SUMMARY
The prefrontal cortex (PFC), a cortical region that was once thought to be functionally insignificant, is now known to play an essential role in the organization and control of goal-directed thought and behavior. Neuroimaging, neurophysiological, and modeling techniques have lead to tremendous advances in our understanding of PFC functions over the last few decades. It should be noted, however, that neurological, neuropathological, and neuropsychological studies have contributed some of the most essential, historical, and often prescient, conclusions regarding the functions of this region. Importantly, examination of patients with brain damage allows one to draw conclusions about whether a brain area is necessary for a particular function. Here, we provide a broad overview of PFC functions based upon behavioral and neural changes resulting from damage to PFC in both human patients and non-human primates.
Introduction
The functions of the frontal lobes until recently have been shrouded in mystery. Several early studies in humans and monkeys reported that large portions of prefrontal cortex (PFC) could be removed without severe losses of mental capacity or changes in behavior (Hebb, 1939; Petrie, 1952; Teuber et al., 1951), leading to the notion that PFC was cognitively “silent” and was therefore not essential for normal functioning. This view may have contributed to the widespread use of psychosurgery (e.g., lobotomy, leucotomy; Fig. 1) as a treatment for numerous psychiatric disorders in the first half of the 20th century. However, case studies published throughout the last two centuries have also described profound behavioral and personality changes following frontal lobe damage in individual patients. Harlow first referred to this collection of symptoms as “frontal lobe syndrome” (Harlow, 1868).
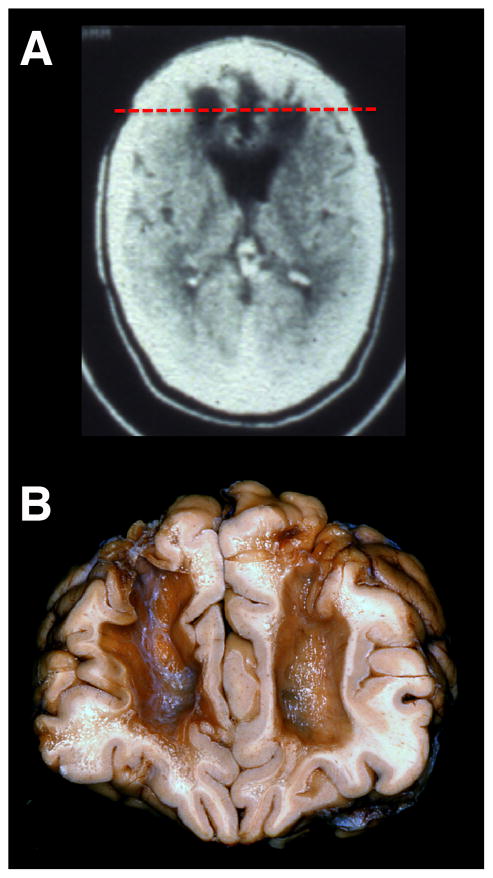
Figure 1. Effects of Psychosurgery.
(A) A computed tomography (CT) axial image of a patient who received a bilateral frontal leucotomy for a psychiatric disorder in the 1950s. The CT was obtained 30 years later. (B) A post-mortem specimen of a patient who received a bilateral frontal leucotomy for a psychiatric disorder in the 1950s. Note the massive subcortical white matter damage from the leucotomy disconnecting multiple PFC regions from the rest of the brain. The coronal slice from the neuropathological specimen is shown in relation to the axial CT scan in (A) (red dashed line). Neuropathological specimen compliments of Professor John Woodard, UC Irvine (Woodard, 2002).
Researchers now agree that the frontal lobes are not cognitively “silent”, but instead play an essential role in the organization and control of goal-directed thought and behavior (Fuster, 1989; Luria, 1966; Stuss and Knight, 2013). These functions are often collectively referred to as cognitive, or executive, control and can be broadly divided into several core cognitive components, including mental set-shifting, inhibition, information updating, working memory, response monitoring, and temporal coding. The PFC has extensive reciprocal connections with nearly all cortical and subcortical structures (Croxson et al., 2005; Ilinsky et al., 1985; Selemon and Goldman-Rakic, 1988), placing it in a unique position to orchestrate a wide range of cognitive and affective neural functions.
The advent of neuroimaging and advanced neurophysiological and modeling techniques to study brain function has lead to tremendous advances over the past few decades in our understanding of PFC functions. However, it is important to note that many of the clue s to our understanding of these functions were provided by neurological and neuropsychological studies of brain damaged patients conducted well over a century ago. For example, after removing large portions of the frontal lobes of monkeys, the British neuropsychologist David Ferrier concluded in 1876 that
“The animals retain their appetites and instincts, and are capable of exhibiting emotional feeling. The sensory faculties, sight, hearing, touch, taste and smell, remain unimpaired… And yet, notwithstanding this apparent absence of physiological symptoms, I could perceive a very decided alteration in the animal’s character and behaviour… Instead of, as before, being actively interested in their surroundings, and curiously prying into all that came within the field of their observation, they remained apathetic, or dull, or dozed off to sleep, responding only to the sensations or impressions of the moment…While not actually deprived of intelligence, they had lost, to all appearance, the faculty of attentive and intelligent observation”
(Ferrier, 1876, pp. 231–232).
In another seminal paper originally published in 1895 and based upon observations of animals with frontal cortex damage, the Italian neuropathologist Leonardo Bianchi concluded that
“the frontal lobes are the seat of coordination and fusion of the incoming and outgoing products of the several sensory and motor areas of the cortex… [to] sum up into series the products of the sensori-motor regions, as well as the emotive states which accompany all the perceptions, the fusion of which constitutes what has been called the psychical tone of the individual”
(Bianchi, 1895, p. 521).
Thus, lesion observations have contributed numerous important, and often prescient, theoretical contributions to our understanding of PFC function. In the current perspective, we aim to provide a broad overview of what is currently known about PFC functions based upon evidence from human patients and non-human primates with brain damage caused by stroke, traumatic brain injury (TBI), or surgical resection.
Lesions to Dorsolateral PFC
Lesions of the dorsolateral PFC (DLPFC), consisting of Brodmann areas (BA) 9 and 46 in the human (Fig. 2, left), can result in deficits across a wide range of functions, including working memory, rule-learning, planning, attention, and motivation (Fig. 3).
Figure 2. Subdivisions of Prefrontal Cortex.
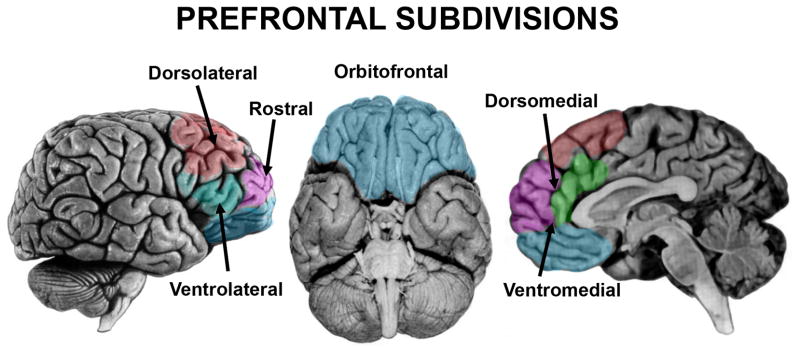
Subdivisions of the prefrontal cortex are color-coded and labeled based upon their approximate anatomical locations in the human brain.
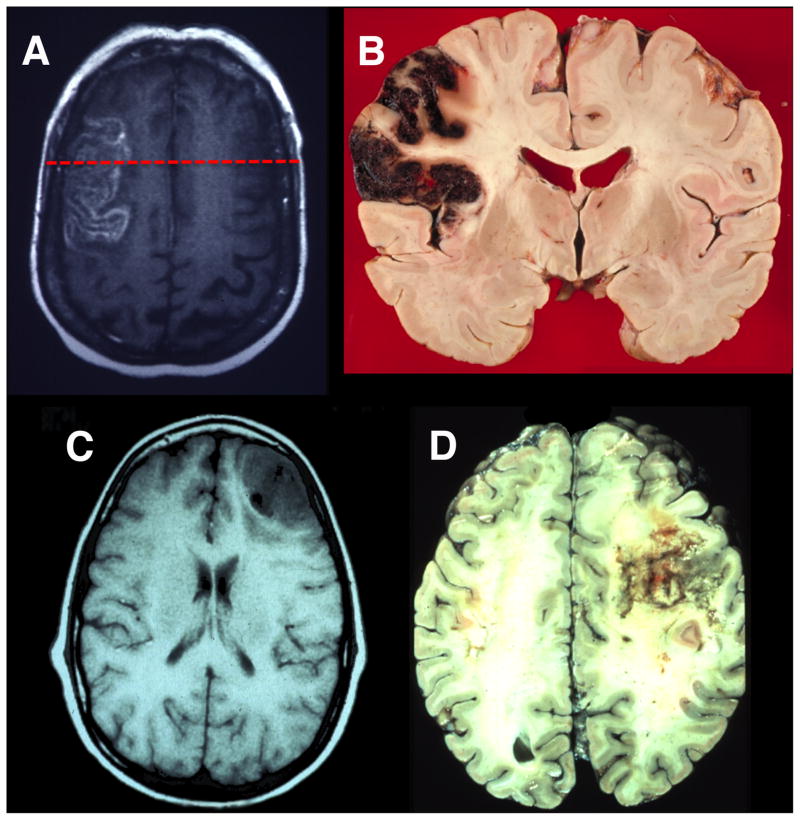
Figure 3. Lesions to Lateral Prefrontal Cortex.
(A) An axial T2 magnetic resonance imaging (MRI) image showing an acute stroke in the left lateral prefrontal cortex. (B)A coronal neuropathological specimen of an infarct in the left lateral PFC (note the hemorrhagic conversion in the cortical mantle). The red dashed line on the MRI in (A) shows the approximate site of the post-mortem coronal slice. (C) An axial T1 MRI image showing infiltrating glioblastoma in the right lateral frontal cortex. (D)A post-mortem axial slice of an infiltrating glioblastoma in the right lateral frontal lobe. Neuropathological specimen in (B) compliments of Professor Dimitri Agamanolis, Akron Children’s Hospital (http://neuropathology-web.org/).
Working memory
Jacobsen conducted seminal studies demonstrating severe impairments on delayed-response tasks following lateral PFC lesions in primates centered in the sulcus principalis. He concluded that “the basic change associated with lesions of the prefrontal area is the loss of capacity for immediate or for recent memory” (Jacobsen, 1935, p. 564). These studies provided the first evidence that the DLPFC is important for the online maintenance of recent memories, a function currently referred to as working memory. Numerous subsequent studies have reported single neurons in monkey DLPFC that are active throughout the delay period during delayed-response tasks (Funahashi et al., 1989; Fuster and Alexander, 1971), suggesting that these neurons are actively maintaining the memory trace. Lesions to DLPFC regions in the monkey where these delay cells are found lead to an inability to perform delayed-response tasks (Funahashi et al., 1993; Passingham, 1985).
