear translocation in response to unopsonized Pneumocystis, AM from healthy individuals (n = 3) were incubated with unopsonized organisms for 0 to 120 min and nuclear translocation was determined by EMSA (Fig. 1A). By quantitative analysis, nuclear translocation of NF-κB was optimal by 60 min, so all subsequent experiments were performed for 60 min. Nuclear translocation of NF-κB was found to be optimal at a multiplicity of infection (MOI) of 5:1 (Pneumocystis cells to AM) by quantitative analysis (Fig. 1B). Data from EMSA with supershift were obtained for five healthy subjects. In all cases, following Pneumocystis stimulation, the NF-κB DNA binding complex was composed predominantly of p50 and p65 subunits (Fig. 1C). Immunofluorescence microscopy demonstrated NF-κB p65 localization predominantly in the cytoplasm of unstimulated AM and confirmed NF-κB p65 translocation to the nucleus in response to Pneumocystis (Fig. 1D).
FIG. 1.
AM NF-κB nuclear translocation in response to Pneumocystis organisms. (A) Time course for NF-κB nuclear translocation in AM from healthy individuals in response to Pneumocystis. The gel is from one healthy individual and is representative of three subjects examined. AM were incubated with unopsonized Pneumocystis for the indicated times, and EMSA was performed on AM nuclear extracts with a biotinylated (nonradioactive) NF-κB probe. Quantitative analysis (n = 3) performed by densitometry (values are means ± standard errors of the means) indicates optimal NF-κB nuclear translocation at 60 min. RU, relative units. (B) AM NF-κB nuclear translocation with different Pneumocystis MOIs. The gel is from one healthy individual and is representative of three subjects examined. AM were incubated with Pneumocystis (Pc) organisms for 1 h. Quantitative analysis performed by densitometry (values are means ± standard errors of the means) demonstrates optimal NF-κB nuclear translocation at an MOI of 5:1 (Pneumocystis/AM ratio). Unstim, unstimulated. (C) Detection of NF-κB subunits in response to Pneumocystis organisms (n = 5). EMSA with supershift from one healthy subject is shown and is representative of five subjects examined. The results demonstrate a supershift of NF-κB bands in the presence of antibodies directed against NF-κB p50 and p65 subunits but not p52, RelB, or c-Rel. (D) AM nuclear translocation of NF-κB detected by fluorescence microscopy with Cy3 (red) staining following a specific antibody recognizing the NF-κB p65 subunit. In unstimulated AM, the p65 staining is predominantly cytoplasmic, whereas incubation with Pneumocystis organisms for 1 h results in nuclear localization of NF-κB p65 staining. N, nucleus.
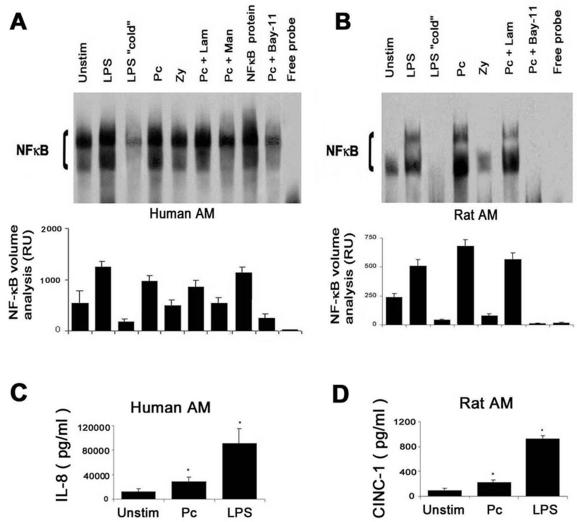
To further characterize the Pneumocystis-mediated AM NF-κB activation, EMSA data were then obtained for AM from 11 healthy subjects. Adherent unstimulated AM demonstrated moderate constitutive nuclear translocation of NF-κB (Fig. 2A). Following LPS stimulation for 1 h, NF-κB nuclear translocation was increased in all subjects. As a control for the assay, recombinant human NF-κB was included in one lane to confirm the resolution of the NF-κB band. As an additional control, the NF-κB signal was undetectable in the presence of a 50-fold excess of unlabeled NF-κB probe (i.e., cold probe), free probe (Fig. 2A), or an irrelevant probe (AP-1) (data not shown).
FIG. 2.
Characterization of NF-κB nuclear translocation in human and rat AM in response to innate immune receptor stimulation. (A) The gel is from one healthy subject and is representative of the 11 subjects. Isolated AM were incubated with LPS, Pneumocystis (Pc), or zymosan (Zy) for 1 h and compared to unstimulated (Unstim) AM. AM were also stimulated with Pneumocystis in the absence and presence of inhibitors of mannose receptor (mannan [Man], 1 mg/ml) and β-glucan receptor (laminarin [Lam], 500 μg/ml). Nuclear extracts were isolated and resolved by EMSA. The lane labeled “cold” refers to competition of the labeled probe with a 50-fold excess of unlabeled NF-κB probe, and the lane labeled “free probe” contains biotinylated NF-κB probe but no AM nuclear extract. NF-κB protein represents recombinant human NF-κB (positive control for the EMSA). Bay-11 is an I-κB inhibitor. Quantitative analysis was performed by densitometry, representing combined data from 11 subjects, and results are expressed as relative units (RU) (values are means ± standard errors of the means). (B) The gel is from one rat experiment and is representative of two experiments. Quantitative analysis was performed by densitometry, and results are expressed as relative units (values are mean ± standard errors of the means). (C) IL-8 release into culture supernatants by human AM in response to rat-derived Pneumocystis or LPS after 24 h (n = 3). Values are mean ± standard errors of the means. (D) Release of CINC-1 (an analogue to human IL-8) into culture supernatants by rat AM in response to rat-derived Pneumocystis or LPS after 24 h (n = 3). Values are mean ± standard errors of the means.
NF-κB nuclear translocation was increased following incubation with unopsonized Pneumocystis. In the presence of mannan (a competitive inhibitor of the mannose receptor), NF-κB nuclear translocation in response to Pneumocystis was reduced to levels similar to those for unstimulated AM. In the presence of laminarin (a competitive inhibitor of the β-glucan receptor [3]), NF-κB nuclear translocation was not significantly reduced in response to Pneumocystis. Pneumocystis-mediated NF-κB nuclear translocation was also decreased in the presence of Bay 11-7082 (which inhibits NF-κB nuclear translocation by inhibiting I-κBα phosphorylation). Incubation of AM with mannan or laminarin alone (i.e., without Pneumocystis) did not significantly influence NF-κB nuclear translocation (data not shown). Collectively, these data demonstrate that unopsonized Pneumocystis activates nuclear translocation of AM NF-κB p50 and p65 subunits in a manner dependent on time and Pneumocystis MOI. Inhibition of Pneumocystis-mediated NF-κB nuclear translocation by mannan (but not laminarin) suggests a role for the mannose receptor.
