Abstract
Neisseria meningitidis acquires iron through the action of the transferrin (Tf) receptor, which is composed of the Tf-binding proteins A and B (TbpA and TbpB). Meningococci can be classified into isotype I and II strains depending on whether they harbor a type I or II form of TbpB. Both types of TbpB have been shown to differ in their genomic, biochemical, and antigenic properties. Here we present a comparative study of isogenic mutants deficient in either or both Tbps from the isotype I strain B16B6 and isotype II strain M982. We show that TbpA is essential in both strains for iron uptake and growth with iron-loaded human Tf as a sole iron source. No growth has also been observed for the TbpB− mutant of strain B16B6, as shown previously, whereas the growth of the analogous mutant in M982 was similar to that in the wild type. This indicates that TbpB in the latter strain plays a facilitating but not essential role in iron uptake, which has been observed previously in similar studies of other bacteria. These data are discussed in relation to the fact that isotype II strains represent more than 80% of serogroup B meningococcal strains. The contribution of both subunits in the bacterial virulence of strain M982 has been assessed in a murine model of bacteremia. Both the TbpB− TbpA− mutant and the TbpA− mutant are shown to be nonvirulent in mice, whereas the virulence of the TbpB− mutant is similar to that of the wild type.
Neisseria meningitidis is a virulent human pathogen, and infections can lead to death within several hours if untreated (4). The resulting meningococcal disease continues to be a major cause of mortality throughout the world (23). There is so far no universal vaccine against serogroup B meningococci available, which would undoubtedly be a major breakthrough in the control and prevention of this disease.
The identification and characterization of the proteins essential for virulence are of high importance in the study of the pathogenesis of infectious agents. Such information is furthermore helpful for the definition of an effective vaccine strategy.
The two subunits of the transferrin (Tf) receptor, Tf-binding proteins A and B (TbpA and TbpB), were shown to be candidates for such vaccines (17, 31). Through the action of this receptor, N. meningitidis is able to acquire iron, an essential element for bacterial growth. The Tf receptor has the capacity to specifically bind human TF (hTf) and to extract and internalize bound iron (11). TbpA (100 kDa) is thought to be a porin-like integral membrane protein, which is proposed to serve as a channel for the transport of iron across the outer membrane. TbpA shares sequence similarities with FepA and FhuA (16). Both proteins have been shown to form an antiparallel β-barrel (6, 18), and TbpA is thought to have a similar structure (30). TbpB (65 to 90 kDa) is considered to be an outer membrane protein, which is anchored to the membrane via the lipidated N-terminal part of the protein (11). The association of both proteins to the receptor has been demonstrated (5).
TbpB of N. meningitidis strain B16B6 was shown in vitro to play an essential role in iron acquisition from hTf (12). The authors demonstrate that isogenic mutants deficient in either TbpA or TbpB have reduced Tf-binding activity, resulting in the inability to use hTf as a sole iron source for their growth.
The increasing amount of sequence information available on TbpB of N. meningitidis has lead to the definition of two isotypes (26, 28). Both isotypes share sequence similarities but differ substantially in size. TbpBs of isotype I, such as the above-mentioned strain B16B6, typically have a molecular mass of around 68 kDa, whereas isotype II proteins are characterized by a molecular mass of between 80 to 90 kDa. It has been shown recently that the thermodynamic mode of hTf binding is fundamentally different for isotype I and II TbpBs (15). The evolutionary reasons leading to this differentiation are unknown. However, the genetic analysis of a collection of diverse N. meningitidis strains reveals that bacteria harboring an isotype II TbpB are more frequently found (82%) than isotype I-containing strains (28). Furthermore, TbpB sequences of Neisseria gonorrhoeae have a higher degree of sequence identity to N. meningitidis isotype II than to isotype I (8), which might indicate that the isotype II TbpB is the predominant form in pathogenic Neisseriae. Therefore, experiments on TbpB isotype I strains, such as B16B6, cannot be considered as being fully representative for N. meningitidis.
Isogenic TbpA and TbpB mutants have also been prepared for other pathogens such as N. gonorrhoeae (2), Haemophilus influenzae (10), and Moraxella catarrhalis (19). The three studies concur in the result that TbpA, but not TbpB, is essential for iron acquisition from Tf, and a facilitating role in iron uptake has been proposed for the latter protein. These data are in strong contrast to the situation described for the N. meningitidis gene tbpB of isotype I strain B16B6 in which both receptor subunits were found to be essential for growth on hTf as a sole iron source (12).
In the light of these differences and the fact that N. meningitidis isotype II is more representative for serogroup B meningococcal strains, we have readdressed the question of the role of TbpB and TbpA in iron uptake by using the isotype II strain M982. Here we present a comparative analysis of isogenic mutants deficient in either or both Tbps of the isotype I strain B16B6 and the isotype II strain M982. Moreover, this study is the first analysis of the contribution of each individual Tbp subunit to the virulence of the meningococcus.
MATERIALS AND METHODS
Plasmids and bacterial strains.
The plasmids and bacterial strains used in this study are listed in Table 1. Meningococcal tbpB isotype I and II strains were grown on Mueller-Hinton Agar (MHA) plates or Mueller-Hinton broth (MHB) medium with or without the addition of different concentrations of an iron chelator, as described previously (28). Erythromycin-resistant N. meningitidis cells were selected on MHA plates containing the antibiotic at a concentration of 4 μg/ml. Recombinant Escherichia coli strains were cultured on Luria-Bertani plates containing appropriate antibiotics (29).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and phenotype | Source or reference |
|---|---|---|
| N. meningitidis strains | ||
| B16B6 | tbpB-isotype I wild type | C. Frasch |
| N16T2K | B16B6 tbpB::aphA-3 (Kmr) | 12 |
| N16T1E | B16B6 tbpA::erm (Ermr) | 12 |
| N16T12EK | B16B6 tbpB::aphA-3 tbpA::erm(Kmr Ermr) | 12 |
| M982 | tbpB-isotype II wild type | C. Frasch |
| PMC202 | M982 tbpB::ermAM inserted in orientation 1 (Ermr) | This study |
| PMC203 | M982 tbpB::ermAM inserted in orientation 2 (Ermr) | This study |
| PMC205 | M982 ΔtbpB | This study |
| PMC206 | M982 tbpA::ermAM (Ermr) | This study |
| PMC207 | M982 ΔtbpB tbpA::ermAM (Ermr) | This study |
| E. coli strain XL-1 Blue Kanr | Stratagene | |
| Plasmids | ||
| pTG1265 | Low-copy-number plasmid | 16 |
| pTG3720 | pTG1265 with XbaI fragment including tbpB and tbpA genes | 16 |
| pMGC10 | Source for ermAM (Ermr) cassette | X. Nassif |
| pIM200 | pTG1265 with tbpB 3′ region | This study |
| pIM201 | pIM200 with tbpB 5′ region | This study |
| pIM202 | pIM201 with ermAM in orientation 1 | This study |
| pIM203 | pIM201 with ermAM in orientation 2 | This study |
| pIM204 | pUC19 with tbpA 5′ region | This study |
| pIM205 | pIM204 with tbpA 3′ region | This study |
| pM1201 | pIM200 with tbpB 5′ region before its ATG | This study |
| pM1206 | pIM205 with ermAM in orientation 1 | This study |
Construction of two TbpB-defective M982 mutants by deletion and replacement of the tbpB gene with an antibiotic resistance marker.