Human lesion studies have reinforced the notion that DLPFC is essential for working memory function (Barbey et al., 2013; Manes et al., 2002; Owen et al., 1990; Tsuchida and Fellows, 2009). However, while a majority of primate lesion studies have implicated DLPFC in working memory maintenance, other studies have found working memory maintenance is not substantially altered following damage to human DLPFC (D’Esposito et al., 2006; D’Esposito and Postle, 1999). Rather, DLPFC seems to be important for the monitoring and manipulation of working memory content (e.g., Barbey et al., 2013; Petrides and Milner, 1982; Tsuchida and Fellows, 2009). For instance, monkeys with lesions constrained to the DLPFC (BA 9, 46 only) and humans with focal lesions to the analogous region have difficulty performing self-ordered working memory tasks, during which they are presented with arrangements of stimuli and must choose a different stimulus for every trial until all of the stimuli have been chosen once (Petrides, 1995; Petrides and Milner, 1982). These studies suggest that the role of the DLPFC might be to rearrange, transform, or track the relative status of stimuli or events within working memory, rather than to simply actively maintain the representation of memories. These findings highlight the recurring observation that many complex functions associated with the PFC require several distinct operations for implementation.
Endogenous attention
Several studies have reported decreased event-related potential (ERP) amplitudes during attention to visual stimuli over ipsilesional visual cortex following unilateral damage to DLPFC (Barcelo et al., 2000; Voytek et al., 2010). These decreased extrastriate neural responses were accompanied by impairments in the ability to detect targets presented in the contralesional visual field. Diminished neural responses have been similarly reported in DLPFC patients during selective auditory attention (Bidet-Caulet et al., 2014). These studies suggest that the DLPFC plays an important role in attention by providing top-down facilitatory input to sensory cortices. Previous studies have also shown that DLPFC damage leads to increased distractibility (Chao and Knight, 1998; Woods and Knight, 1986). For example, DLPFC patients exhibit increased interference effects on trials where distracting sounds are inserted between a cue and target and enhanced ERP responses to the distracting sounds as compared to age-matched controls (Chao and Knight, 1998). Therefore, increased distractibility could partially explain the attentional deficits observed following DLPFC damage.
Declarative memory
Focal PFC damage does not lead to a severe amnestic disorder, such as that observed following medial temporal lobe damage. However, PFC damage does lead to deficits in certain aspects of declarative memory. The lateral PFC, specifically the DLPFC, is thought to be particularly important for the encoding and retrieval of episodic memory (i.e., memory that is associated with a particular episode, or context). For example, patients with lateral frontal damage sometimes demonstrate source memory deficits, in which they mistake where and/or when information was learned (Duarte et al., 2005; Janowsky et al., 1989). DLPFC patients demonstrated memory impairments when asked to recall remote events, such as public events and famous faces (Mangels et al., 1996). These studies suggest that one of the roles of lateral frontal lobes is to link facts to the context in which they were learned. However, episodic memory deficits resulting in confabulatory behavior are also associated with orbital and ventromedial PFC damage (Schnider and Ptak, 1999; Turner et al., 2008).
Lateral PFC patients also show impairments in free recall (for example, they perform poorly during free recall of learned word lists) (Eslinger and Grattan, 1994; Gershberg and Shimamura, 1995; Jetter et al., 1986), although their recall performance improves with cueing (Jetter et al., 1986; Milner et al., 1991). These deficits can occur at both the encoding and the retrieval stages (Gershberg and Shimamura, 1995). Furthermore, lateral PFC patients show impairments in the temporal ordering of events (Milner et al., 1991; Shimamura et al., 1995), which may be related to impairments in strategic retrieval processes (Mangels, 1997). These studies suggest that patients with lateral PFC damage, especially to the DLPFC, are unable to organize learned information to facilitate their recall. It has been proposed that most of these deficits result from a failure of the PFC to inhibit unwanted information or to select among competing memories. As a result, recently activated memories can interfere with the ability to retrieve more distant memories (Shimamura et al., 1995; Warrington and Weiskrantz, 1974).
Although recall deficits are more commonly reported following lateral PFC damage, a few studies have also reported deficits in recognition memory (Alexander et al., 2003; Stuss et al., 1994). In particular, DLPFC patients reveal deficits in familiarity-based recognition only when the lesioned hemisphere is forced to perform the encoding (Duarte et al., 2005). In summary, the lateral PFC is important for the monitoring and control of memory processes, both at the time of encoding and at the time of retrieval.
Rule learning and task switching
Patients with DLPFC damage have difficulties performing the Wisconsin Card Sorting Task (WCST). The WCST requires patients to sort cards based upon a rule (e.g., place cards in piles based upon color, shape, or number). At some point during the task, the rule is changed. DLPFC patients are often unable to switch to a new rule and instead continue to follow the original rule (Milner, 1963; Shallice and Burgess, 1991). Notably, PFC patients make random errors in addition to perseverative errors that may result from transient lapses of attention (Barcelo and Knight, 2002). Several studies have demonstrated that bilateral lesion of the principal sulcus and surrounding tissue impairs behavioral performance of monkeys in modified versions of the WCST (Buckley et al., 2009; Moore et al., 2009) (see also Dias et al., 1996b; Walker et al., 2009 for further evidence using similar tasks in monkeys). This perseverative behavior following damage suggests that DLPFC is important for internally maintaining relevant behavioral rules to control actions and for flexibly switching among these rules when it is behaviorally necessary (i.e., shifting between attentional sets).
Planning and problem solving
Patients with lateral damage are often able to perform an individual action that is part of a sequence in isolation, but are unable to perform the same actions in a particular temporal order. Instead, they often omit or perseverate on actions or perform actions in the incorrect order (Duncan, 1986; Grafman, 1989). These patients often struggle with planning in everyday life situations, which has been termed ‘strategy application disorder’ (Burgess, 2000). The Tower of Hanoi (TOH) and the Tower of London (TOL) are two tasks traditionally used to assess the ability to plan several moves ahead in order to reach a goal (Shallice, 1982; Simon, 1975). Both tasks require subjects to move colored disks arranged on several pegs from a starting position to a predetermined end position. Patients with lateral PFC damage are much slower and require more moves when solving the TOH and TOL tasks (Goel and Grafman, 1995; Manes et al., 2002; Owen et al., 1990; Shallice, 1982). Patients have particular difficulty when making a necessary move that initially appears to take them farther away from the ultimate goal and this phenomena has been interpreted as a failure to inhibit a prepotent response (Goel and Grafman, 1995). This deficit highlights the point that multiple cognitive operations, including inhibition and working memory, are required for successful planning and problem solving.
The results from these studies should be interpreted with caution for several reasons. First, the patients observed in many of these studies had diffuse damage to frontal cortex and underlying white matter, making it difficult to discern which frontal areas are particularly involved in planning and problem solving. However, neuroimaging studies often report DLPFC activations in healthy subjects that are engaged in solving the TOL (Unterrainer and Owen, 2006). Second, although the TOH and TOL are superficially similar, differences in physical characteristics and administration directions between the two tasks likely result in the use of different strategies, and consequently engage different cognitive operations, for successful completion. For example, working memory and inhibition have been shown to strongly predict performance on the TOL, while working memory and inhibition account for little performance variability on the TOH (Welsh et al., 1999). In addition, it is possible to perform the TOH and TOL using a step-by-step perceptual strategy, rather than developing an overall plan, for successful completion. It is therefore unclear whether these tasks measure planning abilities, complicating the conclusions that can be drawn from studies using the TOL and TOH tasks.
Novelty detection and exogenous attention
The ability for humans or animals to detect, respond to, and remember novel stimuli in their environment is fundamental to survival and new learning. Many neurophysiological studies in healthy humans have demonstrated that unexpected, novel stimuli generate an early latency, frontally-distributed P3 potential, referred to as the novelty P3, which has been hypothesized to reflect an involuntary attentional orienting response (Courchesne et al., 1975). Patients with DLPFC damage show markedly decreased P3 amplitudes in response to unexpected, novel stimuli in all sensory modalities compared to age-matched controls (Daffner et al., 2000b; Yamaguchi and Knight, 1991) and novelty P3 amplitude decrements correlate with measures of apathy, as measured by the Apathy Scale, in DLPFC patients (Daffner et al., 2000b). Furthermore, both humans and monkeys with DLPFC damage have impaired recollection-based and familiarity-based recognition memory and fail to exhibit memory advantages for novel stimuli (Kishiyama et al., 2009; Parker et al., 1998), perhaps because DLPFC patients spend less time inspecting novel stimuli than controls (Daffner et al., 2000a). These studies suggest that DLPFC is important for novelty-seeking behavior, which is closely tied to attentional orienting, memory encoding, and motivation (also see following section).
Motivation
Blumer and Benson (1975) identified a “pseudodepressive” syndrome that is often associated with damage to DLPFC and is characterized by loss of initiative and diminished motivation, flattened affect, outward display of apathy and indifference, reduced verbal output, and behavioral slowness (symptoms that are clinically characterized as abulia). Bilateral lesions to anterior portions of PFC in monkeys also lead to apathy, blunted affect, and a lack of curiosity and interest in the environment (Ferrier, 1876). The symptoms of abulia following DLPFC damage are directly tied to the inability of these patients to plan and maintain sequences of goals and actions. Indeed, DLPFC patients often show disregard for task requirements, even though the requirements are understood and remembered, a phenomenon referred to as ‘goal neglect’ (Duncan et al., 2008). DLPFC has been proposed to be one area within a network, also including supplementary motor cortex, anterior cingulate cortex (ACC), basal ganglia, and the thalamus, that controls willed actions (Jahanshahi and Frith, 1998). Thus, when DLPFC is damaged, patients lack the intention to act. It is important to note that the symptoms discussed here are also commonly observed following bilateral damage to the anterior cingulate cortex (ACC) (Damasio and Van Hoesen, 1983; Devinsky et al., 1995), which is discussed in greater detail in the ‘Lesions to Medial PFC’ section.
Lesions to Ventrolateral PFC
Lesions of the ventrolateral PFC (VLPFC), consisting of BA 44, 45, and 47 of the human inferior frontal gyrus (IFG) (Fig. 2, left), cause deficits across a range of seemingly disparate functions, including spatial attention, inhibitory control, and language.
Spatial attention
Visuospatial neglect is a disorder that is characterized by the inability to orient towards or attend to the contralateral side of space, objects, or one’s own body. Although neglect is most often associated with damage to posterior parietal cortex and the temporo-parietal junction (Vallar, 2001), damage to the inferior (BA 44) and middle frontal gyri may also result in neglect symptoms (e.g., Husain and Kennard, 1996; Stone et al., 2011). However, neglect following damage to DLPFC (BAs 8, 9, 46) has also been reported (Ptak and Schnider, 2010; Verdon et al., 2010). Several studies have suggested that compared to parietal patients, frontal patients display greater deficits in the motor component than the perceptual component of neglect (Bisiach et al., 1990; Daffner et al., 1990). For example, frontal neglect patients display decreased initiation of motor movements (directional hypokinesia) and decreased exploratory motor movements towards contralesional space, sometimes with few perceptual deficits (e.g., without signs of visual extinction) (Daffner et al., 1990). However, both perceptual and motor components of neglect can be observed following frontal or parietal damage (Mattingley et al., 1998), so the functional differences that distinguish frontal from parietal neglect still remain unclear. There is strong evidence that neglect symptoms are more persistent and severe following damage to the right than the left hemisphere (Stone et al., 1992). Interestingly, the area of damage most often leading to frontal neglect, the right inferior frontal gyrus, is remarkably similar to the region known as Broca’s area in the left hemisphere (see below).
