Abstract
Purpose
This study aims to quantify trajectories of overall health pre- and post-diagnosis of cancer, trajectories of overall health among cancer-free individuals, and factors affecting overall health status.
Methods
Overall health status, derived from self-rated health report, of Atherosclerosis Risk in Communities (ARIC) cohort participants diagnosed with incident cancer (lung (N=400), breast (N=522), prostate (N=615), colorectal (N=303)), and cancer-free participants (N=11,634) over 19 years was examined. Overall health was evaluated in two ways: 1) overall health was assessed until death or follow-up year 19 (survivorship model) and 2) same as survivorship model except that a SRH value of zero was used for assessments after death to follow-up year 19 (cohort model). Mean overall health at discrete times was used to generate overall health trajectories. Differences in repeated measures of overall health were assessed using linear growth models.
Results
Overall health trajectories declined dramatically within one-year of cancer diagnosis. Lung, breast, and colorectal cancer were associated with a significant decreased overall health score (β) compared to the cancer-free group (survivorship model: lung −7.00, breast −3.97, colorectal −2.12; cohort model: lung −7.63, breast −5.07, colorectal −2.30). Other predictors of decreased overall health score included low education, diabetes, cardiovascular disease, and age.
Conclusions
All incident cancer groups had declines in overall health during the first year post-diagnosis, which could be due to cancer diagnosis or intensive treatments. Targeting factors related to overall health declines could improve health outcomes for cancer patients.
Keywords: cancer, survivorship, health-related quality of life, longitudinal studies, epidemiology
Purpose
The impact of cancer on health status is often assessed utilizing clinical endpoints (e.g., remission and survival). However, these measures do not fully capture the impact on an individual’s general wellbeing. Little quantitative information is available about general wellbeing across the cancer diagnosis and survivorship continuum. Utilizing self-rated health (SRH) trajectories to elucidate disease progression and inform intervention efforts might ameliorate wellbeing decline associated with a cancer diagnosis (1). The majority of studies utilize lengthy instruments to capture the multi-dimensional nature of cancer patients’ health-related quality of life (HRQoL) (2–6). While a few recent studies measure general wellbeing across the cancer continuum (1, 2, 5), most studies are limited to highly selected patient populations, have short follow-up time, or assess measurements after the diagnosis has occurred (3, 4, 6). To our knowledge, no studies are published that examine long-term follow-up of SRH trajectories among multiple incident cancer types pre- and post-cancer event and a group of cancer-free individuals, which provides a comparison group for SRH declines due to aging.
The single-item SRH measure is a validated measure of general wellbeing (7), predictive of all-cause morbidity and mortality (7–11). In this study, we used data from a large community-based cohort study, to quantify trajectories of overall health, derived from SRH report, pre- and post-incident cancer diagnosis, trajectories of overall health among cancer-free individuals, and factors affecting overall health status. This study provides valuable information on factors that could be targeted in an effort to improve health outcomes, specifically HRQoL, in cancer patients.
Methods
Participants
The Atherosclerosis Risk in Communities (ARIC) study has been described previously (12). Briefly, the cohort was established through probability sampling of four US communities located in Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland (12). This prospective cohort of 15,792 men and women, 45–64 years of age at baseline (1987–1989) has been followed through ongoing event surveillance, annual follow-up telephone interviews, and in-person examinations.
Of the 15,006 ARIC study members participating in the ancillary cancer study, we excluded those with self-reported race other than white or black (n=48), blacks residing in Minnesota or Maryland (n=53), participants with prevalent cancer (self-report on baseline interview, n=885), participants with incident cancer other than lung, breast, prostate, or colorectal cancer (n=1,135), individuals with an unclear date of cancer diagnosis (n=9), and one individual with two incident cancers diagnosed on the same date (n=1). The final cohort for this analysis included 13,474 participants with a mean follow-up time of 16.3 (range 1–19) years. The study was approved by the Institutional Review Board at the University of North Carolina–Chapel Hill and all participating institutions.
Self-rated Health Measurement
SRH was assessed at baseline by in-person interview and on each annual follow-up telephone call. Participants were asked, “Over the past year, compared to other people your age, would you say that your health has been excellent, good, fair or poor?” We transformed the SRH responses into overall health scores (13), where scores represented the estimated probability of being healthy in the future based on participant’s current SRH response when alive: 95 for excellent, 80 for good, 30 for fair, and 15 for poor. For individuals that were deceased, individuals were assigned an overall health score of 0 for dead (1, 13).
