Abstract
Listeria infection during pregnancy can cause the death of both mother and fetus. Previous studies established that immunostimulatory CpG oligodeoxynucleotides (ODN) increase the resistance of healthy adult mice to many infectious pathogens, including Listeria monocytogenes. This study examines whether the innate immune response elicited by CpG ODN can reduce the susceptibility of pregnant mice to lethal listeria challenge. The results indicate that CpG ODN treatment significantly improves maternal survival and reduces pathogen transmission to offspring. CpG ODN administered during pregnancy did not induce abortion, birth defects, or reduce the size or health of litters. These findings suggest that CpG ODN may provide a safe and effective means of improving the health of mothers and fetuses during pregnancy.
Pregnancy leads to a generalized suppression of the adaptive immune system, typified by significantly decreased cell-mediated immunity and reduced Th1 responsiveness (25, 31, 33, 38, 39). This immunosuppressed state prevents maternal rejection of the fetus (31, 33) but has the unfortunate consequence of increasing maternal susceptibility to certain infectious agents (24, 35).
Listeria monocytogenes is a gram-positive intracellular bacterium that is a common cause of maternal infection (37, 40). This pathogen can be transmitted from mother to fetus, substantially increasing the risk of abortion, stillbirth, congenital malformations, and neonatal mortality and morbidity (2, 26, 40). In the absence of preexisting maternal vaccination, there is no widely accepted strategy for preventing infection, since immunomodulatory therapy runs the risk of adversely affecting the neonate.
Rapid activation of the innate immune system can limit the early spread of listeriae, allowing the host to develop sterilizing adaptive immunity (17, 21). The innate immune system is activated when Toll-like receptors (TLR) expressed by immune cells recognize pathogen-associated molecular patterns (PAMPs) expressed by infectious microorganisms (1, 28). CpG motifs present in bacterial DNA act as PAMPs (11, 18, 22, 41), interacting with TLR-9 to trigger an innate immune response in which lymphocytes, dendritic cells, and macrophages are stimulated to produce immunoprotective cytokines and chemokines (3, 9, 11, 14, 18, 22, 34, 36). Treatment with CpG oligodeoxynucleotides (ODN) can improve the resistance of normal adult mice to a variety of bacterial, viral and parasitic pathogens (6, 7, 15, 23, 30, 32, 42). This immunoprotective effect peaks several days after CpG ODN administration and persists for several weeks (6, 19, 23).
The current study was undertaken to determine whether CpG ODN can be of benefit during pregnancy, improving survival after listeria infection. The results indicate that CpG ODN treatment effectively stimulates the innate immune system of pregnant mice, improves maternal survival, and prevents pathogen transmission to offspring.
MATERIALS AND METHODS
Reagents.
CpG (GCTAGACGTTAGCGT) and control (GCTAGAGCTTAGGCT) phosphorothioate ODN were synthesized at the CBER core facility. All ODN were free of endotoxin and protein contamination, as determined by chromogenic Limulus amoebocyte lysate and bicinchoninic acid protein assays (Pierce Biotechnology, Rockford, Ill.). Polypeptides from the listeriolysin O (LLO) protein encoding a CD8-specific epitope (LLO 91-99) and a CD4-specific epitope (LLO 189-200) were also synthesized at the CBER core facility.
Animals.
BALB/c mice were purchased from the National Cancer Institute (Frederick, Md.). Animals were housed in sterile microisolator cages in a barrier environment in the CBER specific-pathogen-free animal facility. All experiments were approved by the local Animal Care and Use Committee. Mice were bred at 8 to 16 weeks of age. Appearance of a vaginal plug marked day 0 of pregnancy, at which time male mice were removed. Pregnant mice were injected intraperitoneally (i.p.) with 150 μl of phosphate-buffered saline (PBS) or 150 μg of ODN on day 10. Three to six days later, the animals were challenged i.p. with 50 50% lethal dose(s) (LD50) (i.e., 105 PFU) of L. monocytogenes, as previously described (6, 19, 23). Survival was monitored for 3 weeks.
Preparation of bacteria.