Response inhibition
Numerous studies have demonstrated that the IFG, particularly in the right hemisphere, plays an important role in the inhibitory control and flexible adjustment of movement plans. Two tasks that are often used to study inhibition or control of motor responses are the Go/No-Go task and the Stop-Signal Reaction Time (SSRT) task, both which require subjects to make speeded responses to stimuli on a majority of trials and to withhold their responses on a minority of trials. Patients with right IFG lesions were significantly slower to stop when performing the SSRT task than age-matched controls and the volume of damage to BA 44 and BA 45 predicted the time it took for each patient to initiate a stop, with greater damage leading to slower reaction times (Aron et al., 2003). Further evidence is provided by a monkey lesion study demonstrating that damage to the inferior prefrontal convexity, which is anatomically comparable to the IFG in humans (Petrides and Pandya, 2002), lead to an increased number of errors on no-go trials while monkeys performed a Go/No-Go task (Iversen and Mishkin, 1970). Although these studies provide evidence for the role of the IFG in inhibitory motor control, they have not ruled out the possibility that the responses to infrequent stop events are a result of attentional capture (Kramer et al., 2013), especially when taking into consideration the role of the IFG in spatial attention (see previous section). It has been postulated that, in addition to a role in response inhibition, the IFG may play a more general role in inhibitory control (Cohen et al., 2013). However, little evidence from the lesion literature exists to support this claim. A few studies to date have demonstrated that VLPFC lesions in humans may also lead to deficits in inhibitory oculomotor control (Hodgson et al., 2007) and increased risk-taking behavior (Floden et al., 2008), although patients in the latter study had additional damage to orbitofrontal cortex (OFC).
Language
One of the most famous neurological cases, first reported by Pierre Paul Broca, is that of patient ‘Tan’, who was only able to utter the word “tan” following a lesion to the left posterior inferior frontal gyrus (Broca, 1861). This was the first neuropsychological evidence to suggest that this region, encompassing the pars opercularis and pars triangularis (BA 44/45) and now commonly referred to as Broca’s area, was important for language production. Patients with Broca’s aphasia, or non-fluent aphasia, commonly exhibit slow, halting speech, impairments in lexical access (i.e., they often struggle to find words), and agrammatism (speech characterized by short phrases with simple syntactic structures and omissions of grammatical markers and function words) (Caplan, 2003). Besides difficulties with language production, Broca’s aphasics also demonstrate some impairment in language comprehension, especially when dealing with more syntactically complex sentences whose meaning cannot be inferred (Drai and Grodzinsky, 2006; Zurif et al., 1972).
Although it was originally believed that Broca’s aphasia resulted from isolated damage to Broca’s area (as demonstrated by Broca’s original patients), current evidence now suggests a more complicated picture. For instance, lesions isolated to Broca’s area do not result in Broca’s aphasia, and patients with symptoms consistent with Broca’s aphasia do not necessarily have lesions to Broca’s area (Dick et al., 2001; Dronkers et al., 1992). A recent re-examination of the brains of Broca’s original two cases revealed that his patients also had substantial damage to the insula and arcuate fasciculus (Dronkers et al., 2007) and patients with persistent articulatory deficits often have lesions in these structures (Borovsky et al., 2007; Dronkers, 1996). Dronkers and colleagues (2004) utilized voxel-based lesion symptom mapping (VLSM), a technique to make voxel-wise statistical comparisons between lesion location and neuropsychological test performance across a group of patients (Bates et al., 2003), to demonstrate that PFC damage outside of Broca’s area can produce impairments of syntactically-based sentence comprehension (see also Vanier and Caplan, 1990 for similar conclusions for agrammatism). One consistent finding is that language functions are left hemisphere dominant in most individuals (Toga and Thompson, 2003). Thus, Broca’s area (BA 44/45) is likely to be just one part of a much larger left hemisphere network involved in language production, comprehension, and monitoring (Ries et al., 2013).
One alternative theory proposes that the left IFG is not specialized for language, per se, but is more generally important for selecting among competing representations, especially when there is a need to override a prepotent response or when there is a large number of equally probable response options (Novick et al., 2005; Thompson-Schill et al., 1998). There is evidence from patients with focal left IFG lesions (including BA 44/45) to support this theory. For example, left IFG patients show particular difficulty with incongruent trials when performing the Stroop Task (Stroop, 1935) and other tasks that require the patients to override a prepotent response or to resolve representational conflict (Hamilton and Martin, 2005; Thompson-Schill et al., 2002). It should be noted that these patients do not show impairments when overriding prepotent motor responses (Hamilton and Martin, 2005), suggesting that damage to left IFG impairs internal conflict resolution, rather than response inhibition. Thus, this theory predicts the linguistic tasks that lead to impairments in patients with left IFG damage are also the tasks that require the most resolution of internal conflict.
Other theories propose that the left IFG may be specialized to process hierarchical structures across many functions (e.g., Fiebach and Schubotz, 2006). Whether Broca’s area is specialized for language processing, involved in more domain-general functions, or serves both functions, is still open to debate.
Lesions to Orbitofrontal Cortex
Lesions to the OFC, which includes portions of BA 10 (frontal pole), 11 (spanning both ventral medial and lateral surfaces), 12 (on the ventral medial surface), 47 (on the ventral lateral surface) in the human (Fig. 2, middle; see also (Wallis, 2012) for a different OFC parcellation scheme), are generally associated with a loss of inhibitory and emotional control and an inability to effectively function in the social domain (Fig. 4). These symptoms are best typified by the famous neurological patient, Phineas Gage, who underwent tremendous behavioral and personality changes following PFC damage resulting from a penetrating head injury (Damasio et al., 1994). Gage’s physician, John Harlow, noted these changes following Gage’s recovery(Harlow, 1848). Previous to the accident, Gage was a responsible and socially well-adjusted individual. Following the accident, Harlow described Gage as “capricious”, “vacillating”, and “impatient of restraint” (Harlow, 1848). The damage site was proposed to be bilateral OFC (BA 10, 11, 12) (Damasio et al., 1994), although a recent reanalysis suggests that damage was confined to the left hemisphere, with extensive white matter disconnection (Van Horn et al., 2012). In addition, inspection of the Gage skull shows prominent damage to the sphenoid ridge, suggesting additional anterior temporal/amygdala damage. Since this landmark case, many studies have reported inflexibility, impulsive behavior, and emotional disturbances following OFC damage in humans and monkeys.
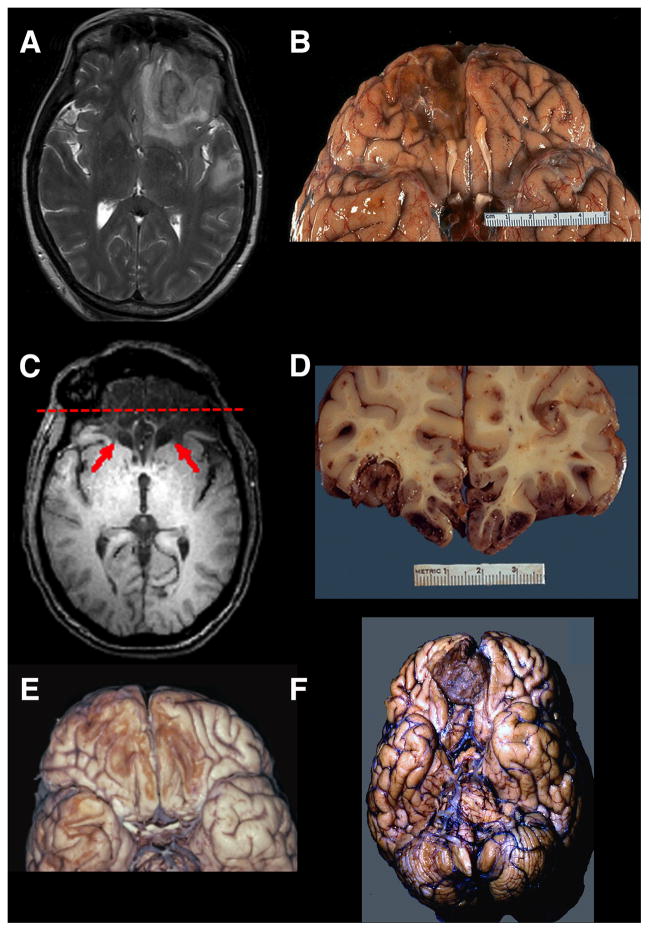
Figure 4. Lesions to Orbitofrontal Cortex.
(A) A T2 axial MRI image of an acute right OFC contusion resulting from trauma (accidental fall down stairs). (B) A ventral view of a postmortem specimen showing chronic residual loss of tissue in right OFC. Note also the damage to the olfactory bulb. (C)A T1 axial MRI image showing extensive bilateral damage to the OFC (red arrows). (D) Acoronal post-mortem slice of a patient with fatal traumatic brain injury due to extensive OFC contusions. If this patient had survived, his/her MRI image would resemble (C). The red dashed line on the MRI in (C) shows the approximate site of this post-mortem coronal slice. (E) A ventral view of a post-mortem specimen showing chronic bilateral loss of tissue in the OFC due to trauma. (F) A post-mortem specimen (ventral view) of an unsuspected right meningioma in a patient with behavioral changes. Neuropathological specimens in (B) and (D) are compliments of Professor Edward C. Klatt, University of Utah (http://library.med.utah.edu/WebPath/CNSHTML/CNSIDX.html). Neuropathological specimen in (E) is compliments of Professor Walter Finkbeiner, UC San Francisco.
Inhibitory control and decision-making
Numerous lesion studies in the monkey and the human have suggested that OFC is involved in decision-making, especially when reward value must be taken into account (Wallis, 2012). Damage to OFC in monkeys disrupts performance on tasks that require inhibition of a pre-potent response, especially in the context of reward reversal and reversal learning (Baxter et al., 2000; Dias et al., 1996a; Izquierdo et al., 2004) and Go/No-Go reward (Iversen and Mishkin, 1970). For example, monkeys with OFC lesions have intact initial learning, but continue to respond to stimuli that were formally, but are no longer rewarded (Dias et al., 1996a) and continually respond to unrewarded No-Go trials (Iversen and Mishkin, 1970). Thus, OFC damage results in both reinforcement- learning deficits and behavioral disinhibition. Several studies have attempted to functionally parcellate monkey OFC further using selective lesioning techniques to identify valuable double dissociations. For example, Rudebeck and Murray (2011) determined that the central portion of OFC is important for stimulus-value representations, while medial OFC is important for successful extinction behavior (i.e., to stop responding to previously rewarded stimuli). Noonan and colleagues (2010) determined that lateral OFC is more involved in reward-guided learning, while medial OFC is more involved in reward-guided decision-making (where the animal must choose among multiple options).
