Abstract
While the pathological events evoked by infection are commonly described, effective host responses to bacteria and their products should primarily be protective. Heat shock protein (Hsp) expression is upregulated by many stimuli and serves to maintain intracellular protein integrity. The ability of the prototypic superantigen, Staphylococcus aureus enterotoxin B (SEB) to induce Hsps was investigated with BALB/c mice and by in vitro addition to the murine small intestinal epithelial cell line MSIE. SEB-treated (5 or 100 μg intraperitoneally) mice revealed increased Hsp25 and Hsp72, but not Hsc73, in jejunal lymphocytes and epithelial cells. A similar Hsp response to SEB occurred in MSIE cells and was preceded by activation of the ERK1/2 and p38 mitogen-activated protein kinases but not the SAPK/JNK pathway; pharmacological inhibition of ERK1/2, but not p38, significantly reduced SEB-induced Hsps. Moreover, SEB-treated MSIE cells were protected against oxidant-induced cytotoxicity (measured by 51Cr release) and F-actin depolymerization. Thus, SEB exposure results in a rapid induction of the Hsp25 and Hsp72 in intestinal epithelial cells, both directly and through lymphocyte activation, and we suggest that this event is important in protecting the gut from damage by Staphylococcus infection or in the reparatory process and may be a generalized response to lumen-derived bacterial toxins.
As exceptionally potent T-cell stimuli, bacterial superantigens (SAgs) have an obvious proinflammatory potential and have been implicated in both human inflammatory and autoimmune disease. Bacterial SAgs are small, protease-resistant, pyrogenic peptides produced by a variety of species, particularly Staphylococcus aureus and Streptococcus pyogenes (7, 17), that are unique in their ability to polyclonally activate T cells. Thus, SAgs bypass the classical route of antigen processing and presentation by antigen-presenting cells and bind directly to a domain of major histocompatibility complex (MHC) class II molecules and a specific region on the variable portion of the β-chain of the T-cell receptor outside the antigen-specific site (3, 7). SAg-producing organisms can, on occasion, be identified in the gut, and indeed, Staphylococcus-derived SAgs are a familiar cause of food poisoning. Moreover, we and others, by using S. aureus enterotoxin B (SEB) as a model SAg, have shown that the enteric effects of SAg exposure extend beyond an emetic response and can result in altered jejunal architecture, local immune cell activation, increased epithelial chemokine synthesis, and altered epithelial ion transport that could contribute to a diarrheal response (1, 2, 6, 15, 18, 19, 20).
The epithelial lining of the gastrointestinal tract is an important component of innate immunity. It represents a first-line defensive barrier against lumen-derived antigens and pathogens and has the ability to modulate adaptive immune responses. Numerous investigators have demonstrated that epithelial cells can recognize and respond to bacteria, bacterial products, and inflammatory mediators elicited by bacterial exposure via alterations in physiology and in the production and secretion of cytokines and other messenger molecules (4, 35).
Analyses of the effects of bacteria, or their products, on gut function tend to focus on defining pathological or proinflammatory mechanisms, with reparatory or endogenous defensive mechanisms often being overlooked. Heat shock proteins (Hsps) are produced in response to a vast array of stimuli (e.g., heat, heavy metals, oxidants, protein synthesis, and degradation inhibitors) (14) and in a broad sense serve as scaffolding or chaperone proteins to preserve the integrity of essential intracellular proteins during times of stress. Hence, inducible Hsps provide the cell with a natural mechanism of protection from environmental stressors. It has long been noted that induction of Hsps by conditions of mild stress (for example thermal stress from fever) then confers protection from an otherwise lethal stress, a phenomenon known as stress tolerance (14, 34). Enteric epithelial cells upregulate the expression of Hsp25 and Hsp72 as a consequence of exposure to a number of different agents, including short chain fatty acids, interleukin-11 (IL-11), and Escherichia coli lipopolysaccharide (LPS) (11, 26, 27), which may confer protection against oxidant and tumor necrosis factor alpha-mediated injury. Therefore, we hypothesized that a facet of the T-cell-driven enteropathy induced by SAg exposure (1, 18) would be an increase in Hsp production in the intestine and possibly in the epithelium. The data described herein support this hypothesis and go further by identifying an epithelial mitogen-activated protein kinase (MAPK) response to direct SAg exposure and showing that the increase in Hsp72 and Hsp25 induced by SEB treatment protects the enterocyte from oxidant-induced F-actin depolymerization and concomitant cytotoxicity.
MATERIALS AND METHODS
Animals and experimental treatments.
Male BALB/c mice (6 to 8 weeks old; Charles River Laboratories, Wilmington, Mass.) were housed in conventional facilities. Mice received a single intraperitoneal injection of 5 or 100 μg of SEB (Sigma Chemical Company, St. Louis, Mo.) as representative low- and high-dose treatments (2, 5, 6). Gram-positive bacteria do not produce LPS; however, stocks were checked with the Limulus amoebocyte assay (Cambrex, Gaithersburg, Md.) and were found to be negative. Jejuna were harvested from mice at times from 4 to 48 h. One section was fixed in 10% neutral buffered formalin for immunohistochemistry, and the mucosa was sheared off an adjacent tissue portion by blunt dissection with glass slides. The mucosa was homogenized in a Teflon pestle homogenizer in lysis buffer (10 mM Tris [pH 7.4], 5 mM MgCl2, 50 U of DNase and RNase/ml, and Complete protease inhibitor cocktail [Roche Molecular Biochemicals, Indianapolis, Ind.]). Samples were incubated on ice for 10 min, a small aliquot was removed for protein determination (by the bicinchoninic acid procedure) (30), and a one-half volume of 3× Laemmli stop solution was added to the remainder. Samples were heated at 65°C for 10 min and analyzed for Hsps by Western blotting.
