Abstract
Culture filtrate protein 10 (CFP-10) from Mycobacterium tuberculosis is a well-characterized immunodominant 10-kDa protein antigen known to elicit a very potent early gamma interferon response in T cells from M. tuberculosis-infected mice and humans. The sequence of the Mycobacterium leprae homologue of CFP-10 shows only 40% identity (60% homology) at the protein level with M. tuberculosis CFP-10 and thus has the potential for development as a T- or B-cell reactive antigen for specific diagnosis of leprosy. Antisera raised in mice or rabbits against recombinant M. leprae and M. tuberculosis CFP-10 proteins reacted only with homologous peptides from arrays of overlapping synthetic peptides, indicating that there was no detectable cross-reactivity at the antibody level. Sera from leprosy and tuberculosis patients were also specific for the homologous protein or peptides and showed distinct patterns of recognition for either M. leprae or M. tuberculosis CFP-10 peptides. At the cellular level, only 2 of 45 mouse T-cell hybridomas raised against either M. leprae or M. tuberculosis CFP-10 displayed a cross-reactive response against the N-terminal heterologous CFP-10 peptide, the region that exhibits the highest level of identity in the two proteins; however, the majority of peptide epitopes recognized by mouse T-cell hybridomas specific for each protein did not cross-react with heterologous peptides. Coupled with the human serology data, these results raise the possibility that peptides that could be used to differentiate infections caused by these two related microorganisms could be developed. Immunohistochemical staining of sections of M. leprae-infected nude mouse footpads resulted in strongly positive staining in macrophages and dendritic cells, as well as weaker staining in extracellular areas, suggesting that M. leprae CFP-10, like its homologue in M. tuberculosis, is a secreted protein.
The number of cases of leprosy worldwide has been reduced dramatically through aggressive World Health Organization-sponsored multidrug therapy, from approximately 10 million cases in 1985 to a little fewer than 700,000 cases today (46-48). However, with the unabated emergence of more than 600,000 new cases per year, there is the possibility that the source of infection is not being addressed (16). The single greatest need in leprosy research is the development of definitive diagnostic tools that identify sources of leprosy and routes of transmission. The recent completion of the genome sequences of both Mycobacterium tuberculosis (8) and Mycobacterium leprae (9) provides an opportunity to identify leprosy-specific antigens that might serve this purpose. A similar comparative approach has allowed identification of genes in the RD1 region that are deleted from Mycobacterium bovis BCG but present in M. tuberculosis and can therefore distinguish between infection with M. tuberculosis and vaccination with BCG (22). Among the deleted antigens are two low-molecular-weight proteins, culture filtrate protein 10 (CFP-10) (Rv3874) and ESAT-6 (Rv3875) (3, 14). Comparative genomic analysis has revealed that genes encoding ESAT-6- and CFP-10-like proteins are found in tandem as part of a cluster of conserved genes in several species of Mycobacterium, including M. leprae, Mycobacterium avium, M. bovis, Mycobacterium paratuberculosis, and Mycobacterium smegmatis, and in the relatively high-G+C-content gram-positive organisms Corynebacterium diphtheriae and Streptomyces coelicolor (40). In M. tuberculosis, the region containing the ESAT-6 and CFP-10 genes has been duplicated five times (designated regions 1 to 5). In M. leprae, only regions 1 and 3 are intact. Region 1 contains the M. leprae homologues of prototype genes encoding ESAT-6 and CFP-10 (ML0049 and ML0050, respectively), while region 3 contains the ML2531 and ML2532 genes, which have been identified as homologues of the genes encoding ESAT-6 and CFP-10, respectively, since they are located in the genome at positions analogous to the positions of the corresponding M. tuberculosis genes (42). In region 4 of the M. leprae genome, only the single ESAT-6-like gene ML0363 remains, while most of the genes in regions 2 and 5 are pseudogenes. Although in M. leprae the genes encoding ESAT-6- and CFP-10-like proteins in regions 3 and 4 exhibit relatively high levels of identity with their M. tuberculosis counterparts (ML2532 and Rv0287, 76% identity; ML2531 and Rv0288, 70% identity; and ML0363 and Rv3444c, 71% identity), region 1 appears to lie in an area prone to genetic change, as the levels of identity for both homologues are much lower at the amino acid level (ML0049 and Rv3875, 36% identity; and ML0050 and Rv3874, 40% identity).
As a consequence of the low level of homology between the prototypic M. leprae and M. tuberculosis ESAT-6 proteins, polyclonal antisera raised against either protein did not cross-react with the other protein, either at the level of the whole molecule or at the level of synthetic overlapping peptides (35). In addition, the dominant B- and T-cell epitopes, as defined by monoclonal antibodies and T-cell hybridomas, were found to reside in different regions of the corresponding proteins. In order to explore the potential of the M. leprae and M. tuberculosis CFP-10 homologues as future diagnostic reagents, we performed a comparative immunological analysis by raising and using polyclonal antiserum reagents and T-cell hybridomas and by examining leprosy and tuberculosis (TB) patient sera.
MATERIALS AND METHODS
Production of recombinant M. leprae and M. tuberculosis CFP-10.
The DNA sequence coding for full-length M. leprae CFP-10 (previously identified as the M. leprae lhp gene) was cloned from M. leprae genomic DNA by using Vent Pfu DNA polymerase (New England Biolabs, Beverly, Mass.). PCR amplification was carried out with forward primer 5′-CCATATGGCAGAAATGATCACCGAGGC-3′ and reverse primer 5′-TTAAGCTTGAAGTTCATCTTCGAGGACAAC-3′) designed to introduce NdeI and HindIII sites, respectively (underlined), into the 5′ and 3′ ends of the open reading frame. The PCR product was digested with restriction enzymes NdeI and HindIII and cloned into expression vector pET 28a(+) (Novagen, Madison, Wis.), which uses a T7 promoter-driven system to achieve high levels of expression of recombinant protein with a C-terminal six-histidine tag. The DNA sequence of each recombinant clone was confirmed by automated nucleotide sequencing (ABI model 377) at the Macromolecular Resources Laboratory, Colorado State University. Ligated products were introduced into the Escherichia coli expression host BL21(DE3) (Invitrogen, Carlsbad, Calif.) by transformation. To obtain soluble recombinant CFP-10 (rCFP-10), a single positive colony was grown to the log phase (optical density at 600 nm, 0.5) in Luria-Bertani medium containing 50 μg of kanamycin per ml and was induced with 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma Chemical Co., St. Louis, Mo.) at 15°C for 16 h. The cells were lysed by enzymatic digestion with lysozyme (0.5 mg/ml) and sonication in 0.5 M NaCl-50 mM Tris-HCl (pH 8.0) buffer with protease inhibitors (2 μg of aprotinin per ml, 1 μg of leupeptin per ml, 1 μg of pepstatin per ml, 1 mM phenylmethylsulfonyl fluoride) and then centrifuged to separate the supernatant and the cell pellet fraction. The supernatant was applied onto Talon nickel affinity chromatography resin (Clontech, Palo Alto, Calif.) and eluted with 50 mM imidazole (24); this was followed by dialysis. Purified proteins were passed over a Detoxi-Gel column (Pierce Chemical, Rockford, Ill.) to remove endotoxin. The lipopolysaccharide content as measured by the Limulus amoebocyte lysate test (BioWhittaker, Walkersville, Md.) was less than 2 ng/mg of protein. The protein concentration was estimated by using the bicinchoninic assay (Pierce) (32), and the recombinant protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (21). Purified M. tuberculosis rCFP-10 (7) was a gift from Marila Gennaro of the Public Health Research Institute, Newark, N.J.
