Abstract
Background
Our purpose was to identify physicians’ individual characteristics, attitudes, and organizational contextual factors associated with higher enrollment of patients in cancer clinical trials among physician participants in the National Cancer Institute’s Community Clinical Oncology Program (CCOP). We hypothesized that physicians’ individual characteristics, such as age, medical specialty, tenure, CCOP organizational factors (i.e., policies and procedures to encourage enrollment), and attitudes towards participating in CCOP would directly determine enrollment. We also hypothesized that physicians’ characteristics and CCOP organizational factors would influence physicians’ attitudes towards participating in CCOP, which in turn would predict enrollment.
Methods
We evaluated enrollment in National Cancer Institute sponsored cancer clinical trials in 2011 among 481 physician participants using structural equation modeling. The data sources include CCOP Annual Progress Reports, two surveys of CCOP administrators and physician participants, and the American Medical Association Masterfile.
Results
Physicians with more positive attitudes towards participating in CCOP enrolled more patients than physicians with less positive attitudes. In addition, physicians who practiced in CCOPs that had more supportive policies and practices in place to encourage enrollment (i.e., offered trainings, provided support to screen and enroll patients, gave incentives to enroll patients, instituted minimum accrual expectations) also significantly enrolled more patients. Physician status as CCOP Principal Investigator had a positive direct effect on enrollment, while physician age and non-oncology medical specialty had negative direct effects on enrollment. Neither physicians’ characteristics nor CCOP organizational factors indirectly influenced enrollment through an effect on physician attitudes.
Conclusions
We examined whether individual physicians’ characteristics and attitudes, as well as CCOP organizational factors, influenced patient enrollment in cancer clinical trials among CCOP physicians. Physician attitudes and CCOP organizational factors had positive direct effects, but not indirect effects, on physician enrollment of patients. Our results could be used to develop physician-directed strategies aimed at increasing involvement in clinical research. For example, administrators may want to ensure physicians have access to support staff to help screen and enroll patients or institute minimum accrual expectations. Our results also highlight the importance of recruiting physicians for volunteer clinical research programs whose attitudes and values align with programmatic goals. Given that physician involvement is a key determinant of patient enrollment in clinical trials, these interventions could expand the overall number of patients involved in cancer research. These strategies will be increasingly important as the CCOP network continues to evolve.
Keywords: Cancer, Clinical trial enrollment, Community Clinical Oncology Program, National Cancer Institute Oncology Research Program, Organizational Design Features, Structural Equation Modeling
Background
Cancer clinical trials are instrumental for developing innovative cancer treatments and expanding current diagnostic, control, and prevention techniques [1,2]. Despite the potential for positive health outcomes, only 3–5% of U.S adults with cancer participate in cancer clinical trials [3]. To increase patient participation in trials, the Community Clinical Oncology Program (CCOP), a cancer focused provider-based research network administered by the National Cancer Institute, engages community physicians in clinical research to enhance the translation of research results into practice [4]. Since its inception in 1983, the CCOP network has generated over 50% of the enrollment in National Cancer Institute sponsored cancer prevention and control trials and 30% of the enrollment in National Cancer Institute sponsored cancer treatment trials [5].
Although the CCOP network has successfully increased overall cancer clinical trial enrollment, individual physicians vary in their enrollment of patients in clinical trials. Many participating physicians enroll no patients in a given year, while others enroll dozens. In 2011, approximately 40% of CCOP physicians enrolled no patients (mean: 3; range: 0–88). Variation in physician enrollment has occurred since the program’s inception, yet the reasons have not been systematically investigated. Research to date has focused on identifying the organizational and environmental contextual factors that drive clinical trial patient enrollment at the CCOP level [6– 9]. No research has examined physician and organizational contextual factors associated with individual physicians’ success in enrolling patients. These findings are critical to determine the context within which we can increase enrollment of cancer patients in National Cancer Institute sponsored clinical trials and, in turn, the pace at which we identify and disseminate innovative therapies. Understanding physician factors that drive patient enrollment will be critical in the organizational design of the new National Cancer Institute Community Oncology Research Program, for example, by setting minimum expectations for enrollment, recognizing high enrolling physicians, or providing physicians with support [10]. Findings can also inform physician recruitment efforts for National Cancer Institute Community Oncology Research Program.
This study seeks to identify the specific CCOP-affiliated physicians’ characteristics and CCOP organizational contextual factors associated with higher enrollment of patients in National Cancer Institute sponsored cancer clinical trials. The hypothesis is that organizational contextual factors, such as trainings, support to enroll patients, expectations for enrollment, physicians’ attitudes towards participating in clinical trials, and individual characteristics, such as age, tenure, medical specialty will directly and indirectly affect their enrollment of patients in trials.