Overall health scores were measured for 226,153 total observations, of which 6,567 (2.9%) were missing. Missing observations with overall health values for both the previous and subsequent years were imputed by averaging these overall health values (1). As a result, approximately 18% (N=1,188) of the missing observations were imputed, for a total of 220,774 overall health observations. If overall health was missing and unable to be imputed, the observation remained a missing value. We assigned a zero for missing overall health occurring the year in which the cohort member died. All analyses were run with and without imputation; no differences were noted and only imputed results are presented.
Cancer Ascertainment
Incident primary cancers among ARIC cohort members occurring between January 1, 1987, and December 31, 2005, were ascertained by two methods: linkage to a state cancer registry and medical record review (12, 14). For individuals diagnosed with multiple cancers, only the first cancer diagnosis was included for analysis. Overall health was analyzed among participants who were cancer-free throughout the study period (N=11,634), and who developed lung (N=400), female breast (N=522), prostate (N=615), and colorectal cancer (N=303).
“Event” was defined as the date of cancer diagnosis for the cases. Members of the cancer-free group were assigned an “event” date using a random number generator (1, 15). The first allowed event date was the baseline interview date, and the last allowed event date was the date of death or December 31, 2005, whichever came first.
Covariate Measurement and Categorization
Factors influencing pre- and post-event overall health trajectories were evaluated. Age at annual follow-up centered at 65 years (average age of cancer diagnosis), gender, and race/community were included. Annual follow-up visits were categorized as pre-event (referent), within 1-year post-event, 1–5 years post-event, or ≥5 years post-event. Variables assessed at baseline included body mass index (BMI, kg/m2), classified into underweight (<18.5), normal (referent, 18.5–<25), overweight (25–<30) or obese (≥30); hypertension, present if systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or if taking hypertensive medication within the previous two weeks; cardiovascular disease (CVD) including coronary heart disease, heart failure, or stroke determined through a combination of self-report at baseline interview, medical record abstraction, and clinical diagnosis; self-reported chronic lung disease (chronic bronchitis, emphysema or asthma); self-reported diabetes; self-reported high cholesterol; any type of health insurance; current, former, or never (referent) drinker; current, former, or never (referent) smoker; physical activity score, ranging from 1 (low) to 5 (high) for physical activity during leisure time (16); educational attainment categorized as less than high school, high school graduate, and greater than high school (referent); and frequency of routine physical examination categorized as at least once a year (referent), at least once every five years, or less often than once every five years/no routine physicals. Period effects [1987–1992, 1993–1999 and 2000–2005 (referent)] captured secular trends (17) that may influence the cancer-overall health relationship (e.g., changes in health behaviors and disease treatments).
Statistical Analysis
Evaluation of overall health trajectories was performed in two ways. First, we captured the overall health of all individuals surviving post-event by including observations through the year of a cohort member’s death or study follow-up year 19, whichever came first (survivorship model). Secondly, overall health of the entire cohort, not just the survivors, was captured through follow-up year 19, by assigning an overall health score of zero for each follow-up year occurring after a cohort member’s death (cohort model). The cohort model resulted in an additional 16,565 observations being assigned a score of zero. The survivorship model evaluated the impact of factors on overall health among survivors, and the cohort model examined the factors associated with the overall health among the entire cohort.
Cancer-specific overall health, assessed three years pre-event through ten years post-event, was regressed at each year interval on study covariates to generate estimates of adjusted overall health and standard errors (PROC GLM, SAS 9.2, Cary, NC) (18). For overall health changes occurring between the event and three years prior to event, one-year post-event, and five years post-event, linear regression models were used to evaluate differences between the cancer groups and the cancer-free group. Change in age-adjusted overall health between the year of event and one-year later was used to calculate how much of the one-year post-event overall health decline was attributable to death. To determine model fit, plots of mean overall health across age were examined and were linear. Additionally, a quadratic term for age was not statistically significant. Therefore, linear models were utilized.
We also fit individual unconditional linear growth models, accounting for repeated measures of overall health (PROC MIXED, SAS 9.2, Cary, NC), to examine the associations between covariates and overall health and to capture differences in overall health changes among the four cancer groups and the referent cancer-free group. This model allows examination of individuals’ longitudinal data over time, where the individual growth parameters are examined as random effects and covariates are entered as fixed effects to determine their impact on the dependent variable (19). The variance-covariance matrix is unstructured. To determine model fit, we tested a quadratic term for age. While age-squared was statistically significant, it did not contribute in a meaningful way to the analysis. Therefore, the linear growth models were utilized for data analysis. The null model likelihood ratio test was examined, which compares the fitted model to the null model (containing only the fixed effects) and it was statistically significant (p<0.0001).