L. monocytogenes strain EGD (ATCC 15313; American Type Culture Collection, Manassas, Va.) was grown in brain heart infusion broth (Becton Dickinson, Sparks, Md.). Aliquots were frozen at −70°C and thawed immediately prior to use. The severity of infection was monitored by culturing serial 10-fold dilutions of homogenized liver preparations in brain heart infusion agar plates at 37°C (6).
Cytokine assays.
A total of 2 × 105 splenocytes/well were incubated with CpG or control ODN in flat-bottom microtiter plates (Costar, Corning, N.Y.) in RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum, 1.5 mM l-glutamine, and 100 U of penicillin-streptomycin/ml for 48 h. Levels of gamma interferon (IFN-γ), interleukin-12 (IL-12), and IL-6 in the supernatants of these cultures were quantified by enzyme-linked immunosorbent assay as previously described (18). In brief, 96-well Immulon H2B plates (Thermo LabSystems, Franklin, Mass.) were coated with cytokine-specific antibody and then blocked with PBS-1% bovine serum albumin. Plates were incubated with culture supernatant, washed, and treated with biotinylated anti-cytokine antibody, followed by streptavidin-alkaline phosphatase. Cytokine concentrations were determined colorimetrically by comparison to standard curves generated with recombinant cytokines. The detection limit of these assays was 0.05 U/ml for IFN-γ, 0.3 ng/ml for IL-12, and 0.1 ng/ml for IL-6. All assays were performed in triplicate or quadruplicate.
Cytokine ELIspot assays.
Single-cell suspensions were prepared from mouse spleens. A total of 5 × 105 splenocytes/well were stimulated for 8 to 10 h with heat-killed listeriae (5 × 105 CFU/ml) in flat-bottom 96-well Immulon-2 plates previously coated with monoclonal anti-cytokine antibody (20). Alternatively, 5 × 105 cells were cultured in round-bottom 96-well microtiter plates with 10−6 M CD4- or CD8-specific LLO peptides for 6 to 8 h and then transferred to anti-IFN-γ antibody-coated plates. Cells were incubated overnight at 37°C with 10−6 M CD4- or CD8-specific LLO peptides. The plates were then washed and treated with biotinylated anti-IFN-γ antibody, followed by streptavidin-alkaline phosphatase. Spots were visualized by the addition of BCIP (5-bromo-4-chloro-3-indolylphosphate) phosphatase solution (Sigma, St. Louis, Mo.) in low-melting-point agarose (Sigma) (20) and counted manually under ×40 magnification. The number of cytokine-secreting cells was determined by a single-blinded reader, who analyzed the number of spots in three separate wells per sample.
Statistics.
Differences in survival, abortion, and infection rates were determined by using the chi-square and Wilcoxon rank-sum tests. All experiments involving cytokine production were repeated at least twice and involved at least two independently studied mice per group per experiment. The statistical significance was evaluated by using the two-tailed Student t test. P values of <0.05 were considered significant.
RESULTS
Immune cells from pregnant mice respond to CpG ODN.
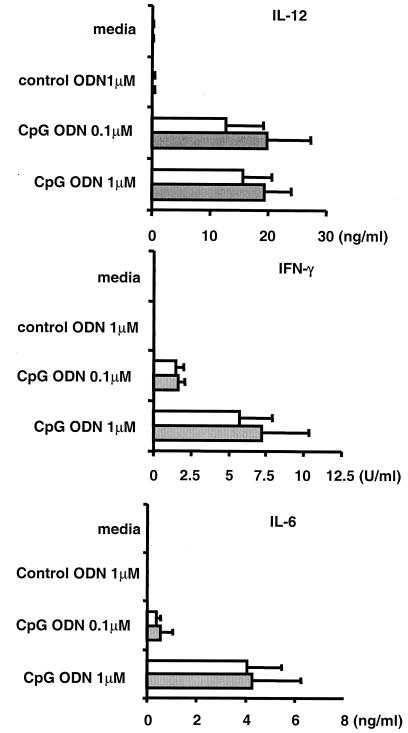
The capacity to mount an antigen-specific Th1 response is suppressed during pregnancy (5, 12). To evaluate whether activation of a TLR-9-mediated innate immune response is similarly compromised, spleen cells from pregnant BALB/c mice were stimulated in vitro with CpG and control ODN. As seen in Fig. 1, spleen cells from pregnant and virgin female mice responded similarly to low and high concentrations of CpG ODN by increasing their production of the Th1 and proinflammatory cytokines IL-12, IFNγ, and IL-6. Control ODN had not such effect, establishing that the induction of cytokine secretion was CpG specific.