These studies have lead researchers to conclude that OFC is important for reward monitoring or for the inhibition of previous responses to rewards. However, monkeys with OFC lesions also have difficulties associating new abstract rules with reward in tasks that do not utilize reward reversal or extinction (Buckley et al., 2009). A recent study suggests that the OFC is necessary to guide contingent learning, rather than for the functions previously hypothesized (Walton et al., 2010).
Humans with OFC damage also have inhibition deficits that are often manifested during real-life decision-making. These deficits have occasionally been characterized as impulsivity, although more recent studies have suggested that these deficits are better characterized by increased risk-taking behavior (Floden et al., 2008; Shiv et al., 2005). Bechara and colleagues (1994) devised the Iowa gambling task (IGT) to study deficits in real-life decision making in the laboratory. In the IGT, subjects must choose cards from four different decks in order to accumulate as much money as possible. Some cards allow the player to win money, while other cards cause the player to lose money. The cards are unequally distributed, with “good” decks(leading to an overall net win money) and “bad” decks (leading to an overall net loss of money) and subjects must figure out the optimal strategy for winning the most money. OFC patients make more risky, maladaptive decisions than controls when performing the IGT (Bechara, 2004; Bechara et al., 1994; Fellows and Farah, 2005a) and OFC damage leads to increased risk-taking even when outcome probabilities are made explicit (Clark et al., 2008). A recent study using VLSM across a large number of patients with diffuse damage determined behavioral deficits on the IGT were unique to patients with medial OFC/ventromedial PFC and frontal pole damage as compared to patients with lesions elsewhere (Glascher et al., 2012). It should be noted that the IGT is a complex task that requires multiple cognitive operations and engages multiple brain areas. For instance, several studies have reported IGT deficits in patients with damage to lateral, but not orbital, PFC (Clark et al., 2003; Fellows and Farah, 2005a; Manes et al., 2002) and patients with focal OFC damage who perform normally on the IGT (Manes et al., 2002). Thus, the IGT may not be an ideal task to probe behavioral deficits specifically associated with OFC damage.
OFC patients, like monkeys, demonstrate deficits in reversal learning, where they continue to respond to stimuli that are no longer rewarded (Clark et al., 2004; Fellows and Farah, 2003; Hornak et al., 2004), even after verbally acknowledging the changes in reward contingencies. These patients are particularly impaired at learning to use negative feedback, but perform better when using positive feedback to change their actions (Tsuchida et al., 2010; Wheeler and Fellows, 2008). The inability of OFC patients to use negative feedback may also be tied to their inability to regulate or inhibit responses to aversive or painful stimuli (Roberts et al., 2004). In summary, these studies suggest that the OFC is important for learning and relearning stimulus- reward/punishment associations and for updating behavior based upon these associations in order to reach goals.
Emotional and social control
Monkeys with OFC damage demonstrate abnormalities in social and emotional behavior, including hypoactivity to environmental stimulation, increased aversive reactions, decreased aggression, loss of maternal behavior, decreased grooming, decreased time spent with conspecifics, and decreased frequency and variability of facial expressions, vocalizations, and social communicative gestures (Butter et al., 1970; Franzen and Myers, 1973).
Damage primarily restricted to medial orbital and ventromedial PFC in humans is associated with profound changes in social and affective behavior (Anderson et al., 2006; Eslinger and Damasio, 1985; Hornak et al., 2003). This includes lack of affect or poorly modulated emotional reactions and disinhibited or socially inappropriate behavior and decision-making (Barrash et al., 2000; Blumer and Benson, 1975; Tranel, 1994). OFC damage also results in impaired insight and difficulty in inferring the mental states of others (Beer et al., 2003; Stone et al., 1998), failure to use emotions to guide decisions (Bechara, 2004), impaired recognition of emotional expressions (Hornak et al., 2003; Tsuchida and Fellows, 2012), and defective social and moral reasoning (Anderson et al., 1999). A recent study demonstrated an anatomical double dissociation in which deficits in cognitive empathy (e.g., perspective taking) result from medial OFC/ventromedial PFC damage, while deficits in emotional empathy (e.g., responding with appropriate emotion to the mental states of others) result from IFG damage (Shamay-Tsoory et al., 2009).
Other impairments
OFC patients also demonstrate impairments in attention, especially to emotional stimuli (Hartikainen and Knight, 2003) and impairments in temporal context memory (Duarte et al., 2010; Gilboa et al., 2006) that often result in confabulatory behavior (Schnider and Ptak, 1999; Turner et al., 2008).
It is important to note that many of the studies reviewed in this section report results from patients with damage extending into more dorsal portions of medial frontal cortex (e.g., BA 24, 25, and 32), making it difficult to determine the exact lesion location that leads to the observed deficits. This is further complicated by the fact that researchers often refer to OFC (or medial OFC) and ventromedial PFC interchangeably in the patient literature. However, a majority of studies point to the importance of the ventromedial surface of the OFC in humans (Wallis, 2012), suggesting that this location is critical for inhibitory control of social and emotional information.
Lesions to Medial PFC
The medial surface of the PFC is generally divided into two sections (Fig. 2, right). The dorsomedial section includes portions of BA 8, 9, 10, 24 (ventral anterior cingulate), and 32 (dorsal anterior cingulate). The ventromedial section includes portions of BA 10, 12, 14, 25, and ventral portions of 24 and 32. Damage to dorsomedial PFC (particularly the ACC) has been associated with the inability to detect errors, difficulty with resolving stimulus conflict, emotional instability, inattention, and abulia or akinetic mutism (Devinsky et al., 1995; Posner and DiGirolamo, 1998) (Fig. 5). In contrast, ventromedial damage disrupts social behavior as well as social, emotional, and value-based decision-making (Anderson et al., 2006; Eslinger and Damasio, 1985; Fellows and Farah, 2003). Note that a clear cytoarchitectural boundary between the dorsal and ventral aspects of medial PFC does not exist, although there is some evidence that it can be functionally subdivided (Steele and Lawrie, 2004), and there is often a considerable amount of lesion overlap between patients described as having dorsomedial damage or ventromedial damage, which could contribute to the overlap of functions attributed to each region.
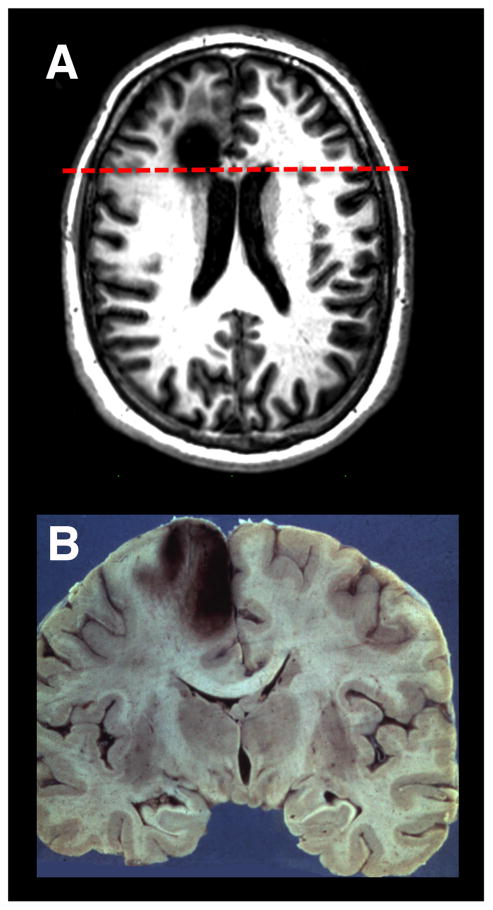
Figure 5. Lesions to Medial Prefrontal Cortex.
(A) An axial T1 MRI image of a patient with a chronic left medial frontal lobe lesion due to the resection of a low-grade glioma. (B)A coronal neuropathological specimen of an infarct in the left medial PFC (note the hemorrhagic conversion in the cortical mantle). The dashed red line on the MRI in (A) shows the approximate site of the post-mortem coronal slice. Image in (A) is compliments of Professor Marianne Løvstad, University of Oslo. Neuropathology specimen in (B) is compliments of Professor Dimitri Agamanolis, Akron Children’s Hospital (http://neuropathology-web.org/).
Motivation and emotion
Papez (1937) noted tumors pressing on or infiltrating the cingulate cortex in humans resulted in “loss of spontaneity in emotion, thought, and activity” and an “indifference to environment, change in personality or character, drowsiness, [and] stuporous or comatose state” (p. 736). These observations are consistent with more recent evidence that ACC lesions in the human lead to abulia, or its more severe form, akinetic mutism (Damasio and Van Hoesen, 1983; Devinsky et al., 1995). For example, patients with ACC damage have been reported to lack the will to move or talk, even though they are capable of both (Damasio and Van Hoesen, 1983). This is supported by a recent study demonstrating that direct ACC stimulation induces feelings of perseverance (Parvizi et al., 2013). Cohen and colleagues (1999) noted emotional and motivational changes in a group of patients following bilateral ACC resection for pain management. As a side effect of the ACC ablations, these patients exhibited a decrease in spontaneous, self-initiated behaviors, including spontaneous response production (Cohen et al., 1999). ACC damage also leads to abnormal autonomic arousal (Critchley et al., 2003), which in turn may affect the motivation level of these patients. Damage to ventral portions of the cingulate also result in altered motivation and emotion. For example, subcallosal cingulate gyrus (ventral BA 24/25/32) damage or malfunction often leads to major depression (Hamani et al., 2011). Taken together, these studies suggest that the cingulate cortex is critical for regulation of motivational and emotional behavior, which is in agreement with the conclusions made by Papez (1937) many decades earlier.
Executive attention
The notion of intentional or voluntary control over behavior has been characterized as executive attention (Posner and DiGirolamo, 1998), supervisory attention (Norman and Shallice, 1986), or attention for action (Posner et al., 1988). Posner and DiGirolamo (1998) theorized that dorsomedial PFC, specifically the ACC, was at the center of the executive attention system and was necessary for tasks involving error detection, novelty, difficult processing, or conflict. The ACC has been linked to deficits in executive or supervisory attention. For example, dorsomedial lesions result in global slowing and increased response variability on a range of different tasks requiring cognitive control (Shallice et al., 2008; Stuss et al., 2005). This inability to react quickly or to keep pace with behavioral demands has been interpreted as a deficit in executive attention (however, it could also result from a lack of motivation). The notion that ACC is necessary for executive attention has now given way to more specific theories about the role of the ACC in error detection (Holroyd and Coles, 2002), conflict monitoring (Botvinick et al., 2004), and hierarchical reinforcement learning (Holroyd and Yeung, 2012).
Decision-making and learning
Medial PFC has been associated with decision-making and learning in both social and non-social (cognitive) contexts. Neuroimaging studies have implicated dorsal ACC (dACC) in decision-making tasks requiring cognitive control, especially when there is conflict between responses (Botvinick et al., 2004) or when there is a need to update behavior to reflect action-reinforcement contingencies (reinforcement learning; Holroyd and Yeung, 2012). However, lesions of the dACC yield inconsistent behavioral results in both monkeys and humans. Initial studies in monkeys found damage to dACC did not lead to impairments in reinforcement learning (Pears et al., 2003) and lead to only modest impairments in response competition and error detection (Rushworth et al., 2003). However, more recent studies have found evidence that dACC damage or inactivation causes deficits in reward strategy (Amiez et al., 2006; Kennerley et al., 2006). For example, monkeys with dACC lesions do not show impaired learning immediately after making an error, but are less likely to repeat a response that leads to a reward and show impaired reinforcement learning over time (Kennerley et al., 2006).