Tests of significance were two-sided, and a p-value of 0.05 was considered statistically significant. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Baseline demographics are provided in Table 1. The baseline mean SRH score for the cancer-free group was 74.9, whereas lung cancer cases had the lowest SRH score of 68.4. The cancer groups were older than the cancer-free group. The proportion of current smokers and individuals with low educational attainment was similar between the cancer-free and cancer groups, with the exception of lung cancer (67.5% current smokers, 35.5% less than high school education). Not undergoing routine physical exams was less common in the cancer-free group (32.9%) versus the lung (39.1%) or prostate cancer group (34.4%). Colorectal cancer cases had the highest percentage of uninsured (14.2%).
Table 1.
Baseline characteristics of participants: The ARIC Study.
| Cancer-free N=11,634 | Cancer
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung N=400 | p* | Breast N=522 | p* | Prostate N=615 | p* | Colorectal N=303 | p* | ||
|
|
|
|
|
|
|||||
| Baseline SRH, mean (sd) | 74.9 (24.4) | 68.4 (26.5) | <0.0001 | 75.6 (23.9) | 0.5 | 75.3 (23.9) | 0.7 | 72.4 (25.5) | 0.08 |
| Excellent, n (%) | 3,978 (34.2) | 85 (21.3) | 177 (33.9) | 212 (34.5) | 90 (29.7) | ||||
| Good, n (%) | 5,378 (46.3) | 202 (50.5) | 252 (48.3) | 285 (46.3) | 143 (47.2) | ||||
| Fair, n (%) | 1,886 (16.2) | 94 (23.5) | 73 (14.0) | 106 (17.2) | 59 (19.5) | ||||
| Poor, n (%) | 386 (3.3) | 19 (4.7) | <0.0001 | 20 (3.8) | 0.5 | 12 (2.0) | 0.3 | 11 (3.6) | 0.3 |
| Age, mean (sd) | 53.7 (5.7) | 56.8 (5.5) | <0.0001 | 54.6 (5.8) | 0.0004 | 56.3 (5.6) | <0.0001 | 55.6 (5.5) | <0.0001 |
| Gender, n (%) | |||||||||
| Male | 5,050 (43.4) | 263 (65.7) | 0 (0.0) | 615 (100.0) | 157 (51.8) | ||||
| Female | 6,584 (56.6) | 137 (34.3) | <0.0001 | 522 (100.0) | <0.0001 | 0 (0.0) | <0.0001 | 146 (48.2) | 0.004 |
| Race/Community, n (%) | |||||||||
| Black/NC | 374 (3.2) | 15 (3.8) | 16 (3.1) | 21 (3.4) | 5 (1.7) | ||||
| Black/MS | 2,914 (25.0) | 79 (19.7) | 109 (20.9) | 174 (28.3) | 82 (27.1) | ||||
| White/NC | 2,576 (22.1) | 104 (26.0) | 122 (23.4) | 127 (20.7) | 61 (20.1) | ||||
| White/MN | 2,865 (24.6) | 88 (22.0) | 140 (26.8) | 157 (25.5) | 68 (22.4) | ||||
| White/MD | 2,905 (25.0) | 114 (28.5) | 0.04 | 135 (25.9) | 0.3 | 136 (22.1) | 0.3 | 87 (28.7) | 0.2 |
| Body Mass Index, n (%) | |||||||||
| Underweight | 102 (0.9) | 6 (1.5) | 4 (0.8) | 1 (0.2) | 3 (1.0) | ||||
| Normal | 3,723 (32.1) | 171 (42.8) | 184 (35.5) | 159 (25.9) | 81 (26.7) | ||||
| Overweight | 4,547 (39.1) | 142 (35.5) | 166 (32.0) | 296 (48.2) | 115 (38.0) | ||||
| Obese | 3,243 (27.9) | 81 (20.2) | <0.0001 | 165 (31.8) | 0.01 | 158 (25.7) | <0.0001 | 104 (34.3) | 0.07 |
