Abstract
Background
Diet and exercise interventions for cancer survivors result in health benefits; however, few studies have examined health outcomes in relation to adherence.
Purpose
We examined associations between adherence to components of a diet–exercise intervention and survivors’ physical and mental health.
Methods
A randomized controlled trial tested a telephone and mailed print intervention among 641 older, overweight, long-term survivors of breast, prostate, and colorectal cancer. Dietary and exercise behaviors were assessed at 14 time points throughout the year-long intervention; health outcomes were examined postintervention.
Results
Telephone session attendance had significant indirect relationships with health outcomes through intervention-period exercise and dietary behavior. Attendance showed positive indirect relationships with physical function (β= 0.11, p<0.05), basic and advanced lower extremity function (β=0.10, p<0.05/β=0.09, p<0.05), and mental health (β= 0.05, p<0.05), and a negative indirect relationship with body mass index (β=−0.06, p<0.05).
Conclusions
Session attendance is vital in facilitating improvement in health behaviors and attendant outcomes (Clinicaltrials.gov number NCT00303875).
Keywords: Adherence, Intervention, Randomized controlled trial, Cancer, Exercise, Diet
Introduction
The number of cancer survivors is rapidly increasing with over 13.7 million survivors in the US alone [1]. Approximately 6.6 million of those survivors have been diagnosed with breast, prostate, or colorectal cancers, which have 5-year survival rates of 90% or higher at early stages [2]. Although the increase in cancer survivorship is encouraging, survivors are at greater risk than others for secondary cancers and comorbidities such as cardiovascular disease, diabetes, and osteoporosis [3–6]. Furthermore, the majority of cancer survivors are older adults (≥65 years of age), many of whom are overweight or obese and experience decrements in physical functioning that threaten their ability to live independently [7–9]. Thus older, overweight, long-term (≥5 years postdiagnosis) survivors of breast, prostate, and colorectal cancer represent a large group that must face the interrelated challenges of cancer survivorship and aging [10].
Interventions to promote exercise and a healthy diet show potential to reduce health risks and slow functional decline in cancer survivors. Specifically, randomized controlled trials among cancer populations have found that increased strength and endurance exercise improves health-related outcomes such as physical function, lower extremity function, mental health, and body mass index [11–15]. Findings from randomized controlled trials also suggest that dietary interventions improve fruit and vegetable intake and reduce fat intake and body weight in cancer survivors [16–18]. Although experimental evidence linking healthy dietary changes to improved mental health and physical function in cancer survivors is lacking, two cross-sectional studies with this population have found a relationship between improved diet quality (i.e., increased fruit and vegetable intake and decreased saturated fat intake) and better health-related outcomes (i.e., increased physical function and basic and advanced lower extremity function, better mental health, and reduced body mass index) [19, 20]. Conversely, another cross-sectional study did not find significant relationships between cancer survivors’ fruit and vegetable intake and their mental or physical health [21]. In recent years, a number of combined diet and exercise intervention trials for older adult cancer survivors also have been conducted [22–25]. These interventions have resulted in increased physical activity, improved diet quality, and reduced body mass index and functional decline [22–25].
When interpreting results of diet and exercise intervention trials, it is important to examine adherence to the intervention protocols. The traditional definition of adherence is the extent to which individuals follow specific treatment instructions [26]. To date, most research on adherence to diet and exercise randomized controlled trials among older adults and cancer survivors has focused on demographic, medical, and psychosocial predictors of adherence [27–30]. An important gap, which we propose to address, is the study of adherence in relation to health outcomes. Identifying the health effects of specific intervention components could inform changes to future diet and exercise interventions to enhance their efficacy.
To our knowledge, researchers have not reported the relationship between adherence to a combined diet and exercise randomized controlled trial and health-related outcomes among cancer survivors. Furthermore, published studies of cancer and noncancer populations have not examined the relationship between adherence to interventions solely targeting dietary habits and health-related outcomes. However, researchers have identified predictors of increased adherence to diet interventions (defined as treatment goal achievement) in healthy adult populations. These predictors have included self-monitoring dietary intake and counseling session attendance [27, 31, 32]. Demographic factors have shown mixed associations with diet intervention adherence [31, 32].
With respect to exercise alone, three randomized controlled trials with older adults have supported a positive effect of adherence on health-related outcomes [33–35]. For example, Fielding et al. [35] examined the relationship between adherence to a 12-month physical activity intervention and physical function among older adults with functional limitations. Adherence was defined as reaching a minimum threshold of 150 min of moderate physical activity per week. Among participants randomly assigned to the intervention, physical function did not differ between those classified as adherent and nonadherent at 6 months postbaseline. At 12 months postbaseline, however, participants who were adherent over the past 6 months showed greater improvement in physical function compared to those who were nonadherent. Another randomized controlled trial for overweight, older adults with osteoarthritis in the knee found that greater exercise adherence (defined as the percentage of exercise sessions attended) was associated with significant improvement in physical function and mental health as well as reduced body mass index and less pain at 6 and 18 months postbaseline [33]. Furthermore, in an exercise randomized controlled trial for sedentary older women, greater caloric expenditure per week was related to improvement in all aspects of self-reported physical and mental health with the exception of bodily pain [34]. In contrast, an exercise and yoga intervention study with older adults yielded no significant effect of adherence (defined as class attendance) on self-reported health or objective measures of physical functioning [36]. Further examination of the relationship between diet and exercise intervention adherence and health-related outcomes among older adults is needed due to the limited number of studies and mixed results.
