Abstract
Dystonia is a neurologic disorder characterized by sustained involuntary muscle contractions. Lesions responsible for unilateral secondary dystonia are confined to the putamen, caudate, globus pallidus and thalamus. Dysfunction of these structures is suspected to play a role in both primary and secondary dystonia. Recent evidence has suggested that the cerebellum may play a role in the pathophysiology of dystonia. The role of the cerebellum in ataxia, a disorder of motor incoordination is well established. How may the cerebellum contribute to two apparently very different movement disorders? This review will discuss the idea of whether in some cases, ataxia and dystonia lie in the same clinical spectrum, and whether graded perturbations in cerebellar function may explain a similar causative role for the cerebellum in these two different motor disorders. The review also proposes a model for cerebellar dystonia based on the available animal models of this disorder.
Introduction
Dystonia is a neurologic disorder characterized by sustained involuntary muscle contractions, which forcefully distort the body into typical postures [1, 2]. Voluntary movement exacerbates the abnormal movements. Only in severe cases is muscle activity recorded in subjects at complete rest [2]. Since lesions responsible for unilateral secondary dystonia are confined to the putamen, caudate, globus pallidus and thalamus, dysfunction of these structures is suspected to play a role in both primary and secondary dystonia [3]. Primary generalized dystonia lacks clear degenerative neuropathology, making the anatomic substrates for dystonia in these cases difficult to identify [4]. Limited autopsy specimens have been examined from subjects with primary dystonia. In a series of autopsy cases of DYT1 dystonia, the most common inherited form of dystonia that results from mutations in torsinA, neuronal loss was not evident in any brain region [5–7]. In one report, ubiquitin and torsinA-positive inclusions were seen in brainstem neurons, although no neuronal loss was reported [8]. The neuropathology supports a functional rather than degenerative etiology of early onset torsion dystonia. Alterations in neuronal activity in the basal ganglia are seen in patients with primary dystonia [9].
Several recent lines of evidence have implicated the cerebellum in the pathophysiology of some forms of dystonia. A reduction in cerebellar gray matter has been reported in patients with focal dystonia [10]. A series of patients have been described, with marked focal or generalized dystonia in association with mild cerebellar signs. Imaging in these patients has shown variable cerebellar and brainstem atrophy. [11–13]. Cervical dystonia is more often associated with lesions of the cerebellum and brainstem than lesions of the basal ganglia [14]. Many of the degenerative spinocerebellar ataxias, where the most prominent neuropathology is in the cerebellum, can have dystonia as a clinical feature [15]. Increased metabolic activity in the cerebellum has previously been described in patients with primary dystonias [16]. Structural and functional imaging has suggested the involvement of the cerebellum in both focal and generalized dystonia [17–19].
Animal models of dystonia provide further evidence for the role of the cerebellum in dystonia. Genetic causes of dystonia in rodents where the cerebellum appears to be the cause of the motor symptoms include tottering mouse [20], a splice mutation in the neuronal sodium channel Scn8a [21] and the genetically dystonic rat [22]. Microinjection of low doses of kainic acid into the cerebellar vermis of mice elicits dystonic postures of the trunk and limbs [23]. The role of the cerebellum in these models of dystonia is suggested by the fact that cerebellectomy in the genetically dystonic rat as well as the tottering mouse abolishes the dystonia [20, 24]. Together, these data suggest that the cerebellum can play a role in producing dystonia.
This review will explore the idea of whether ataxia and dystonia, two very different motor disorders, can lie in a similar clinical spectrum and if so, how the cerebellum could contribute to generation of these different patterns of movement.
Cerebellar circuits responsible for normal agonist and antagonist control
Cerebellar Purkinje neurons and nuclear neurons are autonomously active with high baseline firing rates [25, 26]. Further, Purkinje neuron projections, the major input to the neurons of the cerebellar nuclei, are inhibitory [27]. Input into the cerebellum is predominantly through excitatory mossy fibers, which excite Purkinje neurons through granule cells. Mossy fiber collaterals also directly excite cerebellar nuclear neurons. Bidirectional modulation of Purkinje neuron firing frequency is possible through excitation mediated by parallel fiber synapses and inhibition mediated by cerebellar cortical interneurons [28]. One set of Purkinje neurons can be excited with simultaneous inhibition of flanking sagittally oriented Purkinje neurons by a distinct patch of granule cells [29]. Distinct Purkinje neurons are presumed to receive excitatory input representing distinct agonist muscle groups and inhibitory input from antagonist muscles [30]. Similarly, cerebellar nuclear neurons receive input from distinct groups of Purkinje neurons, whose inhibitory input would be expected to reduce their firing frequency. Bidirectional modulation of cerebellar nuclear neuron firing frequency would be expected through release of inhibition (and stimulation through mossy fiber collaterals) and increased inhibition through Purkinje neurons.