All genetic constructions required for homologous recombination in the strain N. meningitidis M982 (B:9:P1.9) were produced by PCR amplification by using Tfu polymerase (Qbiogene) and the plasmid pTG3720 as a matrix. The plasmid pTG3720 (16) contains a 5,971-bp XbaI fragment harboring both the tbpB gene (ATG at position 762 to TAA at position 2898) and the tbpA gene (ATG at position 2984 to TAA at position 5720).
A fragment corresponding to the tbpB recombigenic 3′ region including a DNA uptake sequence has been amplified by PCR from pTG3720 (position 2878 to 3378) by using the oligonucleotides 5′-AAAGGATCCCGCCAACAGCCTGTGCAATAAG-3′ and 5′-AAAACTGCAGCGTCCCGCCCAATGCCGC-3′ which harbor restriction sites for BamHI or PstI, respectively. This fragment has been cloned into plasmid pTG1265 (16), linearized by BamHI and PstI. The resulting plasmid pIM200 (Table 1) was then linearized by EcoRI and BamHI for the insertion of the tbpB recombigenic 5′ region; this region corresponds to a PCR fragment amplified from plasmid pTG3720 (position 827 to 1323) by using the oligonucleotides 5′-AAGAATTCCTGGGCGGCGGCGGCAG-3′ and 5′-ATTGGATCCACCGTGATAGAAATAACC-3′ harboring restriction sites for EcoRI and BamHI, respectively. The resulting plasmid was named pIM201 (Table 1). This plasmid was digested by BamHI, dephosphorylated with calf intestine phosphatase (Biolabs), for subsequent cloning of the erythromycin cassette previously amplified from plasmid pMGC10 (kindly provided by X. Nassif) by using the following oligonucleotides including a BamHI restriction site: 5′-AAGGATCCGGAAGGGCCGAGCGCAGAAGT-3′ and 5′-AAGGATCCCAACTTACTTCTGACAACGATCGG-3′. Two plasmids were selected, termed pIM202 and pIM203 (Table 1), which contained the 1.2-kb erythromycin resistance marker inserted in either the sense of tbp transcription (orientation 1) or in the opposite orientation (orientation 2), respectively.
The strain M982 was then transformed according to a modified version of a previously described method (22) with 10 μg of either plasmid pIM202 or pIM203, both linearized by PstI. The resulting M982 transformants were named PMC202 and PMC203, respectively (Table 1). These mutants were selected for their ability to grow on MHB medium supplemented with erythromycin at a concentration of 4 μg/ml. Three Ermr transformants were analyzed by Western blotting (see description in legend of Fig. 2) to verify the absence of TbpB production. One mutant was retained after genetic characterization at the tbpB chromosomal locus both by Southern blotting with an erythromycin-based probe and by PCR analysis of genomic DNA isolated from each mutant (data not shown).
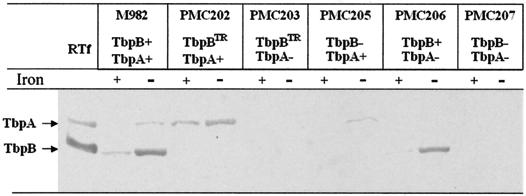
FIG. 2.
Production of TbpA and TbpB in the different meningococcal TbpB-, TbpA-, or Tbp-defective M982 mutants under iron-rich (+) or iron-poor (−) growth conditions, which correspond to culture media without and with EDDHA, respectively. The detection after Western blot analysis was performed with a rabbit anti-Tbp complex purified from strain M982. The analysis of purified Tf receptor (RTf) is shown as a reference in the first lane. Each lane has been charged with the same number of cells, and data can be regarded to be semiquantitative.
Construction of a TbpB-defective M982 mutant without the introduction of an antibiotic resistance marker.
To avoid any production of truncated TbpB, a third TbpB− M982 mutant was successfully constructed by removing the residual fragment of the tbpB gene including the ATG codon.
The new tbpB recombigenic 5′ region was amplified with an Expand Long Template PCR kit (Roche) on pTG3720 from position 207 to 761 by using the oligonucleotides 5′-AAGAATTCGCTTGTGGGTATTTACCGG-3′ and 5′-ATTGGATCCATAAACCCAATTCAATTAAAGAATGATAAGG-3′, which include restriction sites for EcoRI and BamHI, respectively. The corresponding PCR fragment was cloned into pCR2.1 TOPO (Invitrogen), which was validated by DNA sequencing. The resulting recombinant PCR2.1 was digested by EcoRI and BamHI, and the tbpB 5′ recombigenic region was cloned into pIM200 previously opened with the same enzymes. The resulting plasmid was named pIM1201 (Table 1). Plasmid pIM1201 (10 μg) was linearized by EcoRI and used to transform strain M982 by homologous recombination (Table 1). A resulting mutant, termed PMC205, was selected after colony blotting and hybridization with the following [γ32-P]dATP-labeled oligonucleotide: 5′-TGGGTTTATGGATCCGCGCAACAGT-3′. This oligonucleotide overlaps both the region at position 753 to 761 (TGGGTTTATGGATCC) just before the tbpB ATG codon, including an added BamHI restriction site, and the region at position 2878 to 2886 (GCGCAACAGT) of the 3′ tbpB recombigenic region. The deletion at the chromosomal locus of tbpB in PMC205 was validated by PCR analysis of genomic DNA isolated from this mutant (data not shown).
Construction of TbpA-defective and Tbp-defective M982 mutants.
