Abstract
Coating stability is increasingly recognized as a concern impacting the long-term effectiveness of drug eluting stents (DES). In particular, unstable coatings have been brought into focus by a recent published report (JAMA 2012). Towards the goal of overcoming current challenges of DES performance, we have developed an endothelium mimicking nanomatrix coating composed of peptide amphiphiles that promote endothelialization, but limit smooth muscle cell proliferation and platelet adhesion. Here, we report a novel water evaporation based method to uniformly coat the endothelium mimicking nanomatrix onto stents using a rotational coating technique, thereby eliminating residual chemicals and organic solvents, and allowing easy application to even bioabsorbable stents. Furthermore, the stability of the endothelium mimicking nanomatrix was analyzed after force experienced during expansion and shear stress under simulated physiological conditions. Results demonstrate uniformity and structural integrity of the nanomatrix coating. Preliminary animal studies in a rabbit model showed no flaking or peeling, and limited neointimal formation or restenosis. Therefore, it has the potential to improve the clinical performance of DES by providing multifunctional endothelium mimicking characteristics with structural integrity on stent surfaces.
1. Introduction
Recent studies have reported that cracking, flaking, and detachment of particles from polymeric coatings may play a major role in the limitations of current FDA approved DES, as described in JAMA 2012 (Denardo et al., 2012). Several of the evaluated stent coatings come loose during the enormous mechanical stress of stent expansion and could cause additional vessel wall injury. Damage to polymeric coatings during balloon expansion has also been reported before (Otsuka et al., 2007, Basalus et al., 2009). Case reports of embolization of polymeric fragments from other intravascular devices have also been reported and correlate with adverse events (Mehta et al., 2010). In addition, the process of elimination of these polymeric particles and associated vascular injuries could lead to local inflammatory reactions, which is a major concern with DES. Stent coating damage and detached microparticles could therefore contribute to DES-associated complications, including thrombosis, restenosis, and microvascular and endothelial dysfunction. Therefore, it is critical to address coating stability as a component of a multifunctional strategy designed to simultaneously tackle all the challenges faced by current drug eluting stents.
Towards this ultimate goal, we have developed a self-assembled endothelium mimicking peptide amphiphile (PA) based nanomatrix by incorporating laminin derived endothelial cell adhesive ligands, nitric oxide donors, enzyme mediated degradable sites, and water based self-assembly (Kushwaha et al., 2010, Andukuri et al., 2011). As described by Otsuka et al (Nat Rev Cardiol 2012), a healthy endothelium has been recognized as critical for long term vascular health in stenting (Otsuka et al., 2012), and this nanomatrix has demonstrated improved endothelial cell adhesion and proliferation, endothelial progenitor cell adhesion and differentiation, reduced smooth muscle cell proliferation and platelet adhesion (Kushwaha et al., 2010, Andukuri et al., 2011, Andukuri et al., 2013). These multiple bioactive functions in addition to controlled nitric oxide release and the unique water evaporation based self-assembled nanomatrix coating could therefore simultaneously prevent all the limitations of DES by reconstituting an endothelium mimicking on the stent surface.
In light of the emerging significance of coating stability, the goal of this study is to analyze and validate the stability of this endothelium mimicking nanomatrix as a coating for drug eluting stents. To coat the nanomatrix, we developed a rotational coating technique as shown in Figure 1A. The PA solution is held in an open top reservoir and the stent, held on a mandrel is rotated to ensure uniform coating. This technique allows the PAs to self-assemble onto the stent surface without organic solvents, and therefore can be easily applied to even promising bioabsorbable stents. To evaluate stability of the coatings, two critical factors that need to be addressed were considered – Shear stress and the force experienced during expansion. These processes can cause flaking, peeling, and cracking of the coating, thereby contributing to adverse events. We hypothesized that the unique water evaporation based self-assembly into a uniform multilayered nanofibrous matrix (Kushwaha et al., 2010, Andukuri et al., 2011) will endow the nanomatrix with sufficient stability in response to expansion and shear stress. Proof of stability in addition to previously mentioned bioactive properties will demonstrate the potential of this endothelium mimicking nanomatrix as a coating for next generation DES and pave the way for preclinical and clinical studies.
Figure 1.