Normal agonist and antagonist control by the cerebellum
Recordings from non-human primates performing simple movements, demonstrate characteristic patterns of activity in Purkinje neurons and cerebellar nuclear neurons. Simple spikes of most Purkinje cells occur at high maintained frequencies of about 70 Hz, with the monkey at rest. During flexion and extension movements at the wrist, Purkinje neuron simple-spike frequency alternates between rates higher (up to 400–500 Hz) and lower than the resting rate with a consistent temporal relationship to each successive movement cycle [30]. In a different set of experiments, monkeys were trained to perform a maintained isometric grip of the thumb and forefinger that elicited a contraction of the antagonist muscles of the forearm. During the maintained isometric wrist position, the agonist muscles were reciprocally active or silent. The activity of Purkinje cells that were reciprocally active during isometric wrist flexion and extension was related to whether agonist or antagonist muscles were active during the flexion and extension wrist movements [31]. At least a small percentage of Purkinje neurons exhibit simple spike firing that significantly correlates with the grasp force or rate of force production [32, 33]. Most nuclear cells also fire at high maintained frequencies of ~ 40 Hz under resting conditions. During wrist flexion-extension movements, for a cell related to these movements, the discharge frequency also alternates between rates up to 400–500 Hz and lower than the resting rate consistently in time with the movement [30]. In a subset of neurons, correlation coefficients between nuclear cell discharge frequency and the amplitude of the surface EMGs of the flexors and extensors of the wrist and fingers during the wrist-movement task showed positive correlations with activity in one of the two forearm muscle groups. In contrast Purkinje cells recorded during the same task demonstrated low-order correlations that were negative in sign between discharge frequency and one of the two forearm EMGs [34]. In a different motor task, monkeys tracked a small spot presented on a screen in front of them. While the monkey was tracking, recordings were made from Purkinje neurons in floccular complex of the cerebellum. Strong correlations were detected between small trial-by-trial variations in the simple-spike activity of individual PCs and the eye movements at the initiation of pursuit [35]. The relationship between Purkinje neuron firing and eye movements can be modeled by an inverse-dynamics representation where Purkinje cells contribute dynamic command signals to ocular motor neurons and hence extraocular muscles [36].
These data suggest that cerebellar neurons increase (or decrease) firing frequency in relation to whether agonists (or antagonist) muscles are active during a motor task. Distinct Purkinje (and downstream nuclear neurons) are likely involved in the timing of agonist and antagonist muscle activity. Since the majority of cerebellar nuclear neurons are excitatory, it suggests that an increase in firing of cerebellar nuclear neurons corresponds temporally with facilitation of activity of muscle activity and a decrease in firing frequency reduces facilitation of muscle groups. Although the firing of some Purkinje neurons correlates positively with muscle activation, it is unclear how these data may be integrated into models of cerebellar control of limb movement [37]. Only a subset of cerebellar output neurons are presumed to directly regulate motor activity of the limb, with others that discharge in relation to joint position, and the direction of the intended next movement [38]. Although some investigators favor the view that the cerebellum solely plans and controls movements in a kinematic framework, without directly encoding force-related parameters [39], at least a subset of cerebellar output neurons do appear to directly activate muscle groups as evidenced by electrical stimulation of interposed and dentate nuclei in primates [40]. Also, optogenetic manipulation of Purkinje neurons in mice shows that inhibiting Purkinje cell firing can cause muscle contraction[41, 42]. Additionally, an interaction between the cerebellar and basal ganglia motor control systems may allow cerebellar output neurons to influence muscle activity [43, 44].
Physiological correlates of ataxia
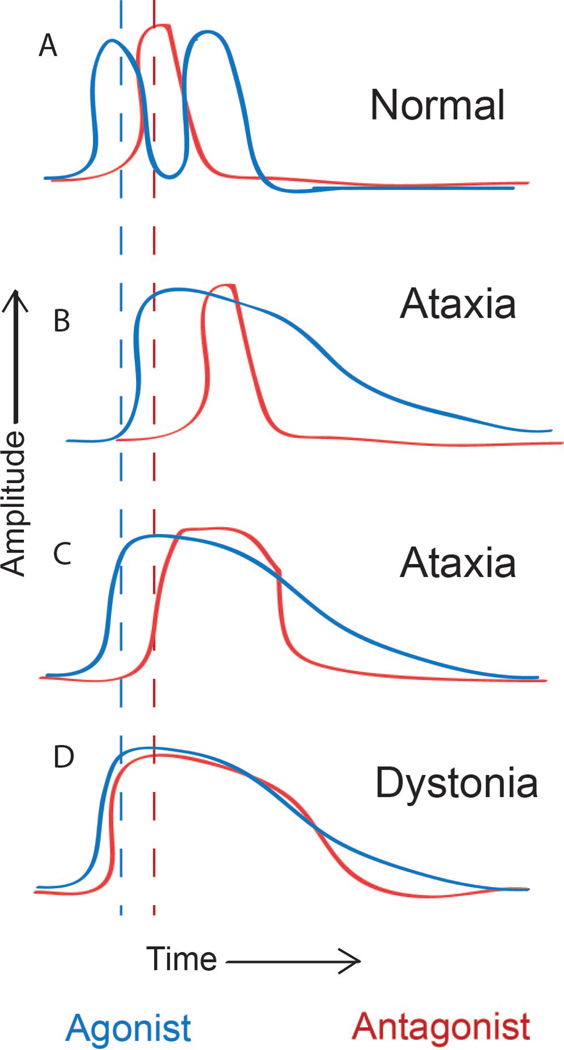
In order to understand the motor phenomena of ataxia and dystonia, it is necessary to characterize the disorders at the site of the effectors; i.e. muscle contractions that are the cause of movement. In this section, the patterns of muscle activity in subjects with clinically evident cerebellar ataxia during motor tasks are reviewed. Studies that have looked at agonist and antagonist muscle activity in subjects with cerebellar ataxia have suggested that timing of agonist and antagonist muscle contraction is abnormal. During rapid flexion movements at the elbow, a prolongation of biceps contraction was consistently present [45]. Subjects with cerebellar ataxia performing rapid flexion movements at the elbow, and wrist exhibited hypermetria, more evident for the shortest movements. Electromyographic (EMG) recordings revealed a variable increase in agonist muscle duration and in the most marked cases there was more gradual development of agonist EMG activity. There was a marked delay in antagonist onset associated with hypermetria, without a change in amplitude or duration of antagonist activity (Figure 1) [45]. In other studies, subjects with cerebellar ataxia performing rapid flexion at the elbow, showed either prolongation of agonist activity alone or a prolongation of activity of both agonists and antagonists (Figure 1) [46] [47].