| Smoker, n (%) | |||||||||
| Current | 2,872 (24.7) | 270 (67.5) | 121 (23.2) | 126 (20.5) | 77 (25.4) | ||||
| Former | 3,731 (32.1) | 109 (27.3) | 118 (22.6) | 288 (46.8) | 96 (31.7) | ||||
| Never | 5,021 (43.2) | 21 (5.2) | <0.0001 | 283 (54.2) | <0.0001 | 201 (32.7) | <0.0001 | 130 (42.9) | 1 |
| Drinker, n (%) | |||||||||
| Current | 6,327 (54.7) | 254 (63.5) | 271 (52.1) | 371 (60.8) | 166 (55.1) | ||||
| Former | 2,188 (18.9) | 97 (24.3) | 74 (14.2) | 139 (22.8) | 59 (19.6) | ||||
| Never | 3,055 (26.4) | 49 (12.2) | <0.0001 | 175 (33.7) | 0.0003 | 100 (16.4) | <0.0001 | 76 (25.2) | 0.9 |
| Education, n (%) | |||||||||
| Less than HS | 2,756 (23.7) | 142 (35.5) | 121 (23.2) | 140 (22.8) | 81 (26.7) | ||||
| HS or equivalent | 3,749 (32.3) | 137 (34.3) | 179 (34.3) | 163 (26.5) | 97 (32.0) | ||||
| Greater than HS | 5,105 (44.0) | 121 (30.2) | <0.0001 | 222 (42.5) | 0.6 | 312 (50.7) | 0.002 | 125 (41.3) | 0.4 |
| Comorbidities, n (%) | |||||||||
| CVD | 1,100 (9.5) | 57 (14.3) | 0.001 | 43 (8.2) | 0.4 | 58 (9.4) | 1 | 29 (9.6) | 0.9 |
| Chronic Lung Disease | 1,002 (8.6) | 66 (16.5) | <0.0001 | 46 (8.8) | 0.9 | 45 (7.3) | 0.3 | 29 (9.6) | 0.6 |
| Hypertension | 4,009 (34.6) | 154 (38.7) | 0.09 | 190 (36.5) | 0.4 | 253 (41.4) | 0.0006 | 116 (38.3) | 0.2 |
| Diabetes | 907 (7.8) | 26 (6.5) | 0.3 | 35 (6.7) | 0.3 | 34 (5.5) | 0.04 | 35 (11.6) | 0.02 |
| High Cholesterol | 2,404 (21.2) | 86 (22.3) | 0.6 | 103 (20.4) | 0.6 | 121 (20.0) | 0.5 | 69 (23.2) | 0.4 |
| Access to Care, n (%) | |||||||||
| No Health Insurance | 1,155 (10.0) | 46 (11.5) | 0.3 | 51 (9.8) | 0.9 | 53 (8.6) | 0.3 | 43 (14.2) | 0.02 |
| Physical Activity Score, mean (sd) | 2.42 (0.80) | 2.36 (0.75) | 0.1 | 2.31 (0.75) | 0.002 | 2.57 (0.80) | <0.0001 | 2.46 (0.78) | 0.4 |
| Freq. of Routine Exams, n (%) | |||||||||
| ≥Yearly | 5,041 (43.4) | 150 (37.6) | 240 (46.2) | 223 (36.3) | 128 (42.4) | ||||
| ≥Every 5 year and <Yearly | 2,740 (23.6) | 93 (23.3) | 119 (22.9) | 180 (29.3) | 80 (26.5) | ||||
| <Every 5 years/No routine exams | 3,823 (32.9) | 156 (39.1) | 0.02 | 160 (30.8) | 0.4 | 211 (34.4) | 0.0005 | 94 (31.1) | 0.5 |
p-value comparing the cancer group to the cancer-free group.
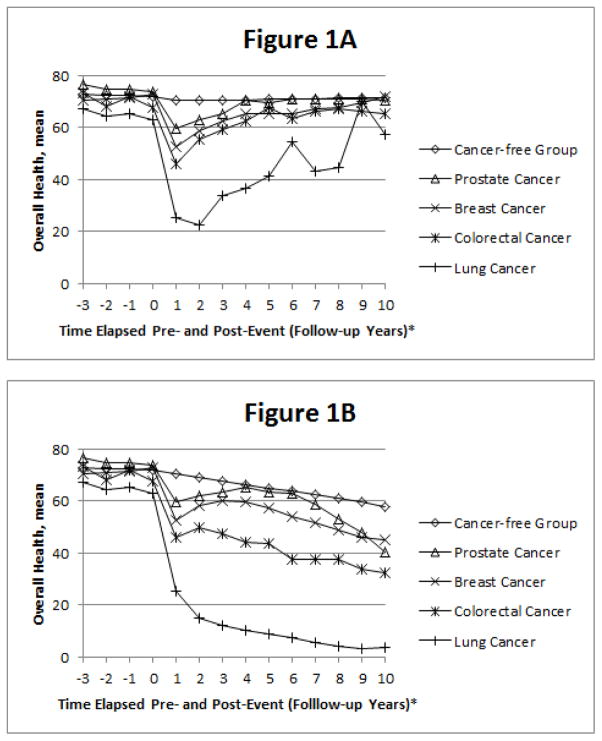
Figure 1 shows mean overall health score among the four cancer groups and cancer-free group pre- and post-event, adjusted for age. Average overall health score three years pre-event was highest in prostate and lowest in lung cancer groups. In the survivorship model (Figure 1A), mean overall health for all cancer groups returned to roughly pre-event levels, with lung cancer excepted. In the cohort model (Figure 1B), one-year post-event overall health increased slightly among the prostate, breast, and colorectal groups followed by subsequent declines.