Methods
Theoretical Framework
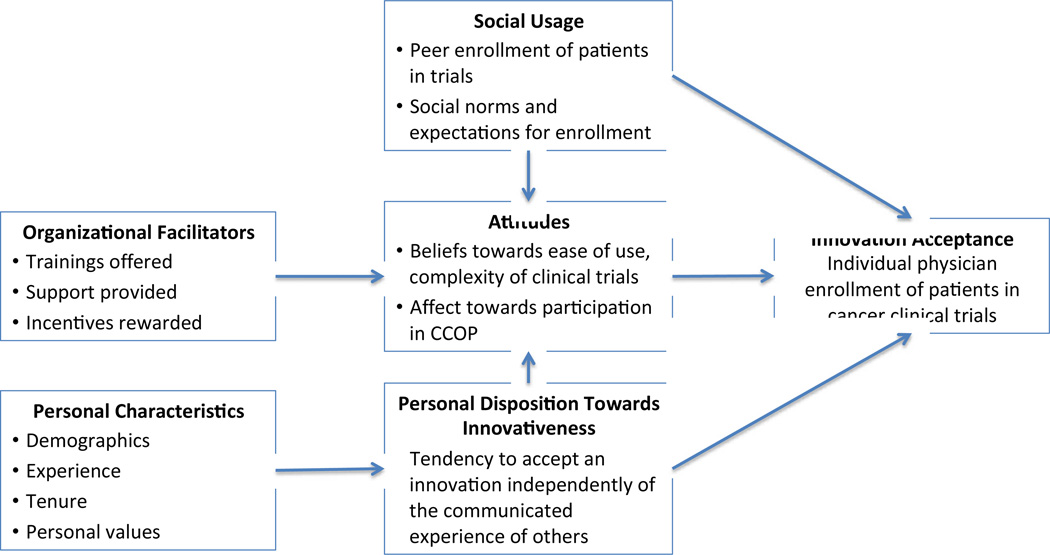
The conceptual model is adapted from the Multilevel Framework of Organizational and Individual Innovation Adoption [11]. Although this framework was developed in the marketing and management literature, it has become a common approach to address innovation implementation in health and human services research as well. For example, the framework has been integrated as part of the Consolidated Framework for Implementation Research, which seeks to advance the implementation of health services research findings into practice [12]. An attractive feature of this framework is that it includes factors at both the organizational and individual levels to predict innovation adoption [11]. In this study we focused on adoption among individual physicians.
The original model as developed by Frambach and applied to this setting is presented in Figure 1. The model postulates that social usage of the innovation, such as social norms, expectations, peer usage, and personal disposition towards innovativeness (i.e., tendency to accept an innovation regardless of others) directly determines individual innovation acceptance. Innovation acceptance in this study is participation in clinical trials, defined as the number of patients CCOP physicians enrolled in National Cancer Institute sponsored cancer clinical trials in 2011. The model also suggests that social usage and personal disposition towards innovativeness determines individuals’ attitudes towards using the innovation, which in turn determines innovation acceptance. Also included in the model are organizational facilitators (e.g., training, support, incentives) and individual characteristics (e.g., demographics, experience) that may also indirectly influence innovation acceptance through individuals’ attitudes and personal disposition towards innovativeness respectively.
Figure 1.
Original Model of Individual Innovation Acceptance in Organizations as Applied to CCOP Physician Enrollment of Patients in Cancer Clinical Trials
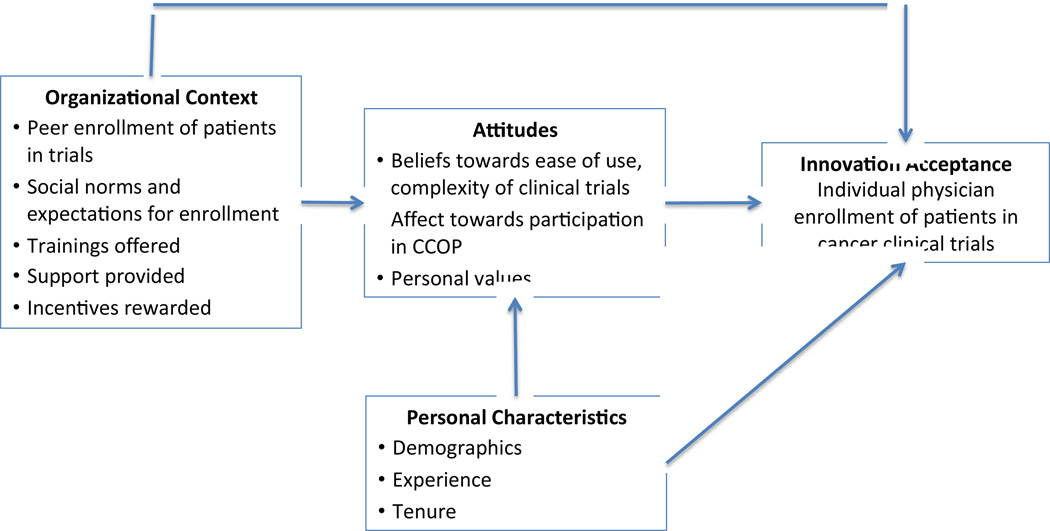
The model we tested adheres to the basic structure of the framework proposed by Frambach [11]; however, based on data availability, theory, and knowledge of CCOP network operation, we made three changes to the original model before analyzing any data. The tested model is presented in Figure 2. First, we combined social usage and organizational facilitators into one construct, organizational context. We did this for two reasons: (1) it makes theoretical sense as all the data used for this construct is at the CCOP level and (2) we only had two observed variables, peer enrollment and expectations, to construct social usage, but the statistical modeling approach required we use at least three observed variables [13]. The second change is that we did not include personal disposition towards innovativeness in our model because we lacked data on this construct. Lastly, we included individual values as a component of attitudes rather than an individual characteristic. We decided to do this because our survey instrument included values, along with general affect, beliefs towards the ease of participation, and complexity of clinical trials as components of attitudes towards innovation adoption. Therefore it made theoretical sense to include values as a component of attitudes versus an individual characteristic.