The current study addressed this gap in the literature by examining the relationship between adherence to the Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW) intervention and health-related outcomes. The main objective of the RENEW trial was to decrease functional decline among older, overweight (body mass index 25.0–39.9), long-term survivors of breast, prostate, and colorectal cancer through a combination of tailored mailed print materials and telephone prompts and counseling regarding diet and exercise [24]. Compared to the delayed-intervention control group, the RENEW intervention group showed less decline in physical function over a 1-year period; moreover, the intervention group reported significant improvement in physical activity, dietary behaviors, basic and advanced lower extremity function, and body mass index compared to the control group at 1 year postbaseline [24]. The intervention was based on social cognitive theory and the trans-theoretical model [37, 38]. From a health promotion standpoint, social cognitive theory proposes five core determinants of health behavior change: (a) knowledge of health risks; (b) self-efficacy or confidence that one can bring about the desired outcome; (c) outcome expectations regarding the benefits and costs of a behavior; (d) health goals that one sets as well as plans for accomplishing goals; and (e) social and structural facilitators and impediments to behavior change [39]. All of these determinants of health behavior change were the focus of the RENEW trial’s telephone counseling sessions. Additionally, certain intervention components were tailored to the participant’s stage of health behavior change based on the trans-theoretical model (i.e., precontemplation, contemplation, preparation, action, and maintenance) [38]. Tailoring interventions to the individual’s stage of change should increase engagement in the intervention.
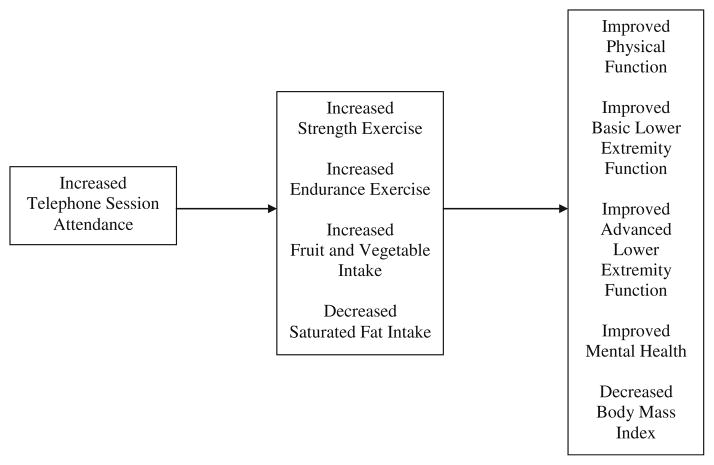
Drawing upon research on older adults’ adherence to diet and exercise interventions [33, 34] and social cognitive theory [37, 39], we proposed a conceptual model of the relationship between adherence to the RENEW intervention and health-related outcomes (see Fig. 1). We hypothesized that increased telephone counseling session attendance would be indirectly related to improved health-related outcomes postintervention (i.e., better physical function, basic and advanced lower extremity function, and mental health and lower body mass index) through increased strength and endurance exercise and healthy dietary behavior (i.e., greater fruit and vegetable intake, reduced saturated fat intake) over the year-long intervention period. Beneficial effects of exercise and dietary behaviors on health-related outcomes were hypothesized based on previous research [11–14, 19, 20].
Fig. 1.
Proposed conceptual model depicting the relationship between adherence to the RENEW diet and exercise intervention and health-related outcomes
Methods
Participants
The Institutional Review Boards of the North Carolina Central Cancer Registry and Duke University Health System approved the trial procedures. Participants were either self-referred or identified from the Duke Cancer Registry and the North Carolina Central Cancer Registry. Inclusion criteria included being an overweight (body mass index=25.0–39.9) older adult (≥65 years of age) who was five or more years postdiagnosis of breast, prostate, or colorectal cancer with no evidence of progressive disease or second primaries. Exclusion criteria included: (1) meeting national physical activity guidelines (i.e., at least 150 min of moderate to vigorous exercise per week); (2) contraindications to unsupervised exercise (e.g., recent heart attack, chronic obstructive pulmonary disease, angina, congestive heart failure, or wheelchair or walker use); (3) contraindications to a high fruit and vegetable diet (e.g., use of warfarin or dialysis); (4) cognitive impairment; (5) hearing impairment; (6) lack of English fluency or literacy; and (7) living in a skilled nursing facility.
Identified physicians were approached for written permission to contact patients about the trial. Additionally, a waiver was obtained to contact patients for whom treating physicians were either no longer practicing or unknown. Following contact approval, a study invitation letter was sent to 20,015 potential participants (see Ref. [40] for CONSORT diagram). Ninety percent (n=17,859) either refused participation (n= 373) or did not respond to the letter (n=17,486). Subsequently, 2,156 potential participants completed consent forms and a screening questionnaire to assess eligibility. Following screening completion, 753 participants (35%) were eligible for the study; however, 112 participants were deemed ineligible after the baseline survey was administered due to their body mass index or physical activity level. Thus, 641 participants were recruited and randomized to the year-long intervention (n= 319) or delayed-intervention control condition (n=322). Block randomization by cancer type, race, and sex was utilized. A wait-list control cross-over design was used; thus, the delayed-intervention arm began the intervention immediately after the 1-year waitlist period. In the intervention arm, 84% (269/319) completed the postintervention follow-up assessment (1 year postbaseline). In the delayed-intervention arm, 90% (289/322) completed the follow-up assessment and began the intervention at 1 year postbaseline. Subsequently, 85% (245/289) of those in the delayed-intervention arm who participated in the intervention completed the postintervention follow-up (2-years postbaseline). Across both groups, reasons for attrition following enrollment included lack of interest (65/127), illness (32/127), loss to follow-up (17/127), and family illness or death (13/127). Study completers and noncompleters did not differ with regard to demographics, number of comorbidities, or baseline levels of physical function, basic lower extremity function, mental health, or body mass index (all p values > 0.05). However, study noncompleters reported slightly worse advanced lower extremity function (mean difference=3.67 on this 0 to 100 scale) at baseline compared to completers, t(639)=2.58, p=0.01.