Figure 1.
Trajectory of Overall Health pre- and post-event in the A) survivorship model and B) cohort model, adjusted for age: The ARIC Study.
* Event date occurs between contact years 0 and 1, which is considered the event follow-up.
We examined differences in overall health slopes among the cancer groups compared to the cancer-free group in the survivorship model. The largest average overall health slope from three years before event up until event is 4.43 overall health points less among the colorectal cancer group compared to the cancer-free group. This difference is statistically significant (p=0.003). Overall health was also significantly different from the cancer-free group for lung cancer (β= −2.86, p=0.03), but not for breast (β=1.86, p=0.11) or prostate cancer (β= −1.85, p=0.07). Statistically significant (p<0.0001) declines in the overall health score were noted among all cancer groups, versus the cancer-free group, in the year following the cancer event. Within the first post-event year, the smallest average decline in the overall health slope occurred among the prostate cancer group (β= −12.69), while the largest decline in the overall health slope occurred among the lung cancer group (β= −35.97). The declines for breast (β= −19.65) and colorectal cancer (β= −19.96) were similar to one another. The percent decline in overall health due to death within one-year of event was 28.3% for lung, 4.3% for breast, 4.3% for prostate, and 14.8% for colorectal cancer. In the five-year post-event period, the largest decline in the overall health slope occurred in lung cancer (β= −17.90, p<0.0001), versus the cancer-free group. Overall health was also significantly different from the cancer-free group for breast (β= −5.97, p<0.0001) and prostate cancer (β= −4.52, p=0.0007), but not for colorectal cancer (β=2.06, p=0.34).
Fitting a linear growth model to the data (Table 2), the average overall health is 7.00 and 7.63 overall health points less among the lung cancer group compared to the cancer-free group for the survivorship and cohort models, respectively, controlling for all available covariates. This difference was statistically significant for both models (p<0.0001). Breast and colorectal cancer were also associated with a significant decline in the overall health score compared to the cancer-free group. Prostate cancer was associated with an increased overall health score for both the survivorship (0.71) and cohort (2.26) models. Diabetes and CVD at baseline were both associated with a decreased overall health score.
Table 2.
Predictors of Overall Health: The ARIC Study.
| Survivorship Model | Cohort Model | |||
|---|---|---|---|---|
|
| ||||
| Estimate | SE | Estimate | SE | |
| Intercept | 77.23* | 0.68 | 77.38* | 0.84 |
| Cancer (vs. cancer-free) | ||||
| Lung | −7.00* | 0.84 | −7.63* | 1.02 |
| Breast | −3.97* | 0.71 | −5.07* | 0.87 |
| Prostate | 0.71 | 0.67 | 2.26* | 0.82 |
| Colorectal | −2.12* | 0.91 | −2.30* | 1.11 |
| Post-event (vs. pre-event)† | ||||
| 5+ years | −3.81* | 0.20 | −8.54* | 0.22 |
| 1–5 years | −3.07* | 0.14 | −6.11* | 0.15 |
| Diagnosis-1 year | −4.75* | 0.18 | −7.95* | 0.18 |
| No Health Insurance (vs. Health Insurance) | −3.99* | 0.48 | −4.86* | 0.59 |
| Body Mass Index (vs. Normal) | ||||
| Obese | −4.50* | 0.37 | −4.58* | 0.45 |
| Overweight | −0.95* | 0.33 | −1.27* | 0.40 |
| Underweight | −6.40* | 1.50 | −7.04* | 1.83 |
| Smoker (vs. Never) | ||||
| Current | −4.45* | 0.36 | −4.55* | 0.44 |
| Former | −0.51 | 0.34 | −0.13 | 0.41 |
| Drinker (vs. Never) | ||||
| Current | 0.40 | 0.37 | −0.21 | 0.46 |
| Former | −2.15* | 0.43 | −2.91* | 0.53 |
| Education(vs. Vocational, College, Graduate school) | ||||
| High School or Less | −9.09* | 0.38 | −8.84* | 0.47 |
| High School Graduate | −3.23* | 0.32 | −3.37* | 0.39 |
| Physical Activity Score | 2.82* | 0.18 | 2.86* | 0.22 |
| Male (vs. Female) | −0.10 | 0.31 | −0.31 | 0.38 |
| Race/Community (vs. White/NC) | ||||
| Black/Mississippi | −8.33* | 0.44 | −9.36* | 0.54 |
| Black/North Carolina | −5.25* | 0.83 | −6.03* | 1.01 |
| White/Maryland | 0.75 | 0.40 | 0.39 | 0.49 |
| White/Minnesota | 1.97* | 0.40 | 1.73* | 0.50 |
| Period (vs. 2000–2005) | ||||
| 1986–1992 | 2.78* | 0.23 | 3.62* | 0.24 |
| 1993–1999 | 1.42* | 0.14 | 1.99* | 0.15 |
| CVD | −10.57* | 0.49 | −11.14* | 0.60 |
| Chronic lung disease | −6.57* | 0.48 | −6.33* | 0.59 |
| Hypertension | −3.66* | 0.31 | −3.46* | 0.38 |