Synthesis of CFP-10 peptides.
Eighteen 15-mer peptides, overlapping by five amino acids and covering the entire 100-amino-acid M. leprae and M. tuberculosis CFP-10 sequences (p1 to p18), were synthesized by using solid-phase pin technology (Mimotopes, San Diego, Calif.). All the peptides were dissolved in sterile distilled water at a concentration of 2 mg/ml and stored frozen at −70°C until they were used. Stock peptide solutions for T-cell assays were diluted to obtain a concentration of 2 μg/25 μl in RPMI medium.
Production of polyclonal antibodies and MAbs to rCFP-10.
Polyclonal antisera were raised in BALB/c mice (Harlan, Indianapolis, Ind.) that were immunized with an emulsion containing 50 μg of either M. leprae or M. tuberculosis rCFP-10 in phosphate-buffered saline (PBS)-incomplete Freund's adjuvant (IFA) (1:1) at two abdominal sites subcutaneously. Booster injections consisting of 50 μg of antigen in an IFA emulsion were administered every 3 weeks, and test bleed samples were obtained and used for an enzyme-linked immunosorbent assay (ELISA) after the first and second booster injections. Rabbit polyclonal antiserum to M. leprae rCFP-10 was raised in an outbred rabbit (Western Oregon Rabbit Co., Philomath, Oreg.) by injecting 100 μg of the protein in an emulsion with IFA at multiple sites subcutaneously or intramuscularly four separate times spaced 2 to 3 weeks apart. Monoclonal antibodies (MAbs) were produced by a standard protocol (49) by using the myeloma B-cell line SP2/0 (30). MAbs 2A6.2C2 (immunoglobulin G1 [IgG1]), 3A10.2C8 (IgG1), and 5B9.1G9 (IgG1) were produced as cell culture supernatants. MAb CS-01 (15), specific for the major 10-kDa antigen GroES (ML0380), was used as a positive control.
Human serum samples.
Sera from both leprosy and TB patients were selected from anonymous serum samples stored at the Department of Microbiology, Yonsei University College of Medicine, Seoul, Republic of Korea. Approval for the use of these serum samples was given by the internal review boards at Yonsei University College of Medicine and Colorado State University, and the sera were used in accordance with federal rules for the use of human sera. A set of serum samples was screened for the presence of antibodies to either the M. leprae or M. tuberculosis CFP-10 whole protein, and only sera showing relatively strong reactivity to each antigen were chosen for further analysis in this study. For leprosy patients, only serum samples from lepromatous patients were included; the clinical diagnosis for four patients was three LL (the bacillary indices were 4.2, 5.0, and 4.8 for patients A, B, and C, respectively) and one BL (the bacillary index was 3.8 for patient D). For TB patients, only sera from pulmonary TB patients whose sputum cultures were positive were used in this study.
ELISA.
For the assay involving animal immune sera, ELISA plate wells (Immulon 4 96-well plates; Dynex Technologies, Chantilly, Va.) were coated with 0.5 μg of M. leprae or M. tuberculosis rCFP-10 or with 5 μg of synthetic peptide in 100 μl of 0.1 M sodium bicarbonate buffer (pH 9.0) overnight at 4°C. The plates were blocked with 1% bovine serum albumin (BSA) in PBS (pH 7.4) containing 0.05% Tween 80 (PBS/T) for 1 h, and this was followed by addition of 100-μl portions of dilutions of primary MAbs or polyclonal antibodies diluted in the same buffer for 2 h. The wells were washed with PBS/T five times and incubated with 100 μl of a 1:2,000 dilution of secondary anti-mouse or anti-rabbit IgG-alkaline phosphatase-conjugated antibody (Sigma) for 2 h. After the wells were washed five times with PBS, 100 μl of p-nitrophenylphosphate substrate (Kirkegaard and Perry Labs, Gaithersburg, Md.) was added, and the plates were incubated until color developed. The plates were read at 405 nm by using a Bio-Rad model 2550 plate reader.
For assays involving human sera, antibodies to recombinant proteins and peptides were detected as described by Voller et al. (43), with minor modifications (6). Briefly, both recombinant antigens and synthetic peptides were diluted to obtain a concentration of 1.0 mg/ml in PBS (pH 7.2) and were solubilized by using a probe sonicator. Each antigen or peptide was then diluted to obtain a concentration of 5 μg/ml in 0.1 M carbonate-bicarbonate buffer (pH 9.6), and 100 μl of the diluted antigen was added to wells of 96-well flat-bottom ELISA plates (Costar, Acton, Mass.) and incubated overnight at 4°C for coating. Unbound antigens and peptides were washed away by using PBS/T four times, and the wells were blocked with a solution containing 1% BSA in PBS/T. Serum samples were diluted 1:200 for leprosy patients and 1:300 for TB patients in PBS/T containing 5% goat serum (Invitrogen). After incubation for 2 h, horseradish peroxidase-conjugated goat anti-human IgG (Calbiochem, San Diego, Calif.) diluted 1:10,000 in PBS/T containing 5% goat serum was added and incubated for 2 h. The substrate o-phenylenediamine (Sigma) was added and allowed to develop. The enzyme reaction was stopped by adding 2.5 M H2SO4 after 15 min, and the absorbance at 490 nm was determined. Each serum was tested in duplicate, and the mean absorbance for control (no antigen) wells was subtracted from that for antigen-coated wells before analysis.
Western blotting.
Approximately 0.5 μg of the recombinant form of each CFP-10 was separated by sodium dodecyl sulfate—15% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes as described previously (37). The native subcellular fractions of M. leprae (cytosol, membrane, and cell wall protein fractions) were prepared as previously described (23) and were loaded onto the membranes at a concentration of 30 μg per lane. The membranes were blocked with 1% BSA in PBS/T, incubated with polyclonal mouse serum (1:2,000), mouse MAb supernatant (1:20), or rabbit polyclonal serum (1:10,000) raised against either M. leprae or M. tuberculosis CFP-10 overnight with gentle rocking. To detect native CFP-10, a purified IgG fraction of rabbit anti-CFP-10 (final dilution of a 1-mg/ml solution, 1:1,000) was used. The membranes were washed with PBS/T and incubated with secondary anti-mouse or anti-rabbit IgG-alkaline phosphatase-conjugated antibody (Sigma). The substrates nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Sigma) were used for color development.
Immunohistological staining of M. leprae-infected nude mouse footpad tissue.