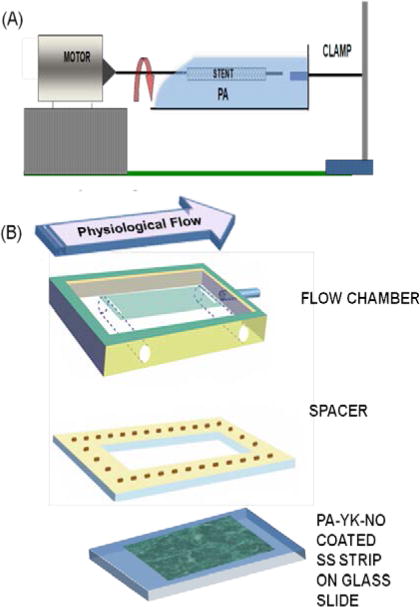
(A) Rotational coating technique for coating stents with the bioinspired multifunctional nanomatrix. (B) Bioreactor setup for simulating physiological shear stress.
2. Methods
2.1. Fabrication of the Endothelium Mimicking Nanomatrix
The nanomatrix was synthesized as previously described (Kushwaha et al., 2010, Andukuri et al., 2011, Andukuri et al., 2013). Briefly, two different PAs were synthesized via Fluorenylmethoxycarbonyl (Fmoc) chemistry. One PA contained an endothelial cell adhesive ligand (YIGSR) (Andukuri et al., 2010) linked to a matrix metalloprotease-2 (MMP-2) degradable sequence (GTAGLIGQ) to form PA-YIGSR. The second PA contained a polylysine nitric oxide (NO) donor (KKKKK) linked to the MMP-2 degradable sequence, forming PA-KKKKK. The two PAs were mixed in a 9:1 ratio as previously described (Kushwaha et al., 2010, Andukuri et al., 2011) and reacted with NO to form PA-YK-NO, the endothelium mimicking nanomatrix. Self-assembly of PA-YK-NO was achieved by a water evaporation method (Kushwaha et al., 2010, Andukuri et al., 2011).
2.2 Rotational Coating Technique
Coating of PA-YK-NO via water-evaporation based self-assembly was achieved by a rotational coating technique, as shown in Figure 1A. PA-YK-NO solution was contained in an open top reservoir to facilitate evaporation and the substrate, which was held by a mandrel attached to a rotating motor, was placed in the solution and rotated for 12 hours. Rotation ensured uniform coating of all surfaces. After immersion for 12 hours, the substrate was allowed to dry for a period of 24 hours.
2.3. Bioreactor Setup
The bioreactor setup is shown in Figure 1B (Walmet et al., 2003). Stainless steel strips (1cm × 3 cm) were coated with 1 wt% PA-YK-NO by the rotational coating technique, followed by self-assembly by water evaporation overnight. Two strips were placed in the parallel plate flow chamber. Phosphate buffered saline was perfused at 10 dynes/cm2 for three days. The flow rate was determined based on physiological shear stress, which ranges from 5 to 10 dynes/cm2 for arteries (Dammers et al., 2003, Sheikh et al., 2003). The coated stainless steel strip was then removed and the coating was analyzed by AFM. A PA-YK-NO coated strip that was not exposed to shear was used as a control surface.
2.4. Expansion of Stents
Coating of PA-YK-NO onto stainless steel stents (Pulse Systems, CA) via water-evaporation based self-assembly was achieved by the rotational coating technique. Coated stents were then expanded to 14 atm in DI water at 37°C, as previously described (Denardo et al., 2012, Basalus et al., 2009). PA-YK-NO coated unexpanded stents were used as controls, and the coatings were analyzed as follows.
2.5. Scanning Electron Microscopy (SEM)
Coated stents were analyzed using a Philips SEM 510 at an accelerating voltage of 20 KeV after being sputter coated with gold/palladium. This enabled visualization of any defects or cracks that arise from handling of the stents. Coated Stainless steel strips were analyzed by FE-SEM (Hitachi S-2500C, Japan) after mounting on aluminium stubs and platinum sputter coating.
2.6. Atomic Force Microscopy (AFM)
Surface characterization was performed using AFM following the procedure described by Booth et al and Lewis et al (Booth et al., 2011, Lewis et al., 2002). Topographical and lateral analysis of the coated surface was performed using contact mode AFM (Park Systems, XE-100, CA). This enabled analysis of the topography and adhesion of the coating to the stainless steel strip.
