Abstract
Homocysteine (Hcy) is a non-protein amino acid derived from dietary methionine. High levels of Hcy, known as hyperhomocysteinemia (HHcy) is known to cause vascular complications. In the mammalian tissue, Hcy is metabolized by transsulfuration enzymes to produce hydrogen sulfide (H2S). H2S, a pungent smelling gas was previously known for its toxic effects in the central nervous system, recent studies however has revealed protective effects in a variety of diseases including hypertension, diabetes, inflammation, atherosclerosis, and renal disease progression and failure. Interestingly, under stress conditions including hypoxia, H2S can reduce metabolic demand and also act as a substrate for ATP production. This review highlights some of the recent advances in H2S research as a potential therapeutic agent targeting renovascular diseases associated with HHcy.
Keywords: Renal remodeling, homocysteine, hydrogen sulfide, extracellular matrix, matrix metalloproteinase, inflammation, mitophagy, hypertension
1. Introduction
Progressive decline of renal function in chronic kidney diseases such as glomerulosclerosis and tubular-interstitial fibrosis impairs the ability of kidney to excrete waste products and maintain water and electrolyte balance. Renal microvascular endothelial injury, vessel calcification and remodeling can increase vascular resistance causing elevation of blood pressure [1; 2],. Clinical data suggests an association between systolic hypertension, renal dysfunction and high levels of plasma homocysteine (Hcy). There is an inverse relationship between plasma Hcy levels and progressive decline in renal function.
In the body, Hcy is metabolized by two distinct pathways: 1) remethylation back to its precursor methionine, and 2) transsulfuration to form H2S. Since the original discovery of H2S biogenesis, it has gained substantial interest in the research community for determining its role in health and disease. Current evidence suggests that H2S regulates a number of physiological processes including but not limited to synaptic transmission, vasorelaxation, pro- and anti-inflammatory effects, angiogenesis, smooth muscle cell proliferation and migration, and autophagy. In hyperhomocysteinemia (HHcy), decreased H2S has been linked to disease progression and morbidity. HHcy and impaired H2S formation is commonly seen in patients with cirrhosis [3]. Further, HHcy in cirrhosis has been shown to cause endothelial dysfunction in rats which was reversed following H2S treatment [3]. In a rat model of HHcy, Wei el al demonstrated marked increase in endoplasmic reticulum (ER) stress in cardiomyocytes and reduction in endogenous H2S production, whereas H2S supplementation decreased the expression of ER stress-associated proteins [4]. Increased Hcy levels and decreased H2S production has been reported in patients undergoing hemodialysis for uraemia [5]. Also, low levels of H2S have been demonstrated in HHcy-induced hypertension wherein both endogenous and exogenous H2S was shown to mitigate high blood pressure suggesting a key role in blood pressure regulation [6]. Taken together, the above studies suggest an inverse relationship between Hcy and H2S in diverse pathologies.
In this review we discuss recent developments on Hcy handling, particularly its synthesis, accumulation, and metabolism in renal vasculature. Furthermore, in light of present literature the roles of H2S are highlighted as a molecule which mitigates renovascular complications and hypertension.
2. Hcy biosynthesis and accumulation
Hcy is a non-protein α-amino acid derived from methionine and is a homologue of amino acid cysteine, differing by an additional methylene group. The normal plasma Hcy levels in humans range from 5–15 μmol/L. In rare inborn errors of metabolism, levels >100 μmol/L have been reported [7]. Based on the plasma concentration, HHcy is categorized into three groups, mild (>15 μmol/L to italic> 30 μmol/L), moderate (> 30 μmol/L to bold> 100 μmol/L) and severe (>100 μmol/L) [8]. A number of studies suggest moderate HHcy as an independent risk factor for vascular diseases including coronary artery disease and venous thromboembolism [9; 10]. To aid US Preventive Services Task Force for finding novel risk factors for coronary heart disease (CHD), a meta-analysis was performed, and the analysis suggests that each increment of Hcy level by 5 μmol/L increases the risk of CHD events by approximately 20 percent [11]. This underlines the clinical significance of HHcy.
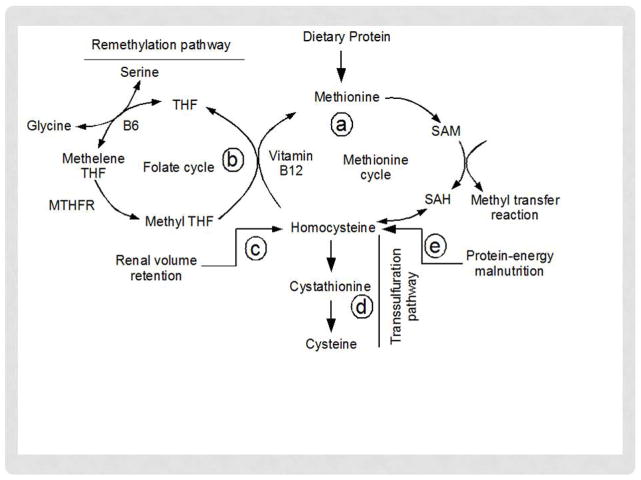
There is however no direct diet source of Hcy; instead it is biosynthesized from methionine. Hcy biosynthesis, accumulation and metabolism in the body depend on many factors and several pathways contribute to regulate plasma Hcy levels. Five major pathways are involved in this process, a) methylation reaction, b) remethylation pathway, c) renal mechanism via volume retention, d) transsulfuration pathway, and e) protein-energy malnutrition (Figure 1). Among the pathways, methylation reaction, renal volume retention and protein-energy malnutrition increase plasma Hcy levels; whereas, remethylation and transsulfuration decrease its level. In cells, methionine by condensation reaction with ATP forms methyl donor, S-adenosylmethionine (SAM) (Figure 1). In the methylation reaction, SAM is transformed into SAH (S-adenosylhomocysteine) by donating its methyl group to other substrates, and in subsequent reversible reaction SAM produces Hcy. Hcy is converted back to methionine through folate and vitamin B12 remethylation pathway. Excess Hcy in circulation is cleared by the kidney and liver. The kidney is capable of filtering as well as metabolizing Hcy [12]. Although the molecular mass of Hcy (135 D) is within the filtration range of glomeruli, major portion of filtered Hcy is absorbed by tubular uptake [12]. In addition, kidney contains transsulfuration enzymes CBS, CSE, 3MST and CAT as well as remethylation enzymes [13; 14; 15; 16]. Therefore, renal pathways of Hcy handling largely depend on filtration, reabsorption and metabolism (remethylation and transsulfuration) ability of kidney [17]. In chronic kidney diseases (CKD) reduced glomerular filtration thus increases plasma Hcy accumulation [12]. In addition, dysregulation in the transsulfuration enzymes further increases plasma Hcy levels [18; 19; 20]. Similar to kidney, the liver remethylates Hcy back to methionine through remethylation (Figure 1) [12; 21], and can also metabolize it by transsulfuration pathway [22; 23]. A derangement in either of the pathways can therefore result in abnormal increase in Hcy. In addition, protein-energy malnutrition is also reported to increase plasma Hcy levels (Figure 1) [24].
Figure 1.
Homocysteine synthesis, accumulation and metabolism pathways. MTHFR, Methelene tetrahydrofolate reductase; SAH, S-adenosyl homocysteine; SAM, S-adenosyl methionine; THF, tetrahydrofolate reductase.
3. Hcy pathobiology in kidney diseases
HHcy is a recognized risk factor for vascular diseases [25; 26]. Individuals with advanced CKD [27] and patients undergoing hemodialysis [28] are reported to have high levels of plasma Hcy, which may further contribute to renovascular injury leading to a vicious cycle [29]. Renal injury has been reported in an experimental weanling rat model of HHcy [29; 30] which is consistent with our own recent findings in mice [19; 31]. A recent systemic review and meta-analysis reported that glomerular filtration rate inversely correlates with Hcy levels with HHcy prevalence of 36–89% in patients with CKD, 70–75% in patients with viable kidney transplants and 85–100% in end-stage renal disease [32]. In vivo studies have demonstrated that HHcy can increase blood pressure by causing arteriolar constriction, arterial stiffness, endothelial damage, and increased sodium absorption [33]. The underlying mechanisms include: a) oxidative stress, b) smooth muscle cell proliferation, c) inflammation, d) autophagy, e) extracellular matrix remodeling, f) formation of Hcy-thiolactone, and g) protein homocysteinylation. Protein modification by homocysteinylation has been implicated in the development of atherogenesis and atherothrombosis [34; 35]. Indeed patients deficient in cystathionine β-synthase (CBS) were shown to have increased levels of prothrombotic N-Hcy-fibrinogen increasing their risk for thrombotic events [35]. In addition, Hcy may homocysteinylate endothelial nitric oxide synthase (eNOS), resulting in decreased nitric oxide (NO) production by endothelial cells including renal vessels. This possibility however is yet to be confirmed.
4. Metabolism of Hcy and formation of H2S
Metabolism of Hcy occurs by two distinct pathways: 1) remethylation wherein Hcy receives a methyl group to form its precursor molecule methionine, and 2) transsulfuration pathway to form cysteine (Figure 1). In the remethylation process, Hcy acquires a methyl group from N-5-tetrahydrafolate in the presence of vitamin B12 catalyzed by homocysteine methyltransferase (HMT) and occurs in all tissues. In the alternate pathway occurring exclusively in the liver, Betaine HMT converts Betaine to dimethylglycine releasing a methyl group which couples with Hcy to form methionine [36].