Figure 1. Schematic patterns of muscle activity in normal, dystonic and ataxic subjects during flexion movements at the elbow.
A. Normal subjects activate the biceps (agonist) followed by the antagonist (triceps). (Middle) B. In some subjects with cerebellar ataxia, agonist contraction is delayed and prolonged. Antagonist contraction is also delayed but is of normal duration. C. In other subjects with cerebellar ataxia, both agonist and antagonist activity is prolonged D. In dystonic subjects agonist contraction is prolonged. Antagonist contraction is premature resulting in co-contraction, as well as prolonged. Modified from [45–47, 54].
In a reaching task, where the force of gravity provided a sufficient extensor torque to decelerate the limb at the end of the flexor movement, extensor muscles were not active in most trials [48]. In this study, subjects with cerebellar ataxia had prolonged biceps activity. More proximal muscles including the anterior deltoid were activated in this task in both normal and ataxic subjects. In subjects with cerebellar ataxia, anterior deltoid activity was abnormally prolonged. When the task was performed with the shoulder fixed, anterior deltoid activity was nearly absent in cerebellar subjects but not in normal controls. This study suggests that under appropriate testing conditions, in subjects with cerebellar ataxia, antagonist muscles do not activate, and spread of activity to more distant muscle groups may be suppressed. Agonist activity was prolonged in this study as in the other studies described above.
In human subjects, the attribution of specific anatomic areas to the observed motor dysfunction can sometimes be difficult. It is therefore informative the describe changes in muscle contraction in primates with induced lesions of the cerebellum. Recordings of movement parameters and EMG activity of cerebellar dysmetric movements induced in primates by local and reversible cooling of the dentate nucleus enabled a comparison to be made of the normal and abnormal activity of the same motor units [49]. Agonist activity in the biceps during elbow movements was less abrupt in onset and more prolonged and variable in duration. There was a delay in the onset of antagonist (triceps) activity but no change in duration of activity. This corresponded to overshoot and an increase in duration of acceleration and a reduction in duration of deceleration [49].
The dysmetria seen in human subjects with cerebellar ataxia therefore can be reproduced by focal lesions in the cerebellum in primates and the electromyographic correlates are similar in humans and primates. Agonist contraction is mistimed and more prolonged. Antagonist activity is mistimed, when present, but of either normal duration or prolonged.
Physiological correlates of primary dystonia
Numerous studies have established a role for the basal ganglia in dystonia [2, 50–52]. Dystonic like posturing can be induced in primates by lesioning or pharmacologic manipulation of the midbrain and basal ganglia [53]. These models are however not informative as to whether and how the cerebellum may contribute to human dystonia. It is therefore important to examine the physiologic correlates of dystonia in human subjects with idiopathic and primary dystonia, where the exact anatomic substrate for dystonia remains undetermined. In subjects with dystonia, a study of rapid elbow flexion movements has shown that movements are slower and more variable. The duration of the first agonist (biceps) EMG burst was longer than in normal subjects moving fast or slow. Patterns of antagonist (triceps) varied from a normal appearance to cocontraction, often with prolonged bursts or tonic EMG activity without recognizable bursts (Figure 1). Many subjects also exhibited a spread of activation into muscles such as the pectoralis major, not acting at the elbow joint, a phenomenon described as “overflow” [1, 54, 55].