2.7. Fourier Transform Infrared Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR) was performed following the procedure described in the paper by Thomas et al (Thomas et al., 2007) to confirm the presence of PA-YK-NO coating on the stainless steel stents. Uncoated stainless steel stents were used as control surfaces. Attenuated total reflection (ATR) mode using a single crystal diamond window was used. The spectra were obtained with 64 scans per sample ranging from 4000–400 cm−1, with a resolution of 4 cm−1.
2.8. Analysis of In Vitro Cell Viability
To analyze cell viability, human umbilical vein endothelial cells (HUVECs) in EGM Media (Lonza) were seeded on stents at a density of 50,000 cells/cm2. After 7 days, the cells were stained with Live/Dead viability assay kit (Molecular probes) and visualized using an epifluorescence microscope.
2.9. Evaluation of In Vivo Performance using Rabbit Iliac Artery Model
In vivo performance of PA-YK-NO coated stents was performed in a rabbit iliac artery model. Using IACUC approved protocols, PEO sterilized coated (2) and uncoated control (2) stents were implanted into the iliac crest arteries of male white New Zealand rabbits (18–20 lbs). In detail, rabbits were anaesthetized with an intramuscular injection of ketamine (35 mg/kg) and xylazine (5 mg/kg). A 6 French Sheath was inserted into the carotid artery and heparin (150 units/kg) and gentamicin (1 mg/kg) were administered intravenously. Under fluoroscopic guidance, a 6 French JR4 coronary guide catheter was advanced over a 0.014” coronary guide wire to the descending aorta. Angiography was performed using approximately 8 ml of Meglumine diaztroate contrast injected via the catheter to determine to diameter of the artery. A 6 French JR4 coronary guide catheter was then advanced to the distal aortic bifurcation, and following angiography, a stent mounted on a delivery balloon was advanced over the guide wire into one iliac artery. The stent was deployed as slightly oversized, with a stent to artery ratio of 1.1 to 1. After 4 weeks, the rabbit was euthanized, and the stented artery sample was collected. It was fixed, sectioned, and stained with Hematoxylin and Eosin stains.
2.10. Statistical Analysis
All in vitro experiments were performed in quadruplicates and repeated at least three times to give a total n value of 12. All the values are expressed as mean±standard deviation. One way ANOVA was used to determine statistical significance and p<0.05 was considered significant using SPSS software.
3. Results and Discussion
PA-YK-NO was successfully fabricated. The bioactive properties of the nanomatrix have been studied earlier. Successful self-assembly into nanofibers, and long term NO release have been demonstrated (Kushwaha et al., 2010, Andukuri et al., 2011). Interestingly, this self-assembly into a multilayered nanomatrix is achieved via water evaporation without any organic solvents or chemical reactions. This is a unique property that highlights the innovation in the endothelium mimicking nanomatrix.
Peptide based stent coatings have been investigated before (Sargeant et al., 2008, Punet et al., 2013), but none of them have been made without the use of chemical reactions or organic solvents. This property enhances the biocompatibility of the nanomatrix, prevents solvent interference with NO release, and ensures that inflammatory reactions are limited. It also allows the utilization of the nanomatrix to functionalize bioabsorbable materials (Andukuri et al., 2011), thereby translating their application to promising bioabsorbable stents. Furthermore, the mechanical properties of PAs can be tuned by incorporating cysteine residues (Hartgerink et al., 2001). The bioactive properties of this endothelium mimicking nanomatrix have been demonstrated earlier. Adhesion and proliferation of endothelial cells, and the adhesion and differentiation of endothelial progenitor cells were promoted (Andukuri et al., 2013). This shows that the nanomatrix has the potential to promote endothelialization. The nanomatrix also inhibited the proliferation of smooth muscle cells, and the attachment of platelets. This shows that the nanomatrix can potentially reduce neointimal hyperplasia and thrombosis, thereby reducing the risk of restenosis. To utilize the unique properties of PA-YK-NO, a rotational coating technique was developed to allow self-assembly by water evaporation without organic solvents. To validate the stability of the nanomatrix, first PA-YK-NO was coated onto stainless steel strips and placed in the bioreactor to analyze the effect of physiological shear stress. Next, PA-YK-NO was coated onto stainless steel stents and the stents were expanded, and the coatings were analyzed. Successful coating by PA-YK-NO was confirmed by Fourier Transform Infrared Spectroscopy (FTIR) as shown in Figure 2. In comparison with uncoated stainless steel stents, the PA-YK-NO coated surfaces showed presence of amide group (1643 cm−1 and 1533 cm−1, N-H bend), carboxylic group (3282 cm−1, O–H stretch), and alkyl group (2925 cm−1 and 2850 cm−1, C–H stretch) confirming the presence of PAs.