FIG. 1.
Cytokine production by CpG-stimulated spleen cells. Spleen cells from virgin (□) and 10 day pregnant mice (░⃞) were incubated with 0.1 to 1 μM ODN. The amount of IL-12, IFN-γ, and IL-6 secreted after 48 h was determined by ELISA. The data represent the means ± the standard deviations of four independently studied mice/group. Note that there was no statistically significant difference in cytokine production between these two groups of mice.
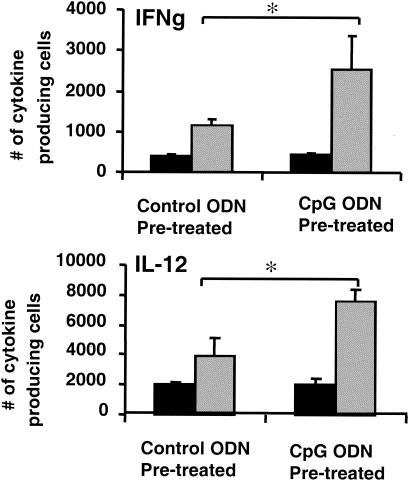
To examine whether CpG ODN treatment might improve the innate immune response to PAMPs, pregnant mice were injected with 150 μg of CpG or control ODN. Three days later, spleen cells from these animals were stimulated in vitro for 10 h with heat-killed listeriae. The exposure to heat-killed listeriae induced significantly greater IFN-γ and IL-12 production from cells of the CpG versus the control ODN-treated group (P < 0.05, Fig. 2).
FIG. 2.
Cytokine responses of spleen cells from ODN-treated mice to stimulation with heat-killed listeriae. Pregnant mice were treated with 150 μg of control (▪) or CpG ODN (░⃞) on day 10 of gestation. Spleen cells were isolated on day 13, and cultured in vitro in the presence of 5 × 105 CFU of heat-killed listeriae/ml. The number of cells activated to secrete IL-12 and IFN-γ (IFNg) was determined by ELIspot assay. The data represent the means ± the standard deviations of three independently studied mice/group and are representative of two similar experiments. ✽, P < 0.05.
Effect of CpG ODN on the susceptibility of pregnant mice to L. monocytogenes infection.
Previous studies established that CpG ODN trigger an innate immune response that can reduce the susceptibility of normal nonpregnant mice to a variety of infectious bacteria, including the model pathogen L. monocytogenes (6, 19, 23).
L. monocytogenes is of particular relevance during pregnancy, since pregnancy increases susceptibility to infection by this pathogen as manifest by a 10-fold reduction in LD50 from 2 × 104 CFU in a naive female mouse to 2 × 103 CFU in a pregnant mouse. Building on studies showing that (i) the induction of IFN-γ by CpG ODN enhances protection from listeria infection, (ii) CpG-induced protection peaks 3 days after ODN administration (6, 19, 23), and (iii) perinatal infection occurs most frequently during the second half of pregnancy (2, 26, 40), BALB/c mice were treated with CpG ODN on day 10 of gestation and challenged with L. monocytogenes 3 to 6 days later.