M. leprae isolate Thai-53 was maintained by serial passage in the footpads of athymic BALB/c nu/nu nude mice (Harlan) (38). The mice were inoculated on the plantar surface of both hind feet with 1 × 107 freshly harvested bacilli in 50 μl of 7H12 medium (Difco, Detroit, Mich.). The tissues used for these studies were harvested after about 6 months of growth, when the footpads were moderately enlarged (0.5 to 1 g) and contained numerous rapidly growing bacilli. To prepare the tissues for histological study, the outer skin was removed, and the lepromatous granuloma on the bottom of each foot was scooped away from the bone with a sterile scalpel. The excised tissue was then fixed in 10% neutral formalin overnight and processed for paraffin sectioning. Uninoculated nude mouse tissue was handled just like the infected material and was used as a normal control. Serial 5-μm sections from both M. leprae-infected and noninfected footpads were cut by using the embedded tissue and placed on glass slides for staining. The paraffin was removed by using EZ-DeWax solution (BioGenex, San Ramon, Calif.), and an antigen retrieval procedure was performed by using Citra antigen retrieval solution (BioGenex) according to the manufacturer's protocol. Endogenous tissue peroxidase was inactivated by incubation with a peroxidase blocking reagent (InnoGenex, San Ramon, Calif.) for 5 min at room temperature, and nonspecific binding was blocked by incubating the sections for 30 min with 5% mouse serum (Sigma) in PBS (pH 7.4). Sections were incubated overnight at 4°C with rabbit polyclonal antibody against CFP-10 (1:2,000 dilution) or normal rabbit IgG antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) diluted in PBS-3% BSA as a control. The sections were washed with PBS-0.5% Tween 20 three times (15 min each). The secondary antibody, a polyclonal anti-rabbit IgG conjugated with the enzyme horseradish peroxidase (Serotec, Raleigh, Calif.) diluted 1:200 in PBS-3% BSA, was incubated for 40 min at room temperature. The sections were washed with PBS-0.5% Tween 20. After washing, specific binding of the antibodies was visualized by using the substrate AEC (BioGenex), which produced a reddish color. Sections were counterstained with hematoxylin (BioGenex) and were mounted by using crystal mount (Biomeda Corp., Foster City, Calif.).
Generation of CFP-10-specific T-cell hybridomas and IL-2 assay.
T-cell hybridomas specific for M. leprae and M. tuberculosis rCFP-10 were generated as previously described (35). Briefly, three mice were immunized with 40 μg of M. leprae or M. tuberculosis rCFP-10 in IFA by injecting 25 μl into the hind footpad and the remainder at the base of the tail. Five days after immunization, the draining lymph nodes (popliteal, inguinal, and periaortic) were harvested to obtain lymph node cells, which were then restimulated in vitro with antigen-pulsed bone marrow-derived dendritic cells by using 5 μg of rCFP-10 per well. After 2 days of culture, the cells were harvested from the wells, fused with the T-cell fusion partner BWα−β− (45), and plated in 10 96-well plates. Clones that grew in individual wells were screened by using a BALB/c mouse-derived B-cell lymphoma line, A20 (19), pulsed with 0.5 μg of rCFP-10 per well. The responding T-cell hybridomas were selected by assaying 24-h culture supernatants by using paired rat MAbs specific for mouse interleukin-2 (IL-2) (Pharmingen/BD, San Diego, Calif.) in a capture ELISA. For antigen presentation studies, we prepared microculture wells containing 250 μl of culture medium (RPMI 1640 supplemented with 10% fetal calf serum, 5 × 10−5 M 2-mercaptoethanol [Sigma], and a nutrient cocktail described previously [18]), 5 × 104 T-cell hybridomas, 5 × 104 antigen-presenting cells (APC), and a known amount of peptide antigen in flat-bottom microtiter wells in a 96-well plate. T-cell hybridomas were also tested with the overlapping M. leprae and M. tuberculosis CFP-10 peptides at a concentration of 2 μg/well (or serial dilutions in dose-response studies) presented by APC bearing defined class II molecules to identify T-cell peptide epitopes and class II restriction elements. Mutant B-cell lymphoma lines bearing either I-Ad alone (M12.B5) or I-Ed alone (M12.A2) (13) were used for this analysis.
RESULTS
Polyclonal antisera to M. leprae and M. tuberculosis CFP-10 show little cross-reactivity.
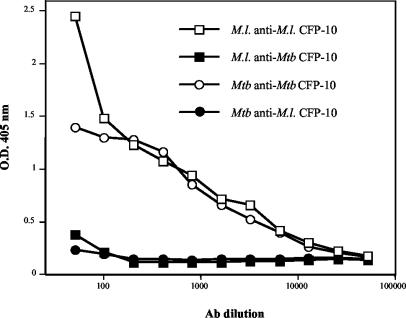
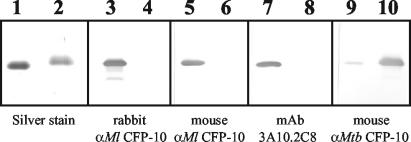
A comparison of the alignment of the sequences of the two 100-amino-acid proteins (Fig. 1) showed that there was only 40% identity (60% homology). Although there was identity at seven of the first nine amino acids at the N terminus (MAEMITEAA; 77% identity; underlining indicates that the amino acids were the same in both proteins), this region is the only region with four or more identical sequential amino acids. The remainder of the sequence alignment showed that there were only one, two, or (in one case at the C terminus) three identical amino acids together, but the sequences of identical amino acids were interrupted by stretches of conserved and nonconserved amino acid differences throughout the proteins. Other than the region at the N terminus, there appeared to be very few conserved regions capable of eliciting cross-reactive antibodies between the two CFP-10 proteins. The reactivity of the mouse antisera to the homologous and heterologous proteins confirmed this conclusion. The polyclonal antisera produced after the third immunization had high titers to the homologous protein immunogen, and these antisera reacted only very weakly to the heterologous proteins as determined by the ELISA (Fig. 2) at low serum dilutions. On the other hand, rabbit antisera raised against M. leprae CFP-10 showed no cross-reactivity against M. tuberculosis CFP-10 as determined by either ELISA (see Table 2) or Western blotting (Fig. 3), even when they were tested at a low dilution (1:500). Native M. leprae CFP-10 was detected by Western blotting in all three native subcellular fractions by using a purified IgG fraction of the rabbit polyclonal anti-CFP-10, which showed that the strongest signal was in the cell wall and the weakest signal was in the membrane fraction (Fig. 4). For comparative purposes, a blot of the 10-kDa GroES protein probed with MAb CS-01 is shown in Fig. 4. No cross-reactivity was detected in corresponding M. tuberculosis fractions of culture filtrate protein or cytosol.
FIG. 1.
Sequence alignment of M. leprae CFP-10 (ML0050) and M. tuberculosis CFP-10 (Rv3874). Identical amino acids are indicated by colons, conservative substitutions are indicated by periods, and nonconservative substitutions are indicated by spaces.
FIG. 2.
Reactivities of polyclonal anti-M. leprae CFP-10 and anti-M. tuberculosis CFP-10 with homologous and heterologous CFP-10 as determined by ELISA. M.l. anti-M.l. CFP-10, M. leprae anti-M. leprae CFP-10; M.l. anti-Mtb CFP-10, M. leprae anti-M. tuberculosis CFP-10; Mtb anti-Mtb CFP-10, M. tuberculosis anti-M. tuberculosis CFP-10; Mtb anti-M.l. CFP-10, M. tuberculosis anti-M. leprae CFP-10; Ab, antibody; O.D. 405 nm, optical density at 405 nm.
TABLE 2.