Western blotting for Hsps.
For various Hsps, different amounts of protein were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and with differing percentages of acrylamide, as follows: Hsp25, 30 μg of protein and 12.5% PAGE; Hsp72, 10 μg of protein and 10% PAGE; Hsc73, 5 μg of protein and 10% PAGE. Proteins were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (Polyscreen; NEN, Boston, Mass.) in 1× Towbin buffer (25 mM Tris, 192 mM glycine [pH 8.8], 10% [vol/vol] methanol). All blots were blocked in 5% (wt/vol) nonfat dry milk in Tween-Tris-buffered saline (T-TBS; 150 mM NaCl, 5 mM KCl, 10 mM Tris [pH 7.4], 0.05% [vol/vol] Tween 20). Blots were incubated overnight at 4°C with the following primary antibodies: anti-Hsp25 (polyclonal; Stressgen, Victoria, British Columbia, Canada), anti-Hsp72 (murine monoclonal antibody [MAb] C92; Stressgen), and anti-Hsc73 (rat MAb 1B5; Stressgen). Each of these antibodies is specific to its respective proteins (manufacturer's specifications and data from our lab). Blots were washed five times with T-TBS, incubated in horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, washed four times with T-TBS, washed once with Tris-buffered saline, and visualized by using an enhanced chemiluminescent system (Supersignal; Pierce Chemical, Rockford, Ill.).
Cell culture.
Mouse small intestinal epithelial (MSIE) cells were a generous gift from Robert Whitehead (Vanderbilt University, Nashville, Tenn.). Cells were grown in RPMI 1640 medium (Life Technologies, Gaithersburg, Md.) supplemented with 5% fetal bovine serum, murine gamma interferon (IFN-γ) (5 U/ml), 50 U of penicillin/ml, and 50 μg of streptomycin/ml (all from Invitrogen), and ITS plus Premix (6.25 mg of insulin/liter, 6.25 mg of transferrin/liter, 6.25 μg of selenous acid/liter, 1.25 g of bovine serum albumin/liter, and 5.35 mg of linoleic acid/liter) (Collaborative Research, Boston, Mass.). The MSIE cells were derived from the transgenic temperature-sensitive simian virus 40 large-T antigen Immortimouse (33) and, as such, were grown at the permissive temperature of 33°C. For experiments, medium was changed to IFN-γ-free medium (the large-T antigen is under a portion of the MHC class I promoter, which is IFN-γ sensitive). To completely stop proliferation, the cells may be switched to 37°C, where the simian virus 40 large-T antigen folds incorrectly. Similar results were obtained with cells in IFN-γ-free medium and 33 and 37°C (data not shown), and therefore, to prevent potential degenerative changes at 37°C, cells were used at 33°C. The MSIE cell line was chosen, since the in vivo effects were investigated in the murine small intestine and we wished to keep the species and segment of the intestine consistent. SEB was added directly to the culture medium. Cells were harvested by scraping in ice-cold saline, pelleting in a microcentrifuge (13,000 × g for 10 s), and resuspending in 50 μl of lysis buffer described above. Cell homogenates were prepared, and Western blots for Hsps were performed as described for the tissues.
For analysis of stress kinase activation, 20 μg of cell homogenate was resolved by SDS-12.5% PAGE and transferred to the PVDF membrane. In all cases, two blots were run, one for analysis with antibody specific for the activated (phosphorylated) forms of the three stress kinases (i.e., p44/42 also known as ERK 1/2, p38, and SAPK/JNK) and another blot for analysis with antibody for the total (active and inactive) kinase. Antibodies for all analysis of stress kinase activation were used according to the manufacturer's instructions (Cell Signaling, Boston, Mass.). Two polyclonal antisera are provided with the kits for p38, p44/42, and SAPK/JNK. One antiserum, in each case, recognizes all forms of the respective kinase(s) and one antiserum reacts only with the phosphorylated, active forms.
For coculture with lamina propria lymphocytes (LPLs), MSIE cells were seeded onto fibrillar collagen inserts (1-μm pore size, 4.2-cm2 surface area; Becton Dickinson, Bedford, Mass.) and grown to confluence at permissive conditions. Twenty-four hours before coculture, MSIE cells were transferred to nonpermissive conditions. LPLs were isolated by a collagenase digestion of small intestinal mucosa of C57BL/6 mice as previously described (13). Lymphocytes (2 × 106 cells/ml of RMPI containing 10% fetal bovine serum) were added to the basal compartment of the coculture well; 48 h later, MSIE monolayers were inserted, and the coculture was maintained for 24 h with or without SEB addition to the basal compartment (i.e., directly to the LPLs). In some experiments, anti-mouse IL-2 MAb (2 μg/ml) was included in the coculture. After 24 h of coculture, protein samples were harvested and analyzed by Western blotting for Hsp expression.
Immunohistochemical staining for Hsp.
Immunostaining was performed on 4-μm-thick paraffin sections of formalin-fixed tissues as previously described (10). Sections were stained for Hsps by using the Vectastain Elite ABC kit according to the manufacturer's instructions (Vector Labs, Burlingame, Calif.). Slides were incubated overnight at 4°C with the same antibodies used for Western blotting and then incubated with biotinylated horse anti-rabbit and anti-mouse or anti-rat immunoglobulin G (Vector Labs). This was followed by incubation with Vector ABC Elite reagent for 30 min and washing five times. Slides were developed by using the diaminobenzidine solution provided for 1 min. All slides were counterstained with hematoxylin and mounted by using DPX mounting agent (Electron Microscopy Services, Ft. Washington, Pa.). An inverted Zeiss Axiophot microscope, a Macintosh G4 computer, and Pixera software were used to image slides.