In another study, wrist flexion movements were studied in subjects with dystonia, some of whom had a mutation in DYT1, a common cause of early onset primary generalized dystonia [56] and where abnormalities in cerebellar outflow are detected by magnetic resonance diffusion tensor imaging [19]. The initial agonist burst was normal in onset, rise time, and amplitude and there was no evidence of excessive co-contraction in the antagonist. The principal abnormality in the EMG pattern identified was a reduced rate of rise and attenuated magnitude of the antagonist burst. Decreased antagonist muscle activity was associated with a significant prolongation and decreased peak amplitude of deceleration, yet there was no significant overshoot of the target. Both normal and dystonic subjects and showed stereotyped patterns of EMG activity in the biceps, triceps, and deltoid muscles, suggesting that overflow of activity to proximal muscles during ballistic movements of the wrist is a common feature of normal movement [57].
The EMG studies from humans with idiopathic and primary dystonia suggest that the agonist burst is prolonged and the antagonist burst, at least in one study, is premature and prolonged resulting in co-contraction of agonists and antagonists. In certain tasks, there is abnormal spread of activity to remote muscles that are not normally activated, consistent with the phenomenon of overflow.
Can dystonia lie in the same clinical spectrum as ataxia?
In order to determine, whether ataxia and dystonia may share a common physiologic basis and whether under some circumstances, dystonia, if it is of cerebellar origin, represents a more “severe” form of cerebellar ataxia, it is important to more fully examine the physiologic underpinnings of ataxia. A complete loss of function of the cerebellum by ablation or lesioning of cerebellar output nuclei in non-human primates leads to irreversible ataxia of variable severity, with limb and gait ataxia with dysmetria [58, 59]. Focal cooling and inactivation of the cerebellar dentate nucleus produces a similar pattern of abnormally mistimed movements [49]. Since a complete loss of cerebellar function mimics the motor changes seen in subjects with cerebellar ataxia, where there are varying degrees of loss of cerebellar neurons in autopsy studies [60], cerebellar ataxia in these cases may be viewed as a negative phenomenon, with a partial loss of cerebellar function. In at least a subset of models of ataxia, however, the movement disorder may be secondary to a gain of cerebellar function due to abnormal cerebellar activity. In a mouse model of episodic ataxia, Purkinje neuron average firing frequency is unchanged, but more irregular, leading to ataxia [61]. An increase in the average firing frequency of cerebellar output neurons causes profound ataxia in mice [62]. An abnormal gain of cerebellar function may also exist in the Spinocerebellar Ataxias, where ataxia and dystonia coexist and the most profound morphological changes are in the cerebellum [15]. Recent work in mouse models of these disorders suggest that abnormally increased excitability of cerebellar neurons may in part explain the motor symptoms [63, 64], indicating that abnormal gain of function of cerebellar neurons may coexist with loss of function due to cerebellar neuronal loss. These data suggest that although clinically similar, cerebellar ataxia may have two distinct underlying physiologic mechanisms, one that involves a loss of cerebellar function and another that involves an abnormal gain of cerebellar function.
As reviewed in prior sections, patterns of muscle activation in subjects with cerebellar ataxia are sometimes variable, with some subjects exhibiting prolonged activity of both agonists and antagonists during motor tasks (Figure 1C) [46]. In other subjects, however, there is a delay in antagonist contraction that is more severe with worse ataxia severity, but the duration of activity is normal [45, 46] (Figure 1B), a pattern that is reproduced by local and reversible cooling of the dentate nucleus in primates. The two different patterns of muscle activity in subjects with cerebellar ataxia performing motor tasks may therefore respectively represent the existence of “gain of function” and “loss of function” ataxias in humans. Interestingly, two of the subjects with cerebellar ataxia with a prolongation of antagonist activity were also reported to have mild dystonia [46]. Although it is unclear whether the origin of ataxia and dystonia in these subjects was from different anatomic sites, it is possible that both the ataxia and dystonia originated in the cerebellum in these subjects. In ataxias presumed to be due to loss of cerebellar function, the patterns of agonist and antagonist activityare distinct from the patterns seen in subjects with dystonia.. In other cases of ataxia, where there is a mistiming and prolongation of antagonist activity, there is a potential overlap in the patterns of muscle activity produced in both these movement disorders. Ataxia of increasing severity that may result from an abnormal gain of cerebellar function may therefore be viewed in a continuum that includes dystonia.