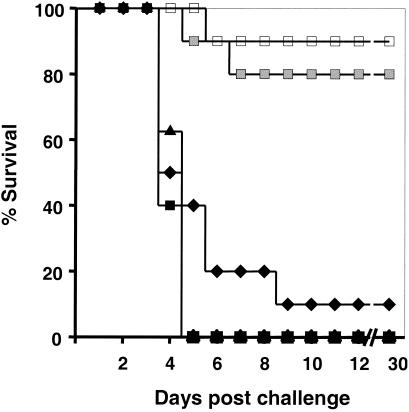
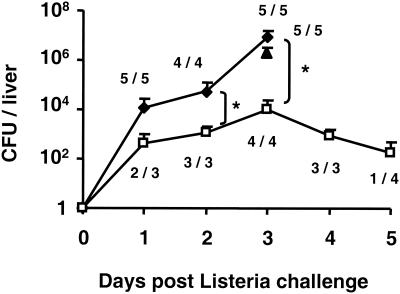
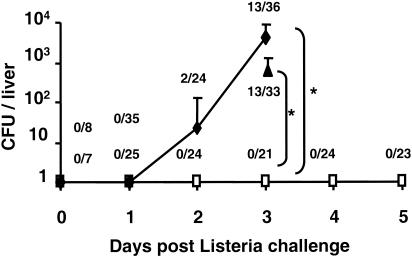
Nearly all pregnant mice treated with PBS or control ODN succumbed to challenge by 50 listeria LD50 (Fig. 3). In contrast, ≥80% of mice treated with CpG ODN 3 to 6 days prior to challenge survived L. monocytogenes infection (P < 0.001). Consistent with studies in normal mice showing that the CpG-mediated protective response requires several days to mature in vivo (6, 19, 23), protection was not observed when ODN treatment was delayed until 1 day after infection (Fig. 3). To evaluate the effect of CpG treatment on pathogen proliferation, the number of bacteria present in the liver of challenged animals (the primary locus of infection) (4) was monitored. The number of microorganisms increased rapidly over time in mice treated with PBS or control ODN, with all animals succumbing to infection by day 4 (Fig. 4). By comparison, the number of infectious organisms in CpG-treated animals rose slowly for 3 days (P < 0.05) and then began to wane. We found 75% of the animals to be free of listeriae by day 5, a result consistent with the 85% survival rate observed above (Fig. 3 and 4).
FIG. 3.
Survival of pregnant mice challenged with L. monocytogenes. BALB/c mice were injected i.p. with PBS (⧫) or with 150 μg of control (▴) or CpG (□) ODN on day 10 of gestation. Most groups were then challenged with listeria on day 13. One group of mice treated with CpG ODN on day 10 was challenged on day 16 (░⃞); another group was challenged with listeria on day 13 and treated with CpG ODN on day 14 (▪). n = 7 to 10 mice/group. ✽, P < 0.001.
FIG. 4.
Bacterial burden after the infection pregnant mice. Pregnant BALB/c mice were treated with PBS (⧫) or with 150 μg of control ODN (▴) or 150 μg of CpG ODN (□) on day 10 of gestation and then challenged i.p. with 50 L. monocytogenes LD50 on day 13. The mean number of viable bacteria in the liver of these mice was monitored, as was the number of infected mice/total. All PBS-treated animals succumbed to challenge by day 4. ✽, P < 0.05.
Effect of CpG ODN on fetal survival.
Maternal infection can result in abortion or the transmission of pathogen to the fetus. The effect of CpG ODN treatment on fetal survival was therefore examined. Infection with 50 listeria LD50 resulted in the loss of all offspring in 16 of 17 pregnant females “treated” with PBS or control ODN (Table 1). In contrast, litters were successfully delivered by 17 of 20 pregnant mice treated with CpG ODN. The mean litter size in the CpG-treated group was 5.9 ± 2.2, which did not differ significantly from the size of litters born to uninfected mothers (5.2 ± 2.3) but was nearly 50% higher than the four pups resulting from the one successful delivery by an infected mother in the absence of CpG treatment (Table 1).
TABLE 1.
Effect of CpG ODN treatment on fetal survivala
| Treatment | n | Day of treatment | Day of listeria challenge | % Litters aborted | Mean litter size ± SD |
|---|---|---|---|---|---|
| None | 10 | 0 | 5.2 ± 2.3 | ||
| PBS | 10 | 10 | 13 | 90 | 4.0 ± 0.0 |
| Control ODN | 7 | 10 | 13 | 100 | 0 |
| CpG ODN | 10 | 10 | 0 | 4.9 ± 2.0 | |
| 10 | 10 | 13 | 20* | 5.8 ± 2.0 | |
| 10 | 10 | 16 | 10* | 6.0 ± 2.3 | |
| 5 | 14 | 13 | 100 | 0 |
Pregnant BALB/c mice were treated i.p. with 150 μg of control ODN, CpG ODN, or PBS on day 10 or 14 of gestation. These mice were infected with 50 LD50 of L. monocytogenes on day 13 or 16, and the numbers and sizes of successfully delivered litters were recorded. *, Significantly different from PBS-treated mice, control ODN-treated mice, and CpG posttreated mice (P < 0.001).