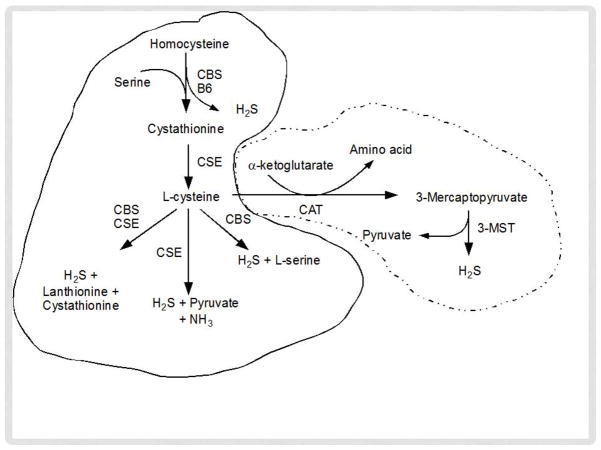
In the transsulfuration pathway, Hcy condenses with serine to from cystathionine (Figure 2). A pyridoxal-5′-phosphate (PLP)-containing enzyme, cystathionine β-synthase (CBS) catalyzes this reaction where vitamin B6 acts as a co-factor. Cystathionine is further hydrolyzed by a second PLP enzyme, cystathionine γ-lyase (CSE) to form cysteine. Cysteine is the main precursor of endogenous H2S formation; thus, Hcy and cysteine synthesize H2S by cytosolic enzymes CBS and CSE, or by the sequential activity of cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfur transferase (3MST) in both the cytosol and mitochondria (Figure 2). H2S synthesis by other mechanisms independent of Hcy has been summarized recently in a review article by Olson et al [37].
Figure 2.
Pathways for hydrogen sulfide (H2S) production from homocysteine (Hcy). Cytosolic enzymes, CBS and CSE metabolizes Hcy to H2S (lined enclosure); whereas cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3MST) metabolizes Hcy to H2S in both cytosol and mitochondria (dotted enclosure).
5. Physiology of H2S in the kidney
H2S has long been known for its neurotoxicity and as an environmental hazard. The ground breaking work by Abe and Kimura in 1996 however demonstrated for the first time that the hippocampus produces H2S, which functions as a neuromodulator [38]. A subsequent study by Kimura H reported the role of H2S as an endogenous smooth muscle relaxant suggesting possible regulation of vascular tone [39]. Subsequent studies by other researchers documented that endogenous H2S has a variety of physiological functions and a decrease in its production was involved in diverse pathological processes, such as oxidative stress, vascular dysfunction, inflammation, neurodegenerative diseases, apoptosis, autophagy, atherosclerosis, hypertension and diabetes [40; 41; 42; 43; 44; 45]. These discoveries further stimulated research into its development as a potential therapeutic agent in diseases attributed to diminished H2S synthesis [46].
In the kidney, H2S is reported in a wide array of physiological functions. For example, Holwerda et al reported that H2S attenuated hypertension and renal damage by upregulating VEGF [47]. In an rat model of unilateral ureteral obstruction (UO), exogenous H2S donor inhibited renal fibrosis by attenuating the production of collagen and other extracellular matrix proteins [48]. In addition, H2S attenuated inflammation by decreasing the expression of inflammatory cytokines and recruitment of macrophages [48]. In another study involving UO model, treatment with with sodium hydrogen sulfide reduced oxidative stress by preserving catalases such as CuZnSOD and MnSOD, and glutathione level [49]. Although these studies did not measure Hcy levels in UO model, decreased levels of H2S were reported in both studies which are consistent with our previous finding in renal pathologies associated diminished H2S and HHcy [18; 19]. Below, we review the beneficial effects of H2S in renal pathology associated with HHcy. Of note, in a recent review Snijder et al summarized in depth on how H2S, along with other two gasotransmitters, NO and carbon monoxide (CO), interact in renal transplantation to exert cytoprotection and reduction of tissue injury in transplanted organ [50].
6. Roles of H2S in Hcy-mediated renovascular pathophysiology
6.1. Normal physiology of renal vasculature and gasotransmitters
Kidney is the filtration unit in vertebrate animals and comprises two major parts: cortex and medulla. The functional unit of kidney is nephron and is grossly divided into two parts; a) renal corpuscle located in the cortex and b) renal tubule that passes from the cortex into the medulla. The major function of the kidney is filtration, reabsorption of glucose, sodium, potassium and other solutes, secretion of hormones and maintaining fluid homeostasis in the body. Endogenous gaseous transmitters, NO, CO and H2S, play immense role in maintaining normal physiological function of kidney. NO is produced by nitric oxide synthase (NOS) and the kidney expresses all three isoforms of NOS, i.e eNOS, nNOS and iNOS [51; 52]. In the kidney NO regulates renal hemodynamics, modulates fluid and electrolyte transport, and may also help minimize renal injury [53]. However, NO generated from iNOS may exacerbates renal injury in concert with oxidative-redox state particularly in the presence of super oxide due to the formation of peroxynitrite [54].
In the kidney, CO is generated during heme degradation by the enzymes heme oxygenase – 1 and -2 (HO-1 and HO-2) or fatty acid oxidation [55]. Studies have demonstrated that CO is a natural vasoprotector against excessive vasoconstriction and promotes natriuresis [55]. H2S is the third gaseous molecule in the group and its production in the kidney is summarized in section 5 above. All the gasotransmitters above have similar physiological effects such as vasodilatory, anti-oxidant, anti-apoptotic, anti-inflammatory, and angiogenic properties through an array of inter- and intracellular signaling cascades [56] however, their mode of action and regulatory function may differ from each other [57]. In addition, recent literature suggests significant crosstalk between these gasotransmitters for synergism and normal physiology [58; 59]. In the following section, we discuss the physiological functions of the gasotransmitters in the kidney in relation to HHcy with an emphasis for using H2S as a potential therapeutic agent.
6.2. Oxidative stress and endothelial dysfunction
A vast majority of literature suggest that Hcy causes oxidative injury to the endothelial cells (EC) [18; 60; 61]. Due to the presence of the highly reactive sulfhydryl group, Hcy can undergo auto-oxidation to generate oxygen radicals [62]. In circulation, the thiol group undergoes rapid metal-catalyzed auto-oxidation leading to generation of super oxide and hydrogen peroxide [60]. In addition to generating oxidant stress, Hcy has indirect effects on vascular redox status by diminishing the expression and activity of anti-oxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (PGx) [62; 63]. The endothelial cells which line the vessel walls are the primary target of these radicals. Since eNOS derived NO is the primary vasorelaxation factor under physiological conditions, the cells are deprived of NO following endothelial injury [64], leading to impaired endothelial function [65; 66]. In a transgenic CBS–deficient mice model Cheng et al reported that HHcy impairs NO– and endothelium-derived hyperpolarizing factor (EDHF)–mediated endothelium-dependent relaxation of small mesenteric arteries (SMAs) [67]. Hcy is also known to promote oxidation of eNOS cofactor, tetrahydro-L-biopterin (BH4), resulting in BH4-eNOS uncoupling and diminished NO production [68; 69; 70; 71]. Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of NO synthase [72], and Hcy is reported to increase ADMA and thus decrease NO production [73]. In contrast to decreased eNOS-derived NO during HHcy, the activity of neural NOS (nNOS) and inducible NOS (iNOS) is increased by HHcy [74; 75]. This paradox, although presumed to have beneficial effects by vasorelaxation, the increase of NO in a highly reactive HHcy-induced oxidative environment can in fact lead to the formation of more potent peroxynitrite radical (ONOO-), known as reactive nitrogen species (RNS) [68]. This can lead to nitration of tyrosine residues in proteins thus impairing their function [76].
Evidence from animal models of HHcy suggest that endothelial dysfunction is largely due to oxidative stress and decreased bioavailability of NO [77]. A decrease in the production and circulation of H2S can further contribute to endothelial-dependent impaired vasorelaxation in these models [18; 19; 20; 43]. This mechanism is partly mediated by interaction between H2S and NO. Both H2S and NO can engage in covalent reactions with proteins thereby temporarily modulating protein structure and function. H2S reacts with Cys residues of the target protein through the formation of persulfide (-SSH) bond and this modification is known as protein S-sulfhydration [78]. Similarly, NO predominantly binds to sulfhydryl groups (-SH) of Cys residues of target protein, and the process is termed as protein S-nitrosylation [79]. It is also reported that H2S functions in concert with NO forming nitrosothiol (RSNO) [80] or reacts with S-nitrosothiols to form thionitrous acid (HSNO) [81]. The latter further metabolizes to form NO [81], and/or H2S displacing NO from S-nitrosothiols to liberate free NO [82]. Thus, it is postulated that impaired production of H2S in HHcy may reduce some of the physiological aspects of NO-signaling. In fact, in a recent finding King et al reported that mice lacking CSE exhibited elevated oxidative stress, diminished NO levels due to dysfunctional eNOS in ischemia/reperfusion (I/R), and exacerbated myocardial injury [83]. These changes were ameliorated following H2S therapy, suggesting H2S-mediated cytoprotective signaling in I/R myocardium is dependent on eNOS activation and NO generation [83]. Using a similar model, Bos et al reported that CSE deficiency increased renal damage and mortality after I/R injury, whereas treatment with exogenous H2S rescued CSE knockout mice from these injuries [84]. Further, overexpression of CSE in in vitro human embryonic kidney 293 cells (HEK293) was found to reduce ROS production [84]. Taken together, the findings above underline the role of endogenous H2S in oxidative stress, and give direction to the mechanisms of H2S related antioxidant effects.
On the other hand, although the role of reduced H2S in CBS-deficiency remains largely unknown, H2S activity is reported to occur partly through sulfhydration [85], a mechanism where NO acts through S-nitrosylation [86]. The literature on the effects of sulfhydration is however limited. It is therefore premature to generalize whether H2S activity occurs through this mechanism. Nonetheless, this process is highly interesting as it could have a major role in physiology, but due to the lack of advanced techniques for accurately measuring sulfhydration it is not certain whether this process occurs or not. Further to H2S generation and mechanism of vascular protection, Bearden et al reported an interesting finding that H2S generating enzyme CBS and CSE are secreted into the bloodstream and generates H2S through extracellular transsulfuration pathway, which protects endothelium from redox stress [87]. Whether these mechanisms are universal or kidney vasculature has additional pathways to handle HHcy-stress is unknown and remains to be elucidated.