Cell viability assay.
MSIE cells were grown to confluence in 24-well plates with or without SEB (1 μg/ml) for 24 h. Cells were loaded with 51Cr (50 μCi/ml; Sigma Chemical Co.) for 60 min, washed, and incubated in media with various amounts of the oxidant monochloramine to induce cell injury. Medium was harvested from the cells after 60 min, and the 51Cr remaining in the cells was extracted with 1 N HNO3 for 4 h. The amounts of 51Cr in the released and cellular fractions were counted by liquid scintillation spectroscopy. The amount of 51Cr released was calculated as the amount released divided by the amount released plus the cellular remainder.
G/F-actin assay.
Plastic-grown confluent cells monolayers were grown at 33°C in IFN-γ-free medium with or without SEB (1 μg/ml). Prior to assessment, cells were treated with phalloidin (30 μg/ml for 2 h; Molecular Probes, Eugene, Oreg.), cytochalasin D (10 μg/ml for 15 min), or the oxidant monochloramine (0.6 mM for 30 min) (10, 23). Cells were rinsed in phosphate-buffered saline, scraped, pelleted (14,000 × g for 20 s at room temperature), and the pellets were resuspended in 200 μl of 30°C lysis buffer [1 mM ATP, 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.9), 50 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5% (vol/vol) glycerol, 0.1% (vol/vol) Nonidet P-40, Tween 20, Triton X-100, and Complete protease inhibitor cocktail]. Cells were homogenized by gently pipetting up and down 10 times and incubated at 30°C for 10 min. Samples were centrifuged at 100,000 × g for 60 min at 30°C, and the supernatants were removed for determination of G-actin. Pellets containing F-actin were resuspended in 200 μl of 4°C distilled water with 1 μM cytochalasin D and left on ice for 60 min. Then 20 μl of each extraction was removed, 6 μl of 3× Laemmli stop solution was added, and the samples were heated to 65°C for 10 min. Samples were resolved by SDS-12.5% PAGE and immediately transferred to PVDF membranes. After transfer, analysis of actin was performed with a polyclonal anti-actin antiserum by Western blotting (Cytoskeleton, Denver, Colo.). Since the F-actin fraction has been depolymerized, only the monomeric 45-kDa form is observed on the Western blots.
Data presentation and analyses.
Numerical data are presented as means ± standard errors of the means and, where appropriate, were compared with Student's t test, and P values of <0.05 were accepted as a level of statistically significant difference.
RESULTS
SEB induces Hsp25 and Hsp72 in intestinal lymphocytes and epithelial cells.
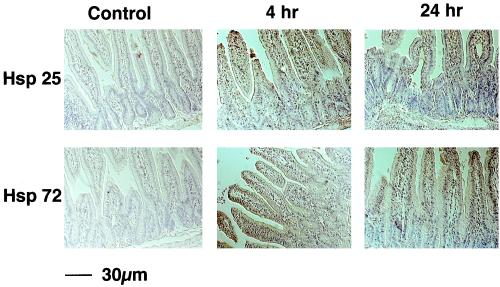
SEB has been found to induce a self-limiting murine enteropathy with both colonic and small intestinal involvement, with effects mediated by CD4+ T cells (2, 18). To determine whether SEB would induce Hsps in specific intestinal cell populations, mice were treated with a single intraperitoneal injection of SEB. Figure 1 shows that with low-dose SEB (i.e., 5 μg), both Hsp25 and Hsp72 expression were induced in a time-dependent manner in lymphocytes and epithelial cells (Fig. 1). Sections of jejunum from saline-treated control mice revealed weak staining for Hsp25 and no Hsp72 positivity. Increased epithelial Hsp25 and Hsp72 immunostaining was apparent 4 h after treatment and was substantially increased after 24 h (Fig. 1) and 48 h (data not shown) post-SEB treatment.
FIG. 1.
Systemic SEB treatment evokes an increase in Hsp25 and Hsp72 in epithelial cells and gut lymphocytes. BALB/c mice were injected intraperitoneally with 5 μg of SEB, and the jejuna were harvested 4 or 24 h later. Tissues were formalin fixed, and immunohistochemistry was performed on paraffin sections as described in Materials and Methods. Images shown are representative of the results from 4 mice.
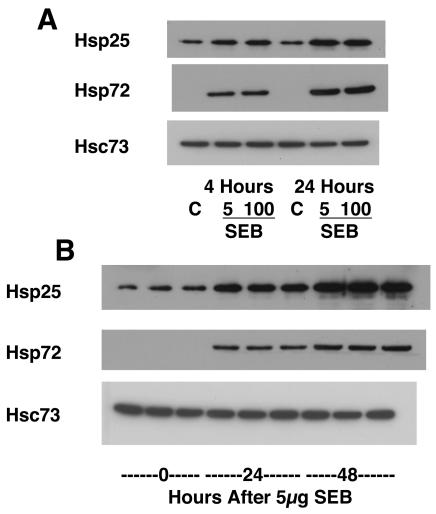
Next, Hsp levels were quantified by Western blotting. When the jejunal mucosa is sheared by glass slides, a separation plane occurs naturally between the muscularis propria and the remainder of the mucosa. Epithelial cells comprise a majority of the cells in the mucosal scrapings and thus represent a majority of the protein in the scrapings. Consistent with the immunostaining, Western blot analyses revealed increases in Hsp25 and Hsp72 4 h post-SEB (5 or 100 μg) treatment (Fig. 2A), and this was further enhanced by 24 and 48 h posttreatment (Fig. 2B). The upregulation of Hsp72 was particularly striking, given that this protein was undetectable by Western blotting in extracts from control mice. Heat shock cognate 73, or Hsc73, is a major endoplasmic reticulum chaperone Hsp which is constitutively expressed in all cell types. Because its level of expression does not change appreciably with stress, Hsc73 serves as a useful control to verify that equal amounts of protein are loaded into each lane in each experiment. There were no significant changes in the expression of Hsc73 (loading control) among the groups.