Mechanism for cerebellar dystonia
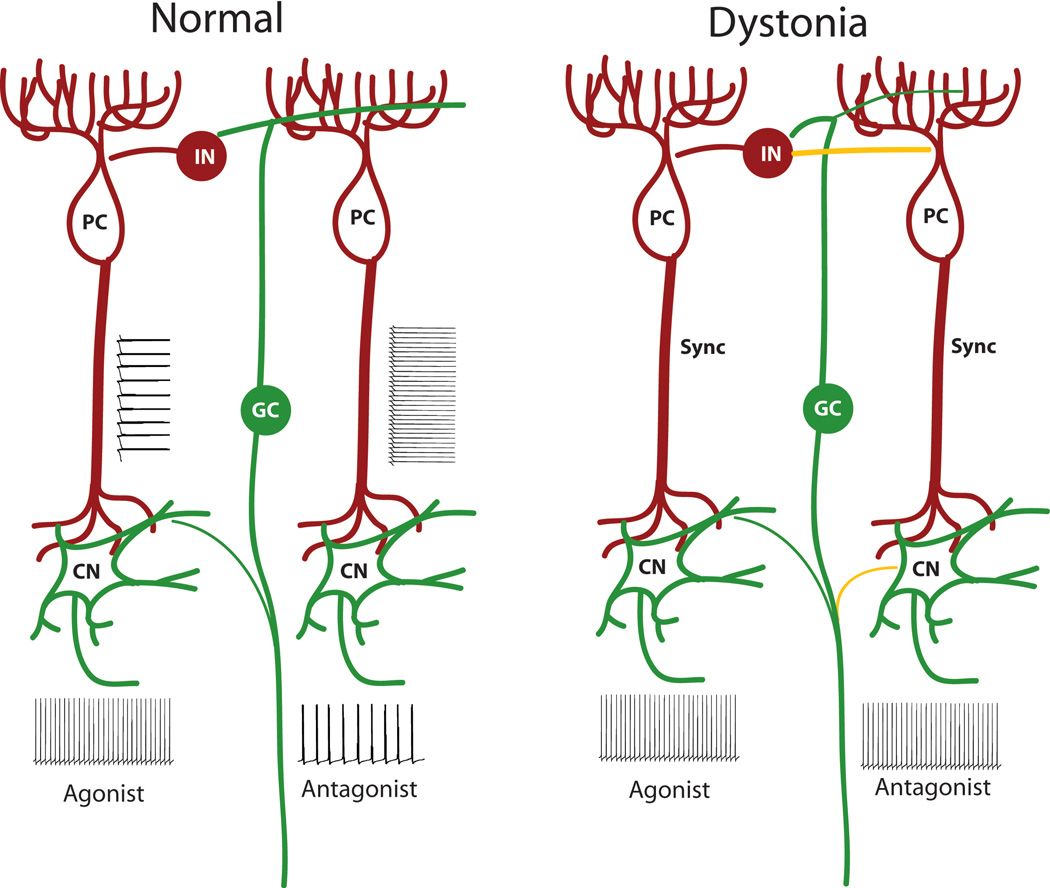
Encoding of information by specific cerebellar firing patterns remains debated [65]. Assuming that firing frequency is an important mode of information coding by cerebellar neurons, co-contraction of agonists and antagonists, with premature onset of antagonist activity would be possible under the following scenarios. A uniform increase in baseline firing rates of cerebellar nuclear neurons either from an increase in intrinsic excitability or synaptically, or increased firing synchrony would result in the interruption of the normal activity related signal separation for agonists and antagonists. Alternatively, a developmental or acquired “miswiring” of the cerebellum may result in input that converges onto cerebellar nuclear neurons that no longer respects the normal separation of signals from agonist and antagonist muscle groups (Figure 2). Normally, a distinct patch of granule cells simultaneously excite one set of Purkinje neurons with inhibition of flanking sagittally oriented Purkinje neurons [29], allowing for the simultaneous reciprocal control of agonist and antagonist muscle groups. Disruption of this “surround inhibition” would potentially allow for simultaneous activation of agonist and antagonist muscle groups (Figure 2). Purkinje neuron synchrony has been postulated to regulate the response of cerebellar nuclear cells to Purkinje neuron inputs [66–68]. Additionally, increased synchrony of Purkinje neurons has been suggested to increase cerebellar nuclear firing frequency [68]. Although it is not clear how important Purkinje neuron firing synchrony is in setting cerebellar output in normal cerebellar processing, factors that globally increase Purkinje neuron synchrony could abrogate the normal separation of signals from agonist and antagonist muscle groups and would therefore be expected to contribute to co-activation of agonists and antagonists. Under all three scenarios outlined above, there would also be more widespread activation of muscle groups explaining the phenomenon of overflow. An important additional consideration is that since dystonia is exacerbated by voluntary movement, a model of cerebellar dystonia takes this into account. This stipulation would be satisfied if the abnormalities in cerebellar activity are driven by the same signals that the cerebellum receives as part of normal motor activity.
Figure 2. Model for cerebellar dystonia.
(Left) Normal cerebellar function relies on separation of signals for agonist and antagonist muscle groups at the level of the cerebellar cortex and cerebellar nuclei. (Right) Model for cerebellar dystonia. Dystonia would be expected to occur under conditions of lack of separation of signals intended for agonist and antagonist muscle groups at the level of the cerebellar nuclei, the sole motor output from the cerebellum. PC: Purkinje neuron, CN: Cerebellar nuclear neuron, GC: Granule cell, IN: Molecular layer interneuron, Sync: Increased synchrony, Green: Excitatory neurons and transmission, Red: Inhibitory neurons and transmission, Yellow: Hypothetical aberrant connections. Strength of connections is indicated by the thickness of the line.