Figure 2.
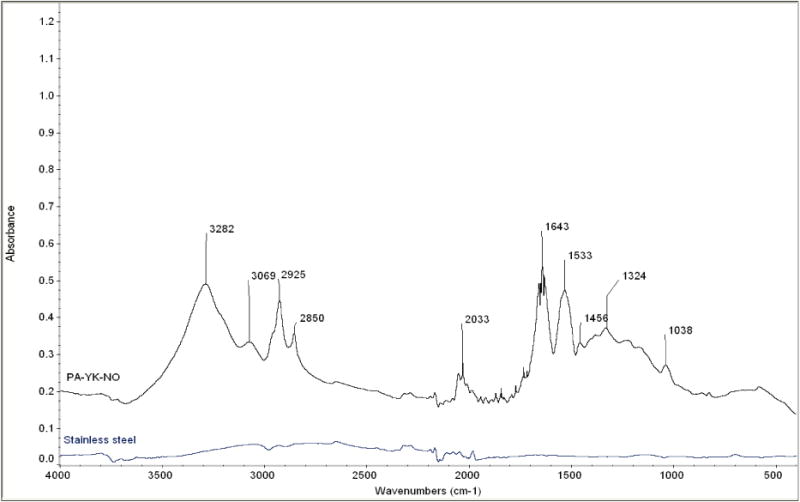
FTIR spectra of PA-YK-NO coated stainless steel stent and an uncoated stent.
The effect of shear stress on PA-YK-NO coatings was analyzed using a bioreactor that simulates physiological shear stress. 1 wt% PA-YK-NO was coated onto stainless steel strips (1 cm × 3 cm) using the rotational coating technique and exposed to shear stress of 10 dynes/cm2. Arterial shear stress ranges from 5–10 dynes/cm2, and the higher end of the range was chosen for these experiments (Dammers et al., 2003, Sheikh et al., 2003). The first three days constitute a critical period in the timeline of vessel healing after stenting. According to Edelman and Rodgers (Edelman and Rogers, 1998), vascular healing after stenting occurs in four phases. Within minutes of stent placement platelets aggregate and activate, secrete various cell-signaling factors and can result in thrombus formation. At this stage, the biocompatible coating on the stent may have a positive effect in reducing the risk of stent-related thrombosis. Over the next few days to weeks, a variety of white cells will gather at the injury site and secrete their own factors and exert their influence in turn on the healing tissue. The inflammatory response can persist for months, over which time the vessel wall is remodeled, largely by migration of smooth muscle cells to the site and creation of extracellular matrix (neointimal hyperplasia). This can act to reduce the vessel lumen size once again. Typical drug delivery strategies target one or more of these phases. PA-YK-NO is designed to deliver NO in a biphasic manner, with an initial burst release that is expected to reduce thrombus deposition, and therefore limit the possibility of thrombosis. Therefore, the first 3 days after stenting is critical for a PA-YK-NO coated stent to maintain its coating stability and uniformity, and thus prevent the aggravation of this initial physiological response to injury. PA-YK-NO coatings were first analyzed by SEM, and the results are shown in Figure 3A. There is no change in the coating surface after exposure to physiological shear stress. Next, topographical analysis of these coatings by AFM is shown in Figure 3B. As shown, there is no evident difference in the two images. There is no peeling, cracking, or flaking of PA-YK-NO. To further confirm the observations, surface roughness, the lateral force required to remove the coating, and depth of coating were analyzed. The results from these studies are shown in Figure 4. There was no statistical difference in surface roughness (Control: 99±21 nm; Test: 90±5 nm), force of removal (Control: 2.9± 0.5 MPa; Test: 3.3 ± 0.5 MPa), or coating depth (Control: 84±9 nm; Test: 72±3 nm) between the test and the control samples. This shows that shear stress is unlikely to cause flaking or peeling of the nanomatrix. Therefore, we can infer that coating stability is not significantly affected by exposure to physiological shear stress. After three days, there are no differences in surface topography, surface roughness, or the strength of adhesion. This indicates that the endothelium mimicking nanomatrix can withstand the shear stress it may experience in the critical period after stent deployment, and therefore, the next step was to analyze the stability of PA-YK-NO coatings on stents.