6.3. Homocysteinylation and protein malfunction
The consequence of high Hcy level has been studied in numerous in vivo and in vitro experimental models. The sulfhydryl group of Hcy forms stable disulfide bonds with cysteine residues in proteins altering their structure and function [62]. Recently, Hubmacher et al demonstrated that homocysteinylation affected functional properties of fibrillin and tropoelastin [88]. Fibrillin and tropoelastin are essential components for the formation of elastic fibers. Fibrillin contains intra-domain disulfide bonds which maintain its structural integrity and functional properties [89]. It is secreted into the extracellular matrix and becomes incorporated into the microfibrils to provide a scaffold for deposition of tropoelastin that is found in the elastic fibers of connective tissues including blood vessels. The N- and C-terminal regions of fibrillin colocalizes to form typical microfibril structure [90], and in this regard disulfide bond-mediated multimerization of the fibrillin-1 C-terminus and N- to C-terminal self-interaction are considered the initial steps for tissue microfibrils formation [91; 92]. Homocysteinylation reduces N- to –C-terminal fibrillin-1 self-interaction properties which is essential for biogenesis of microfibrils [88]. In addition, homocysteinylation on the disulfide bond in tropoelastin changes the self-assembly process which reduces elastic properties of blood vessels [88].
Although the protein modification by Hcy has focused on homocysteinylation, a metabolite of Hcy known as Hcy-thiolactone has the ability to form isopeptide bonds with protein lysine residues leading to a product termed as N-homocysteinylated protein (N-Hcy-protein) [93]. N-Hcy-protein has prothrombotic properties [94], and N-homocysteinylation triggers development of autoimmunity [95]. It is reported that serum levels of anti-N-homocysteinylated antibodies are positively correlated with the levels of plasma Hcy in CBS-deficient and stroke patients compared to their respective control and healthy subjects [34; 35; 96]. Thus protein homocysteinylation and autoimmune response may explain some of the unanswered pathologies found in renal disease patients, even though Hcy levels are at subclinical levels [96]. We and others have shown that H2S supplementation offers renal protection in HHcy, especially by mitigating inflammation and ECM accumulation; however, the subtle mechanism is still incompletely understood. Because H2S has a sulfur molecule, it is possible that H2S may uncouple protein-S-S-Hcy bridge [97] resulting in dehomocysteinylation of protein. Another plausible mechanism is that H2S may prevent the formation of N-homocysteinylated proteins thereby preventing protein modification and malfunction. These possibilities need to be explored in future.
6.4. Smooth muscle cell proliferation and alteration of vascular elastic compliance
The media layer in blood vessels comprise of vascular smooth muscle cells (VSMC) that serve as contractile component to regulate blood pressure. Any morphological or physiological dysregulation in these cells interfere with the vascular elastic compliance leading to alteration of blood pressure. Perry et al reported that H2S inhibits airway smooth muscle cell proliferation through ERK-1/2 and p38 kinase pathway [98]. In an in vitro experimental model Zavaczki et al reported that H2S inhibited phosphate induced osteoblastic transformation and mineralization of VSMC [99]. In patients with chronic kidney disease undergoing hemodialysis, low plasma levels of H2S was associated with decreased CSE enzyme activity. Although the Hcy levels in these patients were not measured, previous clinical studies have reported increased Hcy levels in hemodialysis patients [100; 101; 102]. Decreased CSE activity and low H2S together with calcification and osteoblastic differentiation of VSMC suggests that Hcy may be involved in this process. Further studies are needed to dissect this mechanism.
6.5. Inflammation and autophagy
Reactive oxygen species (ROS) in pathological redox environment mediates tissue injury that facilitates inflammation through activation of pro-inflammatory molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)[18; 19; 103]. While circulating leukocytes adhere to these molecules as a result of host defense mechanism [104; 105], transmigration of leukocytes to the sub-endothelial space depends on cytokines and chemokines which are released from the injured tissue [106]. In chronic inflammatory disorders, sustained elevation of ICAM-1 and VCAM-1 lead to aggregation of macrophages resulting in formation of plaques and atherosclerosis [107]. HHcy is a recognized pathophysiological stimulus of endothelial injury causing increased expression of ICAM-1 and VCAM-1 [108; 109]. In an experimental kidney model, we have shown increased macrophage infiltration, and expression of ICAM-1 and VCAM-1 associated with HHcy [19]. This inflammatory process was partially mitigated by H2S treatment [19]. Since HHcy induced super oxide production and H2S treatment diminished the oxidants in HHcy, it suggests that renal protection was secondary to the anti-oxidant properties of H2S [18; 19].
ROS, including super oxide and hydrogen peroxide are important signaling molecules in normal and pathophysiological processes [110]. One of the age-regulating genes p66shc has recently been shown to exploit intracellular ROS levels by activating membrane-bound NADPH-oxidases, down-regulating antioxidant enzymes synthesis and mitochondrial ROS generation for cellular apoptosis [110]. Knockout mice of p66shc gene and various p66shc-deficient cell lines have exhibited higher resistance to agonist-induced oxidative stress and indicate the importance of p66shc in the stress-mediated apoptotic pathway [111; 112]. In addition, mice lacking p66shc showed increased resistance to ROS-dependent endothelial dysfunction and vascular inflammation compared to their wild type littermates [113; 114]. In a recent study, Kim et al reported that Hcy promotes endothelial dysfunction via p66shc transcription and hypomethylation of specific CpG dinucleotides in the p66shc promoter region [115]. In addition, the same group reported that knockdown of p66shc mitigated Hcy-induced adhesion of monocytes to EC indicating epigenetic mechanism of endothelial dysfunction and inflammation [115]. Whether H2S has any regulatory role in Hcy-induced epigenetic mechanism of inflammatory processes and cellular dysfunction is yet to be investigated.
Oxidative damage of cellular organelles, particularly mitochondria may lead to mitochondrial autophagy or mitophagy [116]. Mitophagy is a natural physiological defense mechanism where damaged mitochondria through a mechanism of selective sequestration and subsequent degradation are removed from the cells and the components recycled [117]. Mitochondria are the major site of oxidative phosphorylation for the production of ATP by oxidizing glucose, pyruvate and NADH [118]. This process of cellular respiration is dependent on oxygen and is known as aerobic respiration. Interestingly, ROS production is a part of aerobic respiration however, excess ROS can be detrimental to the cells [119]. In an earlier study, we demonstrated that HHcy initiated mitophagy through ROS generation and H2S supplementation or delivery of H2S producing gene, CBS, CSE and 3MST mitigated induction of mitophagy markers [43]. The beneficial effects of H2S in Hcy-mediated mitophagy may occur by two different mechanisms: a) protection of mitochondria by scavenging ROS, and/or b) the triple gene (CBS, CSE and 3MST) overexpression metabolizing excess Hcy to produce H2S thus preventing Hcy toxicity [43]. Further studies are needed to guarantee the safety and efficacy of gene delivery method for treating HHcy in pathological renovascular diseases.
6.6. Activation of matrix metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases which cleave a wide variety of substrates including cell surface receptors, adhesion molecules, growth factors, cytokines, in addition to their well-recognized role in matrix components synthesis and degradation [120]. The expression and localization of MMPs in the kidney has been described in a recent review [121]. Resident and latent MMPs are activated by several mechanisms in a variety of renal pathologies including diabetic nephropathy, glomerular-tubulointerstitial fibrosis, inherited kidney disease, acute kidney injury and inflammation [18; 122]. The consequence of aberrantly activated MMPs in physiology are not only restricted to maintain ECM homeostasis, i.e. matrix degradation and synthesis, but also involved in the regulation of a wide array of cellular behaviors, such as proliferation, migration, differentiation, tumor growth and metastasis, epithelial-mesenchymal transition, angiogenesis, and apoptosis [120; 122; 123]. MMPs are synthesized as inactive zymogens that have a conserved cysteine residue which interacts with the active site of zinc rendering the protease inactive [124]. The activity of MMPs depends on disruption of this “cysteine switch”. Proteolytic cleavage of this switch can be accomplished by trypsin, plasmin, other MMPs or even nitrosative and oxidative stress [122]. In particular, cysteine residues within the MMPs propeptide domain are highly sensitive to oxidant-redox status, and ROS oxidizes cysteine residues and activates latent MMPs to active forms [121; 125; 126]. Hcy is known to generate ROS, and thus activate MMPs [18; 127]. In addition, Hcy-generated ROS, especially super oxide (O2−) reacts with NO forming peroxynitrite (ONOO−) that reacts with active sites of MMPs increasing their activity [128]. Through nitration of tyrosine residues within MMPs, Hcy-generated ONOO− can also activate MMPs. In a recent review Steed and Tyagi postulated a plausible mechanism of MMP activation by this mechanism [129].
Since ROS and RNS activate MMPs, antioxidants are natural inhibitors of MMP activation. In this regard, H2S is an antioxidant and free radicle scavenger [130] and therefore the inhibitory mechanism of redox-mediated MMP activity by H2S is largely attributed though its antioxidant properties. Previously, in uninephrectomized one kidney (1-K) CBS+/− model we demonstrated that induction of MMP-2 and -9 correlated with the increased Hcy levels and decreased H2S levels compared with their 2-K littermates [18]. The induction of MMPs was due to increased formation of oxygen radicles in the kidney. Interestingly, H2S supplementation mitigated O2− production and diminished MMP-2 and -9 activities suggesting the mechanism of Hcy-redox-H2S pathway of renal MMP regulation in HHcy [18; 19]. It is also possible that H2S may quench Zn2+ of gelatinases resulting in their inhibition. This Zn2+ quenching mechanism of MMP-2 and -9 inhibitions by H2S has recently been reported by Talei et al in a hamster model of lung remodeling [131].