| Diabetes | −12.79* | 0.54 | −13.73* | 0.65 |
| High Cholesterol | −2.88* | 0.34 | −3.08* | 0.42 |
| Physical Exam (vs. routine physical at least once a year) | ||||
| No routine physicals | 0.04 | 0.33 | −0.15 | 0.40 |
| Physical at least every 5 years | 0.53 | 0.35 | 0.29 | 0.43 |
| Age, centered at 65 | −0.32* | 0.02 | −0.48* | 0.03 |
Significant (p<0.05).
Each member of the cancer-free group was assigned a random “event” date (15).
Discussion
The novel contribution of this study is utilization of long-term community cohort data on both pre- and post-diagnosis overall health trajectories for four cancer types and a cancer-free group of individuals. To date, most studies have SRH cross-sectionally or post-diagnosis (3, 4, 6). The current study also analyzed data separately for survivors and contrasted results to a cohort model, which attempted to capture the overall health of the entire cohort by including observations for deceased members (overall health score=0). These findings illustrate that the impact of cancer is underestimated if only examined among survivors, but this varies by cancer type. By imputing missing overall health values, overestimation of the association may be avoided and provide a more complete picture of overall health (15). Additionally, the health history in the ARIC cohort identified important conditions (e.g., CVD and diabetes) that showed comparable or more severe effects on overall health than a cancer diagnosis. Longitudinal data on health history allowed us to exclude prevalent cancer at baseline that could bias overall health trajectories for incidence cancer.
Our study results align with another longitudinal study which showed a SRH declines pre-event for all cancer types, a steep decline post-event, a leveling-off period, and subsequent declines (15). Similar trajectories for lung cancer and CVD (including cardiac procedure, myocardial infarction, and heart failure) have been reported in the ARIC cohort (1). Another recent longitudinal study did not show SRH as a significant predictor of cancer. However, this study only examined overall cancer and was not able to look at specific types (20), where our study found differences in SRH by cancer type. The current study also collected SRH data every year, whereas this recent study collected data every two years and did not account for death (20). This could partially account for lack of an association between cancer and SRH in this study (20), as we found the largest decline in SRH occurred within the first year of diagnosis.
We believe the overall health decline in the cancer-free group is representative of usual health declines associated with aging. Except for breast cancer, all cancer groups showed greater pre-event decline than the cancer-free group. This substantiates the predictive ability of the overall health measure (15), which is predictive of all-cause morbidity and mortality (7–11). Mammography screening with subsequent detection of early stage breast cancer, before impacting health or causing symptoms, may explain the lack of overall health decline for breast cancer (21). The post-event decline in the cancer-free group may be explained by post-event deaths, aging, and comorbidities. The most dramatic decreases in overall health occur within the first year of cancer diagnosis when most patients are undergoing treatment that can be invasive (e.g., surgery) or have severe side effects (e.g., chemotherapy or radiation). As individuals complete treatment during the first year post-diagnosis, the side effects of treatment begin to resolve. We could not directly test this hypothesis, however, as information on courses of treatment are unavailable. The steep post-event decline is also partially attributable to deaths within one-year of the event; the percentage drop from death within one-year of event ranged from 4.3% for breast and prostate to 28.3% for lung cancer. The higher percentage drop due to death from lung cancer is expected, as relative five-year survival is much lower for lung (16.8%) than for breast (89.2%) or prostate cancer (98.9%) (22). Colorectal cancer also has a more favorable five-year relative survival of 64.7%, compared to lung cancer (22). The poor survival prognosis of lung cancer accounts for the majority of the poorer overall health seen in lung cancer participants, as these individuals often die before being diagnosed with a secondary cancer (23).