FIG. 2.
Western blotting reveals a significant increase in Hsp25 and Hsp72 in jejunal mucosal scrapping from SEB-treated mice. (A) Both 5 and 100 μg of SEB (intraperitoneal) evoked increased expression of Hsp25 and Hsp72 at 4 and 24 h posttreatment; (B) extracts from 3 mice/group showing the time-dependent increase in Hsps following low-dose SEB. Results are representative of data from 6 mice.
SEB induces epithelial cell Hsps directly and indirectly.
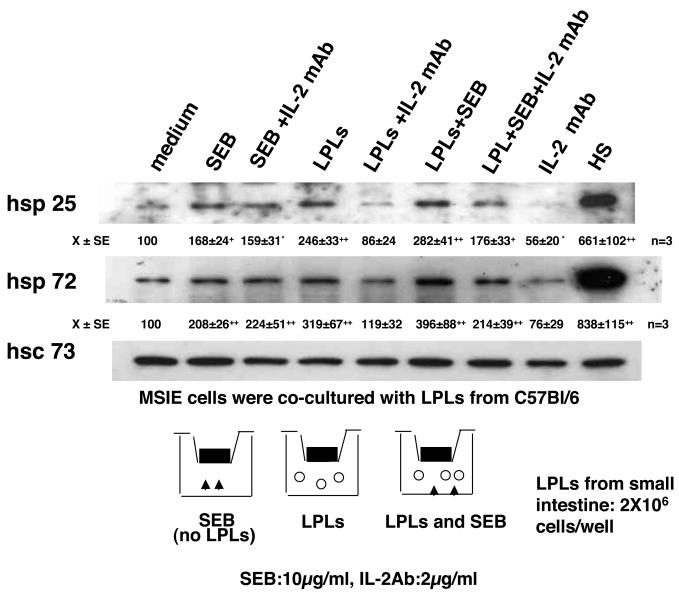
Because SEB is a SAg which causes massive T-cell proliferation and cytokine secretion, the in vivo experiments cannot distinguish between direct and indirect effects of SEB in the induction of Hsp expression, such that the effect of SEB on the epithelium could be via T-cell-antigen-presenting cell activation, resulting in the production of inflammatory mediators. To address this, MSIE cells were grown on permeable supports and cultured with SEB with or without LPLs isolated from the small intestines of C57BL/6 mice. As shown in Fig. 3, SEB applied directly to MSIE cell monolayers caused an increase in Hsp25 and Hsp72 expression. Antibodies against IL-2, one of the most potent Hsp-inducing cytokines described (10), were then added along with SEB. The addition of IL-2 antibodies along with SEB did not affect Hsp induction, indicating that SEB induction of Hsp expression is not mediated by IL-2. In contrast, coculture with nonactivated LPLs evoked MSIE Hsp expression, and this was blocked by the inclusion of a neutralizing MAb against IL-2 (Fig. 3). Densitometric analysis (performed with NIH Image 1.54 software and shown below the panels) shows that the addition of SEB to the LPL-MSIE cocultures resulted in a greater induction of MSIE Hsp72, but not Hsp25, and the former event was eliminated by inclusion of MAb IL-2 in the culture medium (Fig. 3).
FIG. 3.
SEB stimulates epithelial Hsps both directly and indirectly. Representative Western blots (n = 3) showing that SEB alone (10 μg/ml, 24 h) or exposure to LPLs (2 × 106/well) induce MSIE Hsp25 and Hsp72 expression. The latter was inhibited by addition of a neutralizing MAb against IL-2 (2 μg/ml) to the basal compartment of the culture well housing the LPLs. Hsc73 is included as a control for protein loading, and HS (heat shock) is included as a positive control for induction of Hsp25 and Hsp72. Densitometric values for Hsp25 and Hsp72 were obtained with NIH Image 1.54 software and are means ± standard errors (SE) of the results from three experiments. *, P < 0.05; +, P < 0.01; ++, P < 0.001 (compared with medium alone by analysis of variance with Bonferroni corrections) (Instat Software; Graph Pad, San Diego, Calif.).