Examination of the existing animal models of dystonia is informative to determine whether this model could explain the observed changes in physiology in cerebellar dystonia. In the genetically dystonic rat, a physiologically well characterized model of cerebellar dystonia [24], single-unit recordings from the cerebellar nuclei in the awake dystonic rat showed frequent, short bursts of increasing frequency, and rhythmicity with increasing postnatal age [69]. Although cross correlations and synchrony between different units could not be assessed in these studies, the uniformity of the responses in different cerebellar nuclear units would be consistent with the idea of a uniform output signal from the cerebellum for downstream agonist and antagonist muscle groups. In brain slices from a mouse model of gait dystonia, an increase in firing frequency of cerebellar nuclear neurons was observed [70]. In a mouse model of rapid onset dystonia-parkinsonism, when mice developed intermittent dystonic postures, there was a tight correlation between dystonic postures and an increase in amplitude of cerebellar EEG activity [44]. Since an increase in amplitude of signals on surface EEG is thought to represent widespread neuronal synchronization and signal oscillation in a widely coordinated manner [71], it would imply that increased synchronized activity in the cerebellum accompanies dystonia in this model. Finally, in the tottering mouse model of dystonia, where ataxia but not dystonia is present between episodes of prominent generalized dystonia, an increase in variability in the firing pattern of Purkinje neurons is found [61]. Flavoprotein autofluorescence optical imaging demonstrates transient, low-frequency oscillations in the cerebellar cortex of tottering mice in the non-dystonic periods. During dystonia, oscillations are also present in the electromyographic (EMG) activity and this becomes significantly coherent with the oscillations in the cerebellar cortex [72]. These data suggest that the inability of cerebellar nuclear neurons to separate signals intended for specific muscle groups, is associated with abnormal movements in mice similar to human dystonia.
Recently, a structural and functional interaction between the cerebellum and basal ganglia has been identified [43, 44]. In a mouse model of rapid-onset Dystonia-Parkinsonism, aberrant cerebellar activity may cause dystonia by dynamically forcing the dysfunction of the basal ganglia [44]. Since the role of the basal ganglia in causing dystonia is well established, the interaction between the cerebellar and basal ganglia systems provides a unifying mechanism to explain how each of these two brain regions can interact to contribute to the development of dystonia.
Conclusions and Future Directions
Cerebellar ataxia and dystonia have distinct physiological correlates. Patterns of activation of agonist and antagonist muscles during motor tasks reveal that dystonia and ataxia share several important similarities including prolongation of agonist activity and mistimed antagonist activity. Cerebellar ataxia may have two distinct underlying mechanisms, and result from either a gain or loss of cerebellar function. The pattern of muscle activity, in human subjects with ataxia that is presumed to result from a loss of cerebellar function, is distinct from the pattern seen in subjects with dystonia. In a subset of subjects with cerebellar ataxia, however, the patterns of agonist and antagonist activity overlap with that seen in subjects with dystonia. These subjects may represent an ataxia resulting from a gain of cerebellar function. Ataxia of increasing severity that may result from an abnormal gain of cerebellar function may therefore be viewed in a continuum that includes dystonia. Dystonia of cerebellar origin must represent an inability of cerebellar output to correctly distinguish signals intended for agonist and antagonist muscles, which would explain co-contraction and overflow. In models of dystonia, presumed to be of cerebellar origin, this could be examined by simultaneous recordings from multiple Purkinje or nuclear neurons to determine whether there is increased synchrony during dystonic episodes. Although the role of synchrony in normal cerebellar processing is uncertain, alterations in synchrony represent a hypothetical mechanism for cerebellar dystonia. Optogenetic manipulation and synchronization of Purkinje and/or cerebellar nuclear neuron firing should also help clarify the role of the cerebellum in dystonia. A developmental or acquired “miswiring” of the cerebellum could be assessed by determining the pattern of excitation and flanking inhibition of Purkinje neurons by distinct patches of granule cells in dystonic models. In summary, cerebellar ataxia that represents an abnormal gain of cerebellar function, may overlap with dystonia of cerebellar origin but further studies are needed in defined human subjects and animal models to demonstrate that this is the case.
Acknowledgements
The Dystonia Medical Research Foundation and the National Institutes of Health (K08NS072158 and R01NS085054).
Footnotes
Conflicts of Interest
I have no relevant conflicts of interest to declare.