Figure 2.
Tested Model of Individual Innovation Acceptance in Organizations
Study Setting and Sample
The CCOP network is a joint venture between the National Cancer Institute Division of Cancer Prevention, which provides overall direction and funding for community hospitals and physicians to participate in clinical trials, clinical cooperative groups, and community-based physicians and hospitals [14]. The CCOP research bases design and conduct clinical trials, and individual community-based physicians and hospitals assist with patient enrollment, data collection, and dissemination of study findings [5,14]. When the data were collected in 2011, 47 CCOPs operated in 28 states with approximately 3,500 participating community physicians.
The sample is comprised of physicians who responded to the 2011 CCOP Physician Survey. We used a stratified (by CCOP) random sample of 817 physicians across all 47 CCOPs. The final sample included 485 physicians (59.4% of physicians surveyed). The only significant (p<0.05) differences between survey responders and non-responders were that responders enrolled more patients per year (4.7 versus 3.4), were more likely to be a surgeon (10% versus 5%), and were less likely to be a non-specialized general oncologist (11% versus 24%). There were no significant differences between respondents and non-respondents regarding gender, race, age, practice type, training location, and tenure. This study was determined to be exempt from review by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Study Design and Data Sources
The data for this cross-sectional study were obtained from four sources. The 2011 CCOP Progress Reports provided data on physicians’ enrollment activity from June 1, 2011 to February 29, 2012. The 2011 CCOP Administrator Survey and the 2011 CCOP Physician Survey were both administered as part of a larger National Cancer Institute funded-study (5R01CA124402). The Physician Survey supplied data on CCOP physicians’ attitudes towards participation in clinical trials. Responses were collected between October 2011 and January 2012. The Administrator Survey provided information on the CCOP organizational contextual factors. The majority of responses were collected at the annual CCOP meeting in September 2011. Any remaining surveys were completed in October 2011. We achieved a 100% response rate from CCOP Administrators. Lastly, the 2012 American Medical Association Physician Masterfile provided data on CCOP physicians’ individual characteristics.
Measures
Table 1 provides details on our measures. The outcome was the number of patients CCOP-affiliated physicians enrolled in National Cancer Institute sponsored cancer clinical trials in 2011. Physician attitudes, a predictor construct, was composed of questions assessing beliefs related to the complexity of trials, whether trials excluded too many patients, affect towards whether trials explored important issues, and physicians’ values related to participating in clinical trials. Organizational contextual factors, also a predictor construct, included educational trainings offered, support provided by the CCOP to physicians to help screen and enroll patients, incentives provided to physicians, peer usage (i.e., the average number of patients enrolled in National Cancer Institute sponsored clinical trials for physicians within a specific CCOP), and CCOP expectations for enrollment.
Table 1.
Overview of Variables and Measures
| Model Construct | Variable | Measure | Measure Type | Data Source |
|---|---|---|---|---|
| Outcome Variable | ||||
| Innovation Acceptance | Outcome: Enrollment of Patients | No. of patients enrolled in NCI-sponsored cancer clinical trials in 2011 | Continuous | CCOP Progress Reports |
| Predictor Variables | ||||
| Attitudes | Affect | NCI-sponsored trials explore clinical issues that are important in my practice | Continuous: 1=Disagree to 5 = Agree | CCOP-Affiliated Physician Survey |
| Attitudes | Beliefs: Exclude Patients | NCI-sponsored trials exclude too many patients | Continuous: 1=Disagree to 5 = Agree | CCOP-Affiliated Physician Survey |
| Attitudes | Beliefs: Trials Complex | NCI-sponsored trials are too complex to do in my practice | Continuous: 1=Disagree to 5 = Agree | CCOP-Affiliated Physician Survey |
| Attitudes | Personal Values | I value participating in NCI-sponsored clinical trials | Continuous: 1=Disagree to 5 = Agree | CCOP-Affiliated Physician Survey |
| Organizational Context | Training | CCOP sponsor any events where physicians could learn about the latest developments in cancer research, treatment, prevention, or control? | Binary: 0 = N; 1=Y | CCOP Administrator Survey |
| Organizational Context | Support: Screening | Proportion of physicians that have CCOP staff members routinely screen patient charts for potentially eligible patients | Continuous | CCOP Administrator Survey |
| Organizational Context | Support: Enrolling | Proportion of physicians that have CCOP staff members routinely assist with enrollment | Continuous | CCOP Administrator Survey |
| Organizational Context | Incentives | CCOP provide some form of recognition to Type-A physicians with high levels of accrual to NCI-sponsored trials? | Binary: 0 = N; 1=Y | CCOP Administrator Survey |
| Organizational Context | Peer Usage | Average no. of patients enrolled in NCI-sponsored clinical trials by physicians in CCOP | Continuous | CCOP Progress Reports |
| Organizational Context | Persuasion | CCOP expect Type-A physicians to enroll a minimum no. of patients in NCI-sponsored trials? | Binary: 0 = N; 1=Y | CCOP Administrator Survey |
| Individual Characteristics | Age | The current year minus the physicians’ year of birth | Continuous | AMA Provider Masterfile |
| Individual Characteristics | Practice Type | Indicator of present primary employment arrangement (e.g., solo, group, hospital) | Categorical | AMA Provider Masterfile |
| Individual Characteristics | U.S Trained | Indicator if physician trained in the U.S. | Binary: 0 = N; 1=Y | AMA Provider Masterfile |
| Individual Characteristics | PI | Please indicate the PI of the CCOP | Binary: 0 = N; 1=Y | CCOP Progress Reports |
| Individual Characteristics | Medical Specialty | Indicator of physician self-designated primary medical specialty (e.g., medical oncologist, hematologist oncologist, surgeon) | Categorical | AMA Provider Masterfile |
| Individual Characteristics | Tenure | No. of years since graduated medical school | Continuous | AMA Provider Masterfile |
Physicians’ individual characteristics included age, practice type, tenure, physician training location, medical specialty, and whether or not the physician is the CCOP Principal Investigator.