Procedure
The RENEW trial design was published in detail elsewhere (see Ref. [41], trial registration: NCT00303875). To summarize, RENEW was a year-long program of telephone counseling and tailored mailed print diet and exercise materials based on social cognitive theory and the trans-theoretical model [37, 38]. Participants were randomized to the intervention group or a delayed-intervention control group. A delayed-intervention control group was used because previous studies have suggested that an attention control group may be a barrier to recruitment [42]. Once enrolled in the intervention, participants received a package in the mail containing exercise bands of three different strengths, a poster demonstrating lower extremity exercises, a pedometer, a tableware designed to reduce food portions, a T-Factor 2000 Fat Gram Counter booklet (W.W. Norton, New York, NY) to monitor saturated fat intake, and a personalized self-monitoring log for recording daily fruit and vegetable intake and exercise behavior. The intervention was delivered via 15 telephone counseling sessions as well as a tailored workbook, eight automated telephone prompts, and four quarterly progress reports.
The intervention workbook, telephone prompts, and progress reports were tailored to the participants’ exercise and dietary behavior as well as their stage of change, as outlined by the trans-theoretical model [38]. For example, if a participant was in the preparation stage, the tailored components would focus on the importance of developing a diet and exercise plan. Conversely, if a participant was in the action stage, tailored components would encourage them to remain committed to their plan. Once enrolled in the intervention, participants received a workbook which included graphical comparisons of their current diet and exercise behaviors to the following guidelines for cancer survivors (for recommended guidelines, see Ref. [43]): (a) strength exercise every other day for at least 15 min; (b) endurance exercise every day for at least 30 min; (c) average daily consumption of seven to nine servings of fruits and vegetables; and (d) average daily consumption of less than 10% of total calories from saturated fat. Eight automated telephone prompts provided additional reinforcement and encouraged participants to change health behaviors. Finally, four quarterly progress reports reinforced health behaviors through detailed graphs regarding the participants’ overall progress.
During the year-long trial, each intervention participant received up to 15 telephone calls from an exercise physiologist (i.e., health counselor) who received didactic training in the study protocol. The health counselors were assigned participants that they instructed throughout the trial. During the first 3 weeks, counseling sessions were held every week, followed by two biweekly sessions, and concluding with ten monthly sessions. The calls ranged from 15 to 30 min and focused on determinants of health behavior change derived from social cognitive theory such as building social support and enhancing self-efficacy. The health counselors collaborated with the participants to evaluate progress, set goals, overcome barriers to health behavior change, and locate resources. Counseling sessions were standardized through the use of a computer-assisted guide featuring branching algorithms.
Measures
Demographics and Medical Information
At baseline, participants reported their age, gender, race, and education as well as their cancer treatment history (i.e., surgery, chemotherapy, radiation, hormonal therapy, other therapy). Additionally, six common medical conditions were reported and summed including cataracts, high blood pressure, arthritis, circulation difficulties in arms or legs, heart trouble, and osteoporosis [41].
Telephone Counseling Session Attendance
As noted above, participants were contacted via telephone by health counselors during the year-long intervention period. If a participant missed a counseling session, the health counselor would make call back attempts until the time frame for the next phone session; once the next time frame began, if the participant still had not been reached, the counseling session would be coded as missing. The number of telephone counseling sessions attended was used as a measure of intervention adherence, consistent with prior research [31, 33, 35].
Intervention-Period Exercise and Dietary Behavior
Participants reported exercise and dietary behaviors during sessions 2–15 of the 15 telephone counseling sessions. Thus, there was a total of 14 time points for those who attended all sessions. Participants were given a self-monitoring log for recording their exercise behavior and fruit and vegetable intake as well as a fat gram counter booklet to help them track their saturated fat intake. Additionally, participants were instructed to record their behavior immediately after completing the activity.
Strength exercise
Participants were asked to report the number of days and minutes per day of strength exercise over the past week. The average number of minutes of strength exercise across all completed intervention-period assessments was then computed.
Endurance exercise
Participants were asked to report the number of days and minutes per day of endurance exercise over the past week. The average number of minutes of endurance exercise across all completed intervention-period assessments was then computed.
Fruit and vegetable intake
Participants were asked to report their average daily servings of fruits and vegetables over the past week. The average daily servings of fruits and vegetables across all completed intervention-period assessments were then computed.
Saturated fat intake
Participants were asked to report the average number of saturated fat grams eaten per day over the past week. The average number of daily saturated fat grams across all completed intervention-period assessments was then computed.
Physical Function
Physical function was assessed using the ten-item physical function subscale of the Medical Outcomes Study Short-Form 36 [44]. The physical function subscale of the Medical Outcomes Study Short-Form 36 has been tested among older adults and has shown good concurrent and discriminant validity with performance-based measures as well as good reliability and sensitivity to change [45]. Scores are summed and transformed onto a standardized scale from 0 to 100, with higher scores representing better physical function [44].
Basic and Advanced Lower Extremity Function
The basic and advanced lower extremity subscales of the late life function and disability index were administered [46, 47]. Basic lower extremity function refers to activities that require stooping, standing, and walking, whereas advanced lower extremity function refers to activities that require a higher level of endurance and physical ability (e.g., hiking uphill or running half a mile) [47]. These subscales consist of 25 items (11 items for advanced lower extremity function and 14 items for basic lower extremity function) scored on a scale from 0 to 100, with higher scores indicating better functioning. The lower extremity subscales of the late life function and disability index have shown good concurrent and predictive validity with regard to the frequency and severity of disability as well as high test–retest reliability [47–49].