To validate the use of rat-derived Pneumocystis organisms in experiments with human AM, selected experiments were performed with rat AM. Similar to the case for human AM, Pneumocystis increased NF-κB nuclear translocation in rat AM, and NF-κB nuclear translocation was inhibited by Bay-11-7082 and was not influenced by laminarin pretreatment (Fig. 2B). Furthermore, release of the NF-κB-regulated chemokine IL-8 or CINC-1 (the rat analogue of human IL-8) in response to Pneumocystis or LPS was significantly increased, and there were similar patterns for human AM and rat AM (Fig. 2C and D). LPS was a more potent stimulus for AM IL-8 release. These findings suggest that rat-derived Pneumocystis serves as a valid model to investigate human AM NF-κB nuclear translocation.
Influence of mannose receptor gene silencing by siRNA interference on AM NF-κB nuclear translocation.
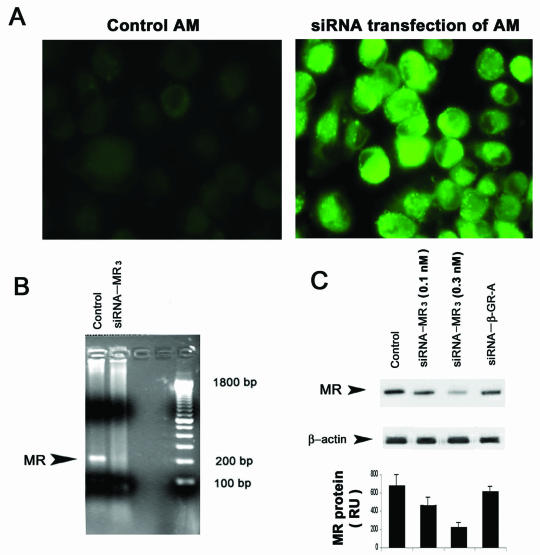
To examine the specific role of the mannose receptor in Pneumocystis-mediated NF-κB nuclear translocation, functional “knock-down” experiments were performed by use of sequence-specific, posttranslational gene silencing with siRNA (14) to specifically target macrophage mannose receptors in AM from healthy individuals. First, the use of fluorescence-labeled nonsilencing siRNA demonstrated that the siRNA oligonucleotides efficiently transfect AM from healthy individuals (Fig. 3A). The siRNA treatment did not significantly influence AM viability. Next, testing of the three 21- to 22-nucleotide siRNA constructs demonstrated that siRNA-MR3 provided the most robust RNA silencing effect on human mannose receptors (data not shown). Next, using siRNA-MR3 on AM from healthy individuals, mannose receptor mRNA was nearly completely suppressed (Fig. 3B). Furthermore, siRNA-MR3 significantly reduced AM mannose receptor protein in a dose-dependent manner in these same AM (Fig. 3C).
FIG. 3.
siRNA gene silencing of the mannose receptor in AM from healthy individuals. (A) Transfection of isolated AM with fluorescein-labeled (nonsilencing) siRNA demonstrates efficient transfection of healthy AM compared to nontransfected controls. (B) Detection of mannose receptor mRNA by RT-PCR in AM following transfection with gene-silencing siRNA targeted to the mannose receptor (siRNA-MR3). The control was AM transfected with blank vector. (C) Detection of mannose receptor protein in AM following transfection with siRNA-MR3 at various concentrations. Gene-silencing siRNA directed against the β-glucan receptor (siRNA-βGRA) does not influence mannose receptor expression.
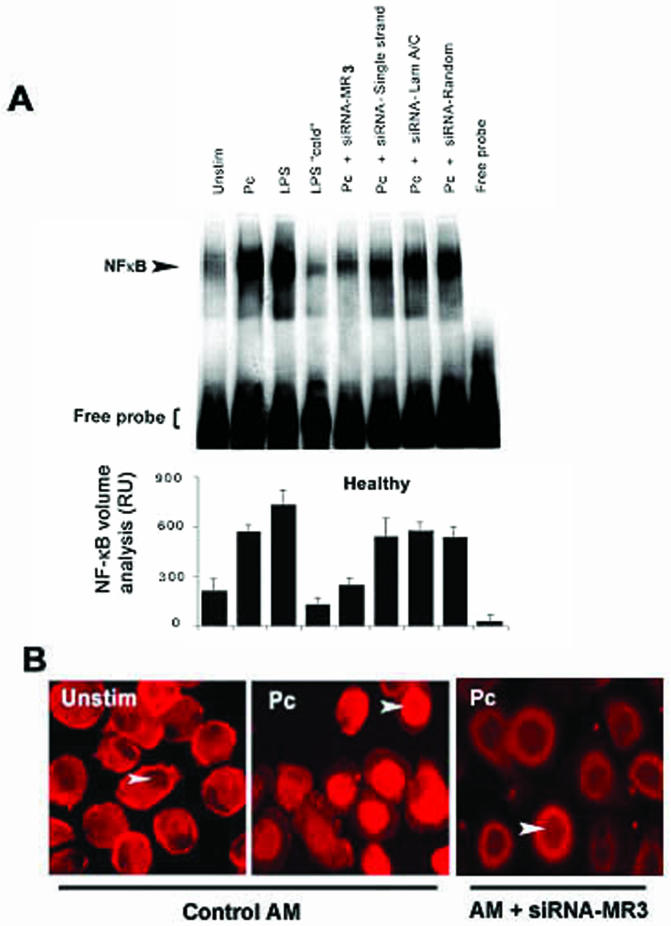
To determine the contribution of the mannose receptor to AM NF-κB nuclear translocation, AM NF-κB nuclear translocation in AM from healthy individuals was assessed by EMSA with the mannose receptor gene silencing siRNA. In the absence of siRNA-MR3, LPS and Pneumocystis increased nuclear translocation of NF-κB at 1 h (Fig. 4A). However, in the presence of siRNA targeted against the mannose receptor (siRNA-MR3), no increase in NF-κB nuclear translocation was observed compared to unstimulated AM (Fig. 4A, compare lane Pc to lane Pc + siRNA-MR3). As a control to determine specificity for the gene suppression experiments, no influence on Pneumocystis-mediated NF-κB nuclear translocation was observed with single-stranded siRNA, laminarin A/C-targeted siRNA, or random siRNA (Fig. 4A). Furthermore, Pneumocystis-mediated NF-κB p65 nuclear translocation was impaired following siRNA-MR3 gene silencing as determined by immunofluorescence microscopy (Fig. 4B). These experiments suggest that healthy AM NF-κB nuclear translocation in response to Pneumocystis organisms is mediated predominately through the macrophage mannose receptor.
FIG. 4.