Statistical Analysis
Structural Equation Modeling with maximum likelihood estimation was used to simultaneously test the effects of the CCOP organizational contextual factors and physician attitudes on enrollment. Structural Equation Modeling is composed of multivariate regression models and can be used to estimate proposed causal relationships [15–18]. We used confirmatory Structural Equation Modeling to test the hypothesized pathways among factors represented in Figure 2 by comparing how well this proposed structure fits the observed data. We elected to use Structural Equation Modeling because it allowed us to test for constructs that are not directly assessed, but are instead composed of observed indicators representing the constructs of interest (e.g., CCOP organizational contextual factors, physician attitudes). We elected to use clustered robust standard errors to account for clustering of physicians within 47 CCOPs. We then evaluated model fit using the Comparative Fit Index and the Tucker-Lewis Index. Comparative Fit Index and the Tucker-Lewis Index values range from 0 to 1, with values ≥ 0.90 representing adequate fit [15]. We also examined the root mean square error of approximation, and the associated confidence interval and p-value. Root mean square error of approximation values < 0.05 and an upper bound of the confidence interval < 0.1 are considered acceptable [15]. Next, we examined the standardized root mean squared residuals, standardized root mean squared residuals with values < 0.08 considered acceptable fit [15]. We also evaluated our model by testing the significance of all standardized estimates, including the direct and indirect effects of variables on the outcome.
Based on these fit statistics for the original model in Figure 2, we elected to re-specify the model to improve its fit. Structural Equation Modeling is an iterative process in which model fit is improved by using theory and modifications indices either to add additional pathways between variables or to allow items to co-vary [15–18]. Modification indices are the minimum that the chi-square statistic is expected to decrease if the corresponding parameter is no longer assumed to be fixed at zero [15]. When revising the model, we tested whether model fit improved by comparing the baseline model with the new model using the Lagrange multiplier test and fit statistics.
Once we achieved a well-fitting model, we tested the significance of all standardized estimates, including direct and indirect effects. Standardized parameter estimates are transformations of unstandardized estimates that remove scaling and can be used for informal comparisons of parameters throughout the model [15]. Direct effects are equal to the regression coefficients (i.e., β) while indirect effects are the product of the two regression coefficients. For example, if X predicts Y and Y predicts Z, then the indirect effect of X on Z equals the product of the two regression coefficients (X on Y and Y on Z). Lastly, to ensure the validity of our Structural Equation Modeling results, we checked our results using negative binomial regression analysis with clustered robust standard errors. Analyses were performed using Mplus 7.
Results
Study Population
The final sample included 481 physicians with complete information. Table 2 provides descriptive statistics for the entire sample. Notably, 74% were male, 75% were White non-Hispanic, and their mean age was 53 years; they have been in practice a mean of 26 years. The vast majority practiced in group practices and trained in the United States; 72% were oncology-based specialists, 10% were surgeons, and 18% reported another medical specialty (e.g., gynecology, pediatrics, internal medicine). Physicians enrolled a mean of 5 patients in 2011 (range: 0–62); approximately 40% of physicians enrolled no patients in the 9-month reporting period.
Table 2.