When pregnant mice were “treated” with PBS or control ODN and then challenged with listeria, transmission of the pathogen to fetuses increased over time, rising from 0% on day 1 to >35% by day 3 (Fig. 5). Similarly, the number of bacteria in the liver of infected fetuses rose significantly over time (Fig. 5). In contrast, there was no pathogen transmission among the 124 fetuses derived from CpG-treated mothers (although 13 of 17 of these mothers had ongoing infection, Fig. 4) and there was no infections among any of the 111 pups born from CpG-treated mothers. These results indicate that CpG treatment significantly reduces the risk of maternal-fetal transfer of listeriae.
FIG. 5.
Transmission of listeria infection from mother to fetus. Fetuses were isolated from the pregnant mice studied in Fig. 3. The mean number of viable bacteria in the liver of these mice is shown, as is the number of infected mice/total. Note that infection rates were significantly higher among fetuses from PBS (⧫)- or control ODN (▴)-treated mothers versus those treated with CpG ODN (□). ✽, P < 0.05.
Effect of CpG ODN treatment on the development of long-term pathogen-specific immunity.
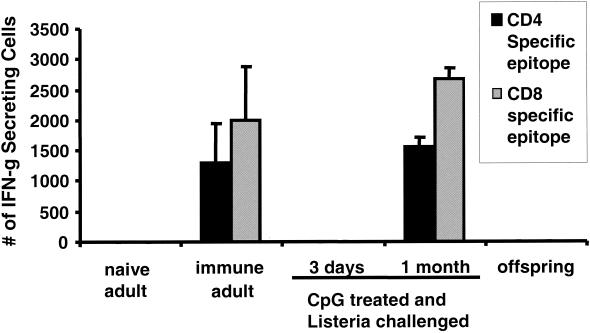
Previous studies established that mice surviving listeria challenge develop long-lasting pathogen-specific immunity (19). Consistent with that finding, spleen cells from normal adult mice exposed to a sublethal listeria dose mount a strong IFN-γ response when stimulated in vitro with peptides that selectively activate listeria-specific CD4 and CD8 T cells (Fig. 6). In contrast, spleen cells from naive adult mice did not respond to these peptides.
FIG. 6.
Frequency of listeria-specific CD4 and CD8 T cells. Spleen cells were stimulated in vitro for 10 h with CD4 (▪)- or CD8 (░⃞)-specific LLO antigen. The number of cells stimulated to secrete IFN-γ (IFN-g) was determined by ELIspot assay. Groups included naive adults, listeria-immune adults (challenged 4 weeks earlier with a sublethal dose of listeria), pregnant mice treated with CpG ODN on day 10 of gestation and challenged with 50 listeria LD50 on day 13 (cells from these mice were isolated either 3 days or 1 month postchallenge), and offspring of listeria-challenged CpG-treated mice (4 weeks after birth). The data show the mean increases ± the standard deviations in the number of cytokine-secreting cells versus unstimulated controls from three individually tested mice/group. The experiment was repeated with similar results. ✽, P < 0.01.
To examine the development of T-cell-mediated immunity in CpG-treated pregnant mice, spleen cells were isolated 3 days or 1 month after listeria challenge. At the 1-month time point, peptide-specific IFN-γ production equal in magnitude to that observed in listeria-immune normal mice was elicited (Fig. 6). These mice were also resistant to listeria challenge (Table 2). In contrast, spleen cells isolated from CpG-treated mice 3 days after listeria exposure did not respond to peptide stimulation (Fig. 6). These findings confirm previous reports showing that CpG ODN trigger an early protective innate immune response but that pathogen exposure leads to the subsequent development of antigen-specific immunity (6, 19).
TABLE 2.