Reactivities of MAb and polyclonal sera to M. leprae and M. tuberculosis whole CFP-10 and homologous peptides
| Antibodya | Reactivity
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. leprae CFP-10 | M. tuberculosis CFP-10 | p1 | p2 | p3 | p4 | p5 | p6 | p7 | p8 | p9 | p10 | p11 | p12 | p13 | p14 | p15 | p16 | p17 | p18 | |
| Mouse anti-M. leprae CFP-10 | +++b | +/− | − | ++ | + | +/− | + | ++ | + | +++ | +/− | +++ | + | +/− | − | + | +++ | + | − | − |
| Rabbit anti-M. leprae CFP-10 | +++ | − | − | ++ | − | ++ | +/− | + | ++ | + | + | +++ | + | + | − | +/− | + | +++ | +++ | − |
| Mouse anti-M. tuber- culosis CFP-10 | +/− | +++ | − | − | +/− | − | − | ++ | ++ | + | − | + | − | ++ | − | +/− | +/− | ++ | +++ | ++ |
| 3A10.2C8 | +++ | − | − | − | − | − | +++ | +++ | − | − | − | − | − | − | − | − | − | − | − | − |
3A10.2C8, mouse MAb specific for M. leprae CFP-10.
+++, optical density as determined by ELISA, >4.00; ++, optical density, 1.50 to 4.00; +, optical density, 0.50 to 1.49; +/−, optical density, 0.20 to 0.49; −, background reading (optical density, <0.20). The polyclonal sera were tested at a 1:500 dilution, and the MAb was tested at a 1:2 dilution. There was no response of either polyclonal antisera or the MAb to any heterologous peptide.
FIG. 3.
Western blot of M. leprae purified rCFP-10 (lanes 1, 3, 5, 7, and 9) and M. tuberculosis purified rCFP-10 (lanes 2, 4, 6, 8, and 10) with MAbs and polyclonal antibodies. Lanes 1 and 2 contained equivalent amounts of the proteins electrophoresed on a 15% polyacrylamide gel and silver stained; lanes 3 and 4 were probed with rabbit polyclonal antiserum against M. leprae CFP-10 (rabbit αMl CFP-10) (1:10,000 dilution); lanes 5 and 6 were probed with mouse polyclonal antiserum against M. leprae CFP-10 (mouse αMl CFP-10) (1:2,500 dilution); lanes 7 and 8 were probed with mouse MAb 3A10.2C8 specific for peptides p5 and p6 (1:20 dilution of culture supernatant); and lanes 9 and 10 were probed with the mouse polyclonal antiserum against M. tuberculosis CFP-10 (mouse αMtb CFP-10) (1:2,500 dilution).
FIG. 4.
Western blot of subcellular fractions of M. leprae to detect native CFP-10. Lanes 1 and 6, M. leprae cytosol; lane 2, M. leprae membrane; lane 3, M. leprae cell wall; lane 4, M. tuberculosis culture filtrate protein; lane 5, M. tuberculosis cytosol. Approximately 30 μg of protein was electrophoresed in lanes 1 to 5, and 1 μg of protein was electrophoresed in lane 6. Lanes 1 to 5 were probed with purified IgG from rabbit polyclonal anti-M. leprae CFP-10, while lane 6 was probed with MAb CS-01 specific for the 10-kDa GroEs protein. mw, molecular weight (in thousands).
Overlapping 15-mer peptides covering the entire M. leprae and M. tuberculosis CFP-10 proteins were synthesized (Table 1) in order to identify B-cell epitopes and to determine if cross-reactive linear B-cell epitopes existed. The polyclonal antibodies revealed distinct reactivity patterns with each of the homologous peptides (Table 2); however, no cross-reactivity of each serum with any of the heterologous peptides was observed (data not shown). Despite this, there was a consistently low level of cross-reactivity of each mouse polyclonal serum with the heterologous whole protein, suggesting that while there was no cross-reactive recognition of linear peptide epitopes, there were conformational epitopes that were similar enough to account for the limited cross-reactivity observed. Certain homologous peptides were recognized by both mouse antisera (notably, peptides p6, p7, p8, p10, and p16), while two peptides, p1 (which had the highest level of identity, 67%) and p13, were not recognized by either antiserum. Most of the peptides recognized by the mouse polyclonal anti-M. leprae CFP-10 serum were also recognized by the rabbit polyclonal serum, although the strength of the reactivity varied with each peptide. These two antisera gave strong and very similar responses to M. leprae CFP-10 peptides p2 and p10, while the rabbit antisera gave additional strong responses to M. leprae peptides p16 and p17. Interestingly, none of the antisera reacted with peptide p18, whereas the mouse polyclonal serum against M. tuberculosis CFP-10 showed a fairly strong response to this C-terminal peptide. The reactive peptides occurred in regions that were more hydrophilic and had potential B-cell epitopes, as predicted by using a Kyte-Doolittle hydrophobicity plot (20) and the Jameson-Wolf antigenic index (17), respectively, while peptide p1 was decidedly more hydrophobic, perhaps accounting for its lack of recognition by all polyclonal antibodies. Of the three MAbs, only MAb 3A10.2C8 showed a response to any of the peptides, responding strongly to homologous peptides p5 and p6, indicating that the linear B-cell epitope recognized is common to both peptides (Table 2), probably lying within peptide 26SQERNFVDSI35. These peptides have low levels of identity (20 and 27%, respectively) to the corresponding M. tuberculosis CFP-10 peptides, but they occur in a region predicted to be very hydrophilic and to have a high probability of containing B-cell epitopes.
TABLE 1.
Synthetic overlapping 15-mer CFP-10 M. leprae and M. tuberculosis peptides
| Peptide | Amino acids | Species | Sequencea | % Identity |
|---|---|---|---|---|
| p1 | 1-15 | M. leprae | MAEMITEAAILTQQA | 67 |
| M. tuberculosis | MAEMKTDAATLAQEA | |||
| p2 | 6-20 | M. leprae | TEAAILTQQAAQFDQ | 47 |
| M. tuberculosis | TDAATLAQEAGNFER | |||
| p3 | 11-25 | M. leprae | LTQQAAQFDQIASGL | 33 |
| M. tuberculosis | LAQEAGNFERISGDL | |||
| p4 | 16-30 | M. leprae | AQFDQIASGLSQERN | 20 |
| M. tuberculosis | GNFERISGDLKTQID | |||
| p5 | 21-35 | M. leprae | IASGLSQERNFVDSI | 20 |
| M. tuberculosis | ISGDLKTQIDQVEST | |||
| p6 | 26-40 | M. leprae | SQERNFVDSIGQSFQ | 27 |
| M. tuberculosis | KTQIDQVESTAGSLQ | |||
| p7 | 31-45 | M. leprae | FVDSIGQSFQNTWEG | 40 |
| M. tuberculosis | QVESTAGSLQGQWRG | |||
| p8 | 36-50 | M. leprae | GQSFQNTWEGQAASA | 40 |
| M. tuberculosis | AGSLQGQWRGAAGTA | |||
| p9 | 41-55 | M. leprae | NTWEGQAASAALGAL | 40 |
| M. tuberculosis | GQWRGAAGTAAQAAV | |||
| p10 | 46-60 | M. leprae | QAASAALGALGRFDE | 47 |
| M. tuberculosis | AAGTAAQAAVVRFQE | |||
| p11 | 51-65 | M. leprae | ALGALGRFDEAMQDQ | 47 |
| M. tuberculosis | AQAAVVRFQEAANKQ | |||
| p12 | 56-70 | M. leprae | GRFDEAMQDQIRQLE | 40 |
| M. tuberculosis | VRFQEAANKQKQELD | |||
| p13 | 61-75 | M. leprae | AMQDQIRQLESIVDK | 27 |
| M. tuberculosis | AANKQKQELDEISTN | |||
| p14 | 66-80 | M. leprae | IRQLESIVDKLNRSG | 20 |
| M. tuberculosis | KQELDEISTNIRQAG | |||
| p15 | 71-85 | M. leprae | SIVDKLNRSGGNYTK | 20 |
| M. tuberculosis | EISTNIRQAGVQYSR | |||
| p16 | 76-90 | M. leprae | LNRSGGNYTKTDDEA | 27 |
| M. tuberculosis | IRQAGVQYSRADEEQ | |||
| p17 | 81-95 | M. leprae | GNYTKTDDEANQLLS | 40 |
| M. tuberculosis | VQYSRADEEQQQALS | |||
| p18 | 86-100 | M. leprae | TDDEANQLLSSKMNF | 53 |
| M. tuberculosis | ADEEQQQALSSQMGF |
Boldface type indicates identity.