References
- 1.Fahn S. The varied clinical expressions of dystonia. Neurol Clin. 1984;2(3):541–554. [PubMed] [Google Scholar]
- 2.Berardelli A, et al. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7):1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 3.Marsden CD, et al. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108(Pt 2):463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 4.den Dunnen WF. Neuropathological diagnostic considerations in hyperkinetic movement disorders. Front Neurol. 2013;4:7. doi: 10.3389/fneur.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furukawa Y, et al. Striatal dopamine in early-onset primary torsion dystonia with the DYT1 mutation. Neurology. 2000;54(5):1193–1195. doi: 10.1212/wnl.54.5.1193. [DOI] [PubMed] [Google Scholar]
- 6.Walker RH, et al. TorsinA immunoreactivity in brains of patients with DYT1 and non- DYT1 dystonia. Neurology. 2002;58(1):120–124. doi: 10.1212/wnl.58.1.120. [DOI] [PubMed] [Google Scholar]
- 7.Rostasy K, et al. TorsinA protein and neuropathology in early onset generalized dystonia with GAG deletion. Neurobiol Dis. 2003;12(1):11–24. doi: 10.1016/s0969-9961(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 8.McNaught KS, et al. Brainstem pathology in DYT1 primary torsion dystonia. Ann Neurol. 2004;56(4):540–547. doi: 10.1002/ana.20225. [DOI] [PubMed] [Google Scholar]
- 9.Vitek JL, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46(1):22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Delmaire C, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology. 2007;69(4):376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 11.Le Ber I, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67(10):1769–1773. doi: 10.1212/01.wnl.0000244484.60489.50. [DOI] [PubMed] [Google Scholar]
- 12.Kuoppamaki M, et al. Slowly progressive cerebellar ataxia and cervical dystonia: clinical presentation of a new form of spinocerebellar ataxia? Mov Disord. 2003;18(2):200–206. doi: 10.1002/mds.10308. [DOI] [PubMed] [Google Scholar]
- 13.van de Warrenburg BP, et al. The syndrome of (predominantly cervical) dystonia and cerebellar ataxia: new cases indicate a distinct but heterogeneous entity. J Neurol Neurosurg Psychiatry. 2007;78(7):774–775. doi: 10.1136/jnnp.2006.105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18(1):60–69. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- 15.Manto MU. The wide spectrum of spinocerebellar ataxias (SCAs) Cerebellum. 2005;4(1):2–6. doi: 10.1080/14734220510007914. [DOI] [PubMed] [Google Scholar]
- 16.Eidelberg D, et al. Functional brain networks in DYT1 dystonia. Ann Neurol. 1998;44(3):303–312. doi: 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- 17.Sadnicka A, et al. The cerebellum in dystonia - help or hindrance? Clin Neurophysiol. 2012;123(1):65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Niethammer M, et al. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42(2):202–209. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argyelan M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29(31):9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DB, Hess EJ. L-type calcium channels contribute to the tottering mouse dystonic episodes. Mol Pharmacol. 1999;55(1):23–31. doi: 10.1124/mol.55.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Sprunger LK, et al. Dystonia associated with mutation of the neuronal sodium channel Scn8a and identification of the modifier locus Scnm1 on mouse chromosome 3. Hum Mol Genet. 1999;8(3):471–479. doi: 10.1093/hmg/8.3.471. [DOI] [PubMed] [Google Scholar]
- 22.Lorden JF, et al. Neuropharmacological correlates of the motor syndrome of the genetically dystonic (dt) rat. Adv Neurol. 1988;50:277–297. [PubMed] [Google Scholar]
- 23.Pizoli CE, et al. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22(17):7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993;120(2):302–310. doi: 10.1006/exnr.1993.1064. [DOI] [PubMed] [Google Scholar]
- 25.Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20(24):9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17(12):4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito M, et al. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- 28.Mittmann W, Koch U, Hausser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol. 2005;563(Pt 2):369–378. doi: 10.1113/jphysiol.2004.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dizon MJ, Khodakhah K. The role of interneurons in shaping Purkinje cell responses in the cerebellar cortex. J Neurosci. 2011;31(29):10463–10473. doi: 10.1523/JNEUROSCI.1350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- 31.Frysinger RC, et al. Cerebellar cortical activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. J Neurophysiol. 1984;51(1):32–49. doi: 10.1152/jn.1984.51.1.32. [DOI] [PubMed] [Google Scholar]
- 32.Smith AM, Bourbonnais D. Neuronal activity in cerebellar cortex related to control of prehensile force. J Neurophysiol. 1981;45(2):286–303. doi: 10.1152/jn.1981.45.2.286. [DOI] [PubMed] [Google Scholar]
- 33.Espinoza E, Smith AM. Purkinje cell simple spike activity during grasping and lifting objects of different textures and weights. J Neurophysiol. 1990;64(3):698–714. doi: 10.1152/jn.1990.64.3.698. [DOI] [PubMed] [Google Scholar]
- 34.Wetts R, Kalaska JF, Smith AM. Cerebellar nuclear cell activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. J Neurophysiol. 1985;54(2):231–244. doi: 10.1152/jn.1985.54.2.231. [DOI] [PubMed] [Google Scholar]
- 35.Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci. 2007;27(25):6832–6842. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shidara M, et al. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365(6441):50–52. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- 37.Holdefer RN, Miller LE. Dynamic correspondence between Purkinje cell discharge and forelimb muscle activity during reaching. Brain Res. 2009;1295:67–75. doi: 10.1016/j.brainres.2009.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thach WT. Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of intended next movement in motor cortex and cerebellum. J Neurophysiol. 1978;41(3):654–676. doi: 10.1152/jn.1978.41.3.654. [DOI] [PubMed] [Google Scholar]