Descriptive Statistics: Physician Level Variables
| CCOP Survey Respondents n=481 | ||
|---|---|---|
| Mean or Proportion of Sample | Range | |
| Outcome | ||
| 2011 Patient Enrollment | 4.7* (8.1) | 0, 62 |
| Descriptive Variables | ||
| Gender | ||
| Male | 74% | |
| Female | 26% | |
| Race | ||
| White | 75% | |
| Asian | 15% | |
| African-American | 1% | |
| Other | 9% | |
| Variables included in Model Attitudes | ||
| Affect | 4.6 (0.7) | 2,5 |
| Beliefs: Exclude Pts. | 3.4 (1.2) | 1,5 |
| Beliefs: Complexity of Trials | 2.4 (1.2) | 1,5 |
| Values | 4.7 (0.6) | 1,5 |
| Personal Characteristics | ||
| Age | 52.6 (9.8) | 34,82 |
| Practice Type | ||
| Group Practice | 78% | |
| Hospital-Based | 12% | |
| Solo Practice | 4% | |
| Other/None Listed | 6% | |
| Training Location | ||
| U.S Trained | 80% | |
| Non U.S Trained | 20% | |
| Tenure (Yrs. In Practice) | 25.7 (10.1) | 8, 57 |
| Medical Specialty | ||
| Hematology Oncology | 40% | |
| Radiation Oncology | 21% | |
| Other Specialty | 18% | |
| Medical Oncology | 11%* | |
| Surgery | 10%* | |
| Principal Investigator | 9% | |
Standard deviations in parentheses
Indicates significant difference between survey respondents and non-survey respondents
Other race includes American Indian, Native Hawaiian/Pacific Islander, More than one race, or unknown
Hematology oncology includes blood banking, hematology oncology, hematology
Radiation Oncology includes diagnostic radiology, nuclear medicine, radiation oncology, radiology, vascular and interventional radiology
Other specialist includes general practice, gynecological oncology, pediatrics, pediatric hematology, cardiovascular disease etc.
Surgery includes colon and rectal surgery, critical care sugary, general surgery, neurological surgery, surgical oncology, urological surgery etc.
Structural Equation Modeling Analysis
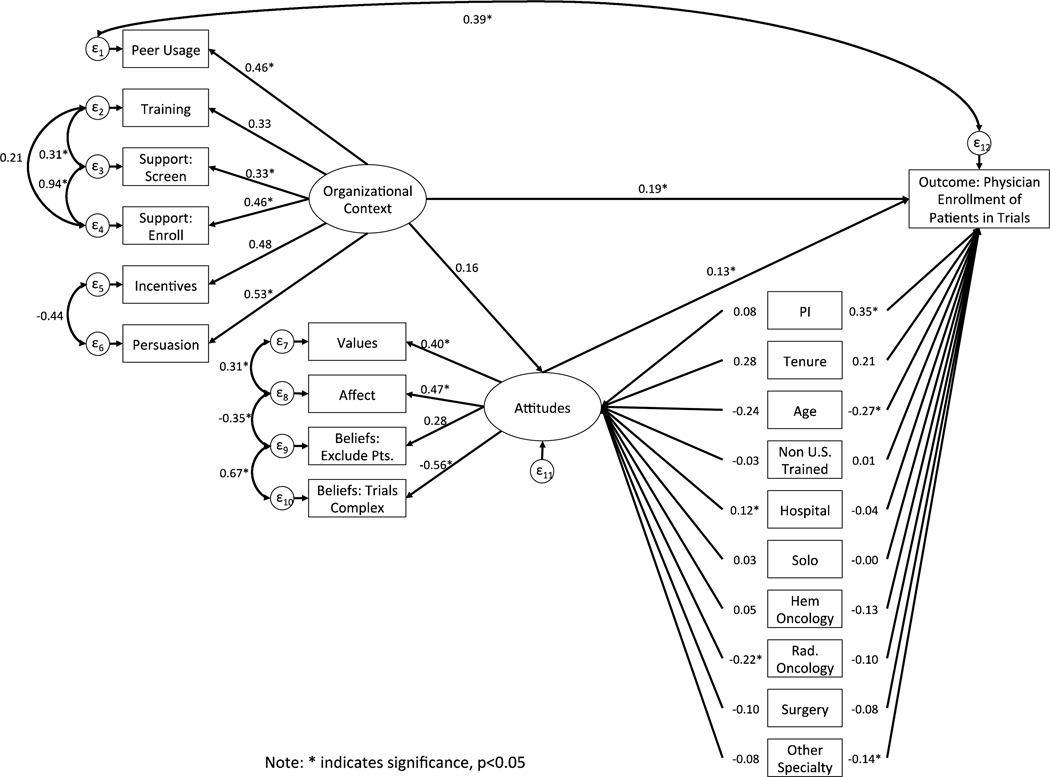
The fit statistics and modification indices for the fixed parameters of the original model tested in Figure 2 suggested that we re-specify the model to improve fit (Comparative Fit Index = 0.648; Tucker-Lewis Index = 0.560; Root mean square error of approximation = 0.067; Standardized root mean squared residuals = 0.061). Therefore, we added seven post-hoc modifications that were theoretically justified and improved model fit. Figure 3 presents the final model with all post hoc modifications and standardized estimates. For these modifications, we allowed the error terms of the following measures to co-vary higher than with other variables. For example, the percentage of doctors supported in screening and enrolling patients, likely share common variation that is not explained by any of the proposed relationships in the model.
-
1
Peer-usage with the outcome: Peer-usage is based on the individual physicians’ enrollment within a specific CCOP. We co-varied the error terms as they likely share common variation that is not explained by relationships in the model.
-
2
The percentage of doctors supported in screening and enrolling patients: The same support staff generally preform both functions within a CCOP.
-
3
Trainings offered with the percentage doctors who are supported in screening and enrolling patients: The number of trainings offered relates to the number of support staff available.