Human serological reactivity to CFP-10 peptides.
Leprosy and TB patient sera that exhibited the greatest responses to M. leprae CFP-10 and M. tuberculosis CFP-10, respectively, were used to examine the reactivity with the 18 peptides. The leprosy patient sera reacted specifically only against the homologous M. leprae CFP-10 protein and peptide set, while the TB patient sera similarly reacted only against the homologous protein and peptides (Tables 3 and 4). Although the sera of the majority of individuals recognized only one or a few peptides, certain peptides were recognized by sera from multiple patients. Two of four of the leprosy patient serum samples recognized peptides p6 and p16, while peptide p15 was recognized by three individual samples. The TB patient sera seemed to react with more peptides overall, and at least two patient samples reacted with peptides p3, p5, p6, p10, p14, and p18. All of the sera of TB patients in this group reacted with peptide p18. As was the case with the animal sera, peptides p1 and p13 showed no response with either leprosy or TB patient sera, except for a fairly weak response against p1 by serum from a single TB patient.
TABLE 3.
Representative leprosy (n = 4) and TB (n = 1) patient serum responses to M. leprae and M. tuberculosis rCFP-10 and to M. leprae CFP-10 peptides p1 to p18
| Antigen | Serum responsea
|
||||
|---|---|---|---|---|---|
| Leprosy patient A | Leprosy patient B | Leprosy patient C | Leprosy patient D | TB patient A | |
| M. leprae CFP-10 | 1.94 | 1.87 | 0.96 | 1.81 | 0.11 |
| M. tuberculosis CFP-10 | 0.07 | 0.01 | 0.01 | 0.01 | 1.39 |
| M. leprae p1 | 0.02 | 0.01 | 0.01 | 0.03 | 0.01 |
| M. leprae p2 | 0.08 | 0.08 | 0.04 | 0.01 | 0.01 |
| M. leprae p3 | 0.09 | 0.09 | 0.10 | 0.01 | 0.01 |
| M. leprae p4 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. leprae p5 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. leprae p6 | 0.46 | 0.44 | 0.10 | 0.06 | 0.01 |
| M. leprae p7 | 0.23 | 0.11 | 0.11 | 0.01 | 0.01 |
| M. leprae p8 | 0.01 | 0.01 | 0.11 | 0.01 | 0.01 |
| M. leprae p9 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
| M. leprae p10 | 0.06 | 0.01 | 0.19 | 0.01 | 0.01 |
| M. leprae p11 | 0.01 | 0.01 | 0.14 | 0.02 | 0.01 |
| M. leprae p12 | 0.01 | 0.01 | 0.01 | 0.77 | 0.01 |
| M. leprae p13 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
| M. leprae p14 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. leprae p15 | 1.46 | 1.32 | 0.59 | 0.01 | 0.01 |
| M. leprae p16 | 0.44 | 0.42 | 0.03 | 0.01 | 0.01 |
| M. leprae p17 | 0.05 | 0.05 | 0.01 | 0.11 | 0.01 |
| M. leprae p18 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
Values are optical densities at 490 nm; boldface indicates positive responses.
TABLE 4.
Representative TB (n = 8) and leprosy (n = 1) patient serum responses to M. leprae and M. tuberculosis rCFP-10 and to M. tuberculosis CFP-10 peptides p1 to p18
| Antigen | Serum responsea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TB patient A | TB patient B | TB patient C | TB patient D | TB patient E | TB patient F | TB patient G | TB patient H | Leprosy patient A | |
| M. tuberculosis CFP-10 | 1.41 | 1.66 | 1.44 | 1.73 | 1.24 | 1.38 | 0.99 | 1.52 | 0.01 |
| M. leprae CFP-10 | 0.01 | 0.05 | 0.01 | 0.13 | 0.06 | 0.01 | 0.01 | 0.01 | 1.86 |
| M. tuberculosis p1 | 0.01 | 0.01 | 0.01 | 0.08 | 0.01 | 0.01 | 0.01 | 0.26 | 0.01 |
| M. tuberculosis p2 | 0.03 | 0.13 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p3 | 0.57 | 1.32 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p4 | 0.01 | 0.01 | 0.01 | 0.01 | 0.36 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p5 | 0.18 | 0.05 | 0.68 | 0.01 | 0.01 | 0.24 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p6 | 0.01 | 0.59 | 0.91 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 |
| M. tuberculosis p7 | 0.01 | 0.02 | 0.01 | 0.03 | 0.11 | 0.01 | 0.01 | 0.07 | 0.01 |
| M. tuberculosis p8 | 0.01 | 0.01 | 0.01 | 0.01 | 0.08 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p9 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p10 | 0.93 | 0.22 | 0.29 | 0.52 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p11 | 0.01 | 0.74 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p12 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p13 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p14 | 0.01 | 0.27 | 0.01 | 0.01 | 0.01 | 0.37 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p15 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p16 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p17 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| M. tuberculosis p18 | 0.57 | 1.54 | 0.27 | 0.16 | 0.17 | 1.26 | 0.51 | 1.31 | 0.01 |
Values are optical densities at 490 nm, boldface indicates positive responses.
Comparison of T-cell epitopes of M. leprae and M. tuberculosis CFP-10.
Fifteen T-cell hybridomas produced IL-2 when they were presented with M. leprae CFP-10, and all of them used the I-Ad class II molecule to present the processed whole molecule. Four of these T-cell hybridomas were able to recognize peptides from the panel of overlapping 15-mers (Table 5). Two of the hybridomas, 5D4 and 8D11, recognized peptide p2 (amino acids 6 to 20), while hybridomas 1G4 and 6G7 recognized peptides p1 (amino acids 1 to 15) and p2, although both exhibited greater responses to peptide p1, suggesting that a portion of the epitope in p2 is truncated. Hybridoma 1G4 was also able to produce IL-2 in response to M. tuberculosis CFP-10 peptide p1, but it did not respond at all to M. tuberculosis CFP-10 peptide p2. These results suggest that the critical amino acid residues necessary for either binding to class II or interacting with the T-cell receptor reside in M. tuberculosis peptide p1 but that changes in p2 disrupt one of these interactions.
TABLE 5.
Reactivities of M. leprae CFP-10-specific T-cell hybridomas to M. leprae CFP-10 and M. leprae and M. tuberculosis peptides p1 and p2
| T-cell hybridoma | Reactivity
|
||||
|---|---|---|---|---|---|
| M. leprae CFP-10 | M. leprae p1 | M. leprae p2 | M. tuber- culosis p1 | M. tuber- culosis p2 | |
| 1G4 | +a | ++ | + | + | − |
| 6G7 | + | +++ | ++ | − | − |
| 5D4 | + | − | ++ | − | − |
| 8D11 | ++ | − | ++ | − | − |
+++, optical density, >4.00; ++, optical density, 1.00 to 3.99; +, optical density, 0.20 to 0.99; −, background reading (optical density, <0.20; corresponds to <1 pg of IL-2 per ml). An optical density of >1.00 indicates production of >20 pg of IL-2 per ml by T-cell hybridomas.