6.7. Extracellular matrix accumulation, glomerulosclerosis and renal dysfunction
Proteinuria is described as loss of protein from glomerular vasculature into urinary space and is the hallmark of ESRD [132; 133; 134]. Protein loss occurs due to disruption of slit diaphragm which is the final barrier of glomerular filtration consisting of interdigitating podocyte foot process with the neighboring cells [135; 136]. Podocytes are highly differentiated glomerular epithelial cells located in the glomerulus [137; 138]. In recent years, NADPH-derived ROS production has been implicated as a key mechanism triggering podocyte injury [139; 140; 141]. ROS activates matrix metalloproteinase in the glomerulus [142], and activated MMPs degrade matrix components including collagen/elastin [143]. However, since the turnover of collagen is faster than elastin [144; 145; 146; 147; 148] in a given time frame, excess collagen renders the vessel stiffer as the disease progresses. In addition, collagen in oxidative redox environment oxidizes [149], and renal peroxidation products deposits in the interstitial space leading to vascular fibrosis including glomerulo-tubulointerstitial fibrosis [150]. The early stages of glomerulosclerosis may be asymptomatic but proteinuria and fluid retention in the body indicates disease progression towards ESRD. Patients in ESRD require dialysis or kidney transplantation since other treatments for glomerulosclerosis are limited such as immunosuppressive drugs that prevent proteinuria [151]. Anti-hypertensive drugs such as ACE inhibitors are currently in use to preserve kidney function [152]. However, these drugs may cause further damage to the kidney. Therefore, developing an effective strategy that prevents early progression of kidney diseases associated with glomerulosclerosis may offer better management of this disease, particularly kidney failure associated with diabetes and hypertension. Since elevated Hcy level is known to be strongly associated with diabetes and hypertension induced nephropathy by deposition of excess ECM proteins in the glomerular-tubulointerstitial space, faster metabolism of Hcy or counteracting its effect in the microenvironment could be an important therapeutic tool for prevention or even slowing down disease progression. Using CBS+/− animal model of HHcy we have shown that uninephrectomy (1-kidney model) further increased Hcy levels in these mice and were associated with lower plasma H2S levels and sign of proteinuria than their 2-kidney littermates [18]. Blood pressure was significantly increased along with a decline in renal function [19]. In addition, increased ROS, particularly superoxide production and ratio of glutathione-to-oxidized glutathione (GSH/GSSG) were increased in CBS+/− uninephrectomized mice with increased expression and activity of MMP-2 and -9 [18; 19]. Furthermore, increased collagen deposition and expression of inflammatory molecules ICAM-1 and VCAM-1, caused macrophage infiltration to sites of injury resulting in glomerular remodeling and a decrease in the filtration rate [19]. H2S supplementation in these animals normalized microarchitecture and improved renal function suggesting that H2S as a potential therapeutic agent for countering the adverse effects of HHcy [18; 19].
6.8. Renal mechanism of hypertension
In chronic kidney disease, low levels of plasma H2S is often associated with a concomitant increase in plasma Hcy levels [5; 153]. The cause and effect relationship of HHcy in renal disease can therefore adversely affect the final outcome. Because Hcy is a precursor of H2S [154; 155], changes in the Hcy metabolism and therefore H2S synthesis can have a significant impact on HHcy-induced pathology. However, till date the mechanism by which HHcy causes vascular dysfunction and the role of H2S in renal protection is incompletely understood. Four enzymes, CBS, CSE, 3MST and CAT metabolize Hcy to produce H2S [156]. In renal disease and HHcy, impairment of CBS, CSE and 3MST enzymes leads to deficient H2S production [18; 19; 157; 158]. Available literature suggests that HHcy induces oxidative stress, endothelial injury and causes down regulation of eNOS resulting in decrease NO production [68; 159; 160]. Activation of MMPs in the Hcy-redox environment leads to imbalance in extracellular matrix synthesis and degradation [161; 162]. Excess or oxidized collagen deposition in the renal microvessels causes glomerulosclerosis and tubulointerstitial fibrosis resulting in kidney remodeling, renal dysfunction and hypertension [163; 164; 165; 166]. We have shown that exogenous H2S offers renal protection and mitigates hypertension by reducing oxidative stress, inflammation, and collagen deposition the HHcy kidney [18; 19]. Additionally in ex vivo renal artery, single, double or triple gene delivery of CBS, CSE and 3MST enzymes, we have demonstrated that conversion of Hcy to H2S improves vascular relaxation [31]. However, further studies are needed for deeper understanding of Hcy-mediated pro-fibrotic and pro-inflammatory effects in renal vasculature and beneficial effects of H2S therapy in improving renovascular function in diseases associated with HHcy.
6.9. Aerobic vs. anaerobic vascular relaxation: Roles of NO, CO, H2S and Hcy
NO, CO and H2S are a group of endogenously synthesized gasotransmitters having distinct physiological actions in the human body. Of the three, NO has been extensively studied. Oxygen is an important co-substrate for the generation of both NO and CO. Three isoforms of nitric oxide synthase (NOS), neuronal (nNOS), inducible (iNOS) and endothelial (eNOS) have been recognized to catalyze the formation of NO from L-arginine and all three forms are expressed by the kidney. The activity of NOS requires stimulation by calcium-calmodulin but the dependence of calcium for nNOS and eNOS is higher than that of iNOS [167]. NO thus formed activates guanylate cyclase (GC) to increase the concentration of cyclic guanosine monophosphate (cGMP) which in turn activates cGMP-dependent protein kinase causing dephosphorylation of myosin light chain phosphatase ultimately leading to vessel relaxation [168; 169]. In the vascular smooth muscle cells, the upregulation and fate of cGMP is controlled by phosphodiesterase 5 (PDE 5) [167] [170]. Within the kidney, iNOS is produced in the proximal tubules and medulla under conditions of inflammation or sepsis and can result in oxidant injury [171]. The eNOS is expressed in the arterioles and glomerular capillaries and is involved in regulation of vascular tone.
CO is a product formed during heme degradation by heme oxygenase (HO) system which is mainly distributed in the spleen, liver and kidney. The isoform HO-1 is induced under conditions of oxidative stress, inflammation and sepsis; whereas, HO-2 and HO-3 are constitutively expressed [172]. The formation of CO-guanylate cyclase complex stimulates the production of cGMP leading to cGMP-dependent signal transduction resulting in vasorelaxation [173]. Similar to NO, CO is involved in other actions such as neurotransmission, anti-proliferation of smooth muscle cells and inhibition of platelet aggregation. When compared to NO, CO is a weak vasodilator but under conditions of pathological stress where NO production is impaired, induction of HO-1 and the subsequent contribution of CO may play a significant role [174]. Support for CO induced vasodilation independent of NO production has been shown by the use of NOS inhibitor, Nω-nitro-L-arginine-methylester, (L-NAME) which demonstrated increased levels of cGMP [175]. In contrast, treatment with Tin protoporphyrin, SnPPIX, an inhibitor of HO showed decreased vasorelaxation induced by acetylcholine [176]. Taken together, the above studies suggest CO- cGMP signaling as an important mechanism involved in regulation of vascular tone. Upregulation of HO-1 has been shown to protect the kidneys from ischemia-reperfusion injury during renal transplantation [177]. Further, pre-treatment with molecules that release CO has been shown to increase the graft survival following kidney transplant and this effect has been attributed to its anti-apoptotic and anti-inflammatory activity mediated by cGMP pathway [178; 179].
Unlike NO and CO, the generation of H2S by CBS/CSE/MST enzymes does not require oxygen as a substrate. The mechanism by which H2S induces vasorelaxation involves activation of ATP-sensitive potassium channels (KATP)[180; 181]. In a previous study involving arterio-venous fistula induced cardiac failure, we demonstrated that pretreatment with sodium thiosulfate reduced adverse extra-cellular matrix remodeling and improved cardiac function [182]. In the same study, an increase in H2S production was associated with ventricular relaxation; this effect could be due to hyperpolarization of KATP channels inhibiting Ca++ entry into the cells. A second possible mechanism may involve the chelating property of sodium thiosulfate causing a reduction in free Ca++ intracellular compartments [182]. A significant down regulation of H2S producing enzymes has been observed in chronic kidney disease suggesting that H2S deficiency may contribute to the disease process [157]. Indeed, in an earlier study we found that kidneys from diabetic mice had decreased H2S levels and underwent adverse renal remodeling involving N-methyl-D-aspartate receptor 1 (NMDAR1) mediated upregulation of connexin-40 and -43 [183]. H2S treatment has been reported to reduce renal injury and improve renal function following ischemia-reperfusion injury in murine models [184; 185]. The beneficial effects of H2S treatment is possibly due to its multiple roles in modulating vascular tone, inflammation and oxidative stress.
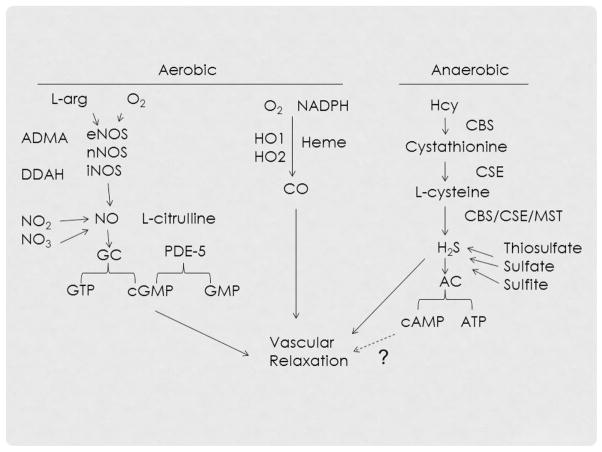
Interestingly in a recent report, Szabo et al found that both H2S and NO are interdependent in mediating vasorelaxation and development of new vessels [186]. In addition, vasorelaxation appears to be secondary to a reduction in PDE5 activity thereby decreasing breakdown of cGMP [186; 187]. H2S is also known to increase NO synthesis by upregulation of eNOS [188]. Together, these studies indicate significant cross-talk between gasotransmitters and appear to maintain reciprocal regulation in various vascular functions. Possible pathways of aerobic vs. anaerobic vascular relaxation and crosstalk between NO, CO and H2S is depicted in Figure 3.