Figure 3.
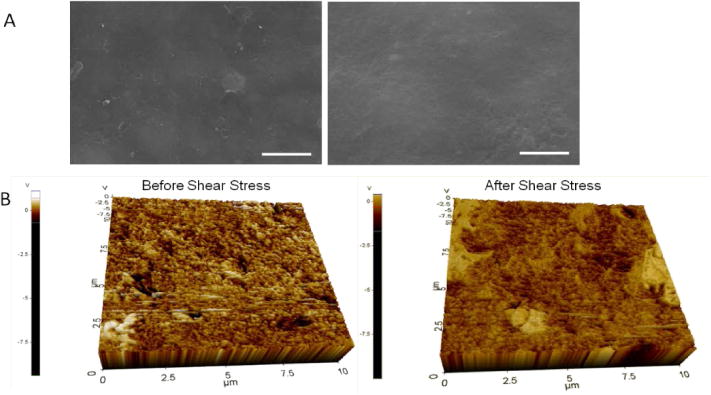
(A) SEM analysis of PA-YK-NO coating before shear stress (Left) and PA-YK-NO coating that was exposed to shear stress (Right). Scale bar = 2μm. (B) Topographical analysis of PA-YK-NO coating exposed to shear stress and PA-YK-NO coating that was not exposed to shear stress.
Figure 4.
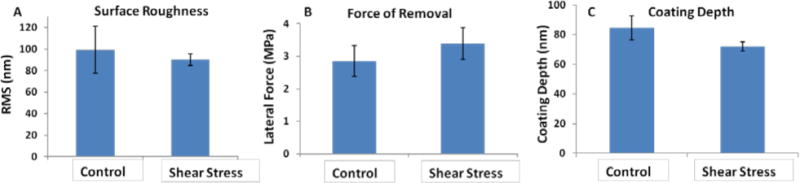
(A) Surface roughness of PA-YK-NO coatings exposed to shear stress. (B) The lateral force required to remove coatings exposed to shear stress. (C) Depth of PA-YK-NO coating before and after shear stress. In all three cases, there is no difference compared to controls.
To analyze this coating stability, stainless steel stents were coated with PA-YK-NO using the rotational coating technique. This coating method allows water evaporation based self-assembly into a uniform nanomatrix. The stents were then crimped, and expanded in DI water at 37°C to 14 atm as previously described (Denardo et al., 2012), and imaged by SEM before and after expansion. The results are shown in Figure 5A. An unexpanded PA-YK-NO coated stent is displayed on the left, while a PA-YK-NO coated stent that was crimped and expanded is displayed on the right. The coating was stable, and no flaking, peeling or cracking is visible after expansion. There are no visible differences between the two conditions. This is critical as finite element modeling has revealed up to 25% plastic deformation in 316L stainless steel stents during expansion (Migliavacca et al., 2002), and recent reports indicate that unstable coatings may contribute to the limitations of DES. PA-YK-NO coatings on expanded stents were further analyzed by contact mode AFM. The results of this study are shown in Figure 5B. As shown in the figure, the topographies of balloon expanded stents and unexpanded stents are similar, with no evidence of cracking, flaking, or peeling. To further confirm this, the roughness and the lateral force of removal were measured. Surface roughness would give a measure of the uniformity of the coating and a risk of flaking, while force of removal would give a measure of the strength of adhesion of the nanomatrix to the stent, as well as a measure of the risk of peeling. As shown in Figure 6A, there is no statistical difference in roughness between expanded (228 ±3.86 nm) and unexpanded stents (219 ±13.68 nm). There was also no significant difference between the unexpanded (77±12 MPa) and expanded stents (59±16 MPa) with respect to force of removal (Figures 6B) indicating that the strength of adhesion was unchanged after balloon expansion. Further, there was no statistical difference in the depth of PA-YK-NO coating on stents before (29±2 nm) and after expansion (33±5 nm) (Figure 6C). From these results, we can infer that expansion of stents does not affect the stability of the PA-YK-NO nanomatrix. There is no change in surface topography, surface roughness, or the strength of adhesion to the stent surface. This is critical for clinical success, as recent reports indicate that balloon expansion of FDA approved DES causes damage to coatings which in turn may contribute to their limitations. Expansion of PA-YK-NO coated stents is therefore unlikely to contribute to adverse events such as restenosis, or affect the bioactive properties of the nanomatrix.