Memory response to listeria infectiona
| Listeria LD50 | No. of surviving mice/total no. challenged |
||
|---|---|---|---|
| Normal adults | CpG-treated mothers | Offspring | |
| 10 | 0/5 | 6/6 | 1/30 |
| 100 | ND | 3/3 | 0/10 |
BALB/c mice were challenged i.p. with 10 to 100 LD50 of L. monocytogenes. Challenge groups included normal adult controls, females treated with CpG ODN and challenged with listeria during pregnancy, and the offspring of these CpG-treated mothers. The latter two groups were challenged 4 weeks postdelivery. Note that the survival of CpG-treated mothers was significantly greater than that of the two other groups (P < 0.001). ND, not determined.
The response of the offspring of CpG treated mothers was also examined. At 1 month of age, spleen cells from these mice did not respond to listeria-specific T-cell peptides (Fig. 6). Moreover, these animals were not resistant to challenge by listeriae (Table 2). These findings suggest that neonatal mice were not exposed to listeria antigens in utero and thus did not develop listeria-specific immunity.
Safety of CpG ODN treatment during pregnancy.
The present findings suggest that CpG treatment may benefit both pregnant mice and their offspring. To examine the safety of such therapy, parameters such as maternal health, abortion rates, litter size, and the birth weight of pups were evaluated. ODN treatment had no discernible effect on the health of the mothers: all remained physically vigorous and gave birth at term. There was also no difference in the number of newborns/litter (Table 1) or in the weight of the newborns derived from CpG ODN-treated (1.31 ± 0.15) versus control (1.34 ± 0.11) animals. None of the offspring had detectable congenital malformations nor did they show any defects and/or delays in development.
DISCUSSION
This study examined the ability of CpG ODN to improve the resistance of pregnant mice to L. monocytogenes infection, and the impact of such treatment on fetal survival. The results indicate that pregnant mice can be treated safely with CpG ODN prior to pathogen exposure, with treatment significantly improving maternal survival after listeria challenge (P < 0.001). CpG treatment also significantly reduced listeria-induced abortion (P < 0.001), pathogen transmission to the fetus (P < 0.001), and fetal death. These effects were clearly attributed to the expression of CpG motifs, since control non-CpG ODN did not mediate immune protection.
Pregnancy reduces antigen-specific Th1 and cell-mediated immune responsiveness (25, 31, 33, 38). This diminution in immunoreactivity helps protect the fetus from rejection (31, 33) but increases maternal susceptibility to infection by pathogens such as listeria (24, 35). Previous studies established that CpG ODN stimulate the innate immune system of normal adult animals, thereby improving host resistance to infection by a variety of bacterial, viral, and parasitic pathogens (6, 7, 15, 19, 23, 27, 29, 30, 32, 42). The present study evaluated whether these ODN could improve the innate immune response in animals whose adaptive immune system was compromised by pregnancy.
Our initial findings demonstrated that immune cells from pregnant mice responded normally to CpG ODN in vitro, as reflected by their production of Th1 and proinflammatory cytokines when stimulated with high or low concentrations of ODN (Fig. 1). Spleen cells from CpG-treated pregnant mice were shown to mount a significantly stronger response to heat-killed bacteria than cells from mice treated with control ODN (Fig. 2). This preceded pathogen challenge and thus must reflect activation of the innate rather than adaptive immune system. A model was developed to monitor the effect of ODN treatment on survival during pregnancy. Mice were treated with ODN on day 10 of gestation and challenged with 50 L. monocytogenes LD50 on day 13. Listeria was selected as the model pathogen both because it causes infection during human pregnancy and is responsive to CpG-mediated immunity (6, 19, 23).
Results show that CpG treatment protected pregnant mice from listeria challenge (Fig. 3). In contrast, protection was not observed when treatment was delayed until after challenge (Fig. 3). This is consistent with findings in normal mice indicating that the immunoprotective cascade elicited by CpG administration requires 3 days to reach optimal activity in vivo (6, 19, 23).