Damage to the dACC in humans does not lead to impairments on tasks commonly used to measure conflict or error detection in neuroimaging studies, such as the Stroop and Go/No-Go tasks, even in patients with extensive bilateral medial lesions (Fellows and Farah, 2005b), nor do dACC lesions lead to deficits in flexible reinforcement learning (Tsuchida et al., 2010). Rather, dACC in the human is needed for associating actions with value. A recent study demonstrated a double dissociation between associations of actions and stimuli with value, such that dACC patients were significantly less likely than healthy controls to repeat an action that had lead to a reward on the previous trial, while OFC patients were significantly less likely than controls to repeatedly choose a stimulus that had been rewarded on the previous trial (Camille et al., 2011). Similar results have been found in the monkey (Kennerley et al., 2006; Rudebeck et al., 2008). These monkey and human lesion studies suggest that dACC may be important for updating goal-directed actions based upon reinforcement history.
Damage to ventromedial PFC leads to poor emotional and social decision-making (Bechara, 2004) as well as impairments in value-based decision-making and learning (Clark et al., 2004; Fellows and Farah, 2003; Hornak et al., 2004). Damage to ventromedial PFC often includes the medial aspects of OFC. As a result, the decision-making and learning deficits associated with ventromedial PFC damage are similar to those already discussed in the ‘Lesions to Orbitofrontal Cortex’ section.
Social cognition
Recent evidence, mostly from neuroimaging, has suggested a role for human medial PFC (BA 8, 9, 10, including the paracingulate cortex, also referred to as the anterior rostral medial PFC) in thinking or having knowledge about oneself or others in a social context (Amodio and Frith, 2006). The anterior portion of the rostral medial PFC is activated in tasks that require subjects to make social judgments (Amodio and Frith, 2006). In particular, it may be important for adjusting initial impressions of individuals (Cooper et al., 2010; Croft et al., 2010) or comparing oneself to others (Ochsner et al., 2005; Tamir and Mitchell, 2010). The medial aspect of BA 10 is often activated in these tasks. Recent theories have suggested that functions related to metacognition, including mentalizing, self-knowledge, or person-knowledge, are specific to BA 10 (Burgess and Wu, 2013; Stuss and Alexander, 2007; see ‘Lesions to Rostral PFC’).
Few lesion studies have specifically investigated the role of medial PFC in social judgment, self- knowledge, or person- knowledge. However, one recent study reported patients with medial PFC damage were unable to adjust their initial judgments of individuals after receiving further personal information (Croft et al., 2010). This suggests that medial PFC is important for updating initial impressions of individuals.
Damage to ventromedial PFC results in deficits in social and emotional behavior, including lack of affect or poorly modulated emotional reactions, lack of empathy, an inability to observe social conventions, and poor social decision-making (Barrash et al., 2000; Blumer and Benson, 1975; Eslinger and Damasio, 1985; Shamay-Tsoory et al., 2009; Tranel, 1994). Damage to ventromedial PFC in early life has lasting effects on social behaviors, including the inability to form lasting friendships and abnormal perception and expression of emotions, such as empathy and regret (Anderson et al., 2006; Anderson et al., 1999). These impairments have been discussed further in ‘Lesions to Orbitofrontal Cortex’.
Lesions to Rostral PFC
The functions of rostral PFC (BA 10; also referred to as the frontal pole) in the human have remained unspecified for many years. Notably, patients with rostral PFC damage often do not exhibit deficits on traditional neuropsychological testing batteries and show normal performance on IQ tests (Goel and Grafman, 2000; Shallice and Burgess, 1991; Uretzky and Gilboa, 2010). In fact, there are reports of improvements on task performance following separation of the rostral PFC from posterior cortex (Petrie, 1952). In a somewhat contradictory finding, neuroimaging studies in healthy subjects report activations in rostral PFC during a wide variety of tasks (Burgess et al., 2005).
Although their performance is normal on traditional neuropsychological tests, patients with rostral PFC damage often show disorganized behavior in situations encountered in everyday life, as evidenced by multiple case studies (Eslinger and Damasio, 1985; Shallice and Burgess, 1991). For example, rostral PFC patients have difficulty making decisions that are encountered in everyday life (e.g., what to wear or what food to buy at the grocery store) and are sometimes unable to maintain their careers following damage (Eslinger and Damasio, 1985).
Researchers have begun to link these seemingly intangible behavioral impairments to underlying function over the last few decades. A recent study (Roca et al., 2010) found rostral PFC damage lead to impairments on a number of (non-traditional) executive tasks that could not be explained by fluid intelligence, including Go/No-Go, the Hayling Sentence Completion Task (Burgess and Shallice, 1997), the Hotel Task (Manly et al., 2002), and the Faux Pas Test (Stone et al., 1998). Some common processing themes that have been suggested to link these deficits are multitasking (Burgess et al., 2000), the ability to switch between cognitive contexts (Badre and D’Esposito, 2009; Koechlin and Summerfield, 2007), or even more generally, “metacognition” (Burgess and Wu, 2013; Stuss and Alexander, 2007).
Mulitasking and prospective memory
Multitasking has been defined as a type of scheduling, which requires one to hold goals in mind while performing or processing secondary subgoals (Burgess et al., 2000), also referred to as ‘cognitive branching’ (Koechlin and Hyafil, 2007). Burgess and colleagues (Burgess, 2000; Shallice and Burgess, 1991) have designed a number of tasks to investigate multitasking ability. For the Multiple Errands Test (Shallice and Burgess, 1991), patients must follow a set of rules while running errands in an actual, but unfamiliar, shopping district. For the Six Element Task (Shallice and Burgess, 1991), and for the Hotel Task that was subsequently developed, patients are required to shift between three different tasks (with two subsections each) by following a set of rules and to finish within an allotted amount of time. This requires patients to effectively manage time while switching tasks. The Greenwich Test (Burgess et al., 2000) is similar to the Six Element Task, but requires patients to follow more rules. Patients with rostral PFC damage perform poorly on all three of these tests (Burgess et al., 2000; Roca et al., 2011; Shallice and Burgess, 1991). Furthermore, the extent of damage to this region positively predicts multitasking deficits (Dreher et al., 2008; Roca et al., 2011). Poor planning or poor memory for task rules cannot explain these deficits (Burgess et al., 2000). Duncan and colleagues (e.g., Duncan et al., 1995) also noted similar impairments in multitasking behavior following PFC damage, but interpreted these impairments as goal neglect, resulting from a loss of general intelligence. However, recent evidence suggests impairments remain even after controlling for fluid intelligence (Roca et al., 2010).
Prospective memory, one element needed for successful multitasking, is the ability “to remember to carry out an intended act in the future, while engaged in another task” (Burgess and Wu, 2013, pg. 531). Several studies have reported deficits in prospective memory following rostral PFC damage (Umeda et al., 2011; Uretzky and Gilboa, 2010; Volle et al., 2011). These deficits are especially evident for time-based prospective memory (i.e., these patients have difficulty carrying out an act at a particular time). Accordingly, rostral PFC patients were also impaired at time estimation compared to patients with damage elsewhere (Volle et al., 2011). Thus, deficits in prospective memory may ultimately lead to poor multitasking performance in patients with lesions to rostral PFC.
Creativity
There is preliminary evidence to suggest rostral PFC damage causes impairments in creativity. A recent study (Shamay-Tsoory et al., 2011) found greater lesion volume in medial BA 10 predicted greater impairments in creativity and original thinking, as measured by the Torrance Test of Creative Thinking (TTCT). Patients with the frontal variant of frontotemporal lobar degeneration, which causes bilateral degeneration of anterior prefrontal and temporal cortex often disproportionatley affecting the ventromedial PFC, are also severely impaired on the TTCT (Cruz de Souza et al., 2010). Furthermore, the amount of hypoperfusion in rostral PFC predicts TTCT scores.
Mentalizing
Several recent studies have suggested that rostral PFC is important for attributing mental states to oneself and others, a concept commonly referred to as mentalizing, or ‘theory of mind’. One common neuropsychological test to measure theory of mind is the Faux Pas Test (Stone et al, 1998). In this test, patients are read a series of stories, half of which contain a faux pas, where one person unintentionally hurts or insults another person, and patients must identify whether someone behaved inappropriately for each story. Roca and colleagues (2010; 2011) demonstrated that only damage to BA 10 leads to deficits in the Faux Pas Test. This suggests that rostral PFC may be an important node in the network used for mentalizing. Note that patients with OFC damage also perform poorly on theory of mind tests (e.g., Stone et al., 1998). Perhaps this is because individual subjects have lesions extending into both OFC and the frontal pole or perhaps OFC and rostral PFC contribute to different aspects of mentalizing ability.
Metacognition
Rostral PFC seems to be involved in a number of functions that can collectively be referred to as metacognition (Burgess and Wu, 2013; Stuss and Alexander, 2007). Neuroimaging studies have implicated rostral PFC in a wide variety of functions that all could be considered metacognitive in nature, including prospection (imagining the future), metamemory and reality monitoring, introspection, mentalizing (already discussed) and self-judgment, analogical reasoning, and switching between attending to the external world and our own inner thoughts (see Burgess and Wu, 2013 for a review).
Translational Applications
Luria suggested that rehabilitation of PFC patients would be difficult (Luria et al., 1975). This was partially based upon his belief that the reorganizational mechanisms for recovery in more posterior cortical regions relied upon the compensatory processes that were carried out by the higher-level systems in the PFC. Thus, if PFC were damaged, the brain lacked the ability for recovery. We now know that this idea is not entirely true. Intact portions of the PFC are able to reorganize following damage or disease, most likely because these parts of cortex are sufficiently flexible. Here, we discuss evidence for the brain’s ability to reorganize following injury, leading to (at least partial) recovery of function, and the various techniques that may be used to assist in this recovery.
Reorganization
There is a growing body of evidence demonstrating network reorganization following brain damage. Several studies have suggested that the intact portions of a cortical network may assume the functions, or compensate, for damaged portions of the network (Corbetta et al., 2005; Rosen et al., 2000; Voytek et al., 2010; Wang et al., 2010). For example, Voytek and colleagues (2010) found increases in theta power over PFC of the intact hemisphere while patients with unilateral DLPFC damage performed a working memory task. Furthermore, the changes in theta power fluctuated on a trial-by-trial basis depending on cognitive load. These data suggest that some PFC regions are able to assume the functions of other regions, at least to some extent, following damage. However, this is not always the case. A recent study found PFC damage resulted in lasting decreases in functional connectivity throughout intact network areas (Nomura et al., 2010). In addition, Anderson and colleagues (2006; 1999) have reported patients with focal unilateral lesions to the ventromedial PFC sustained early in life (< 7 years of age), who show severe and lasting impairments in social cognition and moral reasoning during adulthood. Therefore, whether or not any given PFC area is able to reorganize may depend upon regional factors and age of lesion onset.
