Abstract
Objective
Breast magnetic resonance imaging (MRI) is increasingly being used for both screening and diagnostic purposes. While performance benchmarks for screening and diagnostic mammography have been published, performance benchmarks for breast MRI have yet to be established. The purpose of our study was to comprehensively evaluate breast MRI performance measures, stratified by screening and diagnostic indications, from a single academic institution.
Subjects and Methods
Institutional review board approval was acquired for this HIPAA compliant study. Informed consent was not required. Retrospective review of our institutional database identified all breast MRI examinations performed from 4/1/07 to 3/31/08. After application of exclusion criteria, the following performance measures for screening and diagnostic indications were calculated: cancer detection rate, positive predictive values (PPV), and abnormal interpretation rates.
Results
The study included 2444 examinations, 1313 for screening and 1131 for diagnostic indications. The cancer detection rates were 14 per 1000 screening breast MRI examinations and 47 per 1000 diagnostic examinations (p-value < 0.00001). The abnormal interpretation rate was 12% (152/1313) for screening and 17% (194/1131) for diagnostic indications (p-value = 0.00008). The positive predictive values of MRI were lower for screening (PPV1 = 12%, PPV2 = 24%, PPV3 = 27%) compared to diagnostic indications (PPV1 = 28%, PPV2 = 36%, PPV3 = 38%).
Conclusion
Breast MRI performance measures differ significantly between screening and diagnostic MRI indications. Medical audits for breast MRI should calculate performance measures for screening and diagnostic breast MRI separately, as recommended for mammography.
Keywords: audit, breast MRI, positive predictive value, cancer detection rate, abnormal interpretation rate
Introduction
Since 1992, the Mammography Quality Standards Act (MQSA) has required a mandatory audit of mammography practices. Published in 2003, the fourth edition of the Breast Imaging Reporting and Data System (BI-RADS) atlas suggests additional data to collect and calculate for clinically meaningful mammography audits [1]. More recently, Congress commissioned a study from the Institute of Medicine (IOM) to assess whether additional steps could be taken to further improve breast imaging quality standards. The Institute of Medicine's “Improving Breast Imaging Quality Standards” suggests revision of the MQSA audit to require calculation of abnormal interpretation rate, cancer detection rate, and positive predictive value 2 (PPV2) with each calculation stratified by diagnostic or screening mammography [2]. The IOM report also recommended increased standardization and accreditation of breast magnetic resonance imaging (MRI) practices [2]. To standardize appropriate utilization of breast MRI, the American Cancer Society (ACS) published guidelines for breast MRI screening indications in 2007, and the American College of Radiology (ACR) has outlined recommendations for the use of breast MRI in both screening and diagnostic settings [3, 4]. In addition, accreditation of breast MRI facilities by the ACR began in 2010 [4]. The ACR breast MRI accreditation program requires each facility to establish and maintain a medical outcomes audit program; however, the audit requirements for breast MRI have not yet been formally outlined, as has been done for mammography [1, 4-8].
Although no formal audit requirements for screening or diagnostic breast MRI yet exist, some reports of various breast MRI performance benchmarks, including abnormal interpretation rate, cancer detection rate, and positive predictive values, have been published [9-18]. However, these performance measures are often reported individually, for only screening examinations, or for an aggregate cohort of screening and diagnostic examinations. Because performance measures are affected by the prevalence of cancer, calculations from an aggregate screening and diagnostic cohort should be interpreted with caution [19]. The purpose of our study was to comprehensively evaluate breast MRI performance measures, stratified by screening and diagnostic indications, from a single academic institution.
Subjects and Methods
Study Population
This study was approved by the institutional review board, and its methodology is in compliance with federal HIPAA regulations. A retrospective search of our institution's prospectively populated breast imaging database identified 2,596 breast MRI examinations performed between April 1, 2007 and March 31, 2008. From the preliminary list of 2,596 studies, 152 (5.9%) examinations were excluded for the following reasons: 92 breast MRI deemed non-diagnostic for technical reasons (inadequate fat saturation, contrast extravasation, etc.), 23 non-gadolinium enhanced MRI performed for silicone implant integrity assessment, 4 breast MRI aborted at patient's request, 23 studies with missing dictations in the electronic medical record, and 10 studies performed in patients who were lost to follow up. For each of the 2,444 breast MRI included in the study, the BI-RADS assessment was obtained from the imaging report in the electronic medical record [1]. An examination was assigned an overall BIRADS assessment according to the most actionable BI-RADS lesion category (BI-RADS 5 > 4 > 0 > 3 > 6 > 2 > 1).