The 288-bp tbpA recombigenic 5′ region was obtained by PCR amplification of pTG3720 (position 2696 to 2984) with the oligonucleotides 5′-AAGAATTCCCACCCGCACGCC-3′ and 5′-AAAGGATCCAATGTTTCCCTAATC-3′, including restriction sites for EcoRI or BamHI, respectively. This sequence is located immediately before the tbpA ATG codon and contains an uptake sequence. The resulting PCR fragment was subsequently cloned into pCR-TOPO Blunt (Invitrogen). After digestion of the recombinant pCR-TOPO with EcoRI and BamHI, the tbpA recombigenic 5′ fragment was purified with a GeneClean Concert Kit (Bio 101) and cloned into the plasmid pUC19 previously digested with EcoRI or BamHI. The resulting plasmid was named pIM204 (Table 1). The 226-bp tbpA recombigenic 3′ region was obtained by PCR amplification of pTG3720 (position 5516 to 5742) with oligonucleotides 5′-CGGGATCCGGATGTGTCCGGTTATTACACG-3′ and 5′-AAAACTGCAGGGCATTTGCGGCGTTTGGAC-3′ containing a BamHI or PstI restriction site. This fragment was subsequently cloned into pCR-TOPO Blunt. After digestion of the recombinant PCR-TOPO with BamHI and PstI, the tbpA recombigenic 3′ fragment was purified with a GeneClean Concert Kit and cloned into plasmid pIM204, previously digested with BamHI and PstI. The resulting plasmid was named pIM205 (Table 1). The ermAM cassette harboring at both extremities a BamHI site (see above) was then cloned into pIM205 previously opened by BamHI and dephosphorylated with calf intestine phosphatase. The resulting plasmid pM1206 (Table 1) contains the erythromycin marker in orientation 1, which corresponds to the orientation of tbp transcription. Linearized pM1206 (10 μg) was then introduced into either M982 or PMC205 and erythromycin-resistant transformants were selected on MHA plates containing the antibiotic at a concentration of 4 μg/ml. Three individual Ermr transformants were analyzed to verify the absence of the production of either TbpA or both receptor proteins by Western blotting (see description in legend of Fig. 2). One mutant of each type, termed PMC206 and PMC207, was retained for genetic characterization at the chromosomal locus of tbpA by PCR analysis of genomic DNA isolated from PMC206 or PMC207 (data not shown).
Tf-binding assays. (i) Dot blot with whole cells.
The protocol for the dot blot assay with meningococcal cells and Tf binding has been described previously (27). Briefly, bacterial suspensions (2 × 109 CFU/ml) grown under iron starvation conditions were twofold serially diluted, and 50 μl of each dilution was spotted under vacuum onto nitrocellulose filters (0.45-μm pore size; Schleicher & Schuell). The filters were incubated with hTf-peroxidase (Jackson ImmunoResearch Laboratories), and the reaction was detected with the colorimetric substrate 4-chloro-1-naphtol.
(ii) Growth on different iron sources measured by a disk-diffusion assay.
The ability of tbp isogenic mutants from strains B16B6 and M982 to use various iron sources has been determined by using a disk-diffusion assay adapted from Zhu et al. (32). A bacterial suspension grown in MHB containing 200 μM ethylenediamine di-o-hydroxyphenylacetic acid (EDDHA) was plated onto MHA plates containing 200 μM EDDHA. Sterile filter disks (Prolabo, Fontenay-sous-Bois, France) were placed onto these plates after being impregnated with 10-μl aliquots of the following iron sources: 250 μM iron-loaded (holo-) human lactoferrin (hLf), 250 μM iron-poor (apo-) hTf, and 250 μM holo-hTf. Apo- and holo-hTf were purchased from Intergen, and hLf was from Sigma. Zones of growth around the disks were measured after 24 h of incubation at 37°C in an environment containing 10% CO2.
(iii) Growth with iron sources in broth.
Growth experiments were carried out in MHB medium at 37°C in which iron was limited by the addition of EDDHA. Iron-loaded or iron-poor hTf was added to the cultures in the form of holo-hTf or apo-hTf, respectively. Aliquots were removed every hour to measure the optical density at 600 nm.
Bactericidal assays with rabbit IgG.
The bactericidal activity of purified rabbit immunoglobulin G (IgG) has been determined by using a slightly modified version of the protocol reported by Rokbi et al. (28). Briefly, 50 μl of twofold serial dilutions of IgG preparations was added to 96-well microtiter plates (Nunc) and incubated with 25 μl of an iron-starved meningococcal suspension from each isogenic mutant, adjusted to 2 ×104 CFU/ml, and 25 μl of baby rabbit complement. After 60 min of incubation at 37°C, 20 μl of the mixture from each well was plated onto MHA plates. The plates were incubated overnight at 37°C in an environment containing 10% CO2. The bactericidal titer for each IgG was expressed as the inverse of the last dilution of serum at which ≥50% killing was observed compared to the complement control.
Virulence assay of mutants in a murine model of bacteremia.
A suspension of 0.5 ml of native or mutant N. meningitidis at a concentration of 1.5 × 108 CFU/ml, grown under iron restriction in MHB containing 30 μM EDDHA, was injected into 6-week-old female CD1 outbred mice via the intraperitoneal route. Prior to this injection, all mice received 24 mg of iron-loaded hTf by the intraperitoneal route, as described previously (20). At 3 and 24 h after the infection with the different meningococcal mutants, 50-μl blood samples were taken from the orbital plexus in order to assess the bacteremia. The degree of animal mortality was registered until 48 h after the challenge.
RESULTS
Construction of a TbpB-defective isogenic mutant of N. meningitidis tbpB isotype II strain M982.
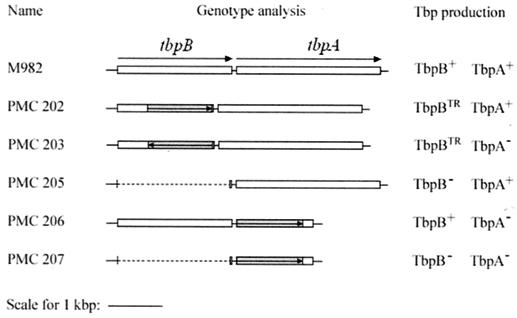
The initial strategy to generate a TbpB-defective mutant was based on the replacement of a large part of the tbpB gene by an erythromycin cassette (ermAM) (Fig. 1). The erythromycin-resistant determinant was amplified by PCR from pMGC10 (see Material and Methods), and the resulting fragment was cloned into pIM201 (Table 1). After transformation with either of the linearized plasmids pIM202 or pIM203, mutant clones were selected for their ability to grow on MHB medium containing erythromycin. TbpB mutants were screened for the loss of reactivity with TbpB-specific antibodies and for the iron-inducible production of TbpA. PMC202 and PMC203 (Fig. 1) were shown to harbor the ermAM gene either in the same or in the opposite orientation with respect to the sense of tbpB transcription, respectively.
FIG. 1.
Schematic representation of the different isogenic TbpB-, TbpA-, or Tbp-defective mutants from N. meningitidis tbpB isotype II strain M982. Gray regions correspond to the positions of the erythromycin cassettes, arrows indicate the orientations of the insertion of the erythromycin cassettes, and dashed lines indicate the deletions of DNA sequences. TR, truncated TbpB.