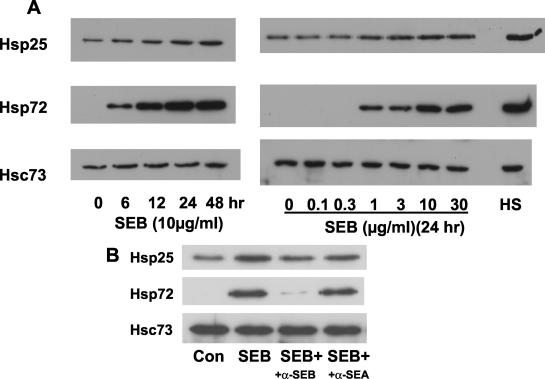
In subsequent experiments the time and dose dependencies of the direct effect of SEB on MSIE induction of Hsp25 and Hsp72 were determined (Fig. 4). Induction occurs as early as 6 h and was elicited at a threshold of 1 μg of SEB/ml, when protein extracts were examined 24 h post-SEB treatment (Fig. 4). Similar results were obtained with colonic epithelial cells from the Immortimouse YAMC cells (data not shown). To determine the specificity of the effects observed, neutralizing antibodies to SEB and staphylococcal enterotoxin A were used (polyclonal antisera specific to each toxin obtained from Sigma-Aldrich). A moderate concentration of SEB was used (3 μg) and was preincubated with 5 μg of antibody of each antiserum or 5 μg of antibody from rabbit preimmune serum. The addition of anti-SEB inhibited SEB induction, whereas incubation of SEB with either anti-staphylococcal enterotoxin A or the preimmune serum had no effect (Fig. 4B; preimmune serum data are not shown). Therefore, the effects of SEB observed on the epithelial cells do appear to be mediated by SEB in the preparation and not by a contaminant.
FIG. 4.
SEB stimulates Hsp expression in cultured MSIE small intestinal epithelial cells. Cells were treated with 10 μg of SEB/ml for various times or various concentrations for 24 h, and total cell protein was analyzed by Western blotting. Heat shock (HS) is shown as a positive control for induction of Hsp25 and Hsp72, and Hsc73 is shown as a loading control. Images shown are representative of the results from three separate experiments. Con, control; +, plus; α, anti; SEA, Staphylococcal enterotoxin A.
SEB activates ERK1/2 and p38 MAPK pathways.
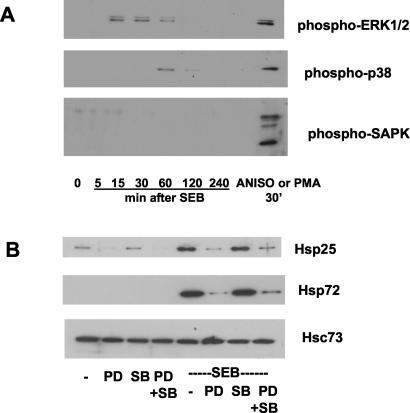
Many bacterial products affect mammalian cell function, including intestinal epithelial cells, through the activation of stress kinases (11, 35). We hypothesized that activation of ERK1/2, p38, or SAPK/JNK would precede SEB induction of Hsps in MSIE cells. SEB was found to elicit early and transient activation of ERK1/2, as shown by phosphorylation, and a comparatively delayed activation of p38 (increased phosphorylation of p38 was apparent at 30 min post-SEB treatment), but it did not result in SAPK/JNK activation (Fig. 5A). This MAPK activation pattern was a consistent finding in three separate experiments with different passages of cells. In separate experiments, the MEK-1 (upstream of ERK1/2) inhibitor, PD98059, but not the p38-directed inhibitor, SB203580, was found to significantly reduce the upregulation of Hsp25 and Hsp72 by SEB treatment of MSIE cells (Fig. 5B). Treatment of MSIE with the MEK-1 inhibitor also decreased basal production of Hsp25. The activities of the pharmacological inhibitors were confirmed by demonstration of inhibition of the respective MAPK activation in response to phorbol-12,13-myristate acetate (data not shown).
FIG. 5.
SEB stimulates the ERK1/2 and p38 MAPK pathways. (A) MSIE cells were treated with SEB (10 μg/ml) for the times indicated, and total protein lysates were harvested. As positive controls, cells were stimulated with anisomycin (ANISO, 10 μg/ml) or phorbol-12,13-myristate acetate (PMA) for 30 min. Activated stress kinases were determined by using antibodies to the phosphospecific forms of the kinases as described in Materials and Methods. Images shown are representative of the results from three separate experiments. Approximately equal protein loading was observed on blots probed for total MAPK levels (data not shown). (B) MSIE cells were pretreated with the MEK-1 inhibitor, PD98059 (PD; 50 μM), the p38 inhibitor, SB203580 (SB; 30 μM), or both (PD + SB) for 2 h prior to treatment with SEB (10 μg/ml, 24 h) or saline vehicle (−). Hsc73 was used as a loading control. Images shown are representative of the results from three separate experiments.
SEB protects MSIE cells from oxidant injury.
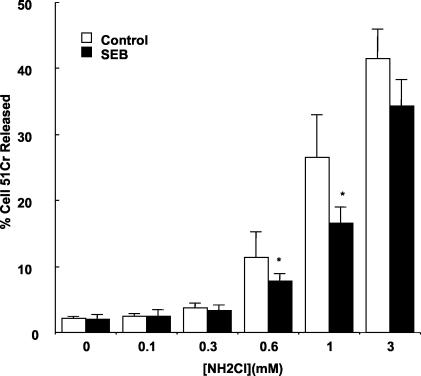
To determine whether SEB induction of Hsps might protect the epithelium from injury, the cell-permeant, long-lived oxidant monochloramine (NH2Cl) was used. Monochloramine was chosen because it is a pathophysiologically relevant oxidant produced in vast quantities when hypochlorous acid, released from innate immune cells within inflamed tissues, reacts with ammonia. Once formed, monochloramine reaches millimolar concentrations and causes loss of tight junction barrier function, mitochondrial injury, cytoskeletal disruption, impaired membrane transport functions, and eventual cell death (23, 24, 26, 27). Cells were treated with SEB (10 μg/ml) for 24 h to induce Hsp expression, and a 51Cr release viability assay was performed. Treatment with SEB diminished the NH2Cl-stimulated 51Cr release at intermediate concentrations of the oxidant (Fig. 6), demonstrating a protective effect. At 0.6 mM NH2Cl, SEB pretreatment decreased the NH2Cl-stimulated 51Cr release and resulted in a mild protective effect. However, a more pronounced protective effect was observed at 1 mM NH2Cl. This effect may be due to SEB induction of Hsp72, as this has been demonstrated to be a potent protective agent against monochloramine in this assay with other intestinal epithelial cell lines (23), whereas Hsp25 appears to have a less profound protective effect (26, 27).