Figure 5.
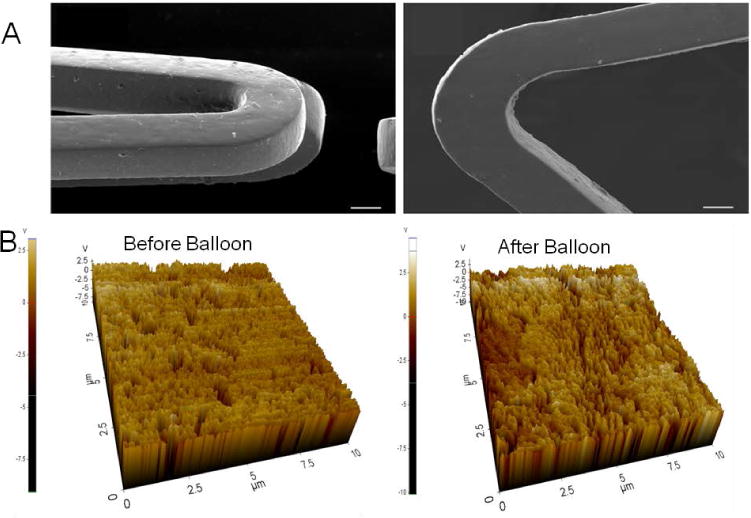
(A) SEM analysis of unexpanded PA-YK-NO (left) coated stent and PA-YK-NO (right) coated stent expanded at 37°C, 14 atm. Scale bar = 50 μm. (B) Topographical analysis of expanded and unexpanded PA-YK-NO coated stents using AFM.
Figure 6.
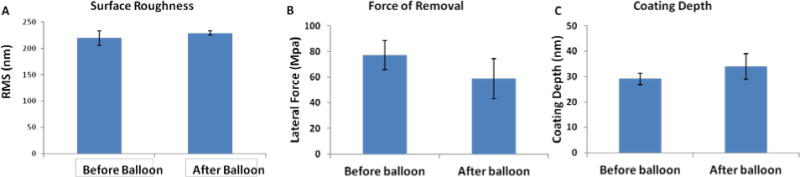
(A) Surface roughness measured using contact mode AFM. (B) Force of removal of coating from the surface measured from AFM. (C) Depth of coating before and after balloon expansion. In all three cases, there was no difference before and after balloon expansion.
Further, the effect of PA-YK-NO coated stents on endothelial cells was investigated by seeding HUVECs on coated stents and analyzing via Live/Dead assay kit at 7 days. As shown in Figure 7A, the cells attach the surface, spread, and achieve confluence. They remain viable, suggesting that PA-YK-NO coated stents support endothelial cell viability.
Figure 7.
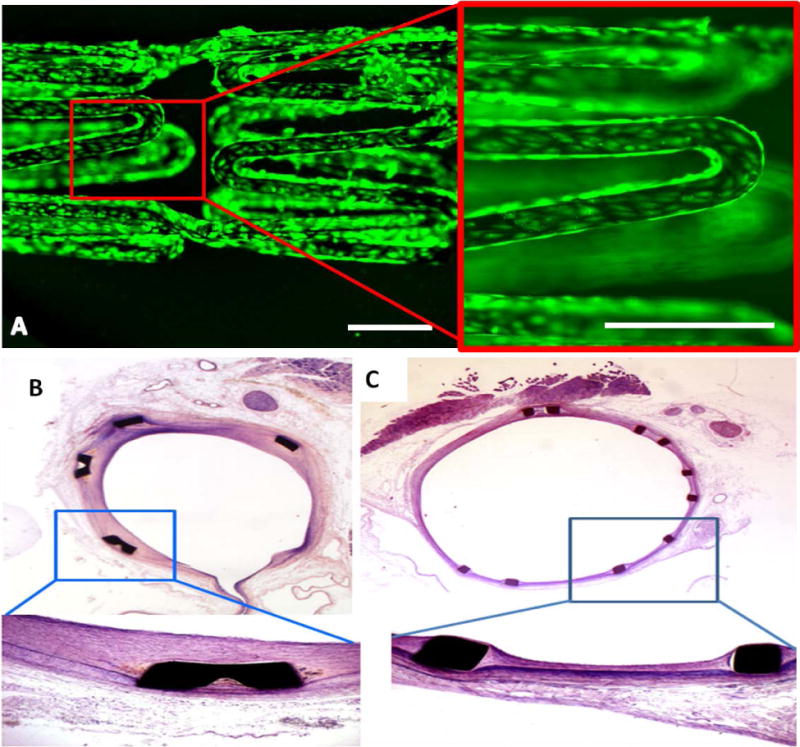
PA-YK-NO coated stents support endothelial cell viability (A). At 7 days, HUVECs seeded on PA-YK-NO coated stents attach, spread, and remain viable. Scale bar = 500 μm. (B) Uncoated control stent implanted in rabbit iliac artery, 4 week time point. (C) PA-YK-NO coated stent implanted in rabbit iliac artery at 4 weeks. There is limited neointimal growth, limited restenosis, and no evidence of flaking or peeling, suggesting the potential stability and efficacy of PA-YK-NO coating.