AM NF-κB nuclear translocation following mannose receptor gene silencing. (A) The EMSA gel is for AM from one healthy individual and is representative of four subjects examined. Pc, Pneumocystis; “cold,” cold competition with unlabeled NF-κB probe; siRNA-MR3, siRNA double-stranded oligonucleotide that provides the most robust gene silencing of the human mannose receptor. Controls include single-stranded siRNA oligonucleotides, siRNA oligonucleotides directed against laminarin A/C (Lam A/C), and random siRNA oligonucleotides. Quantitative analysis was performed by densitometry, and values are expressed as relative units (RU) (values are means ± standard errors of the means). (B) Detection of NF-κB p65 nuclear translocation in AM by immunofluorescence microscopy. Representative microscopic fields of unstimulated human AM (Unstim), demonstrating predominantly cytoplasmic localization of NF-κB p65 (red), and of healthy AM following exposure to Pneumocystis for 1 h, demonstrating predominantly nuclear localization of NF-κB p65, are shown. In contrast, siRNA-MR3 gene silencing of the mannose receptor in healthy AM demonstrates relatively impaired NF-κB p65 nuclear translocation in response to Pneumocystis. Control AM contain blank vector. Arrowheads indicate AM nuclei.
NF-κB nuclear translocation in AM from HIV-infected individuals.
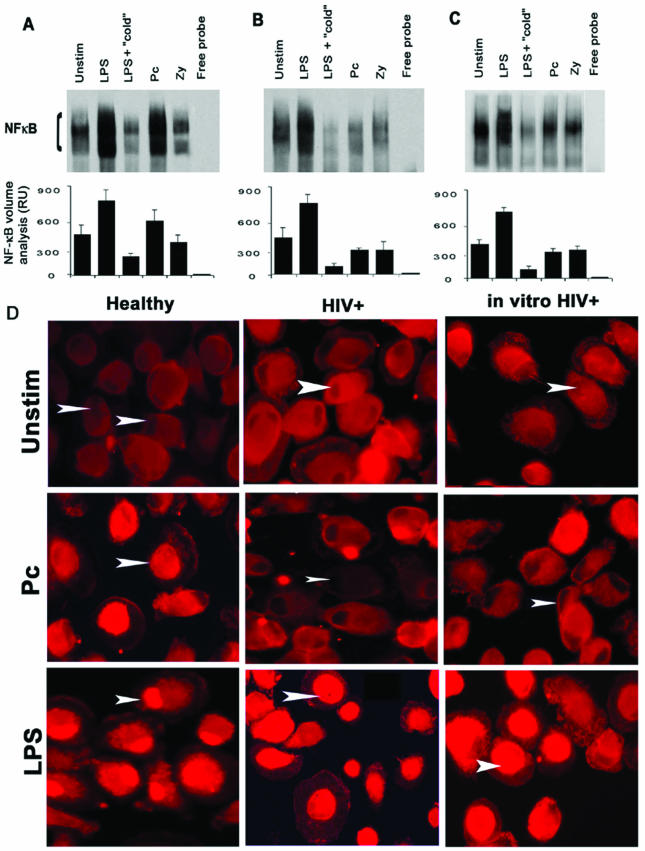
To test that hypothesis that altered mannose receptor expression may influence Pneumocystis NF-κB activation, experiments next examined whether the pathway is intact in AM from HIV-infected subjects (a human disease state associated with altered mannose receptor expression and function). EMSA data were obtained from six HIV-infected subjects. Adherent unstimulated AM from asymptomatic HIV-infected subjects demonstrated moderate constitutive nuclear translocation of NF-κB (Fig. 5A and B). Following LPS stimulation for 1 h, NF-κB nuclear translocation was increased, similar to that in healthy individuals. However, NF-κB nuclear translocation was markedly limited or absent in response to Pneumocystis or zymosan in all HIV-infected individuals examined (Fig. 5). These data demonstrate a specific impairment of NF-κB nuclear translocation in AM from asymptomatic HIV-infected subjects in response to unopsonized Pneumocystis organisms, whereas NF-κB nuclear translocation in response to LPS is intact.
FIG. 5.
Influence of HIV infection on AM NF-κB nuclear translocation. (A to C) Isolated AM were unstimulated (Unstim) or incubated with LPS, zymosan (Zy), or P. carinii (Pc) for 60 min. Nuclear extracts were isolated and resolved by EMSA. (A) The EMSA gel is from one healthy subject and is representative of 11 subjects examined. (B) The EMSA results are from one asymptomatic HIV-infected subject and are representative of six subjects examined. (C) The EMSA results are from one healthy subject following in vitro HIV infection with the monocytotropic 5R isolate of HIV (HIVBal) and are representative of six subjects examined. (The AM in panel C are from the same individual as in panel A). As controls for the assay, the lanes labeled “cold” refer to competition of the labeled probe with a 50-fold excess of unlabeled NF-κB probe, and the lanes labeled “free probe” contain labeled NF-κB probe but no AM nuclear extract. Quantitative analysis was performed by densitometry, and values are expressed as relative units (RU) (values are means ± standard errors of the means). (D) Fluorescence microscopy demonstrates NF-κB p65 nuclear translocation in response to either LPS or Pneumocystis in AM from a healthy individual (left panels). In comparison, NF-κB p65 nuclear translocation was observed in response only to LPS (not to Pneumocystis) in AM from an HIV-infected individual (middle panels) and in AM following in vitro HIV infection (right panels). Arrowheads indicate AM nuclei.
NF-κB nuclear translocation in AM from healthy individuals infected in vitro with HIV-1.
To examine whether the observed differences in the NF-κB response to Pneumocystis in AM from healthy individuals and asymptomatic HIV-infected subjects reflect a direct consequence of HIV-1 infection, EMSA studies were performed following in vitro HIV-1 infection of AM from healthy individuals. Experiments were conducted on cells from five different healthy individuals. Following 2 weeks of culture, adherent unstimulated AM demonstrated moderate constitutive nuclear translocation of NF-κB without appreciable differences between in vitro HIV-infected cells and uninfected controls (Fig. 5A and C). Following LPS stimulation for 1 h, NF-κB nuclear translocation was increased in both in vitro HIV-infected AM and uninfected control cells (Fig. 5C). In response to Pneumocystis, NF-κB nuclear translocation was increased in uninfected control cells (Fig. 5A), whereas NF-κB nuclear translocation was not increased in response to Pneumocystis in in vitro HIV-infected AM (Fig. 5C). AM NF-κB nuclear translocation in response to zymosan was not significant in either AM population. Mannan and laminarin did not significantly further influence Pneumocystis-mediated NF-κB nuclear translocation in in vitro HIV-infected AM (data not shown).