Mental Health
Mental health was assessed using the 14-item mental health summary measure of the Medical Outcomes Study Short-Form 36 [44]. The mental health summary measure is an aggregate of four subscales including (a) four items assessing vitality, (b) two items assessing social functioning, (c) three items assessing role limitations due to emotional problems, and (d) five items assessing general mental health (i.e., depression and anxiety). For each of the four subscales, scores are summed and transformed onto a standardized scale from 0 to 100, with higher scores representing better mental health [50]. Among older adults, the mental health summary measure of the Medical Outcomes Study Short-Form 36 has shown good internal consistency and convergent and discriminant validity [50, 51].
Body Mass Index
Body mass index (kg/m2) was calculated based on self-reported height and weight at baseline and self-reported weight postintervention. Self-reported height and weight have been shown to be highly correlated with measured height and weight among older adults [52].
Analytic Approach
Descriptive statistics and Pearson’s correlations were computed using Statistical Package for the Social Sciences (SPSS) 20.0. Then we performed a measured variable path analysis using LISREL 8.8 to test our hypothesis that increased telephone counseling session attendance would be indirectly related to improved health-related outcomes (i.e., physical function, basic and advanced lower extremity function, mental health, and body mass index) postintervention through improved exercise (i.e., increased strength and endurance exercise) and dietary behavior (i.e., increased fruit and vegetable intake, decreased saturated fat intake) over the intervention period. Health-related outcomes were examined postintervention, defined as 1 year postbaseline for the intervention group and 2 years postbaseline for the delayed-intervention group. Model fit was assessed using the χ2 statistic and the root mean square error of approximation (RMSEA) statistic. A nonsignificant χ2 statistic indicates that the hypothesized model is acceptable because there is no difference between the modeled and the observed patterns of relationships [53]. Additionally, the RMSEA statistic is an adjusted estimate of absolute fit accounting for the parsimony of the model; thus, smaller values represent better fit with values below 0.06 indicative of good fit [54, 55].
Results
Preliminary Analyses
Participant characteristics are shown in Table 1, and means, standard deviations, and Pearson’s correlations for the main study variables are presented in Table 2. Participants attended an average of 62% of the 15 telephone counseling sessions (range=0–15 sessions). During the telephone counseling sessions, participants reported a mean of 27 min/week of strength exercise, which was below the intervention goal of 60 min/week [41]. Furthermore, participants reported engaging in an average of 124 min of endurance exercise per week compared to the intervention goal of 210 min/week [41]. Concerning dietary behavior, participants reported eating an average of six daily servings of fruits and vegetables, which was below the intervention goal of seven to nine servings [41], but better than typical intakes for older adult cancer survivors [19]. Finally, average saturated fat intake was 14 g/day, which tended to be in the recommended range depending on the number of calories consumed per day with the target being less than 10% of total calories from saturated fat [41, 43].
Table 1.
Descriptive information on study sample at baseline (N=641)
| Variable | No. (%) of participantsa |
|---|---|
| Age, mean (SD) | 73.6 (5.1) |
| Race | |
| White | 569 (88.8) |
| African American | 67 (10.5) |
| Other | 5 (1.0) |
| Female sex | 349 (54.4) |
| Some college education | 395 (61.6) |
| No. of comorbidities, mean (SD) | 2.0 (1.2) |
| Cancer type | |
| Breast | 289 (45.1) |
| Prostate | 261 (40.7) |
| Colorectal | 91 (14.2) |
| Years since cancer diagnosis, mean (SD) | 8.6 (2.7) |
| Cancer treatment type | |
| Surgery | 569 (88.8) |
| Radiation | 293 (45.7) |
| Chemotherapy | 167 (26.1) |
| Hormonal therapy | 269 (42.0) |
| Other therapy | 94 (14.7) |
Unless otherwise indicated
Table 2.
Pearson’s correlations, means, and standard deviations for main study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Telephone session attendance | ||||||||||
| 2. Strength exercise | 0.23** | |||||||||
| 3. Endurance exercise | 0.19** | 0.25** | ||||||||
| 4. Fruit and vegetable intake | 0.31** | 0.26** | 0.37** | |||||||
| 5. Saturated fat intake | −0.12** | 0.01 | 0.11* | 0.31** | ||||||
| 6. Physical function | 0.11* | 0.12** | 0.31** | 0.19** | 0.09 | |||||
| 7. Basic lower extremity function | 0.06 | 0.06 | 0.26** | 0.18** | 0.05 | 0.76** | ||||
| 8. Advanced lower extremity function | 0.02 | 0.06 | 0.32** | 0.17** | 0.11* | 0.82** | 0.77** | |||
| 9. Mental health | 0.05 | 0.13** | 0.08 | 0.06 | 0.04 | 0.13** | 0.23** | 0.16** | ||
| 10. Body mass index | −0.09* | −0.03 | −0.21** | −0.09* | 0.02 | −0.28** | −0.26** | −0.24** | 0.03 | |
| Mean | 9.28 | 27.24 | 124.43 | 5.52 | 14.29 | 71.71 | 78.09 | 52.29 | 56.71 | 28.25 |
| Standard deviation | 4.56 | 19.83 | 72.33 | 1.80 | 5.79 | 23.08 | 15.47 | 17.11 | 6.96 | 3.50 |
ns=471–577 due to missingness. Intervention-period exercise and dietary behaviors included strength and endurance exercise in average minutes per week, fruit and vegetable intake in average servings per day, and saturated fat intake in average grams per day. The following outcome measures were administered postintervention: physical function subscale of the Medical Outcomes Study Short-Form 36, basic lower extremity function subscale of the Late Life Function and Disability Index, advanced lower extremity function subscale of the Late Life Function and Disability Index, mental health summary measure of the Medical Outcomes Study Short-Form 36, and body mass index.
p<0.05.
p<0.01
Concerning health-related outcomes (see Table 2), participants’ average score on the physical function subscale of the Medical Outcomes Study Short-Form 36 was similar to that of older adults in the general population [56]. Furthermore, the average score on the basic lower extremity function subscale of the late life function and disability index [47] suggested that participants had only minor troubles with basic performance tasks (e.g., stepping on and off a bus). However, the average score on the advanced lower extremity function subscale of the late life function and disability index [47] was indicative of troubles with more advanced tasks (e.g., running to catch a bus). The average score on the mental health summary measure of the Medical Outcomes Study Short-Form 36 was comparable to that of older adults in the general population [56]. Finally, following the intervention, participants reported an average body mass index that is considered overweight but not obese.