Breast MRI Technique and Interpretation
Breast MRI technique was refined over the study period, but adherence to the basic imaging principles detailed in the following sequences was maintained. At our institution, breast MRI examinations were performed with the patient prone in a 1.5 T magnet with a dedicated breast coil (GE HD 8-channel Breast Array Coil, GE Healthcare). The standard bilateral acquisition protocol included a multi-planar localizing sequence, axial T1-weighted, axial fat-suppressed T2-weighted, and axial and sagittal T1 weighted fat suppressed sequences performed before and after injection of 20 mL gadopentetate dimeglumine (Magnevist, Bayer HealthCare Pharmaceuticals, Wayne, New Jersey). Bilateral axial post gadolinium acquisitions were centered at 1.5 minutes, 3 minutes, and 4.5 minutes, with the bilateral sagittal post gadolinium acquisition centered at 7 minutes. All MRI examinations were post-processed to generate subtraction images and lesion kinetic analysis using commercially available software (CADstream, Confirma).
Each breast MRI examination was interpreted by one of ten board-certified radiologists specializing in breast imaging. Each radiologist had greater than 3 years of breast MRI experience at the time of interpretation.
Data Extraction
For each breast MRI examination, the following data were extracted from the electronic database or electronic medical record: patient age, clinical indication for breast MRI, number and laterality of findings, available clinical follow-up, results of any additional imaging workup at our institution, and biopsy type and pathology, if applicable.
The authors coded each breast MRI study as screening or diagnostic based on the information available from the electronic database and the patient's electronic medical record. Screening examinations were defined as those ordered for the following indications: known BRCA mutation carrier, prior history of breast cancer after completion of treatment, family history of breast cancer, prior breast biopsy with pathology demonstrating atypia or lobular carcinoma in situ, or prior mediastinal radiation. Additional indications assigned to the screening category included patients without a current breast symptom whose medical records indicated that breast MRI was performed secondary to dense breast tissue, fibrocystic breast tissue, a prior history of a papilloma, or a prior history of ovarian cancer. Diagnostic examinations were defined as those performed to evaluate extent of disease in the setting of newly diagnosed breast cancer, examinations performed for additional evaluation of a clinical or imaging finding, or examinations for short interval follow up of probably benign findings on a prior breast MRI. When the breast MRI indication was uncertain, the case was discussed among the authors, and a consensus indication was determined.
Benign versus malignant outcome was determined for each patient with a breast MRI examination performed during the study period. Outcomes were assigned based upon biopsy results, imaging follow up, or clinical surveillance, performed within one year of the breast MRI examination. If a biopsy was performed at our institution or recorded in the electronic medical record, the method of biopsy, percutaneous and/or excisional, was extracted in addition to the pathology results. During the study period, excisional biopsy was routinely recommended for high risk lesions identified on percutaneous biopsy. High risk lesions included flat epithelial atypia, atypical ductal hyperplasia, lobular neoplasia, atypia of any other type, phyllodes tumor, radial scar, or papilloma. Final histopathologic outcomes were assigned as benign (no upgrade to malignancy) or malignant (upgrade to malignancy) based upon the excisional biopsy pathology. Ductal carcinoma in situ (DCIS), invasive lobular carcinoma, and invasive ductal carcinoma were categorized as malignant.
Statistical Analyses
Breast MRI assigned BI-RADS categories 0, 4, or 5 were defined as positive examinations. Breast MRI with suspicious findings were defined as those with a final BI-RADS 4 or 5 after additional imaging evaluation or work-up. A true positive case was defined as one with a diagnosis of malignancy within 1 year after a positive exam. Positive predictive values were calculated based upon BI-RADS recommendations for mammography, using the following definitions as recommended by the American College of Radiology: PPV 1 = true positives divided by positive exams; PPV 2 = true positives divided by exams with suspicious findings for which biopsy was recommended; and PPV 3 = true positives divided by biopsies performed [1]. PPV3 calculations were performed at the examination level, rather than the lesion level. Cancer detection rate was calculated as the number of cancers correctly detected at breast MRI per 1,000 examinations [1]. Abnormal interpretation rate was calculated as the number of positive examinations divided by the total number of examinations [1]. Each calculation was performed for the entire patient cohort and also stratified by screening or diagnostic indication.