Finally, the efficacy of PA-YK-NO coated stents was analyzed by implantation in a rabbit iliac artery. After 4 weeks, the stented arteries were harvested and were histologically analyzed via hematoxylin and eosin staining. The results are shown in Figure 7C. In comparison with control, uncoated stent (Figure 7B), the coated stent displayed significantly reduced neointimal thickness and restenosis. There was no evidence of flaking, peeling, or deposition of thrombus and fibrin. Overall, these results suggest that PA-YK-NO can form a stable coating on stents, which in synergy with multiple bioactive functions can promote efficacy of implanted stents.
4. Conclusion
In light of emerging reports suggesting that insufficient stability of DES coatings may contribute to their limitations, this study aimed to demonstrate the coating stability of an endothelium mimicking nanomatrix in response to critical forces experienced by DES coatings – expansion and shear stress. To coat the nanomatrix onto stents, we have developed a rotating coating technique that allows self-assembly of PAs onto stent surfaces. This technique allows coating without the use of organic solvents and can therefore be easily applied to bioabsorbable stents and scaffolds. Results indicated that there was no evidence of flaking, peeling, or cracking of the nanomatrix. There were no statistical differences between test and control samples that were subjected to expansion and shear stress. In addition, PA-YK-NO coated stents implanted in a rabbit iliac artery model displayed no flaking or peeling and reduced neointimal formation and restenosis. This study demonstrates that this nanomatrix can provide stents with endothelium mimicking characteristics on the surface with structural integrity, and therefore it is an attractive coating for next generation DES.
Acknowledgments
This study was supported by NIH (1R03EB017344-01) for HWJ, NIH (1DP3DK094346-01) for HWJ and YY, and AHA predoctoral fellowship for AA. It was also supported by the Cell Regeneration Program (2012M3A9C6049717) through the Korea NSF funded by the MSIP, Industrial Strategic Technology Development Program (10043971) funded by the MOTIE, and KIST Program (2E24680) funded by MSIP, Korea for DKH.