Preliminary data analyses were run to screen for violations of the assumptions of path analysis. All of the variables were within the recommended normality guidelines; the skewness indices were less than the absolute value of 2.0, and the kurtosis indices were less than the absolute value of 7.0 [53]. Full information maximum likelihood data imputation was used for missing data. This method retains the sample size and estimates parameters based on available data while generating implied values for missing data based on observable data patterns. Thus, full information maximum likelihood data imputation enhances the accuracy of confidence intervals and standard errors relative to other commonly used methods of addressing missing data such as listwise and pairwise deletion and single imputation [53]. Finally, covariates were chosen based on the literature and included age, gender, education, and number of comorbidities [7, 28, 57].
Given that the intervention was delivered first to the intervention group and then 1 year later to the delayed-intervention group, we ran the path analysis with each group separately to determine whether data from the two groups could be combined. The hypothesized model showed good fit to both the intervention and the delayed-intervention group data, χ2 (5, N=319)=7.08, p=0.21, RMSEA=0.04, 90% confidence interval (0.00, 0.09), and χ2 (5, N=322)=7.80, p=0.17, RMSEA=0.04, 90% confidence interval (0.00, 0.10), respectively. Therefore, we combined data from the two groups for the primary analysis.
Primary Analysis
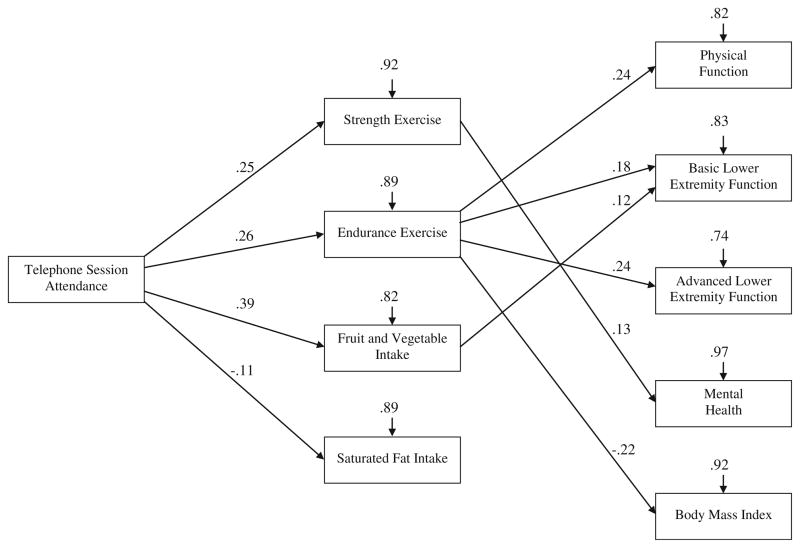
The hypothesized model showed good fit to the combined intervention and delayed-intervention group data, χ2(5, N= 641)=5.55, p=0.35, RMSEA=0.01, 90% confidence interval (0.00, 0.06), and results supported some of the hypothesized pathways (see Fig. 2). Telephone counseling session attendance had a significant indirect relationship with all of the health-related outcomes through intervention-period exercise and dietary behavior, with the exception of saturated fat intake. Specifically, there were positive indirect relationships between telephone counseling session attendance and physical function (β=0.11, p<0.05), basic lower extremity function (β=0.10, p<0.05), advanced lower extremity function (β=0.09, p<0.05), and mental health (β=0.05, p<0.05) as well as a negative relationship with body mass index (β=−0.06, p<0.05). The model accounted for 18% of the variance in physical function, 17% of the variance in basic lower extremity function, 26% of the variance in advanced lower extremity function, 4% of the variance in mental health, and 8% of the variance in body mass index.
Fig. 2.
Test of proposed conceptual model depicting the relationship between adherence to the RENEW diet and exercise intervention and health-related outcomes. Gender, age, education, and number of comorbidities were included in each of the pathways as covariates. Although not depicted in the figure, all mediators (i.e., strength and endurance exercise, fruit and vegetable intake, and saturated fat intake) and outcomes were allowed to covary. Paths are depicted with standardized regression coefficients significant at p<0.05; nonsignificant paths are not depicted. χ2 (5, N=641)=5.55, p=0.35, RMSEA=0.01, 90% confidence interval (0.00, 0.06)
Overall, the tested model provided partial support for the hypothesized pathways. As predicted, telephone counseling session attendance was related to engagement in all of the exercise and dietary behaviors during the intervention period. However, mixed associations were found between exercise and dietary behaviors and health-related outcomes (i.e., physical function, basic and advanced lower extremity function, mental health, and body mass index). When controlling for covariates and the other structural paths: (a) endurance exercise was related to all outcomes with the exception of mental health; (b) strength exercise was only related to mental health; (c) fruit and vegetable intake was only related to basic lower extremity function; and (d) saturated fat intake was not significantly related to any of the outcomes.