Differences in cancer detection rate and abnormal interpretation rate were compared for screening and diagnostic indications using a chi square test. A p-value of less than 0.05 was considered statistically significant. 95% confidence intervals were calculated for cancer detection rates and abnormal interpretation rates. Because positive predictive value depends upon the prior probability of cancer, p values were not calculated to compare the differences in PPVs for screening and diagnostic indications [19].
Results
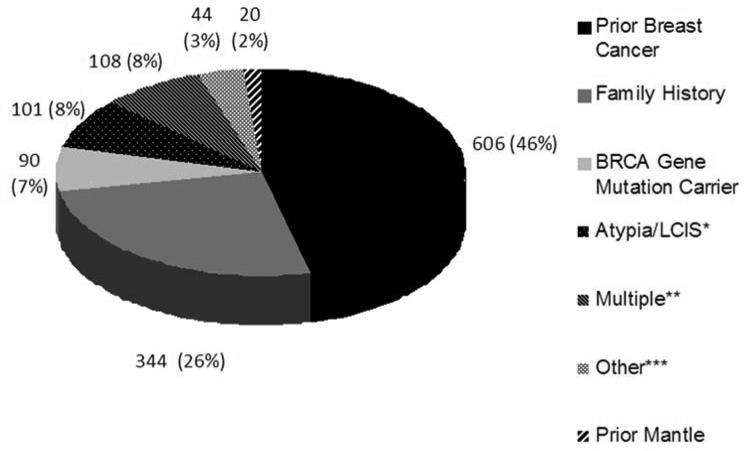
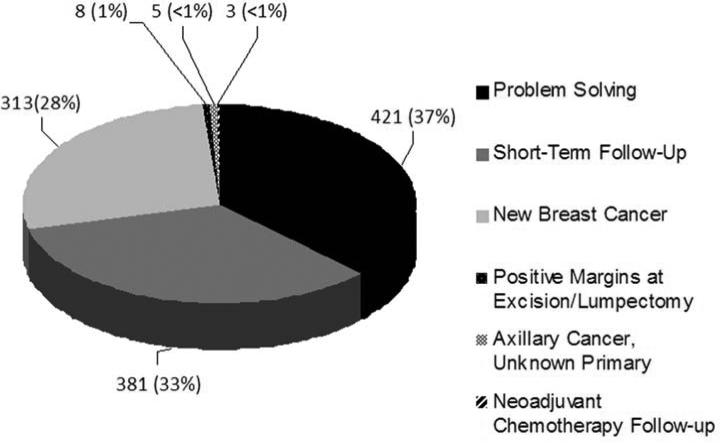
Of 2,444 breast MRI included in the study, the 1,313 screening examinations comprised 54% of the breast MRI performed. The most common reasons for screening MRI included a prior personal history of breast cancer and a family history of breast cancer (Figure 1). The 1,131 (46%) diagnostic breast MRI were most commonly performed for short term follow up of a previously described breast MRI lesion, problem solving, or evaluation of extent of disease in a new breast cancer diagnosis (Figure 2).
Fig 1.
Indications for screening breast MRI
Fig 2.
Indications for diagnostic breast MRI
Abnormal interpretation rate was 12% (95% CI, 10%-13%) for screening and 17% (95% CI, 15%-19%) for diagnostic indications (Table 1), a statistically significant difference (p value =0.00008). Following inter-modality correlation (final BI-RADS 4 or 5 assessment after inter-modality correlation), the biopsy recommendation rate was 6% (75/1313, 95% CI, 4%-7%) for screening and 13% (149/1131, 95% CI, 11%-15%) for diagnostic indications (p-value < 0.00001), with an overall rate of 9% (224/2444, 95% CI, 8%-10%). Following inter-modality correlation, 277 (21%) screening and 314 (28%) diagnostic breast MRI examinations were assigned a final BI-RADS 3 assessment.