The survivorship model shows cancer patients surviving five or more years post-event begin to return to an overall health similar to pre-event. Thus as long-term cancer survivors’ overall health begins to increase, any interventions or efforts that could help them return to their pre-cancer overall health status would be advantageous. The cohort model shows continual declines in overall health for cancer patients and poorer long-term overall health for cancer patients than the cancer-free group due to mortality. Specifically this shows which cancers have the worst overall health post-diagnosis (i.e., lung and colorectal) when accounting for mortality.
A diagnosis of lung, breast, or colorectal cancer was associated with a decreased overall health score compared to the cancer-free group; prostate cancer was not. This is likely due to prostate cancers diagnosed via prostate-specific antigen testing (PSA), which leads to earlier diagnosis and has potential for overdiagnosis (24). Many of these patients exhibit no symptoms at diagnosis; therefore prostate cancer diagnosis would have less impact on overall health. Even though breast and colorectal cancer also have wide-spread screening programs, overdiagnosis is most prevalent in prostate cancer (25).
These results are limited by lack of cancer stage and grade information. Overall health trajectories may differ by stage and grade of the tumor, with later stage and higher grade tumors potentially having greater initial post-diagnosis declines partially due to higher mortality rates and more aggressive treatments. However, a study of lung cancer patients failed to find an association between initial tumor stage and self-reported HRQoL (26). Future studies should examine the effects of cancer on SRH accounting for stage and grade.
Utilizing recoded SRH values (95 for excellent, 80 for good, 30 for fair, 15 for poor, and 0 for death) to capture overall health creates an artificial distribution with an interval-scale that cannot be normally distributed, although this is more interpretable for how overall health most likely operates – that is, there is a larger impact on health outcomes between “good” and “fair” than between “fair” and “poor”. Results were similar when utilizing a coding scheme of 4 for excellent, 3 for good, 2 for fair, and 1 for poor (data not shown).
Participants were asked to report their overall health status over the past year, compared to others individuals of a similar age (i.e., “Over the past year, compared to other people your age, would you say that your health has been excellent, good, fair or poor?”). The question was ascertained this way so 1) participants were not under reporting health status by comparing themselves to their younger self, 2) participants were not under (e.g., due to recent disease diagnosis or treatments) or over reporting (e.g., due to recent disease recovery or remission) health status by thinking only of recent health, and 3) participants provide an average health status over the past year, as follow-up was conducted annually. However, if participants were asked to report a more general health status (e.g., “In general, would you say that your health is excellent, good, fair or poor?”) results may have differed. As this study was conducted utilizing existing data, we were not able to influence the choice of SRH question that was included in the annual ARIC surveys.
While SRH is a good indicator of overall health, the ARIC study did not collect annual measurements of other HRQoL domains. Therefore, the extent to which SRH is associated with physical health, mental health, or a combination of these factors cannot be ascertained. For example, depression is significant predictor of poor HRQoL in elderly cancer patients (27). Therefore, addressing psychosocial issues may improve SRH by enhancing self-management techniques (1).
Other comorbidity predictors of decreased overall health included diabetes, chronic lung disease, and CVD. While CVD has similar impacts on overall health trajectories as cancer, individuals with CVD tend to have lower pre-event overall health scores than cancer patients (1, 15). Therefore, it is not surprising that CVD has larger impact on overall health than cancer. Additionally, CVD patients have some of the lowest post-event overall health scores, which are comparable to being post-cancer event (1, 15).
In conclusion, most patients diagnosed with cancer can expect declines in overall health, but among long-term cancer survivors, overall health begins to return to pre-cancer levels. Declines vary by cancer type and may be affected by comorbidities. Studies have shown that the majority of patients value HRQoL at least as much as length of life (28, 29). Targeting factors related to declines in overall health both pre- and post-event could improve health outcomes, specifically HRQoL, and affect treatment decisions for cancer patients.
Acknowledgments
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Studies on cancer in ARIC are also supported by the National Cancer Institute (U01 CA164975-01).
The authors thank the staff and participants of the ARIC study for their important contributions and Dr. Christy Avery for her statistical expertise. Cancer incidence data have been provided by the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Mental Health and Hygiene, 201 W. Preston Street, Room 400, Baltimore, MD 21201. We acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) for the funds that helped support the availability of the cancer registry data.