Discussion
This study is the first to examine the relationship between adherence to a diet and exercise intervention and health-related outcomes among cancer survivors. Identifying the health effects of specific intervention components may inform the development of highly effective interventions. The current sample consisted of older, overweight, long-term survivors of breast, prostate, and colorectal cancer, a large group at increased risk of disease relative to the general population of older adults [4]. We hypothesized that exercise and dietary behavior over the intervention period would explain the relationship between telephone counseling session attendance and health-related outcomes (see conceptual model in Fig. 1). The hypothesized pathways were informed by social cognitive theory [37, 39] as well as findings from diet and exercise randomized controlled trials [11–14, 22, 23, 25] and surveys of cancer survivors [19, 20]. As predicted, telephone counseling session attendance was related to engagement in all health behaviors over the intervention period. Moreover, engagement in specific health behaviors (i.e., strength and endurance exercise and fruit and vegetable intake) over the intervention period accounted for the relationships between telephone session attendance and certain health benefits.
Endurance exercise was positively related to physical function and basic and advanced lower extremity function and negatively related to body mass index. Exercise randomized controlled trials with cancer survivors have shown the impact of endurance exercise on physical health outcomes [11–13]; however, the relationship between adherence to these interventions and health-related outcomes has not been reported. The current results suggest that survivors who were more adherent to the intervention (i.e., attended more telephone sessions) engaged in greater minutes of endurance exercise, which, in turn, were related to certain health benefits. However, contrary to previous randomized controlled trial findings [15], endurance exercise was not related to mental health. One potential explanation for this finding is that participants were generally reporting good mental health, and this restriction of the variable’s range may have reduced statistical power for detecting a significant relationship.
Increased strength exercise was related to improved mental health, as found in prior research with cancer survivors [14]. However, in this study, strength exercise was unrelated to physical function, basic or advanced lower extremity function, and body mass index. Strength exercise randomized controlled trials with cancer survivors have shown improvement in all of these outcomes, with the exception of body mass index [58–60]. It is likely that the null findings in our study between strength exercise and physical function are due to the low intensity and volume of the exercises performed. Our intervention was unsupervised and home-based without access to high intensity resistive training equipment. Furthermore, participants reported a relatively low volume of strength exercise per week. Concerning strength exercise and body mass index, there has been some empirical support for the notion that strength exercise increases lean body mass and decreases the percentage of body fat [59]; however, there has been limited support for the role of strength exercise in improving body mass index, which may not capture significant change in body composition [61, 62].
With regard to dietary behaviors over the intervention period, increased fruit and vegetable intake was related to improved basic lower extremity function, but it was not related to physical function, advanced lower extremity function, mental health, or body mass index. In addition, saturated fat intake was not related to any of the outcomes. Prior research has also yielded mixed evidence for relationships between dietary variables and health-related outcomes [19–21]. Given the conflicting findings, more experimental research is needed to determine whether diet affects these health outcomes.
The current findings have significant implications for theory and future research. The Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW) telephone counseling sessions were based on social cognitive theory [37, 39], as reflected in their focus on social support, goal-setting, monitoring of progress, and barriers to health behavior change. Increased counseling session attendance was related to improved diet and exercise behaviors, suggesting that theory-driven determinants of behavior change may have led to positive health behavior changes over the intervention period. Thus, these determinants of behavior change warrant further examination in future diet and exercise intervention trials. It is important to note, however, that nonspecific factors related to the telephone sessions (e.g., therapeutic alliance) may have led to the positive behavior changes. Thus, future studies should consider including an attention control condition. In addition, future studies should consider investigating strategies designed to increase session attendance, such as flexible telephone counseling session schedules, incentives for session attendance, or improved staff training and clinical skills.
The present findings also have implications for clinical practice. First, these findings suggest that when addressing lifestyle changes, clinicians should repeatedly inform older adult cancer survivors of the importance of gaining health knowledge, setting health goals, monitoring progress, addressing barriers to health behavior change, and receiving social support. Given findings concerning relationships between exercise and fruit and vegetable intake and health-related outcomes, the importance of regular engagement in these health behaviors should be emphasized.
Limitations of the present study should be noted. First, the tested model is only one potential explanation for the observed patterns of relationships, and, as with any multivariate analysis, alternative models and theories should be explored [53]. For example, observed relationships may reflect an underlying construct such as conscientiousness [63]. That is, participants who were more reliable about taking intervention calls may have also been more reliable in performing expected exercise and dietary behavior. Furthermore, self-report measures are subject to biases such as demand characteristics, recall bias, recency, and saliency. It is important to note, however, that participants were given a fat gram counter booklet to monitor saturated fat intake and a personalized self-monitoring log for recording daily fruit and vegetable intake and exercise behavior to help increase the accuracy of their reports and minimize some of these biases. Future studies should include more objective measures of exercise (e.g., physical performance tests) and dietary intake (e.g., diet-related biomarkers). Additionally, some of the null findings may be attributable to a restricted range in scores for certain assessments such as body mass index and mental health measures. Finally, participants were predominately White and represented a minority of those approached to participate in the study. Thus, findings warrant replication in samples that are representative of the general population of older, overweight, long-term cancer survivors.
The current study also has a number of strengths that should be mentioned. RENEW was the first telephone and mailed print delivered lifestyle intervention for older adult cancer survivors. Moreover, the sample size was large and included participants from 21 US states as well as the UK and Canada with minimal attrition given the study population and the duration of the trial. In addition, the outcome measures were well validated and reliable for use with older adults. Lastly, this study tested a theory and research-based conceptual model using structural equation modeling, which allowed for the accuracy of parameter estimates to be enhanced through full information maximum likelihood data imputation for missing data [53].
Conclusion
Older adult cancer survivors tend to experience accelerated functional decline that results in reduced independence [10]. The RENEW intervention was designed to target functional decline in this vulnerable population by addressing social cognitive theory-based determinants of health behavior change. In contrast to prior research which has examined the relationship between exercise session attendance and health-related outcomes among older adults over 6- and 18-month time periods [33, 36], the current study examined links between telephone counseling session attendance, dietary and exercise behaviors, and health-related outcomes in older adult cancer survivors over a 1-year period. The results showed that increases in some health behaviors (i.e., strength and endurance exercise and fruit and vegetable intake) over the intervention period accounted for the relationship between increased telephone session attendance and certain health benefits. These findings support the importance of targeting social cognitive theory-based determinants of behavior change [37, 39] in health promotion interventions. However, research is needed to further examine the relationship between intervention adherence and health-related outcomes. Future intervention trials could implement a dose–response design testing the relationship between varying doses of the intervention (e.g., number of counseling sessions) and outcomes. This research would help determine the optimum dose of the intervention needed to significantly improve health-related outcomes and thereby increase their efficacy and cost-effectiveness.