Table 1.
Performance measures for screening and diagnostic breast MRI
| Screening | Diagnostic | Overall | |
|---|---|---|---|
| Patient Age Mean (standard deviation) | 51.1 yrs (10.3) | 50.6 yrs (10.6) | 50.9 yrs (10.5) |
| Total # of MRIs performed | 1,313 | 1,131 | 2,444 |
| # Positive MRIs (BI-RADS 0,4,5) | 152 | 194 | 346 |
| Final BI-RADS 4 or 5 | 75 | 149 | 224 |
| Biopsies (lesion level) | 77 | 172 | 249 |
| Biopsies (exam level) | 67 | 139 | 206 |
| Cancer diagnoses (lesion) | 18 | 61 | 79 |
| Cancer diagnoses (exam) | 18 | 54 | 72 |
| Cancer detection rate (per 1000 exams) | 14 | 47* | 29* |
| Abnormal interpretation rate | 152/1313 (12%) | 194/1131 (17%) | 346/2444 (14%) |
| PPV1 | 18/152 (12%) | 54/194 (28%) | 72/346 (21%) |
| PPV2 | 18/75 (24%) | 53/149 (36%)* | 71/224 (32%)* |
| PPV3 (exam level) | 18/67 (27%) | 53/138 (38%)* | 71/205 (35%)* |
One patient underwent a diagnostic breast MRI due to her current history of breast cancer. Her study demonstrated non mass-like enhancement in the contralateral breast, for which correlative mammography was recommended. Inter-modality correlation was negative, so her final BI-RADS category was probably benign (BI-RADS 3). The patient elected to undergo a prophylactic contralateral mastectomy, with pathology demonstrating an invasive carcinoma in the same quadrant as the non mass-like enhancement. Therefore, this patient is excluded from the cancer detection rate, PPV2, and PPV3 calculations.
Table 2 details the pathologic diagnoses in the 249 MR-detected lesions for which biopsies were recommended. Breast MRI performed for diagnostic indications accounted for 69% of the lesions recommended for biopsy, including 77% (61/79) of the biopsies resulting in a diagnosis of malignancy (Table 2). The majority of MR-detected malignancies were invasive carcinomas with no statistically significant difference in invasive cancer size for screening versus diagnostic indications (p-value = 0.62).
Table 2.
Pathologic diagnoses in 249 suspicious breast MRI lesions
| Screening | Diagnostic | Overall | |
|---|---|---|---|
| Total Lesions (%) | 77 | 172 | |
| Malignant | 18 | 61 | 79 |
| Invasive ductal carcinoma | 11/18 (61%) | 27/61 (44%) | 38/79 (48%) |
| Ductal carcinoma in situ | 7/18 (39%) | 20/61 (33%) | 27/79 (34%) |
| Invasive lobular carcinoma | 0/18 (0%) | 3/61 (5%) | 3/79 (4%) |
| Other* | 0/18 (0%) | 11/61 (18%) | 11/79 (14%) |
| Invasive Cancer Size (mm)† | |||
| Mean (Standard Deviation) | 15 mm (13) | 15 mm (15) | 15 mm (13) |
| Benign | 59 | 111 | 170 |
| Fibrocystic changes, stromal fibrosis, or sclerosing adenosis | 18/59 (31%) | 25/111 (23%) | 43/170 (25%) |
| Fibroadenoma | 21/59 (36%) | 33/111 (30%) | 54/170 (32%) |
| Pseudoangiomatous stromal hyperplasia | 3/59 (5%) | 15/111 (14%) | 18/170 (11%) |
| Atypia including lobular neoplasia | 3/59 (5%) | 8/111 (7%) | 11/170 (6%) |
| Hamartoma | 5/59 (8%) | 9/111 (8%) | 14/170 (8%) |
| Radial scar | 3/59 (5%) | 3/111 (3%) | 6/170 (4%) |
| Papilloma | 0/59 (0%) | 3/111 (3%) | 3/170 (2%) |
| Phyllodes tumor | 0/59 (0%) | 0/111 (0%) | 0/170 (0%) |
| Post radiation changes | 2/59 (3%) | 4/111 (4%) | 6/170 (4%) |
| Other‡ | 4/59 (7%) | 11/111 (11%) | 15/170 (9%) |
Included phyllodes with sarcoma, lymph node with metastatic carcinoma, mixed invasive ductal and lobular carcinoma, and lesions missing data on the specific type of malignant diagnosis
Data only available for 75 lesions
Included other benign pathology results and lesions missing data on the specific type of benign diagnosis
The cancer detection rate was significantly lower for screening (14 per 1000 breast MRI, 95% CI 7 -20 per 1000 breast MRI) compared to diagnostic indications (47 per 1000 breast MRI, 95% CI 35 – 59 per 1000 breast MRI) (p-value <0.00001). Positive predictive values were also lower for screening than diagnostic indications (Table 1). PPV1 (cancers detected after positive MRI) was 12% for screening, 28% for diagnostic indications, and 21% overall. PPV2 (cancers detected after biopsy recommended) was 24% for screening, 36% for diagnostic indications, and 32% overall. PPV3 (cancers detected after biopsy performed) was 27% for screening, 38% for diagnostic indications, and 35% overall (Table 1).