Nuclear translocation of the NF-κB p65 subunit was confirmed by immunofluorescent staining (Fig. 5D). In unstimulated AM from each population studied, NF-κB p65 was localized predominantly to the cytoplasm (Fig. 5D, top row). In AM from healthy individuals, nuclear localization of NF-κB p65 was observed following LPS or Pneumocystis incubation for 1 h (Fig. 5D, left panels). In contrast, in HIV-infected subjects, NF-κB nuclear localization did not increase following Pneumocystis stimulation, whereas NF-κB p65 nuclear localization was observed in response to LPS (Fig. 5D, middle panels). Similar to the case for AM from HIV-infected subjects, in vitro HIV infection of AM from healthy individuals demonstrated impaired NF-κB translocation in response to Pneumocystis, but NF-κB p65 nuclear translocation was intact in response to LPS (Fig. 5D, right panels). These data demonstrate that in vitro HIV infection of AM from healthy individuals is sufficient to specifically impair AM NF-κB nuclear translocation in response to unopsonized Pneumocystis organisms.
Cytoplasm I-κBα protein and protein phosphorylation determinations in AM.
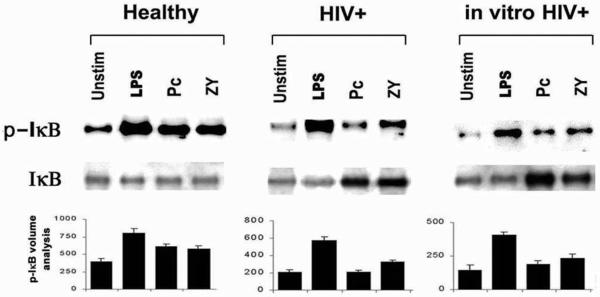
To estimate the activity of IKK, measurements were performed on cytoplasm I-κBα protein (inhibitor) and phosphorylated I-κBα protein (inactivated inhibitor). At 1 h, total I-κBα levels were similar under all conditions examined (Fig. 6). Levels of phosphorylated I-κBα (representing the inactivated form of the inhibitor) in AM from healthy individuals were increased in response to LPS, zymosan, and Pneumocystis. For AM from HIV-infected subjects or in vitro HIV-infected AM from healthy individuals, levels of phosphorylated I-κBα were also increased in response to LPS. However, in contrast to the case for AM from healthy individuals, levels of phosphorylated I-κBα were reduced in response to zymosan or Pneumocystis. These data demonstrate that AM IKK activity is intact in response to LPS but is specifically impaired in response to unopsonized Pneumocystis organisms in the context of HIV infection.
FIG. 6.
Detection of inhibitory and phosphorylated I-κBα protein in AM cytoplasmic extracts. Representative Western blot analyses for AM from one healthy subject (n = 11), AM from one asymptomatic HIV-infected (HIV+) subject (n = 5), and AM from one healthy subject following in vitro HIV infection (n = 4) are shown. Western blots for each group are representative of the subjects examined for each group. Isolated AM were either unstimulated (Unstim) or incubated with LPS, zymosan (Zy), or Pneumocystis (Pc) for 1 h. Cytoplasmic extracts were isolated and resolved by Western blotting for detection of total inhibitory I-κBα protein (I-κBα) and phosphorylated (inactivated) inhibitory I-κBα protein (I-κBα-P) in the AM cytoplasm. Quantitative analysis of phosphorylated I-κB (I-κB-P) was performed by densitometry, and values are expressed as relative units (values are means ± standard errors of the means).
Flow cytometry determination of innate immune receptor surface expression.
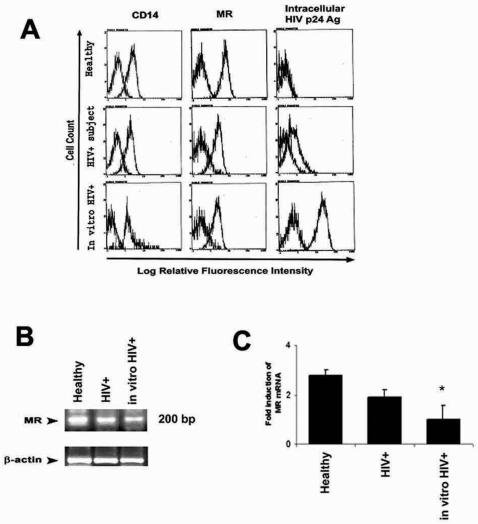
To determine whether NF-κB nuclear translocation is associated with altered surface expression of innate immune receptors, surface expression of the LPS receptor (CD14) and mannose receptor was estimated by flow cytometry. CD14 was expressed on all AM populations examined (Fig. 7A, left panels). The range for mean fluorescence values for AM from healthy individuals (2.4 to 10.4 RFU; n = 10) was similar to that for AM from asymptomatic HIV-infected individuals (3.2 to 6.9 RFU; n = 8), and for AM from healthy persons following in vitro HIV infection (9.2 to 12.1 RFU; n = 5). Mannose receptors were expressed on all AM populations examined (Fig. 7A, middle panels). The range of mean fluorescence values for AM from healthy individuals (5.8 to 8.8 RFU; n = 10), was reduced in AM from HIV-infected subjects (3.6 to 5.2 RFU; n = 6) and in AM from healthy persons following in vitro HIV infection (1.8 to 4.74 RFU; n = 6). Mannose receptor expression demonstrated biological variability in the study subjects.
FIG. 7.
AM surface expression of innate immune receptors and intracellular HIV-1 p24 antigen. (A) Left panels, AM surface expression of CD14 receptor. Each panel shows representative flow cytometry profiles for one individual and is representative of AM from healthy subjects (n = 10), AM from asymptomatic HIV-infected subjects (HIV+) (n = 8), and AM from healthy individuals following in vitro HIV infection (in vitro HIV+) (n = 5). Middle panels, AM surface expression of mannose receptor. Representative flow cytometry profiles for AM from healthy subjects (n = 10), AM from asymptomatic HIV-infected subjects (n = 6), and AM from healthy individuals following in vitro HIV infection (in vitro HIV+) (n = 6) are shown. For CD14 and mannose receptor panels, positively stained cells are compared to PE-labeled isotype antibody, whose profile is similar to AM background autofluorescence. Right panels, AM intracellular HIV-1 p24 antigen expression. Representative flow cytometry profiles for AM from healthy subjects (n = 5), AM from asymptomatic HIV-infected subjects (n = 10), and AM from healthy individuals following in vitro HIV infection (n = 5) are shown. For HIV-1 p24 staining, background refers to AM autofluorescence. (B) Mannose receptor (MR) mRNA detection by RT-PCR, comparing AM from one healthy individual, one asymptomatic HIV-infected subject, and one healthy subject following in vitro HIV infection. β-Actin served as a control. (C) Quantitative measurement of mannose receptor mRNA by real-time PCR for representative samples from AM from a healthy individual, an asymptomatic HIV-infected subject, and a healthy person following in vitro HIV infection. Error bars indicate standard errors of the means. *, P < 0.05.