Mutants have been analyzed by Western blotting with polyclonal anti-Tf receptor antibodies of strain M982 used as probes (Fig. 2). Bands corresponding to TbpA and TbpB are clearly seen when purified Tf receptor was used for a reference (Fig. 2, lane 1). The analysis of wild-type M982 shows that only small amounts of TbpB are produced under iron-rich conditions. However, both proteins are induced following the addition of the chelator EDDHA to the bacterial culture, which results in iron-poor conditions. No TbpB was detected with Tbp-specific antibodies in either the PMC202 or the PMC203 mutant (Fig. 2). In contrast to the wild type, mutant PMC202 produces TbpA both under iron-rich and iron-poor conditions, and mutant PMC203 fails to produce TbpA (Fig. 1 and 2). These data suggest that these two TbpB-defective mutants are not isogenic to their wild-type M982 counterpart since their production of TbpA is different from that of M982.
Western blot analysis is carried out following the separation of proteins in the presence of the denaturing agent sodium dodecyl sulfate, and it cannot be excluded that this treatment alters the properties of the protein. Therefore, the presence of TbpB and TbpA epitopes on the bacterial surface has been evaluated by a determination of the bactericidal activity towards the mutants in the presence of IgG preparations directed against the entire Tf receptor or against different parts of TbpB (Table 2). The presence of bactericidal activity can be regarded as evidence that added IgG samples recognize parts of the Tf receptor on the bacterial surface. Apart from the detection of residual epitopes of the mutated receptor subunit, such analyses permit verification of the correct export and insertion of the second nonmutated receptor subunit into the outer membrane. Furthermore, experiments have been carried out in the presence or absence of iron to verify correct protein induction. Iron starvation leads to the induction of TbpA and TbpB, which in the past has been shown to lead to a more pronounced bactericidal activity of the different IgG preparations (28).
TABLE 2.
Bactericidal activity of Tbp- and TbpB-specific polyclonal IgG against wild type and Tbp mutants
| IgG preparation specific for:a | Titer for strain (phenotype) grown without (−) or with (+) EDDHAb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M982 (TbpB+ TbpA+)
|
PMC202 (TbpBTR TbpA+)
|
PMC203 (TbpBTR TbpA−)
|
PMC205 (TbpB− TbpA+)
|
PMC206 (TbpB+ TbpA−)
|
PMC207 (TbpB− TbpA−)
|
|||||||
| − | + | − | + | − | + | − | + | − | + | − | + | |
| Tbp complex | 64 | 512 | 128 | 512 | <4 | <4 | [4] | 256 | 64 | 512 | [4] | <4 |
| TbpB | 128 | 1,024 | <4 | 32 | <4 | [8] | <4 | <4 | 256 | ≥2,048 | [4] | <4 |
| MBP-TbpB full length | 32 | 256 | <4 | 64 | <4 | [16] | <4 | <4 | 128 | 1,024 | <4 | <4 |
| MBP-TbpB N-terminal domain | ≤4 | 32 | <4 | 64 | <4 | 16 | <4 | <4 | <4 | 32 | <4 | <4 |
| MBP-TbpB C-terminal domain | 32 | 256 | <4 | <4 | <4 | <4 | <4 | <4 | 64 | 2,048 | <4 | <4 |
| MBP alone | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 | <4 |
All IgG preparations were elicited with protein derived from strain M982. MBP-TbpB is a recombinant fusion protein (25).
Bactericidal titers were determined on N. meningitidis M982 wild type and Tbp mutants grown under iron-containing (without EDDHA) and iron-limiting (with EDDHA) conditions. The bactericidal titer is expressed as the reciprocal of the last purified IgG dilution in the presence of which at least 50% of the initial bacterial load was killed. In most cases, IgG preparations were tested at least twice. A titer in brackets indicates incomplete killing. Experiments have been carried out in triplicate. The error observed for all analyses was not superior to one dilution. The values given correspond to the titer, which has been observed in at least two of the three experiments. TR, truncated TbpB.
Polyclonal IgG elicited with the purified Tf receptor (TbpA and TbpB) was bactericidal against wild-type M982, and an increase in bactericidal activity was observed following iron starvation (Table 2). This was also the case when the experiments were repeated with antibodies directed against purified TbpB or a maltose binding protein (MBP)-TbpB fusion protein.
The bactericidal activities observed with IgG raised against the entire receptor (TbpA plus TbpB) are similar to those of the wild-type M982 and mutant PMC202 (Table 2). However, a residual bactericidal activity is noted for antibodies directed against the full-length TbpB and its N-terminal domain, whereas no activity is observed for the anti-TbpB C-terminal domain. This finding indicates that the short fragment corresponding to the TbpB N terminus present in mutant PMC202 (Fig. 1) is folded in a native-like manner and is recognized by anti-TbpB, giving rise to some bactericidal activity (Table 2). Similar residual bactericidal activities were detected for mutant PMC203 by using IgG preparations against full-length TbpB or its N-terminal domain.
From the Western blotting and bactericidal analyses, it can be concluded that mutants PMC202 and PMC203 are not isogenic to their wild type. In PMC202 it appears likely that the ermAM promoter may actually be responsible for the deregulation of TbpA expression. In PMC203 the insertion of the ermAM cassette might cause a polar mutation, which interferes with the expression of TbpA. The construction of a tbpB-deficient mutant has been attempted by an alternative approach, which is not based on the insertion of an antibiotic cassette. This mutant has been obtained after transformation with the linearized plasmid pIM201 (Table 1), which did not contain the 5′ coding region of the tbpB gene. Mutant clones were selected by colony blotting with a radiolabeled 25-mer oligonucleotide which corresponds to the 10 bp immediately before and the 10 bp immediately after the expected deletion (Fig. 1) (see Material and Methods for more details). The selected tbpB deletion mutant PMC205 was characterized by immunoblotting (Fig. 2) and submitted to the bactericidal assay (Table 2). The Western blot analysis of PMC205 (Fig. 2) shows an iron-inducible production of TbpA under the control of the meningococcal tbp promoter, which was comparable to that of the wild type. No Western -blotting reactivity is seen for TbpB. The only IgG shown to induce bactericidal activity against mutant PMC205 was the sample directed against the entire receptor. This activity is clearly due to the action of antibodies directed against TbpA since no anti-TbpB antibodies showed any detectable activity (Table 2). These data illustrate that mutant PMC205 can be regarded as an isogenic TbpB-defective M982 mutant with respect to the wild type.
Construction of isogenic M982 mutants defective for TbpA or Tbp.