Neurorehabilitation
Patient rehabilitation is difficult following PFC injury and little progress has been made towards developing useful therapies to aid those with executive dysfunction (possibly with the exception of language). A number of therapeutic cognitive and/or pharmacological techniques have been suggested and assessed using various patient populations with executive dysfunction caused by a range of different conditions, including stroke, TBI, or neurodegeneration.
Cognitive therapy techniques, techniques that attempt to alter or improve cognitive abilities in patients, can be broadly divided into two categories: compensatory techniques, which are designed to help patients learn new skills in order to compensate for their impairments, and direct interventions, which aim to restore a skill that was previously impaired due to damage. One compensatory technique developed to help patients with disinhibition problems (such as those with OFC damage) is the response-cost procedure (Alderman and Ward, 1991). In this technique, a patient is given tokens that he/she may use to redeem rewards, but these tokens can also be taken away if the patient violates a certain set of rules outlined by the therapist (similar to operant conditioning). This technique has been fairly successful in treating disinhibition problems. Another compensatory technique that has become successful within the last decade is the use of portable electronic memory devices, which aid impairment in prospective memory (O’Neil-Pirozzi et al., 2004).
Numerous direct intervention techniques have focused on various forms of cognitive training to help PFC patients regain some of their lost cognitive abilities (such as attention, working memory, or goal-directed behavior). The hope is that training will transfer to behavioral and cognitive improvement in everyday life situations. Some examples include the Attention Process Training (Sohlberg et al., 2000), which aims to retrain attentional abilities by using a series of auditory or visual exercises, and training in activities of daily living (Carter et al., 1983), which focuses on training of specific skills needed for everyday activities. More recently, improvements in the working memory performance of stroke patients were observed following computer-based training focusing on spatial working memory. These cognitive improvements appeared to transfer to novel working memory tasks performed by patients (e.g., Westerberg et al., 2007). These improvements also correlate with increases in PFC activation and dopamine D1 receptor binding in healthy subjects (McNab et al., 2009; Olesen et al., 2004), suggesting that working memory training could do the same for patients with PFC damage. Increased arousal and mindfulness have also been shown to improve attention and executive behavior following brain injury (Degutis and Van Vleet, 2010; Manly et al., 2002; Novakovic-Agopian et al., 2011). For example, patients with executive dysfunction can be taught to self-regulate their arousal levels, thus learning to self-alert, while performing tasks that require goal-directed behavior (Manly et al., 2002) (see also Goal Management Training (Levine et al., 2011)).
Pharmacological interventions are not widely used at the present time to treat executive dysfunction in patients suffering from PFC damage due to stroke, TBI, or neurodegeneration (the exception being patients with schizophrenia or Parkinson’s Disease, who both show executive dysfunction and are commonly treated using drug intervention). There is some evidence to suggest that dopaminergic drugs could be used to effectively treat behavioral deficits following PFC damage. McDowell and colleagues (1998) demonstrated that bromocriptine, a D2 dopamine receptor agonist, improved the performance of TBI patients on a large number of tasks thought to engage the PFC. But it remains to be seen whether dopaminergic drugs are useful to treat executive dysfunction in patients with more focal PFC lesions.
In summary, it is clear that while many studies have recently begun to focus their efforts on new neurorehabilitation techniques for the treatment of executive dysfunction, further research on this topic is needed. Improved understanding of the physiological basis of PFC functions will hopefully lead to development of new therapies to treat patients with cognitive and executive disorders.
Theories of Prefrontal Function
A longstanding debate in the reviewed literature involves the organization of PFC functions. One central point of disagreement is whether PFC organization is domain-general (i.e., with a single role in which all or many regions participate) or domain-specific (i.e., functions are localized to subregions). Evidence exists to support both views. Proponents of a domain-general theory posit the PFC serves a broad role in executive function, which has been characterized as the active maintenance of goals and the means to achieve them (Miller and Cohen, 2001), adaptive coding and general intelligence (Duncan, 2001), and the representation of temporally complex events (Wilson et al., 2010). Duncan and colleagues have suggested that the PFC is specialized for adaptive behavior, since it contains neurons that are able to code different information depending on current task demands. In support of this view, many of the tasks that engage PFC in humans recruit a similar set of brain regions, including the mid-DLPFC, mid-VLPFC, and dACC and the recruitment of these brain regions has been linked to general intelligence (Duncan, 2010). Single-unit recordings in monkeys have provided ample evidence that substantial portions of PFC cells are ‘pluripotent’ (i.e., able to engage in multiple cognitive tasks) (e.g., Cromer et al., 2010). In addition, many patients with PFC lesions show impairment on a wide variety of tasks, including speeded response choice, episodic memory, and problem solving (Duncan, 2001).
Another set of theories has suggested that PFC is organized hierarchically in a caudal to rostral manner, with information becoming increasingly more abstract in more rostral PFC regions (Badre and D’Esposito, 2009; Koechlin and Summerfield, 2007). Lesion evidence supports this by demonstrating lesion location determines the type of deficits manifested by PFC patients, with more caudal damage resulting in deficits when performing tasks with a lower level of abstractness (i.e., sensorimotor transformation, working memory) and more rostral damage resulting in deficits only when performing more abstract tasks (Badre et al., 2009). Azuar and colleagues (2014) used VLSM across patients with differing lesion locations to demonstrate that more rostral PFC regions rely on the intactness of more caudal regions for normal functioning, but not vice versa, lending further support for a model of hierarchical control across caudal to rostral PFC. It should be noted, however, that this model has only been tested in lateral PFC to date.
Some of the strongest evidence for domain specificity of function is provided by the existence of double dissociations between PFC areas (damage to one region produces a behavioral deficit that is not observed following damage to a different region and vice versa). However, relatively few studies have demonstrated such double dissociations following PFC damage in the human. A majority of studies compare PFC-damaged individuals with age- and education-matched controls or with patients who have lesions outside of PFC. While these studies often provide compelling evidence for functional specificity, they do not necessarily demonstrate that a function is tied to one region only. We have reviewed several studies that demonstrate double dissociations between PFC patient groups (Camille et al., 2011; Shamay-Tsoory et al., 2009; Tsuchida and Fellows, 2009). More examples of double dissociations exist in the primate lesion literature, since lesion size and location are more easily controlled (e.g., Buckley et al., 2009; Dias et al., 1996a; Noonan et al., 2010; Rudebeck and Murray, 2011). Neuroanatomical data suggest regional differences in sensory input to monkey PFC, providing further evidence for domain-specific organization of sensory processing (Romanski, 2007). Thus, some evidence exists to support the domain-specific hypothesis that different PFC subregions are involved in discrete functions.
There are several potential issues preventing researchers from determining the extent of domain specificity within PFC. The first issue is that many studies include patients with large lesions that involve multiple PFC regions, making it challenging to determine functional specificity. The second issue is potential commonalities across seemingly diverse tasks. For example, it is possible that one or a few core cognitive deficits (e.g., working memory, inhibition) could explain impairments on more complex reasoning or social/emotional tasks that require multiple cognitive operations for successful completion. Very few studies have controlled for potential correlations and shared variance among different cognitive operations during task performance. Thus, it is generally unknown how each core cognitive operation might relate to performance across a diverse set of often complex tasks.
Wilson and colleagues (2010) have attempted to resolve the debate by proposing that PFC integrates both domain-general and domain-specific information. That is, although some PFC subregions may be specialized for individual functions, the unifying function of the PFC is greater than the sum of its parts. Our view is that the domain-general and domain-specific theories need not be mutually exclusive. Indeed, we would propose that not only is there domain-specific information, but domain-general cells are intermixed with domain-specific cells within PFC. This would provide the most robust neuroanatomical organization for the rapid and flexible capabilities of the human PFC.
Conclusions
The lesion literature discussed above suggests that major divisions of the PFC control different aspects of executive function and, in turn, make different contributions to goal-directed behavior. Lateral PFC is critical for the selection, monitoring, and manipulation of cognitive task sets, medial PFC is critical for the updating of these task sets, and OFC is critical for assigning social and emotional meaning to these task sets in order to better guide goal-directed behavior. However, one must be cautious in developing a phrenological view of regional PFC capacity. Although the current review has linked specific functions to specific PFC subregions, there are substantial overlapping and interactive functions across these regions (Duncan, 2010), which is not surprising given their extensive interconnectivity (Catani et al., 2012). It is likely that while each major subregion has distinct and partially dissociable functions, the PFC as a whole performs one or more unifying functions. What these unifying functions might be specifically is yet undetermined and should be of theoretical and empirical importance for future PFC research.
Many initial studies, although prescient for their time, were not well controlled and often drew conclusions about PFC functions based upon only one or a few patients with large lesions covering multiple PFC regions. Advances in MRI-based pre-mortem neuroanatomical lesion specificity have allayed some of these concerns. One enduring issue, even for current studies using neuroanatomically well-delineated PFC patients, is small sample sizes. A potential solution to this problem would be to create a consortium, or national database, where researchers would have access to patients, who have specific lesions and are willing to participate in studies across a wide geographic area. Another challenging issue for researchers in this field has been to design behavioral tasks that accurately measure particular functions or clinical phenomena of interest. Many traditional tasks lack construct validity or engage multiple PFC regions simultaneously, making it difficult to accurately measure structure-function relationships. Despite these issues, the field has experienced many important advances. The modern use of patients with more focal lesions, the identification of double dissociations between lesion sites and behavioral deficits, and the development of new methods to link damage to behavior (e.g., VLSM and voxel-based analysis of lesions) have provided both novel insights into PFC functions and crucial information to better interpret data acquired using modern neuroimaging approaches. The use of theory-based behavioral testing in combination with physiological recording techniques in patients with well-delineated PFC damage will increase our understanding of this vast cortical region that is so vital for successful human behavior.