Detection of stable mannose receptor mRNA transcripts revealed relatively reduced levels in AM from HIV-infected subjects and significantly reduced levels in AM following in vitro HIV infection compared to AM from healthy individuals as determined by RT-PCR (Fig. 7B) and as independently confirmed by quantitative real-time PCR (Fig. 7C). Taken together, these results demonstrate that similar CD14 expression is consistent with a preserved AM response to LPS. However, impaired AM NF-κB nuclear translocation in response to unopsonized Pneumocystis organisms is associated in part with reduced mannose receptors surface expression and reduced mannose receptor mRNA.
Flow cytometry determination of AM intracellular HIV-1 p24 antigen expression.
To determine whether reduced NF-κB nuclear translocation in the context of HIV infection is related to HIV expression in macrophages, HIV-1 infection of AM was estimated by measurements of intracellular HIV-1 p24 antigen expression. AM from healthy individuals did not express HIV-1 p24 antigen compared to isotype control antibodies (Fig. 7A, right panels). For HIV-infected persons, 9 of 10 demonstrated positive staining for intracellular p24 antigen (representing AM infected with HIV-1), with a range of mean fluorescence values of 1.1 to 2.6 RFU. For AM from healthy individuals infected in vitro with HIV (n = 5), all were positive for intracellular HIV-1 p24 antigen, with a range of mean fluorescence values of 1.2 to 4.0 RFU. These results demonstrate that impaired AM NF-κB nuclear translocation occurs in cell populations independent of the relative level of HIV-1 infection.
NF-κB-mediated AM release of IL-8 in response to Pneumocystis.
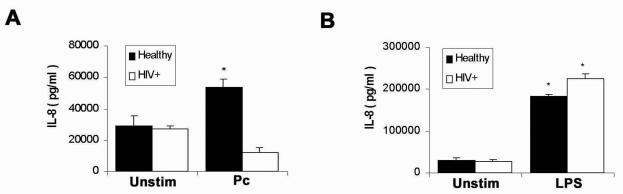
Studies were performed to examine host defense gene regulation mediated by NF-κB nuclear translocation. The measurements focused on IL-8, an inflammatory chemokine with chemoattractant properties for neutrophils and eosinophils involved in the lung inflammatory response, where IL-8 expression is mediated by NF-κB (4). In AM from healthy individuals IL-8 release was significantly increased in response to unopsonized Pneumocystis, whereas IL-8 release was blunted in AM from HIV-infected persons (Fig. 8A). In contrast, in response to LPS, AM IL-8 release was significantly increased and was similar in healthy and asymptomatic HIV-infected subjects (Fig. 8B). In this study, LPS was a more potent inducer of IL-8 release than Pneumocystis. These data demonstrate a functional consequence to the specific impairment of Pneumocystis-mediated NF-κB activation in the context of HIV infection manifest as reduced cellular release of IL-8 (gene regulated by NF-κB).
FIG. 8.
Detection of IL-8 secretion by AM from healthy individuals and AM from HIV-infected persons in response to Pneumocystis or LPS. Isolated AM were incubated with Pneumocystis (Pc) (A) or LPS (B) for 24 h at 37°C with 5% CO2, and cultured supernatants were collected and assayed for IL-8 by ELISA (n = 3). Unstim, unstimulated. Error bars indicate standard errors of the means. *, P < 0.05.
DISCUSSION
This study demonstrates that Pneumocystis organisms can stimulate NF-κB nuclear translocation in AM from healthy individuals. Nuclear translocation involves p50 and p65 NF-κB subunits and is associated with I-κB phosphorylation and IL-8 release. Furthermore, Pneumocystis-mediated NF-κB nuclear translocation is mediated primarily through AM mannose receptors, as NF-κB nuclear translocation is inhibited by soluble mannan or by targeted mannose receptor siRNA gene silencing. The relative role of mannose receptor-mediated signaling is further supported by the observation that Pneumocystis-mediated NF-κB nuclear translocation was reduced in AM from asymptomatic HIV-infected persons and in AM from healthy individuals infected in vitro with HIV (a disease state associated with reduced mannose receptor expression). Furthermore, release of IL-8 (regulated by NF-κB) was impaired in AM from HIV-infected persons compared to healthy individuals. Importantly, the impaired NF-κB response to Pneumocystis appears to be specific, as the NF-κB nuclear translocation and IL-8 release in response to the unrelated ligand LPS appears to be intact in AM from both healthy and HIV-infected persons.
This study is the first to examine the human AM NF-κB signaling response to Pneumocystis organisms. Recovery from Pneumocystis pneumonia in the murine model is associated with increased production of cytokine mRNA (53) and release of proinflammatory cytokines (22), and in vitro Pneumocystis interaction with human macrophages results in the release of IL-1β, IL-6, and TNF-α (24). Recognizing the diverse immunomodulatory functions of NF-κB (including proinflammatory cytokine gene activation) (19) and the central role of AM in intrapulmonary NF-κB activation (29), the findings in the present study suggest that Pneumocystis-mediated NF-κB nuclear translocation may represent an important AM innate immune response in healthy individuals. A report of Pneumocystis pneumonia in an infant with a congenital mutation in a gene encoding an essential component of the NF-κB pathway supports the critical role of NF-κB in the host defense against this opportunistic pathogen (13).
The data in the present study are supported in part by a recent study that demonstrates that a Pneumocystis-derived β-glucan-rich cell wall isolate mediates TNF-α release via NF-κB activation in the RAW 264.7 murine macrophage cell line (28). Although a specific macrophage receptor was not identified (28), the data indicate that the pathway is distinct from LPS (28). The present study demonstrates that Pneumocystis organism-mediated activation of AM NF-κB is distinct from LPS and is dependent predominantly on mannose receptor ligation, as determined by targeted siRNA gene silencing of the mannose receptors. Thus, in addition to phagocytosis and endocytosis effector functions (16, 25), the present study identifies mannose receptors as pattern recognition receptors capable of NF-κB activation in response to infectious non-self challenge.
Prior investigations using rodent macrophages demonstrated that AM recognition of Pneumocystis and Pneumocystis-mediated AM release of TNF-α are inhibited by β-glucans (23) and that rat-derived Pneumocystis cell-wall β-glucan mediates NF-κB nuclear translocation in murine macrophages (28), suggesting that Pneumocystis β-glucan may represent an important and potent activator of NF-κB in rodent AM. In murine models of Pneumocystis pneumonia, macrophage dectin-1 receptors (which recognize β-glucans) mediate Pneumocystis phagocytosis and killing (48). In the present study, the absence of a significant influence of laminarin (an inhibitor of the β-glucan receptor) on NF-κB activation and the suppression of NF-κB activation following targeted mannose receptor gene silencing suggest a β-glucan-independent mechanism in human AM. Thus, in the rodent models, macrophage receptors mediating β-glucan recognition may represent an important component of an effective host response to Pneumocystis, whereas for human AM, Pneumocystis recognition is mediated predominantly by mannose receptors. However, defining the specific role of human AM β-glucan receptors requires further investigation.