FIG. 6.
SEB protects MSIE cells from subsequent oxidant injury. MSIE cells were treated with SEB (10 μg/ml) for 24 h. Cells were labeled with 51Cr for 60 min and stimulated with various concentrations of monochloramine (NH2Cl) for 60 min, and the ratio of released 51Cr to intracellular 51Cr was determined. Results are means ± standard errors for the results from three separate experiments; in each experiment, each group was measured in triplicate. *, P < 0.05 compared to controls.
SEB protects against oxidant-induced actin depolymerization.
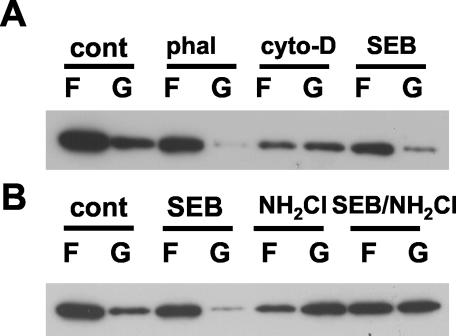
Hsp25 may bind actin filaments and stabilize filamentous actin (22). Therefore, we determined whether SEB induction of Hsp25 is associated with protection of filamentous actin during subsequent exposure to monochloramine (NH2Cl), which rapidly dissociates filamentous actin (23). Cells treated with SEB (10 μg/ml, 24 h) were subjected to treatment with vehicle or NH2Cl. By itself, SEB had little effect on the F/G-actin distribution in MSIE cells (Fig. 7A). Phalloidin, which binds and stabilizes the barbed ends of F-actin filaments, increased the amount of F-actin while cytochalasin D, an F-actin-disrupting agent, greatly increased the amount of G-actin (Fig. 7A). Monochloramine (0.6 mM) stimulated a disruption of F-actin filaments, as demonstrated by a decrease in F-actin and an increase in the G-actin (Fig. 7B). MSIE cells pretreated with SEB and then treated with NH2Cl demonstrated significantly less change in the F/G-actin distribution, indicating a protective effect that is compatible with the actin-stabilizing functions of Hsp25.
FIG. 7.
SEB prevents oxidant-induced actin depolymerization from F- to G-actin form. MSIE cells were treated with control vehicle (cont; 20 μl of ethanol), phalloidin (phal; an F-actin stabilizer as a positive control, 10 μg/ml, 120 min), cytochalasin D (cyto-D; a filamentous actin destabilizer yielding G-actin, 1 μg/ml, 15 min), or SEB (10 μg/ml, 24 h) (A) or control vehicle (cont; 20 μl of saline), SEB (10 μg/ml, 24 h), monochloramine (0.6 mM, 30 min), or SEB (10 μg/ml, 24 h) and then monochloramine (SEB/NH2Cl; 0.6 mM for 30 min after 24 h of treatment with SEB) (B). Cells were processed for globular (G) and filamentous (F) actin, as described in Materials and Methods. Images shown are representative of the results from three separate experiments.
DISCUSSION
The intestinal epithelium exists in a unique environment, always in close proximity to a large antigenic burden and to large numbers of bacteria in the colon. For this reason, it may not be surprising that it has developed unique mechanisms to deal with potentially damaging bacteria and their products. In certain cases, however, noncommensal bacteria which are harmful to the individual establish themselves and may cause pathophysiologic consequences. Many bacteria are known to cause dramatic alterations in intestinal physiology, such as Yersinia, Salmonella, Clostridium, and Staphylococcus (31). These bacteria and their products may stimulate a variety of responses by the intestinal epithelium, such as changes in ion and solute transport and changes in morphology and MHC expression (1, 2, 10, 11, 18, 32, 35).
The present studies demonstrate that a particular toxin, SEB, may induce a defensive mechanism of the epithelial cells. Both in vivo and in vitro, SEB stimulates production of a major class of protective proteins, the Hsps. Whether SEB acts solely on the lumenal or basolateral membranes of intestinal epithelial cells may be a difficult question to resolve. Physiologically, SEB might be derived from lumenal Staphylococcus and therefore be anticipated to act on the lumenal side of intestinal epithelial cells. However, transcytosis of SEB in intestinal epithelial cells has been reported (8, 29). The MSIE cell line used here forms a relatively leaky monolayer which would not effectively exclude SEB and therefore may not answer the question of lumenal or basolateral aspect of action. Nonetheless, SEB stimulates Hsp production, which may be a potential defensive mechanism for the cells. The importance of these evolutionarily conserved stress proteins in cellular defense against stress and injury is well established (12, 16, 34) and has recently been demonstrated by knocking out 70-kDa Hsps and demonstrating increased sensitivity to osmotic stress of the renal medulla (28) or to mitigate ischemic preconditioning of the heart to reperfusion injury (9) as well as susceptibility to infection with Trypanosoma (25).
A variety of bacterial products may stimulate Hsp production by intestinal epithelial cells. The bacterial components LPS and flagellin, as well as short chain fatty acids, which are derived from bacterial fermentation of carbohydrates, primarily stimulate the production of Hsp25 (11, 26). In contrast, SEB also induces Hsp72, suggesting an alternative or additional mechanism(s) of activation. While some chaperone and antiapoptotic functions may be shared between these two Hsps, their actions are not identical. Hsp72 prevents oxidant-mediated cell injury to a greater degree than does Hsp25 (23, 24, 26, 27), and Hsp25 may be more important in actin stabilization and in maintaining cytoskeletal integrity (for a review of multiple actions of Hsp25 in the regulation of actin polymerization, see reference 22).