Acknowledgments
This study was supported by the National Institutes of Health grants R01 CA106919 and P30AG028716 and grant E3386R from the Veterans Affairs Office of Research and Development. The work of the second author was supported by grant KL2 RR025760 (A. Shekhar, PI) from the NCRR. The authors would like to thank Jesse C. Stewart, Richard Sloane, Bernard F. Fuemmeler, and Rebecca N. Adams for their assistance.
Footnotes
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Joseph G. Winger, Catherine E. Mosher, Kevin L. Rand, Miriam C. Morey, Denise C. Snyder, and Wendy Demark-Wahnefried declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Contributor Information
Joseph G. Winger, Email: jgwinger@iupui.edu, Department of Psychology, Indiana University-Purdue University Indianapolis, 402 North Blackford Street, LD 134, Indianapolis, IN 46202, USA
Catherine E. Mosher, Department of Psychology, Indiana University-Purdue University Indianapolis, 402 North Blackford Street, LD 134, Indianapolis, IN 46202, USA
Kevin L. Rand, Department of Psychology, Indiana University-Purdue University Indianapolis, 402 North Blackford Street, LD 134, Indianapolis, IN 46202, USA
Miriam C. Morey, Department of Medicine, Duke University, Durham, NC, USA. Older Americans Independence Center, Duke University, Durham, NC, USA. Geriatric Research, Education and Clinical Center, VA Medical Center, Durham, NC, USA
Denise C. Snyder, Department of Medicine, Duke University, Durham, NC, USA
Wendy Demark-Wahnefried, Department of Nutrition Sciences, Comprehensive Cancer Center, University of Alabama, Birmingham, AL, USA
References
- 1.American Cancer Society. [Accessed 20 December 2013];Cancer facts & Figures 2013. http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures2013.
- 2.National Cancer Institute. [Accessed 20 December 2013];SEER stat fact sheet 2013. http://seer.cancer.gov/statfacts/html/all.html.
- 3.Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin N Am. 2008;22:319. doi: 10.1016/j.hoc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: Findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA. Survivorship: Adult cancer survivors. Prim Care. 2009;36:721–741. doi: 10.1016/j.pop.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Aziz NM. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–432. doi: 10.1080/02841860701367878. [DOI] [PubMed] [Google Scholar]
- 7.Deimling GT, Sterns S, Bowman KF, Kahana B. Functioning and activity participation restrictions among older adult, long-term cancer survivors. Cancer Investig. 2007;25:106–116. doi: 10.1080/07357900701224813. [DOI] [PubMed] [Google Scholar]
- 8.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 9.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: A review of the evidence. J Clin Oncol. 2002;20:3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao AV, Demark-Wahnefried W. The older cancer survivor. Crit Rev Oncol Hematol. 2006;60:131–143. doi: 10.1016/j.critrevonc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 12.Irwin ML, Alvarez-Reeves M, Cadmus L, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity. 2009;17:1534–1541. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Midtgaard J, Rørth M, Stelter R, et al. The impact of a multidimensional exercise program on self-reported anxiety and depression in cancer patients undergoing chemotherapy: A phase II study. Palliat Support Care. 2005;3:197–208. doi: 10.1017/s1478951505050327. [DOI] [PubMed] [Google Scholar]
- 14.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res Treat. 2008;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 15.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Polascik TJ, George SL, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17:3577–3587. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons JK, Newman VA, Mohler JL, et al. Dietary modification in patients with prostate cancer on active surveillance: A randomized, multicentre feasibility study. Br J Urol. 2008;101:1227–1231. doi: 10.1111/j.1464-410X.2007.07365.x. [DOI] [PubMed] [Google Scholar]
- 18.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 19.Demark-Wahnefried W, Clipp EC, Morey MC, et al. Physical function and associations with diet and exercise: Results of a cross-sectional survey among elders with breast or prostate cancer. Int J Behav Nutr Phys Act. 2004;16:1–6. doi: 10.1186/1479-5868-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayne SJ, Baumgartner K, Baumgartner RN, et al. Diet quality is directly associated with quality of life in breast cancer survivors. Breast Cancer Res Treat. 2006;96:227–232. doi: 10.1007/s10549-005-9018-6. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard CM, Stein KD, Baker F, et al. Association between current lifestyle behaviors and health-related quality of life in breast, colorectal, and prostate cancer survivors. Psychol Health. 2004;19:1–13. [Google Scholar]
- 22.Campbell M, Carr C, DeVellis B, et al. A randomized trial of tailoring and motivational interviewing to promote fruit and vegetable consumption for cancer prevention and control. Ann Behav Med. 2009;38:71–85. doi: 10.1007/s12160-009-9140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: A sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 24.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: A randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demark-Wahnefried W, Clipp EC, Morey MC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: Outcomes from project LEAD. J Clin Oncol. 2006;24:3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes RB, Taylor DW, Sackett DL. Compliance in health care. Baltimore: Johns Hopkins University Press; 1978. [Google Scholar]
- 27.Tinker LF, Perri MG, Patterson RE, et al. The effects of physical and emotional status on adherence to a low-fat dietary pattern in the Women’s Health Initiative. J Am Diet Assoc. 2002;102:789–800. doi: 10.1016/s0002-8223(02)90178-1. [DOI] [PubMed] [Google Scholar]