-
4
Incentives with expectations for enrollment: CCOPs that provide incentives may also be more likely to have expectations for enrollment.
-
5
Affect with values: Providers who believe that trials explore important issue are also likely to value participating.
-
6
Belief that trials are too complex with belief that trials exclude too many patients: These relate to an overall negative view of CCOP and may discourage participation.
-
7
Affect with whether physicians believe trials exclude too many patients: Providers who report that trials are important are less likely to think they exclude too many patients.
Figure 3.
Standardized SEM Results for Final Model
With the addition of each error co-variance, we tested the baseline model against the new model for improved model fit. Overall, we achieved a final well-fitting model (Comparative Fit Index = 0.936; Tucker-Lewis Index = 0.914; Root mean square error of approximation = 0.030; Standardized root mean squared residuals = 0.046) (Figure 3).
Table 3 provides standardized indirect, direct, and total effects for all of the variables and constructs included in the model. In general, standardized effects of less than 0.10 constitute a small effect; values greater than 0.30 indicate a medium effect; and values greater than 0.50 indicate a large effect [18]. Overall the effect sizes were fairly small for the latent constructs of organizational context and individual attitudes, which had significant positive direct effects on the outcome. For example, the direct effect of organizational context on enrollment was β=0.19 (p=0.02) and for physician attitudes it was β=0.13 (p=0.04). In addition, physician’s CCOP Principal Investigator status, age, and non-oncologist specialty also had significant direct effects on enrollment. The most significant positive direct effect was whether the physician was the Principal Investigator (β=0.35; p<0.00). Physician age (β= −0.27; p=0.02) and non-oncology specialty (β= −0.14; p=0.03) had significant negative direct effects on enrollment.
Table 3.
Standardized Total, Direct, and Indirect Effects
| Total Effect | Direct Effect | Indirect Effect | |
|---|---|---|---|
| Outcome: Enrollment in NCI-Sponsored Cancer Clinical Trials in 2011 | |||
| Physician Attitudes Construct | 0.130* | 0.130* | N/A |
| Organizational Context Construct | 0.205* | 0.185* | 0.020 |
| Age | −0.301* | −0.271* | −0.030 |
| Hospital-Based^ | −0.024 | −0.040 | 0.016 |
| Solo Practice^ | 0.000 | −0.004 | 0.004 |
| Non U.S. Trained | 0.004 | −0.004 | 0.007 |
| PI | 0.360* | 0.350* | 0.010 |
| Tenure | 0.251* | 0.214 | 0.037 |
| Hematologist Oncology+ | −0.127 | −0.134 | 0.007 |
| Radiation Oncology+ | −0.132 | −0.103 | −0.029 |
| Surgery+ | −0.089 | −0.076 | −0.013 |
| Other Specialty+ | −0.146* | −0.135* | −0.011 |
Model Fit Statistics: CFI=0.936; TLI= 0.914; RMSEA=0.030; SRMR=0.046
Note: Total effects is the sum of direct and indirect effects
Note: Indirect effects are the product of the regression coefficients leading to the outcome. For example for organizational context, context predicts attitudes and attitudes predicts enrollment. The indirect effect of context on enrollment equals the product of the two regression coefficients (From Figure 3)
0.157*0.130=0.020
Statistically Significant (p<0.05)
Compared to Group Practice
Compared to General Non-Specialized Oncology
There was no evidence, however, that CCOP organizational context or any of the physician individual characteristics significantly influenced accrual through their effects on physician attitudes. Finally, training location, practice location, and physicians who are surgeons, hematologists, and radiological oncologists (compared to non-specialized medical oncologists) did not directly affect enrollment. Overall our model explained 21% of the variance in patient enrollment. The robustness check of our Structural Equation Modeling results using negative binomial regression analysis with clustered robust standard errors confirmed our main findings that both organizational context and attitudes were significantly associated with patient enrollment, along with physician status as the CCOP Principal Investigator and medical specialty.
Discussion
We hypothesized that organizational contextual factors, physicians’ attitudes and individual characteristics would have both direct as well as indirect effects operating through attitudes on enrollment of patients in National Cancer Institute sponsored cancer clinical trials. This hypothesis was partially supported as organizational context and physician attitudes had positive significant direct effects on enrollment; however, there were no indirect effects on enrollment operating through attitudes.
Consistent with the literature, we found that physicians’ attitudes towards participating in CCOP directly impacted enrollment [19,20]. This was likely because physicians who viewed participation as more useful and easy, had individual values aligned with CCOP goals, and had more positive feelings were more active in enrolling patients in trials. This finding highlights the importance of recruiting physicians for volunteer research programs who value participating in clinical trials, find participating in trials important, and feel they are able to do so. Recruiting physicians whose attitudes align with the program’s goals is especially important for community sites interested in participating in the new National Cancer Institute Community Oncology Research Program. Interestingly, organizational context did not predict physicians’ attitudes. Changes in organizational context may influence overall enrollment of patients as a supportive environment assists with accrual efforts, but these contextual factors do not appear to impact the attitudes of physicians. This finding further supports recruiting physicians with positive attitudes towards participating in clinical research.