Based on this study, it appears that SEB confers a dually cytoprotective function in that it protects the epithelium against monochloramine-mediated oxidative injury and stabilizes F-actin. Stabilization of the actin cytoskeleton may result in the decreased ability of SEB to penetrate the cell, and in this manner, epithelial cells may protect themselves from further damage due to SEB, to Staphylococcus infection, or even to other inflammatory insults. In support of this hypothesis, another study has demonstrated that administration of SEB to mice successfully inhibited bacterial colonization of E. coli in a murine model of urinary tract infection and accelerated the resolution of infection (21). It is possible that administration of SEB was able to inhibit adherence of E. coli bacteria through a mechanism of cytoskeletal stabilization, and although this group did not measure Hsp levels, they did propose that acute-phase proteins may have played a role in hastening infection resolution. We speculate that the physiological significance of these findings is an adaptive response of the gut epithelium to protect itself, either from further uptake of SEB toxin or from further injury from bacterial insult.
SEB induction of Hsp25 and Hsp72 appears to require stimulation of the ERK stress pathway, since the MEK-1 inhibitor PD98059 potently inhibited this effect (Fig. 5). SEB also stimulated the p38 MAPK pathway; however, inhibition of this kinase with SB203580 had a much less profound and consistent effect on Hsp induction by SEB. The stimulation of the ERK MAPK pathway cannot be the sole mechanism for SEB induction of Hsps, since LPS also stimulates this pathway in MSIE cells but only induces Hsp25. Both LPS and SEB may communicate their signals to the epithelial cells in a complex fashion in vivo. In native tissue, both agents would be expected to stimulate a variety of immune cells, causing production of a number of cytokines and potentially other inflammatory agents. Using a lymphocyte-epithelial cell coculture system, this appears to be the case (Fig. 3).
In summary, we report that SEB, a potent activator of effector T cells which causes a self-limiting enteritis, induces the mucosal expression of Hsp25 and Hsp72. The effect can be observed in both the lamina propria and epithelial cells, although a sustained response is observed in the latter. SEB induces an IL-2-dependent intestinal epithelial Hsp response through activation of mucosal lymphocytes but also through direct actions mediated by the MAPK pathways. We believe that these effects of SEB may represent a mucosal adaptive response to immune and SEB-mediated stress, allowing the gut epithelium to survive and restore critical mucosal functions of the gut following food poisoning.
Acknowledgments
This work was performed in the Martin Boyer Research Laboratories and supported by National Institute of Diabetes and Digestive and Kidney Disease grants DK47722, DK-42086 (Digestive Disease Core grant), and K08 DK064840-01 (to E.O.P.); The Gastrointestinal Research Foundation of Chicago; a Crohn's and Colitis Foundation of America Research grant; and the Canadian Institutes of Health Research MT-13421 (to D.M.M.).
Editor: J. B. Bliska
REFERENCES
- 1.Beery, J. T., S. L. Taylor, L. R. Schlunz, R. C. Freed, and M. S. Bergdoll. 1984. Effects of staphylococcal enterotoxin A on the rat gastrointestinal tract. Infect. Immun. 44:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin, M. A., J. Lu, G. Donnelly, P. Dureja, and D. M. McKay. 1998. Changes in murine jejunal morphology evoked by the bacterial superantigen Staphylococcus aureus enterotoxin B are mediated by CD4+ T cells. Infect. Immun. 66:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake, C. G., and B. L. Kotzin. 1992. Superantigens: biology, immunology, and potential role in disease. Ann. Allergy 12:149-162. [DOI] [PubMed] [Google Scholar]
- 4.Eckmann L., H. C. Jung, C. Schurer-Maly, A. Panja, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1993. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 105:1689-1697. [DOI] [PubMed] [Google Scholar]
- 5.Florquin, S., Z. Amraoui, D. Abramowicz, and M. Goldman. 1994. Systemic release and protective role of IL-10 in staphylococcal enterotoxin B induced shock in mice. J. Immunol. 153:2618-2623. [PubMed] [Google Scholar]
- 6.Jedrzkiewicz, S., G. Kataeva, C. M. Hogaboam, S. L. Kunkel, R. M. Strieter, and D. M. McKay. 1999. Superantigen immune stimulation evokes epithelial monocyte chemoattractant protein 1 and RANTES production. Infect. Immun. 67:6198-6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, H. M., B. A. Torres, and J. M. Soos. 1996. Superantigens: structure and relevance to human disease. Proc. Soc. Exp. Biol. Med. 212:99-109. [DOI] [PubMed] [Google Scholar]
- 8.Hamad, A. R., P. Marrack, and J. W. Kappler. 1997. Transcytosis of staphylococcal superantigen toxins. J. Exp. Med. 185:1447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampton, C. R., A. Shimamoto, C. L. Rothnie, J. Griscavage-Ennis, A. Chong, D. J. Dix, E. D. Verrier, and T. H. Pohlman. 2003. Hsp70.1 and 70.3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am. J. Physiol. 285:H866-H874. [DOI] [PubMed] [Google Scholar]