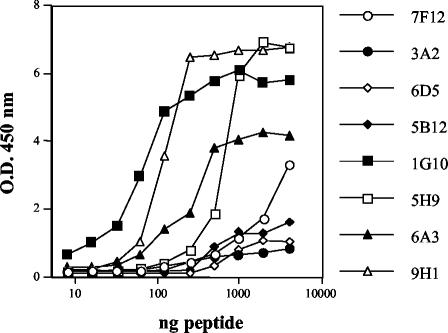
Thirty independently derived T-cell hybridomas were generated that produced IL-2 in response to whole M. tuberculosis CFP-10 presented by APC. Unlike the limited responses to peptides seen with the M. leprae CFP-10-specific T-cell hybridomas, all of the M. tuberculosis CFP-10-specific hybridomas were capable of peptide recognition (Table 6). Four distinct peptide regions were recognized. Eight hybridomas recognized peptides p1 and p2, and like M. leprae CFP-10-specific hybridomas 1G4 and 6G7, all responded more strongly in dose-response presentation assays by producing higher levels of IL-2 in response to p1 than in response to p2. Only one of the hybridomas, 4E9, was capable of cross-reactivity, responding to M. leprae peptide p1 but not to p2. Two hybridomas, 5B12 and 6D5, recognized peptide p4 (amino acids 16 to 30). Nine hybridomas recognized peptide p9 (amino acids 41 to 55), while two other hybridomas, 5B7 and 5D9, recognized an epitope shared by two peptides, p9 and p10. Nine hybridomas responded equally well to peptides p15 (amino acids 71 to 85) and p16 (amino acids 76 to 90), indicating that amino acids common to both peptides were recognized. The relative strengths of the responses of hybridomas to each of the four peptides were examined by preparing a dose-response curve in antigen presentation experiments (Fig. 5). Hybridomas reacting with peptide p9 and with peptides p15 and p16 responded at least 10-fold better than hybridomas reacting with peptide p1 or p4. There were no differences in the dose-response curves for hybridomas reacting with peptides p15 and p16, indicating that the abilities of these peptides to stimulate were equivalent. When mutant B-cell lines expressing either I-Ad (M12.B5) or I-Ed (M12.A2) were used, all of the CFP-10 hybridomas were found to recognize peptides presented by the I-Ad class II molecule (data not shown). Other than hybridomas 1G4 and 4E9, none of the T-cell hybridomas showed any recognition of heterologous peptides (data not shown).
TABLE 6.
Peptide reactivities of 30 M. tuberculosis CFP-10-specific T-cell hybridomas
| T-cell hybridoma(s) | Peptide(s) | Cross- reactivity |
|---|---|---|
| 3A2, 5A5, 5A6, 5A12, 7F12, 8E8, 10A6 | p1 > p2a | No |
| 4E9 | p1 > p2, M. leprae p1 cross-reactive | Yes |
| 5B12, 6D5 | p4 | No |
| 1G10, 2B12, 3C6, 4D12, 4E10, 5H9, 6G10, 8G10, 10G12 | p9 | No |
| 5B7, 5D9 | p9 and p10 | No |
| 2A10, 2H5, 3G11, 5C3, 6A3, 6F5, 9D7, 9H1, 10B8 | p15 and p16 | No |
The optimal peptide concentrations used to induce stimulation of T-cell hybridomas were approximately 7 to 15 μM. The amount of IL-2 secreted in 24-h culture supernatants, as measured by capture ELISA, was >20 pg/ml, while stimulation with the corresponding non-cross-reactive M. leprae peptide resulted in background levels of <1 pg/ml.
FIG. 5.
Comparison of M. tuberculosis CFP-10-specific T-cell hybridoma responses to the four peptide epitopes recognized. T-cell hybridomas were cultured with the antigen-presenting B-cell line A20 pulsed with serial twofold dilutions of peptide (4 μg to 8 ng per well) in a dose-response experiment. The hybridomas examined were 7F12 and 3A2 (peptide p1 specific), 5B12 and 6D5 (p4 specific), 1G10 and 5H9 (p9 specific), and 6A3 and 9H1 (p15 and p16 specific). The IL-2 secretion in response to antigen stimulation was measured in 24-h culture supernatant by a capture ELISA. O.D. 450 nm, optical density at 450 nm.
Immunohistological staining of CFP-10 in M. leprae-infected nude mouse footpads.
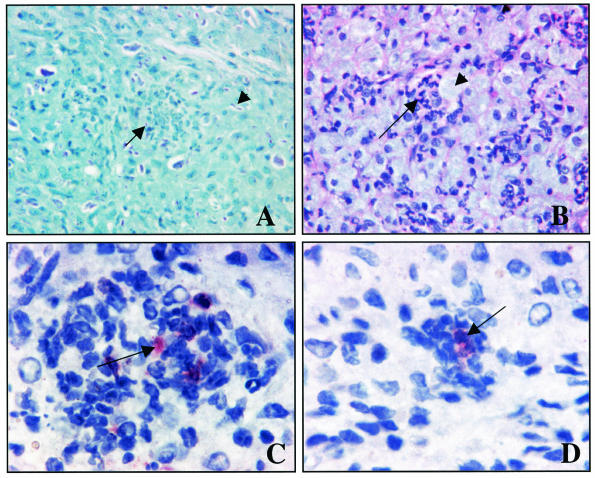
Tissue sections from footpads of M. leprae-infected nu/nu mice were stained with hematoxylin and eosin with or without rabbit polyclonal antibody recognizing M. leprae CFP-10 (Fig. 6). Hematoxylin and eosin staining revealed a non-T-cell type of granulomatous structure, and there were many acid-fast bacilli inside the cells when they were stained with Kinyoun acid-fast stain. Many of the cells within the granuloma were stained with MAb DC205, which is fairly specific for dendritic cells, and MAb MAC-3, which stains cells of the macrophage lineage (data not shown), and these areas were strongly positive for CFP-10. CFP-10 staining was found to be localized both within the cytoplasm of cells and extracellularly, although the extracellular staining was diffuse and weaker than the intracellular staining. There was no background staining of control uninfected nu/nu mouse footpad sections with the rabbit anti-CFP-10 antiserum.
FIG. 6.
Immunohistological staining of M. leprae-infected nu/nu mouse footpad sections with polyclonal rabbit anti-M. leprae CFP-10. Formalin-fixed tissue embedded in paraffin was cut into 5-μm sections and stained by conventional methods. (A) Kinyoun method for acid-fast staining, showing specific acid-fast staining of bacilli within (arrow) and outside (arrowhead) necrotic cell pockets. Magnification, ×200. (B) Hematoxylin and eosin staining, showing a pocket of necrotic cells (arrow) and extensive infiltration of foamy-like macrophages (arrowhead). Magnification, ×200. (C and D) Immunohistochemistry with a rabbit polyclonal antibody against M. leprae CFP-10, indicated by dark red staining in the center of cells within the granuloma (arrows). Magnification, ×400.