In addition, as hypothesized, CCOP organizational contextual factors made a difference. Specifically, organizations that provided support for physicians to consent and enroll patients, offered incentives for enrollment, and mandated expectations for enrollment also increased physician enrollment, perhaps due to a strong sense of organizational commitment and social norms. Program administrators of CCOPs or other voluntary research programs might consider providing support for physicians’ research activities, such as staff to help consent and enroll patients, incentives for enrollment goals (e.g., small tokens of appreciation, public acknowledgment), and trainings to learn about latest developments in research. Such strategies may not directly change physician attitudes, but may provide a supportive organizational context to encourage active physician participation in recruiting patients.
We were surprised that organizational context did not have an indirect effect on enrollment by influencing physicians’ attitudes towards clinical trials. Perhaps physicians’ attitudes were not a significant mediator of organizational context because physicians elect to participate in CCOP. Although implementation of some innovations in healthcare may be mandated, clinical trial participation, however, is not required. It may be that, specific organizational contextual factors do not influence attitudes among physicians who have already agreed to participate and recruit patients to clinical trials. Organizational context may shape attitudes towards participation in other types of settings where participation or implementation of a specific innovation is mandatory and attitudes would likely be more fluid. Therefore, organizational context would have more of an opportunity to determine attitudes towards participation.
In addition, three physician characteristics also significantly effected enrollment. Being a CCOP Principal Investigator was the strongest predictor. Principal Investigators are more likely than non- Principal Investigators to be committed to the CCOP and feel obligated to set a “good example” for their colleagues. Principal Investigators may also be more familiar with available trial protocols and receive greater assistance from support staff to consent and enroll patients. A strong negative predictor of enrollment was whether the physician was a non-oncology specialist. This finding was consistent with the literature [19,21] One reason may be that non-oncologists feel less comfortable and/or familiar with cancer protocols than oncologists. In addition, physician age also had significant negative direct effects on enrollment.
We were surprised that practice location, foreign medical training, and medical specialty (with the exception of non-oncology) did not impact enrollment. Although none of the previous studies exclusively examined enrollment among CCOP physicians, past studies found that practice type (i.e., office-based practice compared to hospital based practice) and medical specialty (i.e., medical oncologists compared to radiation oncologists) increased physician enrollment of patients while foreign-trained oncologists enrolled fewer patients. In our study, practicing at a hospital or as a solo physician (compared to a group practice) may not have had a significant effect because it was difficult to discern a physician’s main practice location [19,21]. In addition, many CCOP physicians travel between different offices, which may make their primary location less relevant. We suspect that foreign medical training did not impact enrollment because we could not determine how long physicians had been practicing in the U.S., which is likely a more relevant predictor of enrollment than training location. In addition, medical specialty may not be as influential on enrollment as there are an abundance of types of cancer clinical trials, including protocols for surgery and radiological interventions. Therefore all cancer-related specialties are comfortable and willing to enroll patients in cancer trials.
Limitations
There are several limitations of our study. First, we only included physicians who participate in CCOPs. These physicians have already agreed at least on some level to participate in CCOP. Therefore our findings suggest the organizational and individual factors that are most relevant to encourage active participation in CCOP. It is important to note, however, that many organizational strategies (e.g., recognition of high achievers, expectations for enrollment) could be implemented by diverse organizations to increase physician participation in clinical research. Second, we are unable to account for variation in the number of potentially eligible patients physicians see. Therefore, we were unable to distinguish physicians failing to offer a cancer clinical trial from patients’ refusal to enroll. We also lacked the data to incorporate patient-level characteristics in the analyses. We cannot account for variations in patients’ cancer stage, co-morbidities, age, or any other factors that may determine eligibility. Ultimately patients are the final decision makers regarding their participation in a cancer clinical trial. However, given that 75% of patients agree to enroll if offered [22], we do not believe this to be a significant limitation of this study. Third, given that we were only able to explain 21% of the variance in enrollment, we were also limited in the data that was available to examine individual physician enrollment. Future studies may want to consider including additional factors, such as patient-level characteristics in the model to increase the amount of variation explained. In addition, more information on physician behaviors and personality traits (e.g., personal disposition to innovativeness, goal-orientation) may also help to explain variance in enrollment in cancer clinical trials.
We believe this study extends the literature in several important ways. First, it is the first study to evaluate physician-level predictors of their success in enrolling patients in CCOP cancer clinical trials. Second, it provides the basis of physician-directed strategies that may effectively promote enrollment of patients in cancer clinical trials. By expanding the number of patients involved in cancer clinical trials, we can accelerate the pace in which we identify promising innovative therapies and novel interventions that can ultimately improve the outcomes of cancer patients.
Conclusions
The findings from this study are important for program administrators looking to increase volunteer physician participation in clinical research as well for new National Cancer Institute Community Oncology Research Program sites. Our results suggest two strategies to increase participation. The first is to ensure physicians attitudes and values align with the programmatic goals. For example, recruiting physicians who value participating in clinical trials, find participating in trials important, and feel they are able to do so is a key determinant of a program’s success. Recruitment of physicians whose values align with program goals is especially important given that CCOP organizational context did influence attitudes towards participation. Second, program administrators should consider providing support for physicians’ research activities, such as staff to help consent and enroll patients, incentives for enrollment goals (e.g., small tokens of appreciation, public acknowledgment), and trainings to learn about latest developments in research. Such strategies may not directly change physician attitudes, but may provide a supportive organizational context to encourage active physician participation in recruiting patients.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (R25CA116339 and R01CA124402). In addition, Dr. Weinberger was funded by a VA HSR&D Senior Research Career Scientist Award (RCS 91-408).