The observed differences in macrophage recognition of Pneumocystis in the present study (using primary human AM) and prior studies (using murine macrophages) (48, 50) may in part reflect differences in sources of macrophages and differences in relative expression and function of receptors such as mannose and β-glucan receptors in macrophages from different species. AM uptake of unopsonized infectious organisms varies greatly and depends on the species origin of the AM (37). In addition, the macrophage recognition of intact organisms may differ from recognition of specific structural or antigenic components of Pneumocystis. The surface of Pneumocystis is composed predominately of the mannose-enriched glycoprotein molecules (including major glycoprotein A or major surface glycoprotein), whereas the β-glucan moiety may be located in deeper layers and primarily in the less-abundant cyst forms of Pneumocystis (42). Pneumocystis β-glucan may indeed represent a potent stimulator of human AM macrophage NF-κB, although this was not specifically examined in the present study.
The cell signaling responses to mannose receptor ligands is controversial, and the specific cellular response to Pneumocystis remains to be defined. In studies with human monocyte-derived dendritic cells, the cellular response to mannose receptor ligands was reported to be stimulatory by some investigators (45, 54) and to be inhibitory by others (6, 38). The present study demonstrates that mannose receptor ligation in human AM is associated with NF-κB nuclear translocation in a concentration-dependent manner. However, the NF-κB activation occurs at relatively low Pneumocystis-to-AM ratios. At higher Pneumocystis-to-AM ratios, NF-κB nuclear translocation is inhibited, suggesting the possibility that critical clustering of mannose receptors may be associated with inhibitory signals as observed in investigations with dendritic cells (38), although this requires further investigation with human AM.
A role for the mannose receptor in the pathogenesis of Pneumocystis pneumonia is supported in part by the observation of increased accumulation of glycoproteins in the lungs of CD4 T-lymphocyte-depleted animals with genetically deleted mannose receptor (50). In addition, a significant accumulation of macrophages and neutrophils in the lungs of these animals (50) suggests a possible role for altered mannose receptor-mediated signal transduction in the genetically deficient animals. Interestingly, genetic deletion of the mannose receptor alone is not sufficient to increase susceptibility to Pneumocystis pneumonia in the murine model (50), underscoring the importance of CD4+ T lymphocytes in disease susceptibility (21, 44). However, the possibility that other members of the mannose receptor family (such as the related Endo 180 [15]) may serve redundant roles in the host response to Pneumocystis remains to be determined. Furthermore, whether the observations in one murine model (50) apply to other animal models (35) or to human disease remains to be established.
The influence of HIV infection on innate receptor-mediated NF-κB activation has not been previously examined. NF-κB is recognized as an important regulatory molecule in HIV replication and HIV gene expression in monocytes/macrophages (20), a process which may be mediated by IKK (1). The findings in the present study are the first to demonstrate that HIV infection is associated with specific alterations in AM innate immune receptor-mediated NF-κB signaling. Although in vitro HIV infection of AM was sufficient to reproduce the specific defect, the level of HIV infection in AM did not account for the observed findings, as impaired NF-κB signaling was observed both in AM with high intracellular HIV-1 p24 antigen expression, as with in vitro infection, and in AM with low HIV p24 expression, as with HIV-infected subjects. Alternatively, HIV infection of AM may result in the release, suppression, or alteration in the pattern of release of soluble factors which may then exhibit autocrine and/or paracrine influences on AM immune receptor expression or signal transduction pathways. These soluble factors could represent specific HIV-1 regulatory or structural gene products, AM cytokines or chemokines, or other factors.
The observation that levels of the inactivated phosphorylated I-κBα protein in cytoplasm were reduced suggests that HIV may directly interfere with innate receptor-mediated IKK function or influence events upstream of IKK. The observed impaired AM NF-κB response to Pneumocystis may in part reflect reduced surface expression of mannose receptors, since HIV infection is associated with reduced AM surface expression (25) and release of the soluble ectodomain of the mannose receptor (18). However, in the present study, mannose receptor surface densities were similar in AM from healthy individuals and from asymptomatic HIV-infected persons, a finding consistent with prior observations (25), as all persons in the present study have CD4 counts of >200 cells/mm3. An influence of receptor shedding and soluble mannose receptor was not specifically investigated and cannot be excluded as a factor.
The observation that the AM NF-κB response to LPS is preserved in AM from asymptomatic HIV-infected persons is consistent with prior observations (33) and suggests that the defect does not represent a global impairment of the NF-κB pathway. The finding that Pneumocystis-mediated NF-κB signaling is markedly impaired in AM from asymptomatic HIV-infected individuals with good clinical responses to highly active antiretroviral therapy (as estimated by peripheral CD4 T-lymphocyte counts of >200 cells/mm3 and undetectable viral loads) suggests that AM function is impaired even in clinically latent periods. However, the clinical consequences manifest by altered AM NF-κB signaling may require coexisting conditions, such as a reduction of CD4 T lymphocytes to critical levels (32).
The roles of other receptors implicated in the AM recognition of P. carinii, including fibronectin (40) and immunoglobulin receptors (31, 52), were not specifically investigated. Other limitations of these studies include the possibility that the observed response of human AM to rodent-derived P. carinii may reflect in part the origin of the organisms. Unfortunately, in the absence of reliable culturing methods, Pneumocystis of human origin is not available (47). However, the observation in the present study that rat-derived Pneumocystis elicited similar responses in human and rat AM in part validates the use of this model. Contaminating rat-derived adherent proteins may be partly responsible for the observed NF-κB activity, although the Pneumocystis organisms used in these studies are relatively free of host proteins such as surfactant protein A (27). As the Pneumocystis preparation is composed primarily of the trophic forms, the present findings may represent the predominant macrophage response to the Pneumocystis trophic form and not the cyst or other forms. Finally, the possibility that in vitro observations may not accurately reflect events in vivo should be considered.