Footnotes
Conflict of Interest Disclosures: The authors declare that they have no conflict of interest.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Foraker RE, Rose KM, Chang PP, et al. Socioeconomic status and the trajectory of self-rated health. Age Ageing. 2011;40:706–11. doi: 10.1093/ageing/afr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–8. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staren ED, Gupta D, Braun DP. The prognostic role of quality of life assessment in breast cancer. Breast J. 2011;17:571–8. doi: 10.1111/j.1524-4741.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 4.Osthus AA, Aarstad AK, Olofsson J, Aarstad HJ. Health-related quality of life scores in long-term head and neck cancer survivors predict subsequent survival: a prospective cohort study. Clin Otolaryngol. 2011;36:361–8. doi: 10.1111/j.1749-4486.2011.02342.x. [DOI] [PubMed] [Google Scholar]
- 5.Reeve BB, Stover AM, Jensen RE, et al. Impact of diagnosis and treatment of clinically localized prostate cancer on health-related quality of life for older Americans: A population-based study. Cancer. 2012 doi: 10.1002/cncr.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray NM, Hall SJ, Browne S, et al. Modifiable and fixed factors predicting quality of life in people with colorectal cancer. Br J Cancer. 2011;104:1697–703. doi: 10.1038/bjc.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh-Manoux A, Dugravot A, Shipley MJ, et al. The association between self-rated health and mortality in different socioeconomic groups in the GAZEL cohort study. Int J Epidemiol. 2007;36:1222–8. doi: 10.1093/ije/dym170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolinsky FD, Tierney WM. Self-rated health and adverse health outcomes: an exploration and refinement of the trajectory hypothesis. J Gerontol B Psychol Sci Soc Sci. 1998;53:S336–40. doi: 10.1093/geronb/53b.6.s336. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy BS, Kasl SV, Vaccarino V. Repeated hospitalizations and self-rated health among the elderly: a multivariate failure time analysis. Am J Epidemiol. 2001;153:232–41. doi: 10.1093/aje/153.3.232. [DOI] [PubMed] [Google Scholar]
- 10.Wolinsky FD, Miller TR, Malmstrom TK, et al. Self-rated health: changes, trajectories, and their antecedents among African Americans. J Aging Health. 2008;20:143–58. doi: 10.1177/0898264307310449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.Diehr P, Patrick DL. Trajectories of health for older adults over time: accounting fully for death. Ann Intern Med. 2003;139:416–20. doi: 10.7326/0003-4819-139-5_part_2-200309021-00007. [DOI] [PubMed] [Google Scholar]
- 14.Kucharska-Newton AM, Rosamond WD, Mink PJ, Alberg AJ, Shahar E, Folsom AR. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol. 2008;18:671–7. doi: 10.1016/j.annepidem.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diehr P, Williamson J, Patrick DL, Bild DE, Burke GL. Patterns of Self-Rated Health in Older Adults Before and After Sentinel Health Events. Journal of the American Geriatrics Society. 2001;49:36–44. doi: 10.1046/j.1532-5415.2001.49007.x. [DOI] [PubMed] [Google Scholar]
- 16.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 17.Rice NE, Lang IA, Henley W, Melzer D. Baby boomers nearing retirement: the healthiest generation? Rejuvenation Res. 2010;13:105–14. doi: 10.1089/rej.2009.0896. [DOI] [PubMed] [Google Scholar]
- 18.Littell RC, Stroup WW, Freund RJ. SAS for linear models. 4. Cary, NC, USA: SAS Institute; 2002. [Google Scholar]
- 19.Johnson M. Individual Growth Analysis Using PROC MIXED. SAS User Group International; 2002. p. 27. [Google Scholar]
- 20.Latham K, Peek CW. Self-rated health and morbidity onset among late midlife U.S. adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:107–16. doi: 10.1093/geronb/gbs104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver DL, Rosenberg RD, Barlow WE, et al. Pathologic findings from the Breast Cancer Surveillance Consortium: population-based outcomes in women undergoing biopsy after screening mammography. Cancer. 2006;106:732–42. doi: 10.1002/cncr.21652. [DOI] [PubMed] [Google Scholar]
- 22.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: [Google Scholar]
- 23.Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC cancer. 2014;14:272. doi: 10.1186/1471-2407-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 25.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 26.Grutters JP, Joore MA, Wiegman EM, et al. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax. 2010;65:903–7. doi: 10.1136/thx.2010.136390. [DOI] [PubMed] [Google Scholar]
- 27.Garrison CM, Overcash J, McMillan SC. Predictors of Quality of Life in Elderly Hospice Patients with Cancer. J Hosp Palliat Nurs. 2011;13:288–97. doi: 10.1097/NJH.0b013e31821adb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113:3459–66. doi: 10.1002/cncr.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. Bmj. 1998;317:771–5. doi: 10.1136/bmj.317.7161.771. [DOI] [PMC free article] [PubMed] [Google Scholar]