Discussion
In this study, we adapted calculations from the MQSA mammography audit to comprehensively analyze performance measures for all breast MRI examinations performed during a single calendar year at a large academic center. This study is one of the largest studies of MRI performance in clinical practice rather than in a clinical trial setting. Because cancer detection rate and positive predictive values are inextricably linked, a single performance measure viewed in isolation may not be meaningful. Another strength of our study is the joint reporting of multiple breast MRI performance measures. Furthermore, our study demonstrates that breast MRI performance measures vary significantly when stratified by screening versus diagnostic indication. Published studies of mammography performance have reported significantly different performance benchmarks for screening and diagnostic mammography, and our study extends those findings to non-mammographic breast imaging [20-23]. Our results of significantly different MRI performance for clinical and diagnostic indications suggests that stratified, rather than aggregate, analyses of performance measures will provide more accurate performance estimates to guide the application of MRI in clinical practice.
In our study, abnormal interpretation rates of 12% and 17% for screening and diagnostic indications, respectively, are consistent with the published literature, which report abnormal interpretation rates for breast MRI ranging from 8-17% for screening MRI and up to 22% for an aggregate screening and diagnostic population [9, 12, 13, 15]. For mammography, abnormal interpretation rate calculations differ for screening and diagnostic indications. Positive screening mammograms include those assigned to BIRADS categories 0, 4, and 5, whereas positive diagnostic mammograms include only BIRADS 4 and 5 because the use of BI-RADS 0 is discouraged in diagnostic mammography [1]. However, utilization of the BI-RADS 0 category differs in mammography and breast MRI, and this difference affects the abnormal interpretation rate calculations. For breast MRI performed at our institution during the study period, the BI-RADS 0 category was used for both screening and diagnostic MRI examinations when the interpreting radiologist recommended inter-modality correlation for a finding identified on MRI. Our usage of BI-RADS 0 during the study period reflected recommendations in the first edition of the BI-RADS MRI atlas published in 2003, which defined BI-RADS 0 as “A recommendation for additional imaging evaluation includes repeating MRI with satisfactory technique, obtaining information from other imaging modalities (mammographic views, ultrasound, etc.), or correlation with prior breast history.” [1]. Therefore, our abnormal interpretation rate calculations presented in Table 1 include BI-RADS 0, 4, and 5 examinations for both screening and diagnostic breast MRI indications. However, the use of BI-RADS 0 is now discouraged for screening or diagnostic breast MRI when inter-modality correlation is recommended [24]. As a result, a contemporary definition of abnormal interpretation rate for screening and diagnostic breast MRI would describe a “positive exam” as one categorized as a BI-RADS 4 or 5. Therefore, in our study, we also calculated abnormal interpretation rates for breast MRI including only examinations with a final BI-RADS 4 or 5 assessment after inter-modality correlation. Using this contemporary definition, the abnormal interpretation rate was 6% for screening and 13% for diagnostic indications. There is discussion that the new edition of the BI-RADS atlas, which remains unpublished at this time, may include BI-RADS 3 examinations as a “positive exam”, in addition to BI-RADS 0, 4, and 5. However, the published version of the BI-RADS atlas, in widespread clinical use at the time of exam interpretation and statistical analyses for this study, currently categorizes a BI-RADS 3 examination as negative [1]. Therefore, in our study analyses and in our clinical practice, BI-RADS 3 breast MRI examinations are currently categorized as negative for auditing purposes. All published studies to date regarding individual performance measures for breast MRI have similarly categorized “positive” and “negative” examinations per the published BI-RADS atlas, permitting the reader to compare our results with the prior literature. Because BI-RADS 3 examinations may be included as “positive” examinations in the upcoming BI-RADS 5 atlas, we included our data regarding the number of BIRADS 3 assessments for both screening and diagnostic breast MRI following inter-modality correlation. The percentage of final BI-RADS 3 assessments was high in our data set, although our BI-RADS 3 assessments over the preceding two years have been low (2.6 – 4.7%, unpublished data). This is consistent with published literature demonstrating that the frequency of BI-RADS 3 assessments decreases with prevalent breast MRI examinations, advances in image quality and interpretation, and radiologist experience [25-29].