In conclusion, these data demonstrate that Pneumocystis organisms stimulate AM NF-κB nuclear translocation and that NF-κB nuclear translocation in human AM is mediated primarily through AM mannose receptors. The significance of these findings relate to AM mannose receptor innate function in lung host defense. First, in addition to phagocytosis and endocytosis effector functions (17), the present study identifies mannose receptors as pattern recognition receptors capable of NF-κB activation in response to infectious non-self challenge, a role associated with pattern recognition molecules of the Toll-like receptor family (34). Second, altered NF-κB activation may in part provide a molecular mechanism for the observed accumulation of proteoglycans and cellular infiltrates that characterizes Pneumocystis pneumonia in a mannose receptor-deficient murine model (50). Third, these data demonstrate that Pneumocystis-mediated NF-κB signaling is markedly impaired in AM from asymptomatic HIV-infected individuals, and, importantly, this impairment appears to be receptor specific and not a global disruption of NF-κB signaling. Impaired mannose receptor-mediated NF-κB activation may contribute to Pneumocystis pathogenesis in HIV-infected persons. Impaired NF-κB nuclear translocation may be multifactorial, involving several mechanisms, including mannose receptor downregulation and impaired IKK-related activation. Given the spectrum of pathogens recognized by mannose receptors (17), specific alterations in AM NF-κB signal transduction pathways in HIV-infected persons may represent another critical impairment of innate immune response in the lungs and, in the setting of CD4 T-lymphocyte depletion, may in part contribute to disease pathogenesis in opportunistic infections by organisms such as Mycobacterium tuberculosis, Cryptococcus neoformans, and Pneumocystis in humans.
Acknowledgments
We gratefully acknowledge the participation of all persons who consented to bronchoscopy and the technical assistance of Robert Garland and Lorraine Gryniuk. We acknowledge the generous gift of Martine Armstrong and Frank Richards, who provided P. carinii organisms for initial studies. We acknowledge the Beth Israel Deaconess Medical Center Real Time PCR Core Facility (director, Victoria Petkova) for performing the quantitative real-time PCR analysis. We also are indebted to Qi He for assistance with the RT-PCR assay and to Naimish Patel, Souvenir Tachado, Lester Kobzik, and R. Alan B. Ezekowitz for their critical review of the manuscript and valuable suggestions.
This study was supported by NIH research grants RO1 HL63655 (to H. Koziel and T. B. Kinane) and F32 HL71372 (to J. Zhang).
Editor: T. R. Kozel
REFERENCES
- 1.Asin, S., J. A. Taylor, S. Trushin, G. Bren, and C. V. Paya. 1999. Ikk mediates NF-κB activation in human immunodeficiency virus-infected cells. J. Virol. 73:3893-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton, E., and W. Campbell. 1969. Pneumocystis carinii in lungs of rats treated with cortisone acetate. Am. J. Pathol. 54:209-236. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, G. D., and S. Gordon. 2001. A new receptor for beta-glucans. Nature 413:36-37. [DOI] [PubMed] [Google Scholar]
- 4.Carter, A. B., M. M. Monick, and G. W. Hunninghake. 1998. Lipopolysaccharide-induced NF-kB activation and cytokine release in human alveolar macrophages is PKC-independent and TK- and PC-PLC-dependent. Am. J. Respir. Cell. Mol. Biol. 18:384-391. [DOI] [PubMed] [Google Scholar]
- 5.Chen, F., and M. Cushion. 1994. Use of an ATP bioluminescent assay to evaluate viability of Pneumocystis carinii from rats. J. Clin. Microbiol. 32:2791-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chieppa, M., G. Bianchi, A. Doni, A. Del Prete, M. Sironi, G. Laskarin, P. Monti, L. Piemonti, A. Biondi, A. Mantovani, M. Introna, and P. Allavena. 2003. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 171:4552-4560. [DOI] [PubMed] [Google Scholar]
- 7.Collins, M. S., and M. T. Cushion. 2001. Standardization of an in vitro drug screening assay by use of cryopreserved and characterized Pneumocystis populations. J. Eukaryot. Microbiol. Suppl. 2001:178-179. [DOI] [PubMed] [Google Scholar]
- 8.Cushion, M., J. Ruffolo, M. Linke, and P. Walzer. 1985. Pneumocystis carinii: growth variables and estimates in the A549 and WI-38 VA 13 human cells lines. Exp. Parasitol. 60:43-54. [DOI] [PubMed] [Google Scholar]
- 9.Cushion, M. T. 1998. Genetic heterogeneity of rat-derived Pneumocystis. FEMS Immunol. Med. Microbiol. 22:51-58. [DOI] [PubMed] [Google Scholar]
- 10.Cushion, M. T., F. Chen, and N. Kloepfer. 1997. A cytotoxicity assay for evaluation of candidate anti-Pneumocystis carinii agents. Antimicrob. Agents Chemother. 41:379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushion, M. T., S. Orr, S. P. Keely, and J. R. Stinger. 2001. Time between inoculations and karyotype forms of Pneumocystis carinii f. sp. carinii influence outcome of experimental coinfections in rats. Infect. Immun. 69:97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, L., M. Kuehl, and J. Battey. 1994. Basic methods in molecular biology, p. 660-669. Appleton and Lange, Norwalk, Conn.
- 13.Dupuis-Girod, S., N. Corradini, S. Hadj-Rabia, J. C. Fournet, L. Faivre, F. LeDeist, P. Durand, R. Doffinger, A. Smahi, A. Israel, G. Courtois, N. Brousse, S. Blanche, A. Munnich, A. Fischer, J. L. Casanova, and C. Bodemer. 2002. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy with incontinentia pigmenti in his mother. Pediatrics 2002:e97. [DOI] [PubMed] [Google Scholar]
- 14.Dykxhoorn, D. M., C. D. Novina, and P. A. Sharp. 2003. Killing the messenger: short RNAs that silence gene expression. Nat. Rev. Mol. Cell Biol. 4:457-467. [DOI] [PubMed] [Google Scholar]
- 15.East, L., and C. M. Isacke. 2002. The mannose receptor family. Biochim. Biophys. Acta 1572:364-386. [DOI] [PubMed] [Google Scholar]
- 16.Ezekowitz, R. A. B., D. J. Williams, H. Koziel, M. Y. K. Armstrong, A. Warner, F. F. Richards, and R. M. Rose. 1991. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 351:155-158. [DOI] [PubMed] [Google Scholar]
- 17.Fraser, I. P., H. Koziel, and R. A. B. Ezekowitz. 1998. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10:363-372. [DOI] [PubMed] [Google Scholar]
- 18.Fraser, I. P., K. Takahashi, H. Koziel, B. Fardin, A. Harmsen, and R. A. B. Ezekowitz. 2000. Pneumocystis carinii enhances the production of soluble mannose receptor by macrophages. Microbes Infect. 2:1305-1310. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 20.Griffin, G. E., K. Leung, T. M. Folks, S. Kunkel, and G. J. Nabel. 1989. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature 339:70-73. [DOI] [PubMed] [Google Scholar]