TbpA- and TbpA-plus-TbpB (Tbp)-defective mutants were constructed by homologous recombination by using linear DNA corresponding to the 5′ region before the coding region of the tbpA gene (first recombigenic region), the ermAM gene, and the 3′ end of tbpA as a second recombigenic region (see Materiel and Methods for more details). The linearized plasmid pM1206 was introduced into strains M982 or PMC205, respectively, giving rise to the TbpA-deficient mutant PMC206 or to mutant PMC207 deficient in Tbp (Table 1 and Fig. 1).
Mutant PMC206 showed no Western blot reactivity with TbpA, whereas the iron-induced production of TbpB was comparable to that of the wild type (Fig. 2). No bands were seen for the Tbp-deficient mutant PMC207. The analysis of bactericidal activity against mutants PMC206 and PMC207 confirmed the mutation. A strong bactericidal activity was observed for mutant PMC206 with IgG specific for full-length TbpB or its recombinant domains (Table 2). It has to be noted that the bactericidal effect achieved by using antibodies against the C-terminal domain was significantly elevated for mutant PMC206, which might be consistent with the exposure of epitopes caused by the absence of TbpA. No IgG preparation had bactericidal activity towards mutant PMC207, confirming that this mutant was devoid of the entire Tf receptor (Table 2).
hTf-binding of the Tbp complex expressed by M982 Tbp mutants: comparison with strain B16B6 Tbp mutants.
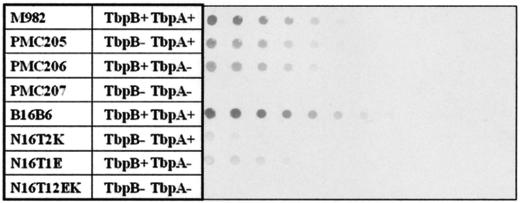
Strains B16B6 and M982 can be considered as representative for TbpB isotype I and II strains, respectively (26). The capacity to bind horseradish peroxidase (HRP)-conjugated hTf has been assessed for native and TbpA- or TbpB-deficient mutants of strains B16B6 and M982 by dot blot analysis (Fig. 3). The construction and analysis of the B16B6 mutants has been reported by Irwin et al. (12).
FIG. 3.
HRP-hTf binding of TbpB-, TbpA-, or Tbp-defective M982 and B16B6 isogenic mutants (indicated at left) by dot blot analysis with whole cells. Serial bacterial dilutions of cells were dotted onto nitrocellulose as described in Materials and Methods. Blots were treated with HRP-conjugated hTf (1μg/ml), partially saturated with iron.
Mutants of both strains in which only one receptor subunit has been expressed were shown to bind Tf, which is consistent with the previous observation that purified TbpA and TbpB can independently bind Tf (7). In both cases, the TbpB-TbpA double mutants were devoid of any hTf binding. Dot blots were probed with a low ligand concentration of 1 μg/ml of HRP-hTf. Dot blot data have to be interpreted with caution since they reflect differences in the affinity and/or differences in protein expression. Assuming that proteins are equally expressed, all single mutants appear to have a lower binding affinity than the wild-type strain. This finding is consistent with a recent proposition (15) that the association of TbpB and TbpA to the receptor is accompanied by an overall increase in affinity for hTf compared to the binding of the ligand to the two individual receptor subunits. Wild-type B16B6 has a higher affinity for hTf than M982. The major difference between both sets of mutants is the dramatic reduction of hTf binding for the B16B6 TbpB-defective mutant N16T2K (Fig. 3). The corresponding TbpB-defective mutant from strain M982 had a much lower impact on hTf binding.
Iron-dependent growth studies of M982 Tbp mutants: comparison with B16B6 Tbp mutants. (i) Growth studies on solid media containing holo-hTf.
Initial information concerning the capacity of the mutants to grow on different iron sources has been obtained by the disk diffusion assay. All strains showed no growth in the presence of apo-hTf and normal growth in the presence of holo-hLf as a sole iron source (Table 3).
TABLE 3.
Growth phenotypes of TbpB- and/or TbpA-defective M982 and B16B6 isogenic mutants with different iron sources
| Strain | Tbp produced | Growth observed after 24 h with:a
|
||
|---|---|---|---|---|
| Holo-hLF | Apo-hTf | Holo-hTf | ||
| M982 | TbpA+ TbpB+ | + | − | + |
| PMC205 | TbpA+ TbpB− | + | − | + |
| PMC206 | TbpA− TbpB+ | + | − | − |
| PMC207 | TbpA− TbpB− | + | − | − |
| B16B6 | TbpA+ TbpB+ | + | − | + |
| N16T2K | TbpA+ TbpB− | + | − | − |
| N16T1E | TbpA− TbpB+ | + | − | − |
| N16T12EK | TbpA− TbpB− | + | − | − |
Growth was determined by a disk diffusion assay. See Materials and Methods and reference 32. +, growth ≥2 mm surrounding the disk; −, no growth.
When holo-hTf was the only iron source, both wild-type strains B16B6 and M982 were found to grow. Among all the mutants tested, only the M982 TbpB− TbpA+ mutant PMC205 grew, which is in strong contrast to the corresponding mutant N16T2K of the isotype I strain B16B6, which showed no growth (Table 3). The incapacity of the B16B6 TbpB− mutant N16T2K to grow on holo-hTf has been reported previously (12).
(ii) Growth studies on liquid media containing holo-hTf.
The growth of the two TbpB− TbpA+ mutants has been analyzed in more detail by using liquid cultures and conditions similar to the method of Irwin et al. (12). Cultures of PMC205 and N16T2K were carried out in the presence of 100 μM EDDHA to chelate free iron and 5 μM holo-hTf, which serves as the sole iron source. Under these conditions, the M982 mutant PMC205 was shown to grow in a similar fashion compared to M982 (data not shown). However, in strong contrast, the B16B6 mutant N16T2K was unable to grow under the conditions described above (data not shown). This result indicates that in M982, TbpA alone is able to acquire iron, which is not the case for the strain B16B6, as has previously been reported by Irwin et al. (12).
(iii) Growth studies on liquid media containing a mixture of holo- and apo-hTf.