- 39.Ebner TJ, Hewitt AL, Popa LS. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum. 2011;10(4):683–693. doi: 10.1007/s12311-010-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rispal-Padel L, Cicirata F, Pons C. Cerebellar nuclear topography of simple and synergistic movements in the alert baboon (Papio papio) Exp Brain Res. 1982;47(3):365–380. doi: 10.1007/BF00239355. [DOI] [PubMed] [Google Scholar]
- 41.Heiney SA, et al. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J Neurosci. 2014;34(6):2321–2330. doi: 10.1523/JNEUROSCI.4547-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witter L, et al. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits. 2013;7:133. doi: 10.3389/fncir.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoshi E, et al. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8(11):1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 44.Calderon DP, et al. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 2011;14(3):357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist, and fingers. J Neurophysiol. 1991;65(3):563–571. doi: 10.1152/jn.1991.65.3.563. [DOI] [PubMed] [Google Scholar]
- 46.Hallett M, Shahani BT, Young RR. EMG analysis of patients with cerebellar deficits. J Neurol Neurosurg Psychiatry. 1975;38(12):1163–1169. doi: 10.1136/jnnp.38.12.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallett M, et al. Physiological analysis of simple rapid movements in patients with cerebellar deficits. J Neurol Neurosurg Psychiatry. 1991;54(2):124–133. doi: 10.1136/jnnp.54.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol. 2000;83(5):3019–3030. doi: 10.1152/jn.2000.83.5.3019. [DOI] [PubMed] [Google Scholar]
- 49.Flament D, Hore J. Movement and electromyographic disorders associated with cerebellar dysmetria. J Neurophysiol. 1986;55(6):1221–1233. doi: 10.1152/jn.1986.55.6.1221. [DOI] [PubMed] [Google Scholar]
- 50.Breakefield XO, et al. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9(3):222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 51.Hallett M. Pathophysiology of dystonia. J Neural Transm Suppl. 2006;(70):485–488. doi: 10.1007/978-3-211-45295-0_72. [DOI] [PubMed] [Google Scholar]
- 52.Lehericy S, et al. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. 2013;28(7):944–957. doi: 10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- 53.Guehl D, et al. Primate models of dystonia. Prog Neurobiol. 2009;87(2):118–131. doi: 10.1016/j.pneurobio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 54.van der Kamp W, et al. Rapid elbow movements in patients with torsion dystonia. J Neurol Neurosurg Psychiatry. 1989;52(9):1043–1049. doi: 10.1136/jnnp.52.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berardelli A, et al. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain. 1996;119(Pt 2):661–674. doi: 10.1093/brain/119.2.661. [DOI] [PubMed] [Google Scholar]
- 56.Ozelius LJ, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATPbinding protein. Nat Genet. 1997;17(1):40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 57.MacKinnon CD, et al. Corticospinal excitability accompanying ballistic wrist movements in primary dystonia. Mov Disord. 2004;19(3):273–284. doi: 10.1002/mds.20017. [DOI] [PubMed] [Google Scholar]
- 58.Carrea RM, Mettler FA. Physiologic consequences following extensive removals of the cerebellar cortex and deep cerebellar nuclei and effect of secondary cerebral ablations in the primate. J Comp Neurol. 1947;87(3):169–288. doi: 10.1002/cne.900870302. [DOI] [PubMed] [Google Scholar]
- 59.Dow RS, Moruzzi G. The physiology and pathology of the cerebellum. Minneapolis: University of Minnesota Press; 1958. p. 675. [Google Scholar]
- 60.Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9(9):885–894. doi: 10.1016/S1474-4422(10)70183-6. [DOI] [PubMed] [Google Scholar]
- 61.Walter JT, et al. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9(3):389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- 62.Shakkottai VG, et al. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J Clin Invest. 2004;113(4):582–590. doi: 10.1172/JCI20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shakkottai VG, et al. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci. 2011;31(36):13002–13014. doi: 10.1523/JNEUROSCI.2789-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasumu AW, et al. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol. 2012;19(10):1340–1353. doi: 10.1016/j.chembiol.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Person AL, Raman IM. Synchrony and neural coding in cerebellar circuits. Front Neural Circuits. 2012;6:97. doi: 10.3389/fncir.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gauck V, Jaeger D. The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. J Neurosci. 2000;20(8):3006–3016. doi: 10.1523/JNEUROSCI.20-08-03006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Zeeuw CI, et al. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. 2011;12(6):327–344. doi: 10.1038/nrn3011. [DOI] [PubMed] [Google Scholar]
- 68.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481(7382):502–505. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeDoux MS, Hurst DC, Lorden JF. Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience. 1998;86(2):533–545. doi: 10.1016/s0306-4522(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 70.Luna-Cancalon K, et al. Alterations in cerebellar physiology are associated with a stifflegged gait in Atcay mice. Neurobiol Dis. 2014;67C:140–148. doi: 10.1016/j.nbd.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 72.Chen G, et al. Low-frequency oscillations in the cerebellar cortex of the tottering mouse. J Neurophysiol. 2009;101(1):234–245. doi: 10.1152/jn.90829.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]