Although the cell types directly responsible for protection in this model were not identified, recent work from our lab indicates that plasmacytoid dendritic cells (pDC) play a key role in CpG-mediated protection against listeria. Studies in normal mice show that CpG ODN stimulate pDC to proliferate, that purified pDC isolated from mice treated with CpG ODN can transfer protection to naive mice, and that CpG treatment fails to protect pDC-deficient mice (work in progress). However, additional cell types also contribute to CpG-mediated immunity, including natural killer cells, macrophages, and B cells (3, 6, 22). In this context, results shown in Fig. 2 demonstrate that CpG treatment enhances the ability of spleen cells to produce IFN-γ and IL-12 when exposed to bacterial antigens in vitro.
These cytokines are known to play an important role in protection against listeriae (10, 13, 16). Further evidence that T cells did not contribute to initial in vivo protection induced by CpG treatment was the inability of cells isolated 3 days after infection to respond to stimulation by a CD4- or CD8-restricted peptide. However, an adaptive immune response was eventually generated after pathogen exposure, as manifested by T-cell-specific immunity to listeria-derived peptides 4 weeks after challenge (Fig. 6). This constellation of findings suggests that CpG ODN induce an early innate immune response that improves host resistance to initial pathogen challenge and promotes the subsequent development of pathogen-specific immunity (6, 17, 19, 21).
Neonatal listeriosis is a life-threatening illness that results from maternal infection during the later half of pregnancy (37). The effect of CpG treatment on the transmission of listeriae to the fetus was evaluated. Whereas >35% of fetuses from infected mothers treated with PBS or control ODN became infected with listeriae by 3 days after maternal challenge, none of the fetuses from CpG-treated mice became infected (P < 0.001; Fig. 5). Further studies indicate that fetal mice in CpG-treated mothers were never exposed to listeriae in utero, since (i) none of these animals died from infection after delivery and (ii) none developed pathogen-specific T-cell immunity after delivery (unlike their mothers; Fig. 6).
Exposure to certain TLR ligands (such as lipopolysaccharide) can increase abortion and fetal resorption rates, effects associated with the production of IFN-γ and tumor necrosis factor alpha (8). In the present study, pregnant mice showed no adverse effects from CpG treatment, remaining healthy and vigorous. This finding is consistent with studies of normal adult animals and ongoing clinical trials in humans indicating that CpG ODN can be administered safely (19, 21). Moreover, none of the pregnant animals treated with CpG ODN aborted, nor did treatment affect the litter size, fetal birth weight, or induce developmental or growth abnormalities in the offspring (Table 1). Thus, these findings support the possibility that CpG ODN may be used safely and effectively to prevent infection during pregnancy.
Acknowledgments
This study was supported in part by Military Interdepartmental Purchase Request #MM8926 and by DARPA.
The findings presented here should not to be construed as official or as reflecting the views of the U.S. Food and Drug Administration.
Editor: F. C. Fang
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., M. L. Cohen, and D. L. Swerdlow. 1997. Emerging food-borne diseases. Emerg. Infect. Dis. 3:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballas, Z. D., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:18401847. [PubMed] [Google Scholar]
- 4.D'Orazio, S. E., D. G. Halme, H. L. Ploegh, and M. N. Starnbach. 2003. Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection. J. Immunol. 171:291-298. [DOI] [PubMed] [Google Scholar]
- 5.Dresser, D. W. 1991. The potentiating effect of pregnancy on humoral immune responses of mice. J. Reprod. Immunol. 20:253-266. [DOI] [PubMed] [Google Scholar]
- 6.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 7.Gramzinski, R. A., D. L. Doolan, M. Sedegah, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddad, E. K., A. J. Duclos, E. Antecka, W. S. Lapp, and M. G. Baines. 1997. Role of interferon-gamma in the priming of decidual macrophages for nitric oxide production and early pregnancy loss. Cell. Immunol. 181:68-75. [DOI] [PubMed] [Google Scholar]
- 9.Halpern, M. D., R. J. Kurlander, and D. S. Pisetsky. 1996. Bacterial DNA induces murine interferon-gamma production by stimulation of IL-12 and tumor necrosis factor-alpha. Cell. Immunol. 167:72-78. [DOI] [PubMed] [Google Scholar]
- 10.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 3:109-117. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi, H., O. Takeuchi, T. Kawai, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 12.Holland, D., P. Bretscher, and A. S. Russell. 1984. Immunologic and inflammatory responses during pregnancy. J. Clin. Lab. Immunol. 14:177-179. [PubMed] [Google Scholar]
- 13.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 19.259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 14.Ishii, K. J., F. Takeshita, I. Gursel, M. Gursel, J. Conover, A. Nussenzweig, and D. M. Klinman. 2002. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J. Exp. Med. 196:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juffermans, N. P., J. C. Leemans, S. Florquin, A. Verbon, A. H. Kolk, P. Speelman, S. J. van Deventer, and P. T. van der. 2002. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect. Immun. 70:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 17.Klinman, D. M., D. Verthelyi, F. Takeshita, and K. J. Ishii. 1999. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity 11:123-129. [DOI] [PubMed] [Google Scholar]
- 18.Klinman, D. M., A. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12, and IFNγ. Proc. Natl. Acad. Sci. USA 93:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinman, D. M., and T. B. Nutman. 1994. ELIspot assay to detect cytokine-secreting murine and human cells, unit 6:19. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober, (ed.), Current protocols in immunology. Greene Publishing Associates, Brooklyn, N.Y.