Acknowledgments
This research was supported by National Institutes of Health grant2R37 NS21135, a grant from the Peder Sather Center for Advanced Study, and the Nielsen Corporation. The authors would like to thank Professor Dimitri Agamanolis, Professor Edward C. Klatt, and Professor Walter Finkbeiner for the use of neuropathological images and Professor Marianne Løvstad for the use of the MRI image in Fig. 5a.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderman N, Ward A. Behavioural treatment of the dysexecutive syndrome: Reduction of repetitive speech using response cost and cognitive overlearning. Neuropsychol Rehabil. 1991;1:65–80. [Google Scholar]
- Alexander MP, Stuss DT, Fansabedian N. California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126:1493–1503. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. J Int Neuropsychol Soc. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Azuar C, Reyes P, Slachevsky A, Volle E, Kinkingnehun S, Kouneiher F, Bravo E, Dubois B, Koechlin E, Levy R. Testing the model of caudo-rostral organization of cognitive control in the human with frontal lesions. NeuroImage. 2014;84:1053–1060. doi: 10.1016/j.neuroimage.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Hoffman J, Cooney JW, D’Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat Neurosci. 2009;12:515–522. doi: 10.1038/nn.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–1205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40:349–356. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. J Pers Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Bianchi L. The Functions of the Frontal Lobes. Brain. 1895;18:497–522. [Google Scholar]
- Bidet-Caulet A, Buchanan KG, Viswanath H, Black J, Scabini D, Bonnet-Brilhault F, Knight RT. Impaired Facilitatory Mechanisms of Auditory Attention After Damage of the Lateral Prefrontal Cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu131. Online pub date: June 12 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach E, Geminiani G, Berti A, Rusconi ML. Perceptual and premotor factors of unilateral neglect. Neurology. 1990;40:1278–1281. doi: 10.1212/wnl.40.8.1278. [DOI] [PubMed] [Google Scholar]
- Blumer D, Benson D. Personality changes with frontal and temporal lobe lesions. In: Benson DF, Blumer D, editors. Psychiatric Aspects of Neurologic Disease. New York: Grune & Stratton; 1975. pp. 151–170. [Google Scholar]
- Borovsky A, Saygin AP, Bates E, Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45:2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Broca PP. Loss of speech, chronic softening and partial destruction of the anterior left lobe of the brain. Bulletin de la Société Anthropologique. 1861;2:235–238. [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Strategy application disorder: the role of the frontal lobes in human multitasking. Psychol Res. 2000;63:279–288. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton tests. Thurston, Suffolk: Thames Valley Test Company; 1997. [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hpothesis of rostral PFC function. In: Duncan J, Phillips L, McLeod P, editors. Measuring the Mind: Speed, Control and Age. Oxford: Oxford University Press; 2005. pp. 215–246. [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Wu H. Rostral prefrontal cortex (Brodmann area 10) In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2013. pp. 524–544. [Google Scholar]
- Butter CM, Snyder DR, McDonald JA. Effects of orbital frontal lesions on aversive and aggressive behaviors in rhesus monkeys. J Comp Physiol Psychol. 1970;72:132–144. doi: 10.1037/h0029303. [DOI] [PubMed] [Google Scholar]
- Camille N, Tsuchida A, Fellows LK. Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. J Neurosci. 2011;31:15048–15052. doi: 10.1523/JNEUROSCI.3164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D. Aphasic syndromes. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; 2003. pp. 14–34. [Google Scholar]
- Carter LT, Howard BE, O’Neil WA. Effectiveness of cognitive skill remediation in acute stroke patients. Am J Occup Ther. 1983;37:320–326. doi: 10.5014/ajot.37.5.320. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’acqua F, Vergani F, Malik F, Hodge H, Roy P, Valabregue R, Thiebaut de Schotten M. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41:1474–1483. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Intentional and incidental self-control in ventrolateral prefrontal cortex. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2013. pp. 417–440. [Google Scholar]
- Cohen RA, Kaplan RF, Zuffante P, Moser DJ, Jenkins MA, Salloway S, Wilkinson H. Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. J Neuropsychiatry Clin Neurosci. 1999;11:444–453. doi: 10.1176/jnp.11.4.444. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Kreps TA, Wiebe T, Pirkl T, Knutson B. When giving is good: ventromedial prefrontal cortex activation for others’ intentions. Neuron. 2010;67:511–521. doi: 10.1016/j.neuron.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Croft KE, Duff MC, Kovach CK, Anderson SW, Adolphs R, Tranel D. Detestable or marvelous? Neuroanatomical correlates of character judgments. Neuropsychologia. 2010;48:1789–1801. doi: 10.1016/j.neuropsychologia.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer JA, Roy JE, Miller EK. Representation of multiple, independent categories in the primate prefrontal cortex. Neuron. 2010;66:796–807. doi: 10.1016/j.neuron.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Souza L, Volle E, Bertoux M, Czernecki V, Funkiewiez A, Allali G, Leroy B, Sarazin M, Habert MO, Dubois B, Kas A, Levy R. Poor creativity in frontotemporal dementia: a window into the neural bases of the creative mind. Neuropsychologia. 2010;48:3733–3742. doi: 10.1016/j.neuropsychologia.2010.09.010. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and FMRI data. J Int Neuropsychol Soc. 2006;12:248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:1303–1315. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ahern GL, Weintraub S, Mesulam MM. Dissociated neglect behavior following sequential strokes in the right hemisphere. Ann Neurol. 1990;28:97–101. doi: 10.1002/ana.410280119. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Holcomb PJ, Calvo V, Acar D, Chabrerie A, Kikinis R, Jolesz FA, Rentz DM, Scinto LF. Disruption of attention to novel events after frontal lobe injury in humans. J Neurol Neurosurg Psychiatry. 2000a;68:18–24. doi: 10.1136/jnnp.68.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000b;123 (Pt 5):927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Van Hoesen GW. Emotional disturbances associated with focal lesions of the limbic frontal lobe. In: Heilman KM, Satz P, editors. Neuropsychology of Human Emotion. New York: Guilford Press; 1983. pp. 85–110. [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Degutis JM, Van Vleet TM. Front Hum Neurosci. Tonic and phasic alertness training: a novel behavioral therapy to improve spatial and non-spatial attention in patients with hemispatial neglect. 2010;4 doi: 10.3389/fnhum.2010.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dick F, Bates E, Wulfeck B, Utman JA, Dronkers N, Gernsbacher MA. Language deficits, localization, and grammar: evidence for a distributive model of language breakdown in aphasic patients and neurologically intact individuals. Psychol Rev. 2001;108:759–788. doi: 10.1037/0033-295x.108.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drai D, Grodzinsky Y. A new empirical angle on the variability debate: quantitative neurosyntactic analyses of a large data set from Broca’s aphasia. Brain Lang. 2006;96:117–128. doi: 10.1016/j.bandl.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS One. 2008;3:e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130:1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Shapiro JK, Redfern B, Knight RT. The role of Broca’s area in Broca’s aphasia. J Clin Exp Neuropsyc. 1992;14:52–53. [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Knight RT, Emery T, Graham KS. Orbito-frontal cortex is necessary for temporal context memory. J Cogn Neurosci. 2010;22:1819–1831. doi: 10.1162/jocn.2009.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J Neurosci. 2005;25:8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. Disorganisation of behaviour after frontal lobe damage. Cognitive Neuropsych. 1986;37:271–290. [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–268. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- Duncan J, Parr A, Woolgar A, Thompson R, Bright P, Cox S, Bishop S, Nimmo-Smith I. Goal neglect and Spearman’s g: competing parts of a complex task. J Exp Psychol Gen. 2008;137:131–148. doi: 10.1037/0096-3445.137.1.131. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM. Altered serial position learning after frontal lobe lesion. Neuropsychologia. 1994;32:729–739. doi: 10.1016/0028-3932(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005a;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005b;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Ferrier D. The Functions of the Brain. London: Smith, Elder, and Co; 1876. [Google Scholar]
- Fiebach CJ, Schubotz RI. Dynamic anticipatory processing of hierarchical sequential events: a common role for Broca’s area and ventral premotor cortex across domains? Cortex. 2006;42:499–502. doi: 10.1016/s0010-9452(08)70386-1. [DOI] [PubMed] [Google Scholar]
- Floden D, Alexander MP, Kubu CS, Katz D, Stuss DT. Impulsivity and risk-taking behavior in focal frontal lobe lesions. Neuropsychologia. 2008;46:213–223. doi: 10.1016/j.neuropsychologia.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Franzen EA, Myers RE. Neural control of social behavior: prefrontal and anterior temporal cortex. Neuropsychologia. 1973;11:141–157. doi: 10.1016/0028-3932(73)90002-x. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. New York: Raven Press; 1989. [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia. 1995;33:1305–1333. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Alain C, Stuss DT, Melo B, Miller S, Moscovitch M. Mechanisms of spontaneous confabulations: a strategic retrieval account. Brain. 2006;129:1399–1414. doi: 10.1093/brain/awl093. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Grafman J. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia. 1995;33:623–642. doi: 10.1016/0028-3932(95)90866-p. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J. Role of the right prefrontal cortex in ill-structured planning. Cogn Neuropsychol. 2000;17:415–436. doi: 10.1080/026432900410775. [DOI] [PubMed] [Google Scholar]
- Grafman J. Plans, actions, and mental states: Managerial knowledge units in the fontal lobes. In: Perecman E, editor. Integrating Theory and Practice in Clinical Neurpsychology. Hillsdale, NJ: Erlbaum; 1989. pp. 93–138. [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Dissociations among tasks involving inhibition: a single-case study. Cogn Affect Behav Neurosci. 2005;5:1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Passage of an iron rod throught the head. Boston Med and Surg J. 1848;39:389–393. [PMC free article] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society (Boston) 1868;2:327–346. [Google Scholar]
- Hartikainen KM, Knight RT. Lateral and orbital prefrontal cortex contributions to attention. In: Polich J, editor. Detection of Change: Event-related Potential and fMRI Findings. Norwell, MA: Kluwer Academic; 2003. pp. 99–116. [Google Scholar]
- Hebb DO. Intelligence in man after large removals of cerebral tissue: Report of four left frontal lobe cases. J Gen Psychol. 1939;21:73–87. [Google Scholar]
- Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, Kennard C. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007;130:1525–1537. doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci. 2012;16:122–128. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Husain M, Kennard C. Visual neglect associated with frontal lobe infarction. J Neurol. 1996;243:652–657. doi: 10.1007/BF00878662. [DOI] [PubMed] [Google Scholar]
- Ilinsky IA, Jouandet ML, Goldman-Rakic PS. Organization of the nigrothalamocortical system in the rhesus monkey. J Comp Neurol. 1985;236:315–330. doi: 10.1002/cne.902360304. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CF. Functions of frontal association area in primates. Arch NeurPsych. 1935;33:558–569. [Google Scholar]
- Jahanshahi M, Frith CD. Willed action and its impairments. Cognit Neuropsychol. 1998;15:483–533. doi: 10.1080/026432998381005. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Jetter W, Poser U, Freeman RB, Jr, Markowitsch HJ. A verbal long term memory deficit in frontal lobe damaged patients. Cortex. 1986;22:229–242. doi: 10.1016/s0010-9452(86)80047-8. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Yonelinas AP, Knight RT. Novelty enhancements in memory are dependent on lateral prefrontal cortex. J Neurosci. 2009;29:8114–8118. doi: 10.1523/JNEUROSCI.5507-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Solbakk AK, Funderud I, Lovstad M, Endestad T, Knight RT. The role of the lateral prefrontal cortex in inhibitory motor control. Cortex. 2013;49:837–849. doi: 10.1016/j.cortex.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Schweizer TA, O’Connor C, Turner G, Gillingham S, Stuss DT, Manly T, Robertson IH. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. doi: 10.3389/fnhum.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. Higher Cortical Functions in Man. New York: Basic Books; 1966. [Google Scholar]