- 10.Kojima, K., M. W. Musch, H. Ren, D. L. Boone, B. A. Hendrickson, A. Ma, and E. B. Chang. 2003. Enteric flora & lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 124:1396-1407. [DOI] [PubMed] [Google Scholar]
- 11.Kojima, K., M. W. Musch, M. J. Ropeleski, D. L. Boone, A. Ma, and E. B. Chang. 2004. E. coli lipopolysaccharide induces heat shock protein 25 in intestinal epithelial cells through MAP kinase activation. Am. J. Physiol. 286:167-176. [DOI] [PubMed] [Google Scholar]
- 12.Kregel, K. C. 2002. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 92:2177-2186. [DOI] [PubMed] [Google Scholar]
- 13.Lefrancois, L., and N. Lycke. 1996. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patches, and lamina propria cells, p. 3.19.1-3.19.6. In J. E. Coligan, A. M. Kruisbeek, D. H. Marguiles, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. Wiley, Brooklyn, N.Y. [DOI] [PubMed]
- 14.Lindquist, S., and E. A. Craig. 1988. The heat shock proteins. Annu. Rev. Genet. 22:631-677. [DOI] [PubMed] [Google Scholar]
- 15.Lionetti, P., J. Spencer, E. J. Breese, S. H. Murch, J. Taylor, and T. T. MacDonald. 1993. Activation of mucosal Vβ3+ T cells and tissue damage in human small intestine by the bacterial superantigen, Staphylococcus aureus enterotoxin B. Eur. J. Immunol. 23:664-668. [DOI] [PubMed] [Google Scholar]
- 16.Malago, J. J., J. F. Koninkx, and J. E. van Dijk. 2002. The heat shock response and cytoprotection of the intestinal epithelium. Cell Stress Chaperones 7:191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:685-686. [PubMed] [Google Scholar]
- 18.McKay, D. M., M. Benjamin, and J. Lu. 1998. CD4+ T cells mediate superantigen-induced abnormalities in murine jejunal ion transport. Am. J. Physiol. 275:G29-G38. [DOI] [PubMed] [Google Scholar]
- 19.McKay, D. M., and P. K. Singh. 1997. Superantigen-activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via interferon- and tumour necrosis factor-alpha. Inhibition of increased permeability, but not diminished secretory responses by transforming growth factor 2. J. Immunol. 159:2382-2390. [PubMed] [Google Scholar]
- 20.Merrill, T., and H. Sprinz. 1968. The effect of staphylococcal enterotoxin on the fine structure of the monkey jejunum. Lab. Investig. 18:114-123. [PubMed] [Google Scholar]
- 21.Morin, M. D., and W. J. Hopkins. 1998. Treatment of mice with staphylococcal enterotoxin B enhances resolution of an induced Escherichia coli urinary tract infection and stimulates production of proinflammatory cytokines. Infect. Immun. 66:2466-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mounier, N., and A. P. Arrigo. 2002. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7:176.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musch, M. W., K. Sugi, D. Straus, and E. B. Chang. 1999. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology 117:115-122. [DOI] [PubMed] [Google Scholar]
- 24.Musch, M. W., B. Kaplan, and E. B. Chang. 2001. Role of increased basal expression of heat shock protein 72 in colonic epithelial c2bbe adenocarcinoma cells. Cell Growth Differ. 12:419-426. [PubMed] [Google Scholar]
- 25.Nakamura, Y., J. Naessens, M. Takata, M. Taniguchi, K. Sekikawa, J. Gibson, and F. Iraqi. 2003. Susceptibility of heat shock protein 70.1-deficient C57BL/6 J, wild-type C57BL/6 and A/J mice to Trypansoma congolese infection. Parasitol. Res. 90:171-174. [DOI] [PubMed] [Google Scholar]
- 26.Ren, H., M. W. Musch, K. Kojima, D. Boone, A. Ma, and E. B. Chang. 2001. Short chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC18 cells. Gastroenterology 121:631-639. [DOI] [PubMed] [Google Scholar]
- 27.Ropeleski, M. R., J. Tang, M. M. Walsh-Reitz, M. W. Musch, and E. B. Chang. 2003. Interleukin-11 induced heat shock protein 25 confers intestinal epithelial specific cytoprotection from oxidant stress. Gastroenterology 124:1358-1368. [DOI] [PubMed] [Google Scholar]
- 28.Shim, E.-H., J. I. Kim, E.-S. Bang, et al. 2002. Targeted disruption of Hsp70.1 sensitizes to osmotic stress. EMBO Rep. 3:857-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shupp, J. W., M. Jett, and C. H. Pontzer. 2002. Identification of a transcytosis epitope on staphylococcal enterotoxins. Infect. Immun. 70:2178-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, P., R. I. Krohn, G. T. Hermanson, A. K. Mallia, P. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 31.Stephen, J. 2001. Pathogenesis of infectious diarrhea. Can. J. Gastroenterol. 15:669-683. [DOI] [PubMed] [Google Scholar]
- 32.Umesaki, Y., Y. Okada, S. Matsumoto, A. Imaoka, and H. Setoyama. 1995. Segmented filamentous bacteria (SFB) are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ free mouse. Microbiol. Immunol. 39:555-562. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead, R. H., P. E. VanEeden, M. D. Noble, P. Ataliotis, and P. S. Jat. 1993. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc. Natl. Acad. Sci. USA 90:587-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xanthoudakis, S., and D. W. Nicholson. 2000. Heat shock proteins as death determinants. Nat. Cell Biol. 2:E163-E165. [DOI] [PubMed] [Google Scholar]
- 35.Yu, Y., S. Lyons, A. Carlson, D. Merlin, A. Neish, and A. T. Gewirtz. 2003. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am. J. Physiol. 285:G282-G290. [DOI] [PubMed] [Google Scholar]