Compared to mammography alone, screening breast MRI in high risk women demonstrates an incremental cancer detection rate of 8 to 67 cancers per 1000 patients [12, 13, 30]. Our cancer detection rate for diagnostic indications (48 per 1000) was significantly higher than for screening indications (14 per 1000), as expected due to the higher prior probability of breast cancer in the diagnostic group. Our results correlate with the higher cancer detection rates in diagnostic versus screening mammography [20, 23]. Our positive predictive value calculations for screening, diagnostic, and combined indications are also consistent with previously published results. Breast MRI PPV1 has been reported as high as 50% for combined screening and diagnostic breast MRI and 24% for screening alone [9, 13, 15, 18]. Previously published breast MRI PPV2 (biopsy recommended) is 42% overall and 22% in a screening population [9, 13]. Prior studies of PPV3 (biopsy performed) for breast MRI report ranges of 20-61% overall, 10-25% for screening indications, and 28-42% for diagnostic indications [9-17].
Different patient populations likely result in different distributions of screening and diagnostic breast MRI indications. In screening and diagnostic mammography, performance measures vary substantially by exam indication due to different prior probabilities of breast cancer in these patient groups. For example, diagnostic mammograms performed to evaluate areas of clinical concern have higher PPVs than examinations performed for short interval follow-up or to evaluate screen-detected abnormalities [20]. Our study's inclusion of overall and stratified cancer detection rates, as well as the distributions of screening and diagnostic exam indications, should enable the reader to evaluate the degree to which our patient population differs from his or her own patient population. The relatively smaller sample size of our single institution study precluded analyses further stratified by individual screening or diagnostic exam indications (e.g. family history of breast cancer, prior history of breast cancer, or short interval follow up examination).
Limitations of this study include the reporting of breast MRI performance measures from a single tertiary care center, which may limit generalizability of the observed findings. Compared to the remainder of the United States, patients in Massachusetts are more likely to be white and have health insurance [31]. Another limitation of our study is our inability to confirm tissue diagnoses of cancer in patients who may have been diagnosed outside of our institution because our patient population is not tracked within a cancer registry. However, greater than 90% of women with a recommendation for biopsy returned to our institution to undergo the biopsy, suggesting that any effect on our analyses from missing pathology results is likely to be minor. An additional limitation of our study is the lack of stratified analyses for incident versus prevalent examinations. Prevalent examinations have higher abnormal interpretation rates compared to incident examinations in previously published reports of screening breast MRI examinations [12].
In summary, we comprehensively analyzed performance measures for breast MRI and identified significant differences in cancer detection rate, abnormal interpretation rate, and PPVs, when stratified by screening and diagnostic indications. We suggest that audits of breast MRI examinations incorporate separate performance benchmarks for screening and diagnostic breast MRI, as recommended for mammography.
Take-Home Points.
Performance measures, including positive predictive value, abnormal interpretation rate, and cancer detection rate, vary significantly for screening and diagnostic breast MRI examinations.
Comprehensive audits of breast MRI examinations should incorporate separate performance benchmarks for screening and diagnostic breast MRI, as recommended for mammography.