- 21.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 22.Krieg, A. M., A. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-548. [DOI] [PubMed] [Google Scholar]
- 23.Krieg, A. M., L. L. Homan, A. K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 24.Krishnan, L., L. J. Guilbert, A. S. Russell, T. G. Wegmann, T. R. Mosmann, and M. Belosevic. 1996. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J. Immunol. 156:644-652. [PubMed] [Google Scholar]
- 25.Lin, H., T. R. Mosmann, L. Guilbert, S. Tuntipopipat, and T. G. Wegmann. 1993. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151:4562-4573. [PubMed] [Google Scholar]
- 26.Low, J. C., and W. Donachie. 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153:9-29. [DOI] [PubMed] [Google Scholar]
- 27.McCluskie, M. J., and H. L. Davis. 1998. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161:4463-4466. [PubMed] [Google Scholar]
- 28.Medzhitov, R., and C. A. Janeway. 1997. Innate immunity: impact on the adaptive immune response. Cur. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 29.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216-1224. [DOI] [PubMed] [Google Scholar]
- 30.Olbrich, A. R., S. Schimmer, and U. Dittmer. 2003. Preinfection treatment of resistant mice with CpG oligodeoxynucleotides renders them susceptible to friend retrovirus-induced leukemia. J. Virol. 77:10658-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priddy, K. D. 1997. Immunologic adaptations during pregnancy. J. Obstet. Gynecol. Neonatal Nurs. 26:388-394. [DOI] [PubMed] [Google Scholar]
- 32.Pyles, R. B., D. Higgins, C. Chalk, A. Zalar, J. Eiden, C. Brown, G. Van Nest, and L. R. Stanberry. 2002. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76:11387-11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghupathy, R. 1997. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today 18:478-482. [DOI] [PubMed] [Google Scholar]
- 34.Roman, M., E. Martin-Orozco, J. S. Goodman, M. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1 promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 35.Sano, M., M. Mitsuyama, Y. Watanabe, and K. Nomoto. 1986. Impairment of T cell-mediated immunity to Listeria monocytogenes in pregnant mice. Microbiol. Immunol. 30:165-176. [DOI] [PubMed] [Google Scholar]
- 36.Takeshita, F., C. A. Leifer, I. Gursel, K. Ishii, S. Takeshita, M. Gursel, and D. M. Klinman. 2001. Cutting Edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167:3555-3558. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wegmann, T. G., H. Lin, L. Guilbert, and T. R. Mosmann. 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14:353-356. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg, E. D. 1984. Pregnancy-associated depression of cell-mediated immunity. Rev. Infect. Dis. 6:814-831. [DOI] [PubMed] [Google Scholar]
- 40.Wing, E. J., and S. H. Gregory. 2002. Listeria monocytogenes: clinical and experimental update. J. Infect. Dis. 185(Suppl. 1):S18-S24. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto, S., T. Yamamoto, T. Katoaka, E. Kuramoto, O. Yano, and T. Tokunaga. 1992. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J. Immunol. 148:4072-4076. [PubMed] [Google Scholar]
- 42.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]