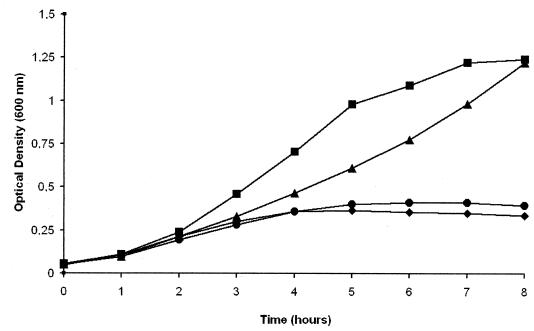
The above growth experiments were carried out with holo-hTf used as a supplement. In human body liquids however, Tf is present as a mixture of holo- and apo-hTf (14). To study the mutants under conditions that were closer to the physiological situation, growth experiments were carried out with a 20:80 (wt/wt) mixture of holo- and apo-hTf as a supplement (Fig. 4). The TbpB+ TbpA− mutant PMC206 did not grow in the presence of EDDHA and the Tf mixture, confirming that no iron acquisition occurs in the absence of TbpA. No growth was equally observed for the M982 wild type grown under iron-poor conditions in the absence of any added Tf. In contrast, a sigmoid growth curve was observed for the wild type grown in the presence of EDDHA and the Tf mixture. Under the same conditions the TbpB− TbpA+ mutant PMC205 grew (Fig. 4). The growth kinetics of PMC205 was, in contrast to that of the wild-type M982, rather exponential since initially a slower growth rate was observed, which was then followed by accelerated growth, reaching after 8 h an optical density similar to that of the wild type.
FIG. 4.
Growth phenotype of the wild-type strain M982 and its TbpB- or TbpA-defective isogenic mutants with a mixture of apo- and holo-hTf used as the sole iron source. Growth in the presence of 200 μM EDDHA supplemented with a mixture of 2 μM holo-hTf and 8 μM apo-hTf. (▪), wild-type M982; (▴), TbpB− TbpA+ mutant PMC205; (♦), TbpB+ TbpA− mutant PMC206; (•), control (growth of M982 in the presence of EDDHA only).
Virulence analysis of N. meningitidis strain M982 and its TbpB- or/and TbpA- defective mutants in a murine model of bacteremia.
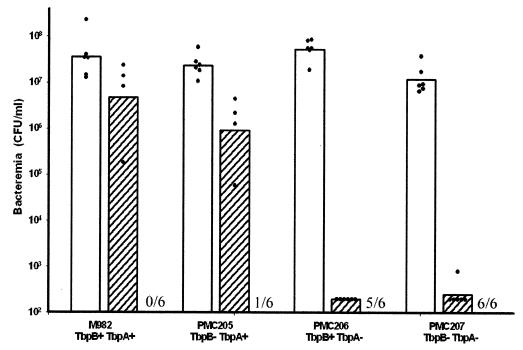
Data presented so far provide evidence that TbpA, but not TbpB, of isotype II strain M982 is essential for growth on hTf as a sole carbon source. Htf is the dominating iron source in human blood, and it can thus be hypothesized that TbpA plays a stronger role in virulence than TbpB. This hypothesis has been verified by animal experimentation. Outbred CD1 mice were injected with the same amount of M982 wild type as well as with mutants PMC205, PMC206, and PMC207. Prior to all injections with meningococcal strains, mice received 24 mg of holo-hTf as described previously by Oftung et al. (20). No mortality was observed when bacteria were injected without previous administration of holo-hTf. Three hours after injection, the three mutants and the wild type caused a similarly high bacteremia (Fig. 5). However, after 24 h of incubation, the bacteremia was found to be dramatically reduced for the TbpB+ TbpA− and the TbpB− TbpA− mutants PMC206 and PMC207. In strong contrast, only a slight reduction in bacteremia is seen in mice infected with the TbpB− TbpA+ mutant PMC205 compared to those infected with the wild-type M982 (Fig. 5). Moreover, two mice died 24 h after injection with both M982 and PMC205, indicating the similar virulence of the TbpB-defective mutant compared to its wild-type counterpart. After a period of 48 h, all animals that received the wild-type strain died, and only one of six mice that were exposed to PMC205 survived (Fig. 5). No or little mortality has been registered for animals that received the Tbp-deficient mutant PMC207 or the TbpA-defective mutant PMC206, respectively (Fig. 5). These data correlate with the growth studies and point to an important role of TbpA in the virulence of N. meningitidis strain M982, whereas the role of TbpB appears to be minor in this murine model of bacteremia.
FIG. 5.
Determination of the extent of meningococcal bacteremia in individual mice following injection of N. meningitidis tbpB isotype II strain M982 and its mutants defective in either or both Tbps. Bacteremia (CFU/milliliter) was determined in individual murine blood samples taken 3 (open bars) and 24 h (striped bars) after injection of each strain. The number of mice (n = 6) who survived was registered 48 h after injection, which is indicated at the bottom of the graph. The bars indicate the geometric average of the bacteremic results.
DISCUSSION
Isogenic mutagenesis studies of TbpA and TbpB in N. gonorrhoeae, H. influenzae, and M. catarrhalis have demonstrated that TbpA is essential for iron uptake from Tf, whereas the absence of TbpB gave rise only to a reduced iron uptake capacity which, however, did not prevent the mutant from growing (2, 10, 19). This finding is consistent with the proposition that TbpA forms a pore through which iron is internalized (30) and that the outer membrane protein TbpB plays only a facilitating role (2).
However, isogenic mutagenesis studies of N. meningitidis tbpB isotype I strain B16B6 do not seem to be in agreement with the above data since both Tbps were found to be essential for iron uptake and growth (12). The aim of the present article is to reassess the role of TbpB in iron uptake by a comparative analysis of Tbp isogenic mutants of tbpB isotype I strain B16B6 and tbpB isotype II strain M982. Further analyses were aimed at evaluating the contribution of both receptor subunits to virulence.
Our results on isogenic mutants of strain B16B6 (Table 3) confirm the data reported by Irwin et al. (12), who have demonstrated that the deletion of either or both Tbps results in a failure to grow on hTf as a sole iron source. This result, however, was in sharp contrast to the data obtained with the tbpB isotype II strain M982. Deletion of TbpA prevented bacterial growth (Table 3), whereas the TbpB-deficient mutant was characterized only by a slower growth kinetics (Fig. 4). These data are in agreement with the above-mentioned reports on N. gonorrhoeae, H. influenzae, and M. catarrhalis (2, 10, 19).
There is now general agreement that TbpA is essential for iron uptake, and we are thus unable to confirm the report by Pintor et al. (24), who demonstrated significant iron uptake and growth of a TbpA-deficient mutant of strain B16B6.
Our data and the studies of N. gonorrhoeae, H. influenzae, and M. catarrhalis demonstrate that significant growth is observed in the absence of TbpB (Fig. 4), which thus raises the question of its functional role. Recent in vitro studies (15) have shown that individual TbpA binds apo-hTf with a significantly higher affinity than holo-hTf. The presence of TbpB at TbpA (as in the complete receptor), however, alters the substrate specificity at TbpA, giving rise to a preferential binding of the holo form with an affinity superior to the binding of apo-hTf to individual TbpA proteins. It has been proposed that the functional role of TbpB is to shift the substrate specificity towards holo-hTf, which facilitates iron uptake (15). This would imply that in a medium containing a mixture of apo- and holo-hTf, thus reflecting the physiological situation, mainly apo-hTf binds at TbpA in the TbpB-deficient mutant PMC205, which might give rise to a reduction in iron uptake and to slower growth. The binding of apo-hTf to TbpA produced in mutant PMC205 is likely to be the cause for the initial lack of growth observed with this mutant (Fig. 4). This preferential binding of apo-hTf, however, did not prevent the mutant from growing and thus did not block iron uptake, as proposed by Baltes et al. (3).