Figure 3.
H2S mechanism of anaerobic vs. NO and CO mechanisms of aerobic vascular relaxation. ADMA, asymmetric dimethyl arginine; ATP, Adenosine triphosphate; cGMP, Cyclic guanosine monophosphate; cAMP, Cyclic adenosine monophosphate; DDAH, dimethylarginine dimethylaminohydrolase; PDE-5, phosphodiesterase type 5; GC, guanylate cyclase; GTP, guanosine triphosphate; NADPH, Nicotinamide adenine dinucleotide phosphate
7. Concluding remarks and perspectives
Hcy is a methionine metabolite non-protein amino acid which is the homologue of cysteine, differing by additional methylene bridge (-CH2-). Although kidney is a major site for Hcy metabolism, a small portion of Hcy is also excreted by the kidney. However, in chronic kidney diseases, as renal function declines, plasma levels of Hcy increases due to volume retention. This in turn, further contributes to renovascular injury through diverse mechanisms which includes oxidative stress, protein homocysteinylation, inflammation, autophagy, activation of latent MMPs, ECM deposition and alteration of renal-vascular elastic properties resulting in exacerbated kidney dysfunction. Renal dysfunction, volume retention and Hcy accumulation is a vicious cycle causing renal mechanism of hypertension.
Interestingly, many of the adverse effects of Hcy are counteracted by the gaseous molecule H2S, which is one of its natural metabolites. H2S is produced by desulfuration of Hcy (or cysteine) in the transsulfuration pathway catabolized by CBS, CSE, 3MST and CAT enzymes. It functions as endogenous signaling molecule to regulate a wide array of physiological processes including cellular oxidative stress. Hcy is known to cause oxidative stress that activates MMPs. In pathological state, active MMPs lead to adverse remodeling causing an imbalance of collage/elastin ratio resulting in glomerular-tubulointerstitial fibrosis which progresses to renal dysfunction. Since H2S is an antioxidant and vasodilator, treatment with H2S reduces oxidative stress and normalizes vascular compliance in kidney diseases associated with HHcy. Although at our current understanding it is not clear whether H2S dehomocysteinylates protein; it is apparent that the benefit of H2S treatment in HHcy is in part by reduction of Hcy-induced oxidant stress irrespective of plasma Hcy levels. In addition, CBS, CSE and 3MST gene therapy can metabolize Hcy to produce H2S and thus render better management of Hcy handling in pathological HHcy.
H2S shares many of the physiological processes rendered by CO and NO, although few fundamental differences exist in terms of their generation and mechanisms of action. For example, unlike NO and CO, generation of H2S does not require oxygen as substrate and therefore sustains mitochondrial ATP generation under hypoxic condition [189]. In a recent report, Modis et al proposed that intramitochondrial H2S producing pathway may serve a physiological role to maintain mitochondrial electron transport and cellular bioenergetics [190]. We previously reported that H2S regulated adenylate cyclase VI (ACVI), an enzyme which converts ATP to cAMP in cardiac tissue [182]. It would be interesting to study whether the conversion of Hcy to bio-friendly H2S in HHcy may lead to ATP generation and subsequent cAMP production, which may vasodilate arterioles to improve renal function under diseased states.
Highlights.
Renal volume retention in chronic kidney disease increases plasma Hcy levels
Hcy metabolism produces physiologically important hydrogen sulfide molecule
H2S production is impaired in chronic kidney disease
H2S deficiency is associated with vascular dysfunction and matrix remodeling
H2S treatment mitigates HHcy-associated renal dysfunction
Acknowledgments
This research was supported, in part, by NIH grant HL-104103.
Footnotes
Conflict of interest:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutton-Tyrrell K, Bostom A, Selhub J, Zeigler-Johnson C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997;96:1745–9. doi: 10.1161/01.cir.96.6.1745. [DOI] [PubMed] [Google Scholar]
- 2.Wollesen F, Brattstrom L, Refsum H, Ueland PM, Berglund L, Berne C. Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney International. 1999;55:1028–1035. doi: 10.1046/j.1523-1755.1999.0550031028.x. [DOI] [PubMed] [Google Scholar]
- 3.Distrutti E, Mencarelli A, Santucci L, Renga B, Orlandi S, Donini A, Shah V, Fiorucci S. The methionine connection: homocysteine and hydrogen sulfide exert opposite effects on hepatic microcirculation in rats. Hepatology. 2008;47:659–67. doi: 10.1002/hep.22037. [DOI] [PubMed] [Google Scholar]
- 4.Wei H, Zhang R, Jin H, Liu D, Tang X, Tang C, Du J. Hydrogen sulfide attenuates hyperhomocysteinemia-induced cardiomyocytic endoplasmic reticulum stress in rats. Antioxid Redox Signal. 2010;12:1079–91. doi: 10.1089/ars.2009.2898. [DOI] [PubMed] [Google Scholar]
- 5.Perna AF, Luciano MG, Ingrosso D, Pulzella P, Sepe I, Lanza D, Violetti E, Capasso R, Lombardi C, De Santo NG. Hydrogen sulphide-generating pathways in haemodialysis patients: a study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrology Dialysis Transplantation. 2009;24:3756–3763. doi: 10.1093/ndt/gfp378. [DOI] [PubMed] [Google Scholar]
- 6.Sowmya S, Swathi Y, Yeo AL, Shoon ML, Moore PK, Bhatia M. Hydrogen sulfide: regulatory role on blood pressure in hyperhomocysteinemia. Vascul Pharmacol. 2010;53:138–43. doi: 10.1016/j.vph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–6. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 8.Kang SS, Wong PWK, Malinow MR. Hyperhomocyst(E)Inemia as a Risk Factor for Occlusive Vascular-Disease. Annual Review of Nutrition. 1992;12:279–298. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 9.Fermo I, Dangelo SV, Paroni R, Mazzola G, Calori G, Dangelo A. Prevalence of Moderate Hyperhomocysteinemia in Patients with Early-Onset Venous and Arterial Occlusive Disease. Annals of Internal Medicine. 1995;123:747. doi: 10.7326/0003-4819-123-10-199511150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Angelo AD, Selhub J. Homocysteine and thrombotic disease. Blood. 1997;90:1–11. [PubMed] [Google Scholar]
- 11.Humphrey LL, Fu RW, Rogers K, Freeman M, Helfand M. Homocysteine Level and Coronary Heart Disease Incidence: A Systematic Review and Meta-analysis. Mayo Clinic Proceedings. 2008;83:1203–1212. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- 12.Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. Journal of the American Society of Nephrology. 2001;12:2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- 13.Bao LM, Vlcek C, Paces V, Kraus JP. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Archives of Biochemistry and Biophysics. 1998;350:95–103. doi: 10.1006/abbi.1997.0486. [DOI] [PubMed] [Google Scholar]
- 14.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014 doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–77. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibuya N, Kimura H. Production of hydrogen sulfide from d-cysteine and its therapeutic potential. Front Endocrinol (Lausanne) 2013;4:87. doi: 10.3389/fendo.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Guldener C. Why is homocysteine elevated in renal failure and what can be expected from homocysteine-lowering? Nephrology Dialysis Transplantation. 2006;21:1161–1166. doi: 10.1093/ndt/gfl044. [DOI] [PubMed] [Google Scholar]
- 18.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. American Journal of Physiology-Renal Physiology. 2009;297:F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen U, Munjal C, Qipshidze N, Abe O, Gargoum R, Tyagi SC. Hydrogen Sulfide Regulates Homocysteine-Mediated Glomerulosclerosis. American Journal of Nephrology. 2010;31:442–455. doi: 10.1159/000296717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perna AF, Ingrosso D. Low hydrogen sulphide and chronic kidney disease: a dangerous liaison. Nephrol Dial Transplant. 2012;27:486–93. doi: 10.1093/ndt/gfr737. [DOI] [PubMed] [Google Scholar]
- 21.Diez J, Frohlich ED. Fourth International Seminar on Cardiovascular Biology and Medicine: Part I. Hypertension. 2001;38:1198–1198. [Google Scholar]
- 22.Mudd SH, Finkelstein JD, Irreverre F, Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965;240:4382–92. [PubMed] [Google Scholar]
- 23.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–77. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 24.Ingenbleek Y, McCully KS. Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition. 2012;28:148–53. doi: 10.1016/j.nut.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Majors A, Ehrhart LA, Pezacka EH. Homocysteine as a risk factor for vascular disease - Enhanced collagen production and accumulation by smooth muscle cells. Arteriosclerosis Thrombosis and Vascular Biology. 1997;17:2074–2081. doi: 10.1161/01.atv.17.10.2074. [DOI] [PubMed] [Google Scholar]
- 26.Anand K, Lee HS, Paik M, Stabler S, Allen R, Sacco RL. Homocysteine is a stronger risk factor for vascular death than stroke in a multiethnic cohort: The Northern Manhattan study. Stroke. 2004;35:313–313. [Google Scholar]
- 27.Francis ME, Eggers PW, Hostetter TH, Briggs JP. Association between serum homocysteine and markers of impaired kidney function in adults in the United States. Kidney International. 2004;66:303–312. doi: 10.1111/j.1523-1755.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- 28.Robinson K. Renal disease, homocysteine, and cardiovascular complications. Circulation. 2004;109:294–295. doi: 10.1161/01.CIR.0000114133.99074.96. [DOI] [PubMed] [Google Scholar]
- 29.Ikegaya N, Yanagisawa C, Kumagai H. Relationship between plasma homocysteine concentration and urinary markers of tubulointerstitial injury. Kidney International. 2005;67:375–375. doi: 10.1111/j.1523-1755.2005.091_1.x. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai H, Katoh S, Hirosawa K, Kimura M, Hishida A, Ikegaya N. Renal tubulointerstitial injury in weanling rats with hyperhomocysteinemia. Kidney International. 2002;62:1219–1228. doi: 10.1111/j.1523-1755.2002.kid558.x. [DOI] [PubMed] [Google Scholar]
- 31.Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, Qipshidze N, Tyagi N, Metreveli N, Tyagi SC. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. American Journal of Physiology-Cell Physiology. 2012;303:C41–C51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, Gallagher MP, Cass A, Strippoli G, Perkovic V. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta-analysis. Bmj-British Medical Journal. 2012;344 doi: 10.1136/bmj.e3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stehouwer CDA, van Guldener C. Does homocysteine cause hypertension? Clinical Chemistry and Laboratory Medicine. 2003;41:1408–1411. doi: 10.1515/CCLM.2003.216. [DOI] [PubMed] [Google Scholar]