DISCUSSION
In this study, we characterized the major B- and T-cell epitopes recognized by immune sera and T-cell hybridomas generated against the M. tuberculosis and M. leprae CFP-10 proteins. At the antibody level, each mouse polyclonal antiserum showed a distinct pattern of recognition of the homologous set of peptides; for each protein there was no cross-reactivity of the serum with any of the peptides of the other protein, and there were only very weak cross-reactive responses against the whole protein. In contrast to the mouse polyclonal antiserum, which showed low levels of cross-reactivity to the heterologous whole CFP-10 protein in ELISA and Western blots, the rabbit polyclonal antiserum showed no cross-reactivity by either method, even when a dilution as low as 1:500 was used. The peptides that induced the greatest responses for each CFP-10 were in regions of the molecule that had the highest hydrophilicity index, indicating that there are sites on the protein that are more likely to be exposed to the surface and thus bear potential B-cell epitopes. Despite the overall similarity in the hydrophilicity profiles for the two CFP-10 proteins, there were peptides that induced preferentially greater responses for each protein. For the M. leprae CFP-10 antiserum, homologous peptides p2, p8, p10, and p15 were much more reactive than the M. tuberculosis CFP-10 antiserum to the corresponding M. tuberculosis peptides; conversely, M. tuberculosis peptides p12, p17, and p18 were more strongly recognized by anti-M. tuberculosis CFP-10 serum than their M. leprae counterparts were. Despite having the highest levels of homology, peptides at the N and C termini (p1, 80% homology; and p18, 67% homology) were not cross-reactive; peptide p1 did not even induce an antibody response, probably due to its greater hydrophobicity.
The lack of immunologic cross-reactivity for peptides at the B-cell level could be useful in differentiating M. leprae infections from M. tuberculosis infections. Indeed, the responses of leprosy and TB serum samples with the M. leprae and M. tuberculosis CFP-10 proteins support the possibility that the differences can be exploited. Both leprosy and TB sera were specific for the homologous CFP-10 and, like the animal antisera, showed no cross-reactivity against any of the heterologous peptides. Not all patient sera produced a strong response against the whole CFP-10 proteins, and only the sera that had a relatively high titer against the whole protein were tested further, as weaker responders gave indeterminate or negative peptide responses. The recognition of peptides by human serum samples was generally weaker overall, but this was not unexpected since our assay detects the antibody response to a single protein among the many antigens produced by the microorganism in a natural infection, as opposed to the stronger peptide responses generated by immunizing animals with a single protein antigen. Nevertheless, some individuals in each group responded well to one or more peptides, and the individuals showed distinct differences in peptide preference. Leprosy patient sera reacted well with M. leprae CFP-10 peptides p15 and p16, which mirrored the mouse and rabbit serum responses, whereas none of the sera from TB patients responded to either of these two peptides. Likewise, mouse polyclonal antisera against M. tuberculosis CFP-10 responded quite well to the homologous peptide p18, and the sera of all of the TB patients in this group reacted with p18, some quite strongly, whereas both rabbit and mouse antisera against M. leprae CFP-10, as well as the four leprosy patient sera, did not respond at all to the homologous peptide p18. One would expect that there are differences in peptide responses against the two forms of CFP-10 at the T-cell level as well, a prospect which we are currently studying.
An analysis of the T-cell responses against the two CFP-10 proteins showed that multiple peptide epitopes were recognized for each protein. Although all 30 of the M. tuberculosis CFP-10-specific T-cell hybridomas generated were found to react with one or more peptides in four distinct regions, only 4 of the 15 M. leprae CFP-10-specific hybridomas were similarly characterized. A similar percentage of hybridomas specific for M. leprae ESAT-6 that remained uncharacterized with regard to peptide epitope specificity has been observed previously (35). It is possible that when the overlapping 15-mer M. leprae peptide set was created, critical amino acids necessary for peptide binding to major histocompatibility complex (MHC) class II or for interactions with the T-cell receptor molecules were omitted, leading to a lack of a response to peptides for most of the hybridomas despite the excellent IL-2 production with the whole protein. Overlapping 15-mer peptides were synthesized in order to maximize the probability of detecting all possible T-cell epitopes since it has been shown previously that native processed peptides eluted from MHC class II molecules are generally 13 to 17 amino acids long (27). Generally, for a given protein of average size, there are usually only one or a few peptides that can be recognized as T-cell epitopes (26, 28), so the discovery of four distinct T-cell epitopes on a fairly small protein was surprising. As was the case with M. leprae ESAT-6 (35), all of the peptide epitopes identified in this study were presented by the MHC class II molecule I-Ad, a bias that may be a reflection of the higher levels of expression of this isotype than of I-Ed on the surface of BALB/c APC (34). As for the peptides recognized, a high percentage of T-cell epitopes that have been described contain amphipathic structures (10), and such structures were identified in all four peptide regions recognized in Rv3874. Such structures were also found within the two recognized peptides of ML0050, but the structures were much shorter. In addition, as determined by using a program developed by Sette et al. (29) which predicts the ability of hexapeptides to bind to the I-Ad class II molecule based on a common structural motif, peptides p4, p15, and 16 from Rv3874 and peptides p1 and p2 from ML0050 contain such motifs. The limited cross-reactivity of peptide p1 was not unexpected, since this region has the highest homology of all of the peptides in the set. Nevertheless, the presence of only one hybridoma that is capable of cross-reactive recognition in each set suggests that the cross-reactive recognition between the M. leprae and M. tuberculosis CFP-10 proteins is fairly limited, giving hope that similar disease-specific epitopes can be defined that may ultimately serve as diagnostic reagents for early detection of leprosy in humans.
CFP-10 is found mainly as an exported protein in the culture filtrate protein fraction of M. tuberculosis. If it is also found mainly as a secreted protein in M. leprae, the likelihood of finding detectable levels of it in any of the subcellular fractions would be small. The method by which M. leprae is purified from infected armadillo tissues includes digestion of the homogenized tissue with collagenase and proteolytic enzymes and treatment with alkali intended to remove host proteins. This process probably leads to destruction of any secreted and cell surface-associated proteins. Although previously M. leprae ESAT-6 was found only in the native cell wall fraction (35), by using a purified IgG fraction of the rabbit polyclonal anti-CFP-10 we were able to detect CFP-10 in all three M. leprae subcellular fractions; the largest amount was found in the cell wall fraction, followed by the cytosol, and the protein was barely detectable in the membrane fraction. There was no reaction with either the culture filtrate protein or the cytosolic fraction from M. tuberculosis. In addition, we performed in situ immunohistological staining of M. leprae-infected nu/nu mouse footpads. When the rabbit polyclonal anti-M. leprae CFP-10 serum was used, this reagent revealed strong staining inside macrophages and dendritic cells within the granulomatous structure, as well as a diffuse pattern of weaker staining of the extracellular matrix. There was no staining of the uninfected control nude mouse footpads. The finding that staining was localized extensively within the cytoplasm of infected cells, as well as extracellularly, and was not just associated with intact bacilli was indirect evidence that the CFP-10 produced by M. leprae is potentially secreted.