Acknowledgments
Dr Lee's participation was supported in part by grant K07 CA128816 from the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Current contact information for Dr. Janie Lee: Seattle Cancer Care Alliance 825 Eastlake Avenue East Seattle, WA 98109 Telephone: 206-288-6241 Fax: 206-288-6556 jmlee58@uw.edu
The authors have no relevant conflicts of interest to disclose.
We comprehensively analyzed performance measures for breast MRI and identified significant differences in cancer detection rate, abnormal interpretation rate, and PPVs, when stratified by screening and diagnostic indications.
Contributor Information
Bethany L. Niell, Massachusetts General Hospital Avon Comprehensive Breast Evaluation Center Wang Building Suite 240 Boston, Massachusetts 02114 Telephone: 617-726-3093 Fax: 617-726-1074 bniell@partners.org
Sara C. Gavenonis, Department of Radiology Christiana Care Health System 4755 Ogletown-Stanton Road Newark, Delaware 19718 Telephone: 302-623-4122 Fax: 302-623-4204 sgavenonis@christianacare.org
Tina Motazedi, University of Texas Health Science Center San Antonio School of Medicine 7703 Floyd Curl Drive San Antonio, TX 78229 Telephone: 713-303-1129 Motazedi@livemail.uthscsa.edu
Jessica Cott Chubiz, Massachusetts General Hospital Department of Radiology Institute for Technology Assessment 101 Merrimac Street, 10th Floor Boston, Massachusetts 02114 Telephone: 617-726-0849 Fax: 617-726-9414 jchubiz@partners.org
Elkan F. Halpern, Massachusetts General Hospital Department of Radiology Institute for Technology Assessment 101 Merrimac Street, 10th Floor Boston, Massachusetts 02114 Telephone: 617-726-0849 Fax: 617-726-9414 elk@mgh-ita.org
Elizabeth A. Rafferty, Massachusetts General Hospital Avon Comprehensive Breast Evaluation Center Wang Building Suite 240 Boston, Massachusetts 02114 Telephone: 617-726-3093 Fax: 617-726-1074 erafferty@partners.org
Janie M. Lee, Contact information at the time of the study: Massachusetts General Hospital Department of Radiology Institute for Technology Assessment 101 Merrimac Street, 10th Floor Boston, Massachusetts 02114 Telephone: 617-726-0849 Fax: 617-726-9414
References
- 1.D'Orsi C, Mendelson E, Ikeda D, et al. Breast Imaging Reporting and Data System: ACR BI-RADS - breast imaging atlas. American College of Radiology; Reston, VA: 2003. [Google Scholar]
- 2.Nass S, Ball J. Improving Breast Imaging Quality Standards. 2005:240. [Google Scholar]
- 3.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 4.ACR Joint Committee on Breast Imaging for Appropriateness Criteria and Practice Guidelines of the Commission on Breast Imaging. ACR practice guideline for the performance of contrast enhanced magnetic resonance imaging (MRI) of the breast. Available at: http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/MRI_Breast.pdf. Accessed: May 21, 2013.
- 5.American College of Radiology Breast magnetic resonance imaging (MRI) accreditation program requirements. doi: 10.1016/j.mri.2020.06.017. Available at: http://www.acr.org/~/media/ACR/Documents/Accreditation/BreastMRI/Requirements.pdf. Accessed: May 21, 2013. [DOI] [PMC free article] [PubMed]
- 6.Linver MN, Osuch JR, Brenner RJ, Smith RA. The mammography audit: a primer for the mammography quality standards act (MQSA). AJR. 1995;165:19–25. doi: 10.2214/ajr.165.1.7785586. [DOI] [PubMed] [Google Scholar]
- 7.Sickles EA. Quality assurance. How to audit your own mammography practice. Radiol Clin North Am. 1992;30:265–275. [PubMed] [Google Scholar]
- 8.Sickles EA, Ominsky SH, Sollitto RA, Galvin HB, Monticciolo DL. Medical audit of a rapid-throughput mammography screening practice: methodology and results of 27,114 examinations. Radiology. 1990;175:323–327. doi: 10.1148/radiology.175.2.2326455. [DOI] [PubMed] [Google Scholar]
- 9.DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR. 2012;198:W373–80. doi: 10.2214/AJR.10.6272. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez RL, Demartini WB, Eby P, Kurland BF, Peacock S, Lehman CD. Clinical indication and patient age predict likelihood of malignancy in suspicious breast MRI lesions. Acad Radiol. 2009;16:1281–1285. doi: 10.1016/j.acra.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Han BK, Schnall MD, Orel SG, Rosen M. Outcome of MRI-guided breast biopsy. AJR. 2008;191:1798–1804. doi: 10.2214/AJR.07.2827. [DOI] [PubMed] [Google Scholar]