- 34.Undas A, Perla J, Lacinski M, Trzeciak W, Kazmierski R, Jakubowski H. Autoantibodies against N-homocysteinylated proteins in humans: implications for atherosclerosis. Stroke. 2004;35:1299–304. doi: 10.1161/01.STR.0000128412.59768.6e. [DOI] [PubMed] [Google Scholar]
- 35.Jakubowski H, Boers GH, Strauss KA. Mutations in cystathionine beta-synthase or methylenetetrahydrofolate reductase gene increase N-homocysteinylated protein levels in humans. FASEB J. 2008;22:4071–6. doi: 10.1096/fj.08-112086. [DOI] [PubMed] [Google Scholar]
- 36.Selhub J. Homocysteine metabolism. Annual Review of Nutrition. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 37.Olson KR, DeLeon ER, Gao Y, Hurley K, Sadauskas V, Batz C, Stoy GF. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2013;305:R592–R603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- 38.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. Journal of Neuroscience. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 40.Predmore BL, Lefer DJ, Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 2012;17:119–40. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang GD, Wu LY, Jiang B, Yang W, Qi JS, Cao K, Meng QH, Mustafa AK, Mu WT, Zhang SM, Snyder SH, Wang R. H(2)S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang TJ, Zhou ZM, Wu LY, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, Qipshidze N, Tyagi N, Metreveli N, Tyagi SC. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am J Physiol Cell Physiol. 2012;303:C41–51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kida K, Yamada M, Tokuda K, Marutani E, Kakinohana M, Kaneki M, Ichinose F. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson’s disease. Antioxid Redox Signal. 2011;15:343–52. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, An G, Chen J. Hydrogen sulfide improves left ventricular function in smoking rats via regulation of apoptosis and autophagy. Apoptosis. 2014;19:998–1005. doi: 10.1007/s10495-014-0978-z. [DOI] [PubMed] [Google Scholar]
- 46.Olson KR. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am J Physiol Regul Integr Comp Physiol. 2011;301:R297–312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- 47.Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ, Karumanchi SA, Lely AT. Hydrogen Sulfide Attenuates sFlt1-Induced Hypertension and Renal Damage by Upregulating Vascular Endothelial Growth Factor. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song K, Wang F, Li Q, Shi YB, Zheng HF, Peng H, Shen HY, Liu CF, Hu LF. Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int. 2013 doi: 10.1038/ki.2013.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung KJ, Jang HS, Kim JI, Han SJ, Park JW, Park KM. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta. 2013;1832:1989–97. doi: 10.1016/j.bbadis.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Snijder PM, van den Berg E, Whiteman M, Bakker SJ, Leuvenink HG, van Goor H. Emerging role of gasotransmitters in renal transplantation. Am J Transplant. 2013;13:3067–75. doi: 10.1111/ajt.12483. [DOI] [PubMed] [Google Scholar]
- 51.Kone BC. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol. 2004;24:299–315. doi: 10.1016/j.semnephrol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. American Journal of Physiology-Renal Physiology. 2003;284:F1121–F1137. doi: 10.1152/ajprenal.00265.2002. [DOI] [PubMed] [Google Scholar]
- 53.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiologica. 2006;187:433–446. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- 54.Heemskerk S, Masereeuw R, Russel FGM, Pickkers P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nature Reviews Nephrology. 2009;5:629–640. doi: 10.1038/nrneph.2009.155. [DOI] [PubMed] [Google Scholar]
- 55.Csongradi E, Juncos LA, Drummond HA, Vera T, Stec DE. Role of Carbon Monoxide in Kidney Function: Is a little Carbon Monoxide Good for the Kidney? Curr Pharm Biotechnol. 2012;13:819–826. doi: 10.2174/138920112800399284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snijder PM, van den Berg E, Whiteman M, Bakker SJL, Leuvenink HGD, van Goor H. Emerging Role of Gasotransmitters in Renal Transplantation. American Journal of Transplantation. 2013;13:3067–3075. doi: 10.1111/ajt.12483. [DOI] [PubMed] [Google Scholar]
- 57.Moody BF, Calvert JW. Emergent role of gasotransmitters in ischemia-reperfusion injury. Med Gas Res. 2011;1:3. doi: 10.1186/2045-9912-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolluru GK, Shen X, Kevil CG. A tale of two gases: NO, HS, foes or friends for life? Redox Biol. 2013;1:313–318. doi: 10.1016/j.redox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R. Shared signaling pathways among gasotransmitters. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8801–8802. doi: 10.1073/pnas.1206646109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starkebaum G, Harlan JM. Endothelial-Cell Injury Due to Copper-Catalyzed Hydrogen-Peroxide Generation from Homocysteine. Journal of Clinical Investigation. 1986;77:1370–1376. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yi F, Jin S, Zhang F, Xia M, Bao JX, Hu J, Poklis JL, Li PL. Formation of lipid raft redox signalling platforms in glomerular endothelial cells: an early event of homocysteine-induced glomerular injury. J Cell Mol Med. 2009;13:3303–14. doi: 10.1111/j.1582-4934.2009.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glushchenko AV, Jacobsen DW. Molecular targeting of proteins by L-homocysteine: Mechanistic implications for vascular disease. Antioxidants & Redox Signaling. 2007;9:1883–1898. doi: 10.1089/ars.2007.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss N, Zhang YY, Heydrick S, Bierl C, Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12503–12508. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lubos E, Handy DE, Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2008;13:5323–44. doi: 10.2741/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–41. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 66.Rocha M, Apostolova N, Hernandez-Mijares A, Herance R, Victor VM. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Current Medicinal Chemistry. 2010;17:3827–41. doi: 10.2174/092986710793205444. [DOI] [PubMed] [Google Scholar]
- 67.Cheng ZJ, Jiang XH, Kruger WD, Pratico D, Gupta S, Mallilankaraman K, Madesh M, Schafer AI, Durante W, Yang XF, Wang H. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118:1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, Devynck MA, David-Dufilho M. Homocysteine induces oxidative, stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radical Biology and Medicine. 2004;36:1532–1541. doi: 10.1016/j.freeradbiomed.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 69.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–91. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhillon B, Badiwala MV, Maitland A, Rao V, Li SH, Verma S. Tetrahydrobiopterin attenuates homocysteine induced endothelial dysfunction. Mol Cell Biochem. 2003;247:223–7. doi: 10.1023/a:1024146501743. [DOI] [PubMed] [Google Scholar]
- 71.Stanger O, Weger M. Interactions of homocysteine, nitric oxide, folate and radicals in the progressively damaged endothelium. Clin Chem Lab Med. 2003;41:1444–54. doi: 10.1515/CCLM.2003.222. [DOI] [PubMed] [Google Scholar]
- 72.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction - Its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 73.Stuhlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569–75. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 74.Woo CW, Cheung F, Chan VW, Siow YLKO. Homocysteine stimulates inducible nitric oxide synthase expression in macrophages: antagonizing effect of ginkgolides and bilobalide. Mol Cell Biochem. 2003;243:37–47. doi: 10.1023/a:1021601512058. [DOI] [PubMed] [Google Scholar]
- 75.Ikeda U, Ikeda M, Minota S, Shimada K. Homocysteine increases nitric oxide synthesis in cytokine-stimulated vascular smooth muscle cells. Circulation. 1999;99:1230–5. doi: 10.1161/01.cir.99.9.1230. [DOI] [PubMed] [Google Scholar]
- 76.Liu T, Hou DD, Zhao Q, Liu W, Zhen PP, Xu JP, Wang K, Huang HX, Li X, Zhang H, Xu HB, Wang W. Phytoestrogen alpha-Zearalanol attenuates homocysteine-induced apoptosis in human umbilical vein endothelial cells. Biomed Res Int. 2013;2013:813450. doi: 10.1155/2013/813450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nature Reviews Molecular Cell Biology. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 79.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89:444–8. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochemical and Biophysical Research Communications. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 81.Filipovic MR, Miljkovic JL, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. Chemical Characterization of the Smallest S-Nitrosothiol, HSNO; Cellular Cross-talk of H2S and S-Nitrosothiols. J Am Chem Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ondrias K, Stasko A, Cacanyiova S, Sulova Z, Krizanova O, Kristek F, Malekova L, Knezl V, Breier A. H2S and HS- donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflugers Archiv-European Journal of Physiology. 2008;457:271–279. doi: 10.1007/s00424-008-0519-0. [DOI] [PubMed] [Google Scholar]
- 83.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A. 2014;111:3182–7. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, Moser J, Hillebrands JL, Ploeg RJ, Yang G, Leuvenink HG, van Goor H. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24:759–70. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu WT, Gazi SK, Barrow RK, Yang GD, Wang R, Snyder SH. H2S Signals Through Protein S-Sulfhydration. Science Signaling. 2009;2 doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–46. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bearden SE, Beard RS, Pfau JC. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. American Journal of Physiology-Heart and Circulatory Physiology. 2010;299:H1568–H1576. doi: 10.1152/ajpheart.00555.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hubmacher D, Cirulis JT, Miao M, Keeley FW, Reinhardt DP. Functional Consequences of Homocysteinylation of the Elastic Fiber Proteins Fibrillin-1 and Tropoelastin. Journal of Biological Chemistry. 2010;285:1188–1198. doi: 10.1074/jbc.M109.021246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kielty CM, Sherratt MJ, Marson A, Baldock C. Fibrillin microfibrils, Fibrous Proteins: Coiled-Coils. Collagen and Elastomers. 2005;70:405. doi: 10.1016/S0065-3233(05)70012-7. [DOI] [PubMed] [Google Scholar]