- 21.Harmsen, A., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmsen, A. G., and W. Chen. 1992. Resolution of Pneumocystis carinii pneumonia in CD4+ lymphocyte-depleted mice given aerosols of heat-treated Escherichia coli. J. Exp. Med. 176:881-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman, O. A., J. E. Standing, and A. H. Limper. 1993. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-medicated mechanism. J. Immunol. 150:3932-3940. [PubMed] [Google Scholar]
- 24.Kandil, O., J. A. Fishman, H. Koziel, P. Pinkston, R. M. Rose, and H. G. Remold. 1994. Human immunodeficiency virus type 1 infection of human macrophages modulates the cytokine response to Pneumocystis carinii. Infect. Immun. 62:644-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koziel, H., Q. Eichbaum, B. A. Kruskal, P. Pinkston, R. A. Rogers, M. Y. K. Armstrong, F. F. Richards, R. M. Rose, and R. A. B. Ezekowitz. 1998. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J. Clin. Investig. 102:1332-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koziel, H., X. Li, M. Y. K. Armstrong, F. F. Richards, and R. M. Rose. 2000. Alveolar macrophages from human immunodeficiency virus-infected persons demonstrate impaired oxidative burst response to Pneumocystis carinii in vitro. Am. J. Respir. Cell Mol. Biol. 23:452-459. [DOI] [PubMed] [Google Scholar]
- 27.Koziel, H., D. S. Phelps, J. A. Fishman, M. Y. K. Armstrong, F. F. Richards, and R. M. Rose. 1998. Surfactant protein-A reduces binding and phagocytosis of Pneumocystis carinii by human alveolar macrophages in vitro. Am. J. Respir. Cell Mol. Biol. 18:834-843. [DOI] [PubMed] [Google Scholar]
- 28.Lebron, F., R. Vassallo, V. Puri, and A. H. Limper. 2003. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-kB activation. J. Biol. Chem. 278:25001-25008. [DOI] [PubMed] [Google Scholar]
- 29.Lentsch, A. B., B. J. Czermak, N. M. Bless, N. Van Rooijen, and P. A. Ward. 1999. Essential role of alveolar macrophages in intrapulmonary activation of NF-kB. Am. J. Respir. Cell Mol. Biol. 20:692-698. [DOI] [PubMed] [Google Scholar]
- 30.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masur, H., and T. C. Jones. 1978. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J. Exp. Med. 147:157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masur, H., F. P. Ognibene, R. Yarchoan, J. H. Shelhamer, B. F. Baird, W. Travis, A. F. Suffredini, L. Deyton, J. A. Kovacs, J. Falloon, R. Davey, M. Polis, J. Metcalf, M. Baseler, R. Wesley, V. J. Gill, A. S. Fauci, and H. C. Lane. 1989. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann. Intern. Med. 111:223-231. [DOI] [PubMed] [Google Scholar]
- 33.Mathys, J. M., S. M. Melanson, D. J. Schiffer-Alberts, J. P. A. Ioannidis, H. Koziel, and P. R. Skolnik. 2000. NF-kB modulates TNF-alpha production by alveolar macrophages in asymptomatic HIV-seropositive individuals. J. Immunol. 164:1588-1594. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 35.Mi, Y., S. D. Shapiro, and J. U. Baenziger. 2002. Regulation of lutropin circulatory half-life by the mannose/N-acetylgalactosamine-4-SO4 receptor is critical for implantation in vivo. J. Clin. Investig. 109:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori, N., Y. Yamada, S. Ikeda, Y. Yamasaki, K. Tsukasaki, Y. Tanaka, M. Tomonaga, N. Yamamoto, and M. Fujii. 2002. Bay 11-7082 inhibits transcription factor NF-kB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 100:1828-1834. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen, B. T., P. K. Peterson, H. A. Verbrugh, P. G. Quie, and J. R. Hoidal. 1982. Differences in phagocytosis and killing by alveolar macrophages from humans, rabbits, rats, and hamsters. Infect. Immun. 36:504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477-7485. [DOI] [PubMed] [Google Scholar]
- 39.O'Riordan, D. M., J. E. Standing, and A. H. Limper. 1995. Pneumocystis carinii glycoprotein A binds macrophage mannose receptors. Infect. Immun. 63:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pottratz, S. T., and W. J. Martin. 1990. Mechanism of Pneumocystis carinii attachment to cultured rat alveolar macrophages. J. Clin. Investig. 86:1678-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roths, J. B., J. D. Marshall, R. D. Allen, G. A. Carlson, and C. L. Sidman. 1990. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Am. J. Pathol. 136:1173-1186. [PMC free article] [PubMed] [Google Scholar]
- 42.Ruffolo, J. J. 1994. Pneumocystis carinii cell structure, p. 25-71. In P. D. Walzer (ed.), Lung biology in health and disease, vol. 69. Pneumocystis carinii pneumonia. Marcel Dekker, Inc., New York, N.Y.
- 43.Salahuddin, S. Z., R. M. Rose, J. E. Groopman, P. D. Markham, and R. C. Gallo. 1986. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood 68:281-284. [PubMed] [Google Scholar]
- 44.Shellito, J. E., V. V. Suzara, W. Blumenfeld, J. M. Beck, H. J. Steger, and T. H. Ermak. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibata, Y., W. J. Metzger, and Q. N. Myrvik. 1997. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor mediated phagocytosis initiates IL-12 production. J. Immunol. 159:2462-2467. [PubMed] [Google Scholar]
- 46.Silverman, N., and T. Maniatis. 2001. NF-kB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321-2342. [DOI] [PubMed] [Google Scholar]
- 47.Sloand, E., B. Laughon, M. Armstrong, M. Bartlett, W. Blumenfeld, M. Cushion, A. Kalica, J. Kovacs, W. Martin, E. Pitt, E. Pesanti, F. Richards, R. Rose, and P. Walzer. 1993. The challenge of Pneumocystis carinii culture. J. Eukaryot. Microbiol. 40:188-195. [DOI] [PubMed] [Google Scholar]
- 48.Steele, C., L. Marrero, S. Swain, A. G. Harmsen, M. Zheng, G. D. Brown, S. Gordon, J. E. Shellito, and J. K. Kolls. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the dectin-1 beta-glucan receptor. J. Exp. Med. 198:1677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stringer, J. R., C. B. Beard, R. F. Miller, and A. E. Wakefield. 2002. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg. Infect. Dis. 8:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swain, S. D., S. J. Lee, M. C. Nussenzweig, and A. G. Harmsen. 2003. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect. Immun. 71:6213-6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vassallo, R., C. F. Thomas, Jr., Z. Vuk-Paklovic, and A. H. Limper. 1999. Alveolar macrophage interactions with Pneumocystis carinii. J. Lab. Clin. Med. 133:535-540. [DOI] [PubMed] [Google Scholar]
- 52.von Behren, L. A., and E. L. Pesanti. 1978. Uptake and degradation of Pneumocystis carinii by macrophages in vitro. Am. Rev. Respir. Dis. 118:1051-1059. [DOI] [PubMed] [Google Scholar]
- 53.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1997. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii-infected mice. Infect. Immun. 17:491-500. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto, Y., T. W. Klein, and H. Friedman. 1997. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1 β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 65:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]