List of References
- ANDUKURI A, KUSHWAHA M, TAMBRALLI A, ANDERSON JM, DEAN DR, BERRY JL, SOHN YD, YOON YS, BROTT BC, JUN HW. A hybrid biomimetic nanomatrix composed of electrospun polycaprolactone and bioactive peptide amphiphiles for cardiovascular implants. Acta Biomater. 2011;7:225–33. doi: 10.1016/j.actbio.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDUKURI A, MINOR W, KUSHWAHA M, ANDERSON J, JUN HW. Effect of endothelium mimicking self-assembled nanomatrices on cell adhesion and spreading of human endothelial cells and smooth muscle cells. Nanomedicine. 2010;6:289–297. doi: 10.1016/j.nano.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDUKURI A, SOHN YD, ANAKWENZE CP, LIM DJ, BROTT BC, YOON YS, JUN HW. Enhanced human endothelial progenitor cell adhesion and differentiation by a bioinspired multifunctional nanomatrix. Tissue Eng Part C Methods. 2013;19:375–85. doi: 10.1089/ten.tec.2012.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASALUS MW, ANKONE MJ, VAN HOUWELINGEN GK, DE MAN FH, VON BIRGELEN C. Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy. EuroIntervention. 2009;5:157–65. doi: 10.4244/eijv5i1a24. [DOI] [PubMed] [Google Scholar]
- BOOTH L, CATLEDGE SA, NOLEN D, THOMPSON RG, VOHRA YK. Synthesis and characterization of multilayered diamond coatings for biomedical implants. Materials (Basel) 2011;4:857–867. doi: 10.3390/ma4050857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMMERS R, STIFFT F, TORDOIR JH, HAMELEERS JM, HOEKS AP, KITSLAAR PJ. Shear stress depends on vascular territory: comparison between common carotid and brachial artery. J Appl Physiol. 2003;94:485–9. doi: 10.1152/japplphysiol.00823.2002. [DOI] [PubMed] [Google Scholar]
- DENARDO SJ, CARPINONE PL, VOCK DM, BATICH CD, PEPINE CJ. Changes to polymer surface of drug-eluting stents during balloon expansion. JAMA. 2012;307:2148–50. doi: 10.1001/jama.2012.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN ER, ROGERS C. Pathobiologic responses to stenting. Am J Cardiol. 1998;81:4E–6E. doi: 10.1016/s0002-9149(98)00189-1. [DOI] [PubMed] [Google Scholar]
- HARTGERINK JD, BENIASH E, STUPP SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- KUSHWAHA M, ANDERSON J, MINOR W, ANDUKURI A, BOSWORTH C, LANCASTER J, BROTT B, ANDERSON P, JUN HW. Native endothelium mimicking self-assembled nanomatrix for cardiovascular devices. Biomaterials. 2010;31:1502–1508. doi: 10.1016/j.biomaterials.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS AL, TOLHURST LA, STRATFORD PW. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials. 2002;23:1697–706. doi: 10.1016/s0142-9612(01)00297-6. [DOI] [PubMed] [Google Scholar]
- MEHTA RI, MEHTA RI, SOLIS OE, JAHAN R, SALAMON N, TOBIS JM, YONG WH, VINTERS HV, FISHBEIN MC. Hydrophilic polymer emboli: an under-recognized iatrogenic cause of ischemia and infarct. Mod Pathol. 2010;23:921–30. doi: 10.1038/modpathol.2010.74. [DOI] [PubMed] [Google Scholar]
- MIGLIAVACCA F, PETRINI L, COLOMBO M, AURICCHIO F, PIETRABISSA R. Mechanical behavior of coronary stents investigated through the finite element method. J Biomech. 2002;35:803–11. doi: 10.1016/s0021-9290(02)00033-7. [DOI] [PubMed] [Google Scholar]
- OTSUKA F, FINN AV, YAZDANI SK, NAKANO M, KOLODGIE FD, VIRMANI R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–53. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- OTSUKA Y, CHRONOS NA, APKARIAN RP, ROBINSON KA. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio, Taxus and Cypher stents. J Invasive Cardiol. 2007;19:71–6. [PubMed] [Google Scholar]
- PUNET X, MAUCHAUFFE R, GIANNOTTI MI, RODRIGUEZ-CABELLO JC, SANZ F, ENGEL E, MATEOS-TIMONEDA MA, PLANELL JA. Enhanced cell-material interactions through the biofunctionalization of polymeric surfaces with engineered peptides. Biomacromolecules. 2013 doi: 10.1021/bm4005436. [DOI] [PubMed] [Google Scholar]
- SARGEANT TD, RAO MS, KOH CY, STUPP SI. Covalent functionalization of NiTi surfaces with bioactive peptide amphiphile nanofibers. Biomaterials. 2008;29:1085–98. doi: 10.1016/j.biomaterials.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEIKH S, RAINGER GE, GALE Z, RAHMAN M, NASH GB. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood. 2003;102:2828–34. doi: 10.1182/blood-2003-01-0080. [DOI] [PubMed] [Google Scholar]
- THOMAS V, DEAN DR, JOSE MV, MATHEW B, CHOWDHURY S, VOHRA YK. Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite. Biomacromolecules. 2007;8:631–7. doi: 10.1021/bm060879w. [DOI] [PubMed] [Google Scholar]
- WALMET PS, ECKMAN JR, WICK TM. Inflammatory mediators promote strong sickle cell adherence to endothelium under venular flow conditions. Am J Hematol. 2003;73:215–224. doi: 10.1002/ajh.10360. [DOI] [PubMed] [Google Scholar]