The comparative growth analysis of mutants from strains B16B6 and M982 (Table 3) indicates that TbpB is essential for isotype I strains, whereas it plays only a facilitating role for the isotype II strains. The genetic, biochemical, and antigenic differences observed for isotype I and II TbpBs are, thus, further reflected in differing functional roles, which still need to be identified.
The conclusion that generally TbpA, but not TbpB, is essential for iron uptake is furthermore confirmed by a report from Ogunnariwo and Schryvers (21), who demonstrate that the Tf receptor of Pasteurella multocida consists only of a single receptor protein, TbpA. The authors were unable to generate any genetic or biochemical evidence for the existence of TbpB in this bacteria.
Several IgG preparations have been used to test their bactericidal activities against the M982 mutants. These experiments have been performed to validate the mutations, but data obtained also provide valuable information on the molecular arrangement of the receptor subunits and their immunogenic potential.
Ala'Aldeen and Boriello (1) have evaluated the bactericidal activity of anti-Tbp sera against wild-type strain B16B6 as well as its mutant strains N16T1E and N16T2K, which are deficient in TbpA and TbpB, respectively. The authors observed similar killing effects for the parent and both mutant strains and concluded that both receptor subunits were surface exposed and capable of inducing bactericidal antibodies. Here we report analogous data on the tbpB isotype II strain M982. In agreement with Ala'Aldeen and Boriello (1), we show that the bactericidal titer produced by using antibodies raised against the Tbp complex is similar for the wild-type M982 and mutant PMC205 and PMC206 strains (Table 2), which confirms the conclusion drawn from the analysis of the B16B6 mutants.
However, the above-cited authors did not rule out that the killing effects observed were due to the presence of antibodies directed against discontinuous epitopes of non-Tbp antigens. To verify this hypothesis, the authors attempted to determine the bactericidal activity of sera towards the Tbp double mutant strain N16T12EK. However, their experiments failed since this mutant was entirely killed by exposure to different sources of human and baby rabbit complement, and it was concluded that the loss of both Tbps might have resulted in a change of the bacterial surface which rendered it sensitive to killing. Here we show that no bactericidal activity is observed for an anti-Tbp serum towards the M982 double mutant PMC207 (Table 2). Moreover, previous bactericidal activity measurements produced by using IgG raised with whole germs of M982 revealed that bactericidal titers for the three mutants were similar to those of the wild-type M982 (data not shown). This finding is additional evidence that the removal of Tf receptor subunits did not result in dramatic changes in the bacterial surface.
Moreover, in the absence of TbpA, the bactericidal activity of sera elicited with the full-length TbpB as well as with the recombinant domains of TbpB was found to be elevated for the mutant PMC206 compared to the corresponding values of the wild-type M982 (Table 3). The significant increase in bactericidal activity of sera directed against the C-terminal domain is consistent with an interaction of this domain with TbpA, which has been shown in vitro (15). The data on the wild type and the PMC206 mutant demonstrate that the bactericidal activity seen with sera against the full-length protein is mainly due to antibodies directed against the C-terminal domain of this protein. These data in combination with the favorable thermodynamic stability of this domain (15) confirm the vaccine potential of this recombinant C-terminal domain.
Mutant PMC202 produces a truncated form of TbpB (Fig. 1) and contains the DNA sequence corresponding to the first 158 amino acids of TbpB. The bactericidal activity of the IgG preparation directed against the N-terminal domain of mutant PMC202 is comparable to that of the wild-type strain (Table 2). This finding indicates that the epitope responsible for the induction of anti-N-terminal domain antibodies is located on the initial 158 amino acids. The data are consistent with the identification by peptide mapping of an immunodominant peptide in TbpB corresponding to amino acids 4 to 17 (our unpublished data).
The acquisition of iron from Tf was shown for several species to be essential for growth, and both receptor subunits are considered virulence factors. Clear evidence that the Tf receptor is an essential virulence factor for N. gonorrhoeae has been obtained by Cornelissen et al. (9). A recent report on Actinobacillus pleuropneumoniae showed that each receptor subunit on its own is essential for virulence in an aerosol infection model (3). In contrast to the latter study, we report here that TbpA but not TbpB is a virulence factor for the meningococcal strain M982 in a murine model of bacteremia in the presence of human holo-hTf (Fig. 5). Our growth studies of the mutants on a medium containing hTf (Fig. 4 and Table 3) correlate well with the virulence results (Fig. 5). Mutants able to grow on hTf were shown to be virulent, whereas the incapacity to use hTf rendered the mutants nonvirulent. This finding demonstrates the direct link between the capacity for iron uptake and virulence.
Baltes et al. (3) showed that the TbpB-defective mutant of the pig pathogen A. pleuropneumoniae was nonvirulent in a pig animal model. This mutant, however, had preserved its capacity to utilize Tf-bound iron in vitro (3). As mentioned above, TbpB binds specifically holo-hTf, whereas TbpA binds both forms of Tf. The authors propose that the reason for this unexpected observation might be that TbpA is saturated in vivo in the absence of TbpB by pig apo-hTf, which blocks the iron uptake system. N. meningitidis is a pathogen specific for humans, and animal models are currently used for experimentation. Tf from mice, the animals used for our virulence studies, does not bind efficiently to the neisserial Tf receptor. According to Baltes et al. (3), one might argue that the virulence of the TbpB− mutant PMC205 is due to the fact that experiments have been carried out in mice where a blocking of TbpA by murine apo-Tf can be excluded. However, our growth studies of mutant PMC205 on a medium containing a physiological mixture of apo- and holo-hTf provide evidence that human apo-Tf does not block the iron uptake at TbpA since bacterial growth is observed (Fig. 4). This underlines the difficulties in studying pathogens specific to humans. Any animal model, including the recently described CD46 transgenic murine model (13), is unable to fully mimic the natural host. However, the combined interpretation of growth and virulence data of mutant PMC205 justifies the conclusion concerning the contribution of TbpB to virulence. The murine model could be improved by generating mice transgenic for hTf. However, first attempts were unfortunately unsuccessful (unpublished data).
Acknowledgments
We are very grateful to A. Schryvers for providing the tbp mutants constructed in N. meningitidis tbpB isotype I strains B16B6, N16T2K, N16T1E, and N16T12EK.