- 90.Yadin DA, Robertson IB, McNaught-Davis J, Evans P, Stoddart D, Handford PA, Jensen SA, Redfield C. Structure of the fibrillin-1 N-terminal domains suggests that heparan sulfate regulates the early stages of microfibril assembly. Structure. 2013;21:1743–56. doi: 10.1016/j.str.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hubmacher D, El-Hallous EI, Nelea V, Kaartinen MT, Lee ER, Reinhardt DP. Biogenesis of extracellular microfibrils: Multimerization of the fibrillin-1 C terminus into bead-like structures enables self-assembly. Proc Natl Acad Sci U S A. 2008;105:6548–53. doi: 10.1073/pnas.0706335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marson A, Rock MJ, Cain SA, Freeman LJ, Morgan A, Mellody K, Shuttleworth CA, Baldock C, Kielty CM. Homotypic fibrillin-1 interactions in microfibril assembly. J Biol Chem. 2005;280:5013–21. doi: 10.1074/jbc.M409029200. [DOI] [PubMed] [Google Scholar]
- 93.Perna AF, Satta E, Acanfora F, Lombardi C, Ingrosso D, De Santo NG. Increased plasma protein homocysteinylation in hemodialysis patients. Kidney Int. 2006;69:869–76. doi: 10.1038/sj.ki.5000070. [DOI] [PubMed] [Google Scholar]
- 94.Jakubowski H, Perla-Kajan J, Finnell RH, Cabrera RM, Wang H, Gupta S, Kruger WD, Kraus JP, Shih DM. Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J. 2009;23:1721–7. doi: 10.1096/fj.08-127548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beard RS, Jr, Bearden SE. Vascular complications of cystathionine beta-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am J Physiol Heart Circ Physiol. 2011;300:H13–26. doi: 10.1152/ajpheart.00598.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Undas A, Kolarz M, Kopec G, Glowacki R, Placzkiewicz-Jankowska E, Tracz W. Autoantibodies against N-homocysteinylated proteins in patients on long-term haemodialysis. Nephrol Dial Transplant. 2007;22:1685–9. doi: 10.1093/ndt/gfm068. [DOI] [PubMed] [Google Scholar]
- 97.Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J. 1999;13:2277–83. [PubMed] [Google Scholar]
- 98.Perry MM, Hui CK, Whiteman M, Wood ME, Adcock I, Kirkham P, Michaeloudes C, Chung KF. Hydrogen Sulfide Inhibits Proliferation and Release of IL-8 from Human Airway Smooth Muscle Cells. Am J Respir Cell Mol Biol. 2011;45:746–752. doi: 10.1165/rcmb.2010-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zavaczki E, Jeney V, Agarwal A, Zarjou A, Oros M, Katko M, Varga Z, Balla G, Balla J. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney International. 2011;80:731–739. doi: 10.1038/ki.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suliman ME, Qureshi AR, Barany P, Stenvinkel P, Filho JC, Anderstam B, Heimburger O, Lindholm B, Bergstrom J. Hyperhomocysteinemia, nutritional status, and cardiovascular disease in hemodialysis patients. Kidney Int. 2000;57:1727–35. doi: 10.1046/j.1523-1755.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 101.Nair AP, Nemirovsky D, Kim M, Geer EB, Farkouh ME, Winston J, Halperin JL, Robbins MJ. Elevated homocysteine levels in patients with end-stage renal disease. Mt Sinai J Med. 2005;72:365–73. [PubMed] [Google Scholar]
- 102.Urquhart BL, Freeman DJ, Cutler MJ, Mainra R, Spence JD, House AA. Mesna for treatment of hyperhomocysteinemia in hemodialysis patients: A placebo-controlled, double-blind, randomized trial. Clinical Journal of the American Society of Nephrology. 2008;3:1041–1047. doi: 10.2215/CJN.04771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25:155–61. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 104.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–45. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McIntyre TM, Prescott SM, Weyrich AS, Zimmerman GA. Cell-cell interactions: leukocyte-endothelial interactions. Current Opinion in Hematology. 2003;10:150–8. doi: 10.1097/00062752-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 106.Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Armstrong PW, Willerson JT. Clinical cardiology: New frontiers. Circulation. 1998;97:1107–1107. [Google Scholar]
- 108.Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–83. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-beta-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1779–87. doi: 10.1152/ajpcell.00207.2007. [DOI] [PubMed] [Google Scholar]
- 110.Afanas’ev I. Signaling of reactive oxygen and nitrogen species in diabetes mellitus. Oxidative Medicine and Cellular Longevity. 2010;3:361–373. doi: 10.4161/oxim.3.6.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 112.Di Stefano V, Cencioni C, Zaccagnini G, Magenta A, Capogrossi MC, Martelli F. p66ShcA modulates oxidative stress and survival of endothelial progenitor cells in response to high glucose. Cardiovasc Res. 2009;82:421–9. doi: 10.1093/cvr/cvp082. [DOI] [PubMed] [Google Scholar]
- 113.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–22. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paneni F, Capretti G, Costantino S, Chiandotto S, Akhmedov A, Di Stasio E, Rocca B, Luscher TF, Volpe M, Cosentino F. The lifespan determinant p66shc drives obesity-induced oxidative stress, mitochondrial dysfunction and vascular inflammation. European Heart Journal. 2013;34:159–159. [Google Scholar]
- 115.Kim CS, Kim YR, Naqvi A, Kumar S, Hoffman TA, Jung SB, Kumar A, Jeon BH, McNamara DM, Irani K. Homocysteine promotes human endothelial cell dysfunction via site-specific epigenetic regulation of p66shc. Cardiovascular Research. 2011;92:466–475. doi: 10.1093/cvr/cvr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochimica Et Biophysica Acta-Molecular Cell Research. 2012;1823:2297–2310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 117.Kubli DA, Gustafsson AB. Mitochondria and Mitophagy The Yin and Yang of Cell Death Control. Circulation Research. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kadenbach B. Introduction to mitochondrial oxidative phosphorylation. Adv Exp Med Biol. 2012;748:1–11. doi: 10.1007/978-1-4614-3573-0_1. [DOI] [PubMed] [Google Scholar]
- 119.Lee J, Giordano S, Zhang JH. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochemical Journal. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Current Opinion in Cell Biology. 2009;21:645–653. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 121.Tan RJ, Liu YH. Matrix metalloproteinases in kidney homeostasis and diseases. American Journal of Physiology-Renal Physiology. 2012;302:F1351–F1361. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302:F1351–61. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. Febs Journal. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 124.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Vanwart HE, Birkedalhansen H. The Cysteine Switch - a Principle of Regulation of Metalloproteinase Activity with Potential Applicability to the Entire Matrix Metalloproteinase Gene Family. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Owens MW, Milligan SA, JourdHeuil D, Grisham MB. Effects of reactive metabolites of oxygen and nitrogen on gelatinase A activity. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1997;273:L445–L450. doi: 10.1152/ajplung.1997.273.2.L445. [DOI] [PubMed] [Google Scholar]
- 127.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 128.Radomski A, Sawicki G, Olson DM, Radomski MW. The role of nitric oxide and metalloproteinases in the pathogenesis of hyperoxia-induced lung injury in newborn rats. British Journal of Pharmacology. 1998;125:1455–1462. doi: 10.1038/sj.bjp.0702216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011;15:1927–43. doi: 10.1089/ars.2010.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Predmore BL, Lefer DJ, Gojon G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxidants & Redox Signaling. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Talaei F, Bouma HR, Hylkema MN, Strijkstra AM, Boerema AS, Schmidt M, Henning RH. The role of endogenous H2S formation in reversible remodeling of lung tissue during hibernation in the Syrian hamster. J Exp Biol. 2012;215:2912–9. doi: 10.1242/jeb.067363. [DOI] [PubMed] [Google Scholar]
- 132.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? Journal of the American Society of Nephrology. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 133.Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrology Dialysis Transplantation. 2002;17:1197–1203. doi: 10.1093/ndt/17.7.1197. [DOI] [PubMed] [Google Scholar]
- 134.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney International. 2003;63:1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 135.Kawachi H, Suzuki K, Miyauchi N, Hashimoto T, Otaki Y, Shimizu F. Slit diaphragm dysfunction in proteinuric states: identification of novel therapeutic targets for nephrotic syndrome. Clinical and Experimental Nephrology. 2009;13:275–280. doi: 10.1007/s10157-009-0162-x. [DOI] [PubMed] [Google Scholar]
- 136.Benzing T. Signaling at the slit diaphragm. Journal of the American Society of Nephrology. 2004;15:1382–1391. doi: 10.1097/01.asn.0000130167.30769.55. [DOI] [PubMed] [Google Scholar]
- 137.Holthofer H. Molecular architecture of the glomerular slit diaphragm: lessons learnt for a better understanding of disease pathogenesis. Nephrology Dialysis Transplantation. 2007;22:2124–2128. doi: 10.1093/ndt/gfm344. [DOI] [PubMed] [Google Scholar]
- 138.Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm-from a thin grey line to a complex signalling hub. Nature Reviews Nephrology. 2013;9:587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- 139.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 140.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE. Mechanisms of Podocyte Injury in Diabetes Role of Cytochrome P450 and NADPH Oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen S, Meng XF, Zhang C. Role of NADPH Oxidase-Mediated Reactive Oxygen Species in Podocyte Injury. Biomed Res Int. 2013 doi: 10.1155/2013/839761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Furuichi K, Hisada Y, Shimizu M, Okumura T, Kitagawa K, Yoshimoto K, Iwata Y, Yokoyama H, Kaneko S, Wada T. Matrix metalloproteinase-2 (MMP-2) and membrane-type 1 MMP (MT1-MMP) affect the remodeling of glomerulosclerosis in diabetic OLETF rats. Nephrology Dialysis Transplantation. 2011;26:3124–3131. doi: 10.1093/ndt/gfr125. [DOI] [PubMed] [Google Scholar]
- 143.Pushpakumar S, Kundu S, Pryor T, Givvimani S, Lederer E, Tyagi SC, Sen U. Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. Journal of Hypertension. 2013;31:2270–2281. doi: 10.1097/HJH.0b013e3283649b33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Humphrey JD, DeLange S. An introduction to biomechanics: solids and fluids, analysis and design. Springer; London: 2003. [Google Scholar]
- 145.C.e.o.c. Dodds, C.M.e.o.c. Kumar, B.T.e.o.c. Veering, Oxford textbook of anaesthesia for the elderly patient.