REFERENCES
- 1.Sorbye H, Pfeiffer P, Cavalli-Bjorkman N, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer. 2009;115(20):4679–4687. doi: 10.1002/cncr.24527. [DOI] [PubMed] [Google Scholar]
- 2.Grunfeld E, Zitzelsberger L, Coristine M, et al. Barriers and Facilitators to Enrollment in Cancer Clinical Trials. Cancer. 2002;95(7):1577–1583. doi: 10.1002/cncr.10862. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute Cancer: Clinical Trials The Basic Workbook. [Accessed 20 November 2013];2002 http://myeloma.org/pdfs/NCI-BasicsWorkbook.pdf. [Google Scholar]
- 4.National Cancer Institute: Cancer Clinical Trials Fact Sheet. [Accessed 20 November 2013];2010 http://www.cancer.gov/cancertopics/factsheet/Information/clinical-trials.
- 5.Minasian LM, Carpenter WR, Weiner BJ, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116(9):4440–4444. doi: 10.1002/cncr.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaluzny AD, Lacey LM, Warnecke R, et al. Predicting the Performance of a Strategic Alliance: An Analysis of the Community Clinical Oncology Program. Health Services Research. 1995;28(2):159–182. [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter WR, Fortune-Greeley AK, Zullig LL, et al. Sustainability and performance of the National Cancer Institute's Community Clinical Oncology Program. Contemporary Clinical Trials. 2012;33:46–55. doi: 10.1016/j.cct.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner BJ, Jacobs SR, Minasian LM, et al. Organizational Designs for Achieving High Treatment Trial Enrollment: A Fuzzy-Set Analysis of the Community Clinical Oncology Program. Journal of Oncology Practice. 2012;8(5):287–291. doi: 10.1200/JOP.2011.000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs SR, Weiner BJ, Minasian LM, et al. Achieving high cancer control trial enrollment in the community setting: An analysis of the Community Clinical Oncology Program. Contemporary Clinical Trials. 2013;34(2):320–325. doi: 10.1016/j.cct.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute: NCI Community Oncology Research Program Approved. [Accessed 20 November 2013];2013 http://ccop.cancer.gov/news-events/news/20130625.
- 11.Frambach RT, Schilewaert N. Organizational innovation adoption a multi-level framework of determinants and opportunities for future research. Journal of Business Research. 2002;55:163–176. [Google Scholar]
- 12.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of healh services research fidnigns into practice: a consodliated framework for advancing implementation science. Implementation Science. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien R. Identification of Simple Measurement Models with Multiple Latent Variables and Correlated Errors. Sociological Methodology. 1994;24:137–170. [Google Scholar]
- 14.Kaluzny AD, Lacey LM, Warnecke R, et al. Accrual of patients to randomized clinical trials. Factors affecting cancer prevention and control research. International Journal of Technology Assessment in Health Care. 1994;10(3):506–516. doi: 10.1017/s0266462300006723. [DOI] [PubMed] [Google Scholar]
- 15.Norman GR, Streiner DL, editors. PDQ Statistics Third Edition. London: BC Decker Inc; 2003. Chapter 17: Path Analysis and Structural Equation Modeling. [Google Scholar]
- 16.Hox JJ, Bechger TM. An Introduction to Structural Equation Modeling. Family Science Review. 2007;11:354–373. [Google Scholar]
- 17.Schreiber JB, Stage FK, King J, et al. Reporting Structural Equation Modeling and Confirmatory Factor Analysis Results: A Review. Journal of Educational Research. 2006;99(6):323–337. [Google Scholar]
- 18.Suhr D. The Basics of Structural Equation Modeling. [Accessed 5 March 2014];Tutorial. 2006 http://www.lexjansen.com/wuss/2006/tutorials/tut-suhr.pdf. [Google Scholar]
- 19.Meropol NJ, Buzaglo JS, Millard J, et al. Barriers to Clinical Trial Participation as Perceived by Oncologists and Patients. Journal of the National Comprehensive Care Network. 2007;5:753–762. doi: 10.6004/jnccn.2007.0067. [DOI] [PubMed] [Google Scholar]
- 20.Mansour EM. Barriers to Clinical Trials Part III: Knowledge and Attitudes of Health Care Providers. Cancer. 1994;74:2672–2675. doi: 10.1002/1097-0142(19941101)74:9+<2672::aid-cncr2820741815>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CL, Keating NL, Potosky AL, et al. A Population-Based Assessment of Specialty Physician Involvement in Cancer Clinical Trials. Journal of the National Cancer Institute. 2011;103:384–397. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albrecht TL, Eggly SS, Gleason MEJ, et al. Influence of Clinical Communication on Patients' Decision Making on Participation in Clinical Trial. Journal of Clinical Oncology. 2008;26(16):2666–2673. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.