Editor: D. L. Burns
REFERENCES
- 1.Ala'Aldeen, A. A., and S. P. Boriello. 1996. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine 14:49-53. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. E., P. F. Sparling, and C. N. Cornelissen. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 176:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltes, N., I. Pauka-Hennig, and G.-F. Gerlach. 2002. Both transferrin-binding proteins are virulence factors in Actinobacillus pleuropneumoniae serotype 7 infection. FEMS Microbiol. Lett. 209:283-287. [DOI] [PubMed] [Google Scholar]
- 4.Begg, N., K. A. V. Cartwright, J. Cohen, E. B. Kaczmarski, J. A. Innes, C. L. S. Leen, D. Nathwani, M. Singer, L. Southgate, W. T. A. Todd, P. D. Welsby, M. J. Wood, et al. 1999. Consensus statement on diagnosis, investigation, treatment and prevention of acute bacterial meningitis in immunocompetent adults. J. Infect. 39:1-15. [DOI] [PubMed] [Google Scholar]
- 5.Boulton, I. C., A. R., Gorringe, N. Allison, A. Robinson, B. Gorinsky, C. L. Joannou, and R. W. Evans. 1998. Transferrin-binding protein B isolated from Neisseria meningitidis discriminates between apo and diferric human transferrin. Biochem. J. 334:269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 7.Cornelissen, C. N., and P. F. Sparling. 1996. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J. Bacteriol. 178:1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen, C. N., J. E. Anderson, and P. F. Sparling. 1997. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect. Immun. 65:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen, C. N., M. Kelly, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611-616. [DOI] [PubMed] [Google Scholar]
- 10.Gray-Owen, S. D., S. Loosmore, and A. B. Schryvers. 1995. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect. Immun. 63:1201-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray-Owen, S. D., and A. B. Schryvers. 1996. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 14:843-850. [DOI] [PubMed] [Google Scholar]
- 12.Irwin, S. W., N. Averil, C. Y. Cheng, and A. B. Schryvers. 1993. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbpA and tbpB, from Neisseria meningitidis. Mol. Microbiol. 8:1125-1133. [DOI] [PubMed] [Google Scholar]
- 13.Johansson, L., A. Rytkonen, P. Bergman, B. Albiger, H. Kallstrom, T. Hokfelt, B. Agerberth, R. Cattaneo, and A. B. Jonsson. 2003. CD46 in meningococcal disease. Science 301:373-375. [DOI] [PubMed] [Google Scholar]
- 14.Kamboh, M. I., and R. E. Ferrell. 1987. Human transferrin polymorphism. Hum. Hered. 37:65-81. [DOI] [PubMed] [Google Scholar]
- 15.Krell, T., G. Renauld-Mongenie, M.-C. Nicolai, S. Fraysse, M. Chevalier, Y. Berard, J. Oakhill, R. W. Evans, A. Gorringe, and L. Lissolo. 2003. Insight into the structure and function of the transferrin receptor from Neisseria meningitidis using microcalorimetric techniques. J. Biol. Chem. 278:14712-14722. [DOI] [PubMed] [Google Scholar]
- 16.Legrain, M., V. Mazarin, S. W. Irwin, B. Bouchon, M.-J. Quentin-Millet, E. Jacobs, and A. B. Schryvers. 1993. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 130:73-80. [DOI] [PubMed] [Google Scholar]
- 17.Lissolo, L., G. Maitre-Wilmotte, P. Dumas, S. Colombani, A. B. Schryvers, and M.-J. Quentin-Millet. 1995. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect. Immun. 63:884-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locher, K. P., B. Rees, R. Koebnik, L. Mitschler, A. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 19.Luke, N. R., and A. A. Campagnari. 1999. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. Infect. Immun. 67:5815-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oftung, F., M. Lovik, S. R. Andersen, L. O. Froholm, and G. Bjune. 1999. A mouse model utilizing human transferrin to study protection against Neisseria meningitidis serogroup B induced by outer membrane vesicle vaccination. FEMS Immunol. Med. Microbiol. 26:75-82. [DOI] [PubMed] [Google Scholar]
- 21.Ogunnariwo, J. A., and A. B. Schryvers. 2001. Characterization of a novel transferrin receptor in bovine strains of Pasteurella multocida. J. Bacteriol. 183:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelilic, V., S. Morelle, D. Lampe, and X. Nassif. 2000. Mutagenesis of Neisseria meningitidis by in vitro transposition of Himar1 mariner. J. Bacteriol. 182:5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltola, H. 1983. Meningococcal disease: still with us. Rev. Infect. Dis. 5:71-91. [DOI] [PubMed] [Google Scholar]
- 24.Pintor, M., J. A. Gomez, L. Ferron, C. M. Ferreiros, and M. T. Criado. 1998. Analysis of TbpA and TbpB functionality in defective mutants of Neisseria meningitidis. J. Med. Microbiol. 47:757-760. [DOI] [PubMed] [Google Scholar]
- 25.Renauld-Mongénie, G., D. Poncet, L. von Olleschik-Elbheim, T. Cournez, M. Mignon, M. A. Schmidt, and M.-J. Quentin-Millet. 1997. Identification of human transferrin-binding sites within meningococcal transferrin-binding protein B. J. Bacteriol. 179:6400-9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rokbi, B., V. Mazarin, G. Maitre-Willmotte, and M. J. Quentin-Millet. 1993. Identification of two major families of transferrin receptors among Neisseria meningitidis strains based on antigenic and genomic features. FEMS Microbiol. Lett. 110:51-58. [DOI] [PubMed] [Google Scholar]
- 27.Rokbi, B., M. Mignon, G. Maitre-Wilmott, L. Lissolo, B. Danve, D. A. Caugant, and M.-J. Quentin-Millet. 1997. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect. Immun. 65:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rokbi, B., G. Renauld-Mongénie, M. Mignon, B. Danve, D. Poncet, C. Chabanel, D. A. Caugant, and M.-J. Quentin-Millet. 2000. Allelic diversity of the two transferring-binding protein B gene isotypes among a collection of Neisseria meningitidis strains representative of serogroup B disease: implication for the composition of a recombinant TbpB-based vaccine. Infect. Immun. 68:4938-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseriae. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 31.West, D., K. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. Gorringe. 2001. Recombinant Neisseria meningitidis transferring-binding protein A protects against experimental meningococcal infection. Infect. Immun. 69:1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu, W., D. J. Hunt, A. R. Richardson, and I. Stojiljkovic. 2000. Use of heme compounds as iron sources by pathogenic Neisseriae requires the product of the hemO gene. J. Bacteriol. 182:439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]