- 28.King AC, Rejeski WJ, Buchner DM. Physical activity interventions targeting older adults: A critical review and recommendations. Am J Prev Med. 1998;15:316–333. doi: 10.1016/s0749-3797(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 29.Courneya KS, Segal RJ, Reid RD, et al. Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J Clin Epidemiol. 2004;57:571–579. doi: 10.1016/j.jclinepi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Courneya KS, Stevinson C, McNeely ML, et al. Predictors of adherence to supervised exercise in lymphoma patients participating in a randomized controlled trial. Ann Behav Med. 2010;40:30–39. doi: 10.1007/s12160-010-9205-5. [DOI] [PubMed] [Google Scholar]
- 31.Schmid TL, Jeffery RW, Onstad L, Corrigan SA. Demographic, knowledge, physiological, and behavioral variables as predictors of compliance with dietary treatment goals in hypertension. Addict Behav. 1991;16:151–160. doi: 10.1016/0306-4603(91)90007-5. [DOI] [PubMed] [Google Scholar]
- 32.Tinker LF, Rosal MC, Young AF, et al. Predictors of dietary change and maintenance in the Women’s Health Initiative dietary modification trial. J Am Diet Assoc. 2007;107:1155–1165. doi: 10.1016/j.jada.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 33.van Gool CH, Penninx BWJH, Kempen GIJM, et al. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthritis Rheum. 2005;53:24–32. doi: 10.1002/art.20902. [DOI] [PubMed] [Google Scholar]
- 34.Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: A randomized controlled trial. Arch Intern Med. 2009;169:269–278. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fielding RA, Katula J, Miller ME, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007;39:1997–2004. doi: 10.1249/mss.0b013e318145348d. [DOI] [PubMed] [Google Scholar]
- 36.Flegal KE, Kishiyama S, Zajdel D, Haas M, Oken BS. Adherence to yoga and exercise interventions in a 6-month clinical trial. BMC Complement Altern Med. 2007;7:37–37. doi: 10.1186/1472-6882-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall: Englewood Cliffs, NJ; 1986. [Google Scholar]
- 38.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 39.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 40.Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet–exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30:2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder DC, Morey MC, Sloane R, et al. Reach out to ENhancE Wellness in older cancer survivors (RENEW): Design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psychooncology. 2009;18:429–439. doi: 10.1002/pon.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madsen SM, Mirza MR, Holm S, et al. Attitudes towards clinical research amongst participants and nonparticipants. J Intern Med. 2002;251:156–168. doi: 10.1046/j.1365-2796.2002.00949.x. [DOI] [PubMed] [Google Scholar]
- 43.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 44.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I: Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 45.Bohannon RW, DePasquale L. Physical Functioning scale of the short-form (SF) 36: Internal consistency and validity with older adults. J Geriatr Phys Ther. 2010;33:16–18. [PubMed] [Google Scholar]
- 46.Jette AM, Haley SM, Coster WJ, et al. Late Life function and disability instrument I: Development and evaluation of the disability component. J Gerontol. 2002;57:209–216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 47.Haley SM, Jette AM, Coster WJ, et al. Late life function and disability instrument II: Development and evaluation of the function component. J Gerontol. 2002;57:217–222. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 48.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 49.Sayers SP, Jette AM, Haley SM, et al. Validation of the late-life function and disability instrument. J Am Geriatr Soc. 2004;52:1554–1559. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 50.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. Br Med J. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walters SJ, Munro JF, Brazier JE. Using the SF-36 with older adults: A cross-sectional community-based survey. Age Ageing. 2001;30 doi: 10.1093/ageing/30.4.337. [DOI] [PubMed] [Google Scholar]
- 52.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: Findings from the third national health and nutrition examination survey, 1988–1994. J Am Diet Assoc. 2001;101:28–34. doi: 10.1016/S0002-8223(01)00008-6. [DOI] [PubMed] [Google Scholar]
- 53.Kline RB. Principles and practice of structural equation modeling. 3. New York: The Guilford Press; 2011. [Google Scholar]
- 54.Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol Methods Res. 1992;21:230–259. [Google Scholar]
- 55.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Str Equine Model. 1999;6:1–55. [Google Scholar]
- 56.Ware JE, Jr, Snow K, Kosinski M, Gandek B. SF-36 Health Survey: Manual and interpretation guide. Lincoln, RI: Quality Metric; 2000. [Google Scholar]
- 57.Rhodes RE, Martin AD, Taunton JE, et al. Factors associated with exercise adherence among older adults: An individual perspective. Sports Med. 1999;28:397–411. doi: 10.2165/00007256-199928060-00003. [DOI] [PubMed] [Google Scholar]
- 58.LaStayo PC, Marcus RL, Dibble LE, Smith SB, Beck SL. Eccentric exercise versus usual-care with older cancer survivors: The impact on muscle and mobility—An exploratory pilot study. BMC Geriatr. 2011;11:5–5. doi: 10.1186/1471-2318-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers. 2005;14:1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 60.Yuen HK, Sword D. Home-based exercise to alleviate fatigue and improve functional capacity among breast cancer survivors. J Allied Health. 2007;36:e257–e275. [PubMed] [Google Scholar]
- 61.Bourke L, Doll H, Crank H, et al. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: A feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20:647–657. doi: 10.1158/1055-9965.EPI-10-1143. [DOI] [PubMed] [Google Scholar]
- 62.Burkhauser RV, Cawley J, Beyond BMI. The value of more accurate measures of fatness and obesity in social science research. J Health Econ. 2008;27:519–529. doi: 10.1016/j.jhealeco.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Courneya KS, Hellsten L-AM. Personality correlates of exercise behavior, motives, barriers and preferences: An application of the five-factor model. Pers Individ Differ. 1998;24:625–633. [Google Scholar]