- Luria AR, Naydin VL, Tsvetkova LS, Vinarskaya EN. Restoration of higher cortical functions following local brain damage. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. New York: Elsevier; 1975. pp. 368–433. [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Gershberg FB, Shimamura AP, Knight RT. Impaired retrieval from remote memory in patients with frontal lobe damage. Neuropsychology. 1996;10:32–41. [Google Scholar]
- Manly T, Hawkins K, Evans J, Woldt K, Robertson IH. Rehabilitation of executive function: facilitation of effective goal management on complex tasks using periodic auditory alerts. Neuropsychologia. 2002;40:271–281. doi: 10.1016/s0028-3932(01)00094-x. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Husain M, Rorden C, Kennard C, Driver J. Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature. 1998;392:179–182. doi: 10.1038/32413. [DOI] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D’Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121 (Pt 6):1155–1164. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. The role of the frontal lobes. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgements. Neuropsychologia. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB. Effects on executive function following damage to the prefrontal cortex in the rhesus monkey (Macaca mulatta) Behav Neurosci. 2009;123:231–241. doi: 10.1037/a0014723. [DOI] [PubMed] [Google Scholar]
- Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D’Esposito M. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci U S A. 2010;107:12017–12022. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: willing and automatic control of behaviour. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self Regulation. New York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Novakovic-Agopian T, Chen AJ, Rome S, Abrams G, Castelli H, Rossi A, McKim R, Hills N, D’Esposito M. Rehabilitation of executive functioning with training in attention regulation applied to individually defined goals: a pilot study bridging theory, assessment, and treatment. J Head Trauma Rehab. 2011;26:325–338. doi: 10.1097/HTR.0b013e3181f1ead2. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: reexamining the role of Broca’s area in sentence comprehension. Cogn Affect Behav Neurosci. 2005;5:263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- O’Neil-Pirozzi TM, Kendrick H, Goldstein R, Glenn M. Clinician influences on use of portable electronic memory devices in traumatic brain injury rehabilitation. Brain Injury. 2004;18:179–189. doi: 10.1080/0269905031000149560. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch Neur Psych. 1937;38:725–743. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Parker A, Wilding E, Akerman C. The Von Restorff effect in visual object recognition memory in humans and monkeys. The role of frontal/perirhinal interaction. J Cogn Neurosci. 1998;10:691–703. doi: 10.1162/089892998563103. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013;80:1359–1367. doi: 10.1016/j.neuron.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE. Memory of monkeys (Macaca mulatta) with lesions in prefrontal cortex. Behav Neurosci. 1985;99:3–21. doi: 10.1037//0735-7044.99.1.3. [DOI] [PubMed] [Google Scholar]
- Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrie A. Personality and the Frontal Lobes. New York: Blakiston; 1952. [Google Scholar]
- Posner MI, DiGirolamo G. Executive attention: conflict, target detection and cognitive control. In: Parasuraman R, editor. The Attentive Brain. Cambridge: MIT Press; 1998. pp. 401–423. [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A. The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. J Neurosci. 2010;30:12557–12565. doi: 10.1523/JNEUROSCI.2722-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries SK, Xie K, Haaland KY, Dronkers NF, Knight RT. Role of the lateral prefrontal cortex in speech monitoring. Front Hum Neurosci. 2013;7:703. doi: 10.3389/fnhum.2013.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NA, Beer JS, Werner KH, Scabini D, Levens SM, Knight RT, Levenson RW. The impact of orbital prefrontal cortex damage on emotional activation to unanticipated and anticipated acoustic startle stimuli. Cogn Affect Behav Neurosci. 2004;4:307–316. doi: 10.3758/cabn.4.3.307. [DOI] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, Manes F, Duncan J. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M, Torralva T, Gleichgerrcht E, Woolgar A, Thompson R, Duncan J, Manes F. The role of Area 10 (BA10) in human multitasking and in social cognition: a lesion study. Neuropsychologia. 2011;49:3525–3531. doi: 10.1016/j.neuropsychologia.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Romanski LM. Representation and integration of auditory and visual stimuli in the primate ventral lateral prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i61–69. doi: 10.1093/cercor/bhm099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Gaffan D, Passingham RE. The effect of cingulate cortex lesions on task switching and working memory. J Cogn Neurosci. 2003;15:338–353. doi: 10.1162/089892903321593072. [DOI] [PubMed] [Google Scholar]
- Schnider A, Ptak R. Spontaneous confabulators fail to suppress currently irrelevant memory traces. Nat Neurosci. 1999;2:677–681. doi: 10.1038/10236. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114 (Pt 2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Multiple effects of prefrontal lesions on task-switching. Front Hum Neurosci. 2008;1:2. doi: 10.3389/neuro.09.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Adler N, Aharon-Peretz J, Perry D, Mayseless N. The origins of originality: the neural bases of creative thinking and originality. Neuropsychologia. 2011;49:178–185. doi: 10.1016/j.neuropsychologia.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Jurica PJ, Mangels JA, Gershberg FB, Knight RT. Susceptibility to Memory Interference Effects following Frontal Lobe Damage: Findings from Tests of Paired-Associate Learning. J Cogn Neurosci. 1995;7:144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- Shiv B, Loewenstein G, Bechara A, Damasio H, Damasio AR. Investment behavior and the negative side of emotion. Psychol Sci. 2005;16:435–439. doi: 10.1111/j.0956-7976.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- Simon HA. The functional equivilence of problem solving skills. Cognitive Psychol. 1975;7:268–288. [Google Scholar]
- Sohlberg MM, McLaughlin KA, Pavese A, Heidrich A, Posner MI. Evaluation of attention process training and brain injury education in persons with acquired brain injury. J Clin Exp Neuropsychol. 2000;22:656–676. doi: 10.1076/1380-3395(200010)22:5;1-9;FT656. [DOI] [PubMed] [Google Scholar]
- Steele JD, Lawrie SM. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. NeuroImage. 2004;21:868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Stone JJ, Reynolds MR, Leuthardt EC. Transient hemispatial neglect after surgical resection of a right frontal lobe mass. World Neurosurg. 2011;76:361 e367–310. doi: 10.1016/j.wneu.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Stone SP, Patel P, Greenwood RJ, Halligan PW. Measuring visual neglect in acute stroke and predicting its recovery: the visual neglect recovery index. J Neurol Neurosurg Psychiatry. 1992;55:431–436. doi: 10.1136/jnnp.55.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo CL, Buckle L, Sayer L, Pogue J. Organizational strategies with unilateral or bilateral frontal lobe injury in word learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of Frontal Lobe Function. New York: Oxford University Press; 2013. [Google Scholar]
- Tamir DI, Mitchell JP. Neural correlates of anchoring-and-adjustment during mentalizing. Proc Natl Acad Sci U S A. 2010;107:10827–10832. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber HL, Battersby WS, Bender MB. Performance of complex visual tasks after cerebral lesions. J Nerv Ment Dis. 1951;114:413–429. [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tranel D. “Acquired sociopathy”: The development of sociopathic behavior following focal brain damage. In: Fowles DC, Sutker P, Goodman SH, editors. Progress in Experimental Personality and Psychopathology Research. New York: Springer; 1994. pp. 285–311. [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30:16868–16875. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Lesion evidence that two distinct regions within prefrontal cortex are critical for n-back performance in humans. J Cogn Neurosci. 2009;21:2263–2275. doi: 10.1162/jocn.2008.21172. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex. 2012;22:2904–2912. doi: 10.1093/cercor/bhr370. [DOI] [PubMed] [Google Scholar]
- Turner MS, Cipolotti L, Yousry TA, Shallice T. Confabulation: damage to a specific inferior medial prefrontal system. Cortex. 2008;44:637–648. doi: 10.1016/j.cortex.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Umeda S, Kurosaki Y, Terasawa Y, Kato M, Miyahara Y. Deficits in prospective memory following damage to the prefrontal cortex. Neuropsychologia. 2011;49:2178–2184. doi: 10.1016/j.neuropsychologia.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Unterrainer JM, Owen AM. Planning and problem solving: from neuropsychology to functional neuroimaging. J Physiol Paris. 2006;99:308–317. doi: 10.1016/j.jphysparis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Uretzky S, Gilboa A. Knowing your lines but missing your cue: rostral prefrontal lesions impair prospective memory cue detection, but not action-intention superiority. J Cogn Neurosci. 2010;22:2745–2757. doi: 10.1162/jocn.2010.21419. [DOI] [PubMed] [Google Scholar]
- Vallar G. Extrapersonal visual unilateral spatial neglect and its neuroanatomy. NeuroImage. 2001;14:S52–58. doi: 10.1006/nimg.2001.0822. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, Irimia A, Torgerson CM, Chambers MC, Kikinis R, Toga AW. Mapping connectivity damage in the case of Phineas Gage. PLoS One. 2012;7:e37454. doi: 10.1371/journal.pone.0037454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier M, Caplan D. CT-scan correlates in agrammatism. In: Menn L, Obler LK, editors. Agrammatic Aphasia. Amsterdam: Benjamins; 1990. pp. 97–114. [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133:880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Volle E, Gonen-Yaacovi G, de Costello AL, Gilbert SJ, Burgess PW. The role of rostral prefrontal cortex in prospective memory: a voxel-based lesion study. Neuropsychologia. 2011;49:2185–2198. doi: 10.1016/j.neuropsychologia.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Davis M, Yago E, Barcelo F, Vogel EK, Knight RT. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron. 2010;68:401–408. doi: 10.1016/j.neuron.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SC, Robbins TW, Roberts AC. Response disengagement on a spatial self-ordered sequencing task: effects of regionally selective excitotoxic lesions and serotonin depletion within the prefrontal cortex. J Neurosci. 2009;29:6033–6041. doi: 10.1523/JNEUROSCI.0312-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Chaozhe Z. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. The effect of prior learning on subsequent retention in amnesic patients. Neuropsychologia. 1974;12:419–428. doi: 10.1016/0028-3932(74)90072-4. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Satterlee-Cartmell T, Stine M. Towers of Hanoi and London: contribution of working memory and inhibition to performance. Brain Cogn. 1999;41:231–242. doi: 10.1006/brcg.1999.1123. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson ML, Bartfai A, Klingberg T. Computerized working memory training after stroke--a pilot study. Brain Injury. 2007;21:21–29. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- Wheeler EZ, Fellows LK. The human ventromedial frontal lobe is critical for learning from negative feedback. Brain. 2008;131:1323–1331. doi: 10.1093/brain/awn041. [DOI] [PubMed] [Google Scholar]
- Wilson CR, Gaffan D, Browning PG, Baxter MG. Functional localization within the prefrontal cortex: missing the forest for the trees? Trends Neurosci. 2010;33:533–540. doi: 10.1016/j.tins.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard J. Digital Atlas of Basic Neuropathology. Temecula, CA: California Medical Publications; 2002. [Google Scholar]
- Woods DL, Knight RT. Electrophysiologic evidence of increased distractibility after dorsolateral prefrontal lesions. Neurology. 1986;36:212–216. doi: 10.1212/wnl.36.2.212. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Anterior and posterior association cortex contributions to the somatosensory P300. J Neurosci. 1991;11:2039–2054. doi: 10.1523/JNEUROSCI.11-07-02039.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurif EB, Caramazza A, Myerson R. Grammatical judgments of agrammatic aphasics. Neuropsychologia. 1972;10:405–417. doi: 10.1016/0028-3932(72)90003-6. [DOI] [PubMed] [Google Scholar]