- 12.Lehman CD. Role of MRI in screening women at high risk for breast cancer. J Magn Reson Imaging. 2006;24:964–970. doi: 10.1002/jmri.20752. [DOI] [PubMed] [Google Scholar]
- 13.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 14.Liberman L, Mason G, Morris EA, Dershaw DD. Does size matter? Positive predictive value of MRI-detected breast lesions as a function of lesion size. AJR. 2006;186:426–430. doi: 10.2214/AJR.04.1707. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C. Positive predictive value of BI-RADS MR imaging. Radiology. 2012;264:51–58. doi: 10.1148/radiol.12110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch GM, Dogan BE, Smith TB, Liu P, Yang WT. Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR. 2012;198:292–299. doi: 10.2214/AJR.11.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viehweg P, Bernerth T, Kiechle M, et al. MR-guided intervention in women with a family history of breast cancer. Eur J Radiol. 2006;57:81–89. doi: 10.1016/j.ejrad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Hillman BJ, Harms SE, Stevens G, et al. Diagnostic performance of a dedicated 1.5-T breast MR imaging system. Radiology. 2012;265:51–58. doi: 10.1148/radiol.12110600. [DOI] [PubMed] [Google Scholar]
- 19.Kopans DB. The positive predictive value of mammography. AJR. 1992;158:521–526. doi: 10.2214/ajr.158.3.1310825. [DOI] [PubMed] [Google Scholar]
- 20.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology. 2005;235:775–790. doi: 10.1148/radiol.2353040738. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology. 2006;241:55–66. doi: 10.1148/radiol.2411051504. [DOI] [PubMed] [Google Scholar]
- 22.Carney PA, Sickles EA, Monsees BS, et al. Identifying minimally acceptable interpretive performance criteria for screening mammography. Radiology. 2010;255:354–361. doi: 10.1148/radiol.10091636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feig SA. Auditing and benchmarks in screening and diagnostic mammography. Radiol Clin North Am. 2007;45:791–800. doi: 10.1016/j.rcl.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Edwards S, Lipson J, Ikeda D, Lee J. Updates and Revisions to the BI-RADS Magnetic Resonance Imaging Lexicon. Magnetic Resonance Imaging Clinics of North America. 2013;21:483–493. doi: 10.1016/j.mric.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Sadowski EA, Kelcz F. Frequency of malignancy in lesions classified as probably benign after dynamic contrast-enhanced breast MRI examination. J Magn Reson Imaging. 2005;21:556 – 564. doi: 10.1002/jmri.20312. [DOI] [PubMed] [Google Scholar]
- 26.Liberman L, Morris EA, Benton CL, Abramson AF, Dershaw DD. Probably benign lesions at breast magnetic resonance imaging: preliminary experience in high risk women. Cancer. 2003;98:377 – 388. doi: 10.1002/cncr.11491. [DOI] [PubMed] [Google Scholar]
- 27.Kuhl CK, Schmutzler RK, Leutner CC, et al. Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology. 2000;215:267 – 279. doi: 10.1148/radiology.215.1.r00ap01267. [DOI] [PubMed] [Google Scholar]
- 28.Eby PR, Demartini WB, Gutierrez RL, et al. Characteristics of probably benign breast MRI lesions. AJR. 2009;193:861 – 867. doi: 10.2214/AJR.08.2096. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein SP, Hanna LG, Gatsonis C, et al. Frequency of malignancy seen in probably benign lesions at contrast-enhanced breast MR imaging: findings from ACRIN 6667. Radiology. 2010;255:731–737. doi: 10.1148/radiol.10081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic Review: Using Magnetic Resonance Imaging to Screen Women at High Risk for Breast Cancer. Ann Intern Med. 2008;148:671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 31.United States Census Bureau Available at: http://www.census.gov/main/www/access.html. Accessed: June 18. 2013.