- 146.Lefevre M, Rucker RB. Aorta elastin turnover in normal and hypercholesterolemic Japanese quail. Biochim Biophys Acta. 1980;630:519–29. doi: 10.1016/0304-4165(80)90006-9. [DOI] [PubMed] [Google Scholar]
- 147.Johnson DJ, Labourene J, Rabinovitch M, Keeley FW. Relative Efficiency of Incorporation of Newly Synthesized Elastin, Collagen into Aorta, Pulmonary-Artery and Pulmonary Vein of Growing Pigs. Connective Tissue Research. 1993;29:213–221. doi: 10.3109/03008209309016828. [DOI] [PubMed] [Google Scholar]
- 148.Satta J, Juvonen T, Haukipuro K, Juvonen M, Kairaluoma MI. Increased turnover of collagen in abdominal aortic aneurysms, demonstrated by measuring the concentration of the aminoterminal propeptide of type III procollagen in peripheral and aortal blood samples. J Vasc Surg. 1995;22:155–60. doi: 10.1016/s0741-5214(95)70110-9. [DOI] [PubMed] [Google Scholar]
- 149.Meng J, Sakata N, Takebayashi S, Asano T, Futata T, Nagai R, Ikeda K, Horiuchi S, Myint T, Taniguchi N. Glycoxidation in aortic collagen from STZ-induced diabetic rats and its relevance to vascular damage. Atherosclerosis. 1998;136:355–65. doi: 10.1016/s0021-9150(97)00238-4. [DOI] [PubMed] [Google Scholar]
- 150.Eddy AA. Interstitial fibrosis in hypercholesterolemic rats: role of oxidation, matrix synthesis, and proteolytic cascades. Kidney Int. 1998;53:1182–9. doi: 10.1046/j.1523-1755.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 151.Schwarz A. New aspects of the treatment of nephrotic syndrome. J Am Soc Nephrol. 2001;12(Suppl 17):S44–7. [PubMed] [Google Scholar]
- 152.Bakris GL. Slowing nephropathy progression: Focus on proteinuria reduction. Clinical Journal of the American Society of Nephrology. 2008;3:S3–S10. doi: 10.2215/CJN.03250807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Perna AF, Sepe I, Lanza D, Capasso R, Di Marino V, De Santo NG, Ingrosso D. The gasotransmitter hydrogen sulfide in hemodialysis patients. J Nephrol. 2010;23(Suppl 16):S92–6. [PubMed] [Google Scholar]
- 154.Chen XL, Jhee KH, Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. Journal of Biological Chemistry. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 155.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011;15:363–72. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kimura H. Production and Physiological Effects of Hydrogen Sulfide. Antioxidants & Redox Signaling. 2014;20:783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aminzadeh MA, Vaziri ND. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol Dial Transplant. 2012;27:498–504. doi: 10.1093/ndt/gfr560. [DOI] [PubMed] [Google Scholar]
- 158.Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, Moser J, Hillebrands JL, Ploeg RJ, Yang GD, Leuvenink HGD, van Goor H. Cystathionine gamma-Lyase Protects against Renal Ischemia/Reperfusion by Modulating Oxidative Stress. Journal of the American Society of Nephrology. 2013;24:759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–56. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 160.Meye C, Schumann J, Wagner A, Gross P. Effects of homocysteine on the levels of caveolin-1 and eNOS in caveolae of human coronary artery endothelial cells. Atherosclerosis. 2007;190:256–63. doi: 10.1016/j.atherosclerosis.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 161.Liu X, Luo F, Li J, Wu W, Li L, Chen H. Homocysteine induces connective tissue growth factor expression in vascular smooth muscle cells. J Thromb Haemost. 2008;6:184–92. doi: 10.1111/j.1538-7836.2007.02801.x. [DOI] [PubMed] [Google Scholar]
- 162.Tyagi SC. Homocysteine redox receptor and regulation of extracellular matrix components in vascular cells. Am J Physiol. 1998;274:C396–405. doi: 10.1152/ajpcell.1998.274.2.C396. [DOI] [PubMed] [Google Scholar]
- 163.Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–21. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boffa JJ, Tharaux PL, Placier S, Ardaillou R, Dussaule JC, Chatziantoniou C. Angiotensin II activates collagen type I gene in the renal vasculature of transgenic mice during inhibition of nitric oxide synthesis: evidence for an endothelin-mediated mechanism. Circulation. 1999;100:1901–8. doi: 10.1161/01.cir.100.18.1901. [DOI] [PubMed] [Google Scholar]
- 165.Araki S, Haneda M, Koya D, Isshiki K, Kume S, Sugimoto T, Kawai H, Nishio Y, Kashiwagi A, Uzu T, Maegawa H. Association Between Urinary Type IV Collagen Level and Deterioration of Renal Function in Type 2 Diabetic Patients Without Overt Proteinuria. Diabetes Care. 2010;33:1805–1810. doi: 10.2337/dc10-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006;55:3112–20. doi: 10.2337/db06-0895. [DOI] [PubMed] [Google Scholar]
- 167.Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–9. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 168.Bennett BM, Schroder H, Hayward LD, Waldman SA, Murad F. Effect of in vitro organic nitrate tolerance on relaxation, cyclic GMP accumulation, and guanylate cyclase activation by glyceryl trinitrate and the enantiomers of isoidide dinitrate. Circ Res. 1988;63:693–701. doi: 10.1161/01.res.63.4.693. [DOI] [PubMed] [Google Scholar]
- 169.Bian K, Murad F. Nitric oxide signaling in vascular biology. J Am Soc Hypertens. 2007;1:17–29. doi: 10.1016/j.jash.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 170.Kass DA, Takimoto E, Nagayama T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75:303–14. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 171.Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol. 2003;284:R628–38. doi: 10.1152/ajpregu.00401.2002. [DOI] [PubMed] [Google Scholar]
- 172.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234–235:249–63. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 174.Wiesel P, Patel AP, Carvajal IM, Wang ZY, Pellacani A, Maemura K, DiFonzo N, Rennke HG, Layne MD, Yet SF, Lee ME, Perrella MA. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1-deficient mice. Circ Res. 2001;88:1088–94. doi: 10.1161/hh1001.091521. [DOI] [PubMed] [Google Scholar]
- 175.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475–9. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci U S A. 1996;93:795–8. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Blydt-Hansen TD, Katori M, Lassman C, Ke B, Coito AJ, Iyer S, Buelow R, Ettenger R, Busuttil RW, Kupiec-Weglinski JW. Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:745–54. doi: 10.1097/01.asn.0000050760.87113.25. [DOI] [PubMed] [Google Scholar]
- 178.Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006;10:650–71. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Caumartin Y, Stephen J, Deng JP, Lian D, Lan Z, Liu W, Garcia B, Jevnikar AM, Wang H, Cepinskas G, Luke PP. Carbon monoxide-releasing molecules protect against ischemia-reperfusion injury during kidney transplantation. Kidney Int. 2011;79:1080–9. doi: 10.1038/ki.2010.542. [DOI] [PubMed] [Google Scholar]
- 180.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–16. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–31. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 182.Sen U, Vacek TP, Hughes WM, Kumar M, Moshal KS, Tyagi N, Metreveli N, Hayden MR, Tyagi SC. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology. 2008;82:201–13. doi: 10.1159/000156486. [DOI] [PubMed] [Google Scholar]
- 183.Kundu S, Pushpakumar SB, Tyagi A, Coley D, Sen U. Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am J Physiol Endocrinol Metab. 2013;304:E1365–78. doi: 10.1152/ajpendo.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bos EM, Leuvenink HG, Snijder PM, Kloosterhuis NJ, Hillebrands JL, Leemans JC, Florquin S, van Goor H. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J Am Soc Nephrol. 2009;20:1901–5. doi: 10.1681/ASN.2008121269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu JX, Kalbfleisch M, Yang YX, Bihari R, Lobb I, Davison M, Mok A, Cepinskas G, Lawendy AR, Sener A. Detrimental effects of prolonged warm renal ischaemia-reperfusion injury are abrogated by supplemental hydrogen sulphide: an analysis using real-time intravital microscopy and polymerase chain reaction. BJU Int. 2012;110:E1218–27. doi: 10.1111/j.1464-410X.2012.11555.x. [DOI] [PubMed] [Google Scholar]
- 186.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–6. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 188.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Sr, Gojon G, Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–27. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A. 2012;109:2943–8. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Modis K, Coletta C, Erdelyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–11. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]