Members of the CFP-10 family have been closely linked to the immunologically important ESAT-6 family; both CFP-10 and ESAT-6 are small proteins that are released into the culture filtrate of M. tuberculosis cultures very early in the growth cycle, despite lacking signal peptide sequences. Two recent studies (25, 36) verified the hypothesis proposed by van Pittius and coworkers (40) that a cluster of conserved genes that flank the esx and lhp genes are involved in a non-Sec-dependent secretion apparatus for the export of ESAT-6 and CFP-10 of M. tuberculosis, in agreement with the lack of signal sequences in these proteins. Genetic complementation of M. bovis BCG with M. tuberculosis genes indicated that at least 11 genes (Rv3867 to Rv3877) are required for secretion of large amounts of ESAT-6 and CFP-10 into the culture supernatant (25). Rv3870 and Rv3871 are AAA+ class ATPases, while Rv3877 is a large transmembrane protein. Mutants of M. tuberculosis lacking these proteins cannot secrete ESAT-6 and CFP-10. Furthermore, both ESAT-6 and CFP-10 must be intact for secretion of either of the proteins (36). All of the 11 genes except Rv3872 are present in the M. leprae genome in the same genetic arrangement. Therefore, it is believed that M. leprae secretes ESAT-6 and CFP-10 in the same manner that M. tuberculosis secretes these proteins. However, because of extensive sequence differences found in the proteins encoded in this region in M. leprae, the only way to prove this would be to conduct similar gene complementation experiments with the genes of M. leprae. Nevertheless, transcripts for M. leprae CFP-10 and ESAT-6 have been shown to exist by reverse transcription-PCR in nu/nu mouse-derived M. leprae by using two different isolates, strains Thai-53 and 4089, and by using a pool of human leprosy patient biopsy material (Diana Williams, National Hansen's Disease Program, Louisiana State University, Baton Rouge, unpublished observations).
Although the function of these proteins is unknown, both elicit potent cell-mediated responses very early during the course of infection (1, 7, 31, 33). Both ESAT-6 and CFP-10 have shown great promise as immunodiagnostic reagents for TB in humans and cattle (2, 5, 39, 44). It has been shown that combining M. tuberculosis ESAT-6 and CFP-10 in a diagnostic test for infection or exposure to TB could provide the level of specificity and sensitivity necessary to detect exposure to TB (2). However, there have been recent reports suggesting that cross-reactive recognition or high background responses to either whole ESAT-6 or CFP-10 or their peptides may be more common in areas where TB and leprosy are endemic, even in healthy controls with minimal exposure to either disease (11, 12, 41). It is possible that in countries where both diseases are endemic, there is much more exposure of otherwise healthy individuals to a variety of pathogenic and environmental mycobacteria that could contribute to this phenomenon. It has been argued that one of the reasons why BCG vaccination appears to be less effective in areas where TB is prevalent is that there is much more exposure to nonpathogenic mycobacteria found in the soil and water, in addition to disease exposure (4). It is important to determine how widespread such cross-reactive responses to ESAT-6 and CFP-10 are in the countries where TB and leprosy are found together and to use the non-cross-reactive peptides identified in this work as disease-specific diagnostic reagents. Thus, the low-molecular-weight proteins of M. leprae, only a few of which are not encoded by pseudogenes, offer great promise as specific diagnostic reagents for leprosy.
Acknowledgments
This work was supported by NIH/NIAID contract AI-55262 and NIH/NIAID grant AI-47197.
We thank Nathan Groathouse (Colorado State University) for technical assistance, Pier Brusasca (Public Health Research Institute) for preparation of M. tuberculosis rCFP-10, John Belisle for the gift of M. tuberculosis CFP-10 and cytosolic proteins (generated through NIH NIAID contract NO1 AI-75320), and Marilyn Hein for preparation of the manuscript.
Editor: F. C. Fang
REFERENCES
- 1.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 2.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. V. Skjot, J. T. van Dissel, and T. H. M. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early secreted antigen target 6 kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 3.Berthet, F.-X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 4.Black, G. F., H. M. Dockrell, A. C. Crampin, S. Floyd, R. E. Weir, L. Bliss, L. Sichali, L. Mwaungulu, H. Kanyongoloka, B. Ngwira, D. K. Warndorff, and P. E. M. Fine. 2001. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J. Infect. Dis. 184:322-329. [DOI] [PubMed] [Google Scholar]
- 5.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-γ test for tuberculosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 6.Cho, S. N., J. S. Shin, I. H. Choi, S. H. Kim, D. I. Kim, and J. D. Kim. 1988. Detection of phenolic glycolipid I of Mycobacterium leprae and antibodies to the antigen in sera from leprosy patients and their contacts. Yonsei Med. J. 29:219-224. [DOI] [PubMed] [Google Scholar]
- 7.Colangeli, R., J. S. Spencer, P. Bifani, A. Williams, K. Lyashenko, M. A. Keen, P. J. Hill, J. Belisle, and M. L. Gennaro. 2000. MTSA-10, the product of the Rv3874 gene of Mycobacterium tuberculosis, elicits tuberculosis-specific, delayed-type hypersensitivity in guinea pigs. Infect. Immun. 68:990-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III., F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, S. Squares, R. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 9.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 10.DeLisi, C., and J. Berzofsky. 1985. T cell antigenic sites tend to be amphipathic structures. Proc. Natl. Acad. Sci. USA 82:7048-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geluk, A., K. E. van Meijgaarden, K. L. M. C. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. M. Ottenhoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geluk, A., K. E. van Meijgaarden, K. L. M. C. Franken, B. Wieles, S. M. Arend, W. R. Faber, B. Naafs, and T. H. M. Ottenhoff. 2004. Immunological crossreactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 59:66-70. [DOI] [PubMed] [Google Scholar]
- 13.Griffith, I. J., N. Nabavi, Z. Ghogawala, C. G. Chase, M. Rodriquez, D. J. McKean, and L. H. Glimcher. 1988. Structural mutation affecting intracellular transport and cell surface expression of murine class II molecules. J. Exp. Med. 167:541-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter, S. W., B. Rivoire, V. Mehra, B. R. Bloom, and P. J. Brennan. 1990. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265:14065-14068. [PubMed] [Google Scholar]
- 16.International Leprosy Association. 2002. Report of the International Leprosy Association Technical Forum. Int. J. Lepr. 70:S3-S62. [Google Scholar]
- 17.Jameson, B. A., and H. Wolf. 1988. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput. Applic. Biosci. 4:181-186. [DOI] [PubMed] [Google Scholar]
- 18.Kappler, J. W., B. Skidmore, J. White, and P. Marrack. 1981. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J. Exp. Med. 153:1198-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, K. J., C. Kanellopoulos-Langevin, R. Merwin, D. Sachs, and R. Asofsky. 1979. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 122:549-554. [PubMed] [Google Scholar]
- 20.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques, M. A. M., S. Chitale, P. J. Brennan, and M. C. V. Pessolani. 1998. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae. Infect. Immun. 66:2625-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porathe, J., J. Carlsson, I. Olsson, and G. Belfrage. 1975. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 258:598-599. [DOI] [PubMed] [Google Scholar]
- 25.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 5:533-539. [DOI] [PubMed] [Google Scholar]
- 26.Rothbard, J. B., and M. L. Gefter. 1991. Interactions between immunogenic peptides and MHC proteins. Annu. Rev. Immunol. 9:527-565. [DOI] [PubMed] [Google Scholar]
- 27.Rudensky, A. Y., P. Preston-Hurlburt, S.-C. Hong, A. Barlow, and C. A. Janeway. 1991. Sequence analysis of peptides bound to MHC class II molecules. Nature 353:622-627. [DOI] [PubMed] [Google Scholar]
- 28.Sette, A., S. Buus, S. Colon, J. A. Smith, C. Miles, and H. M. Grey. 1987. Structural characteristics of an antigen required for its interaction with Ia and recognition by T cells. Nature 328:395-399. [DOI] [PubMed] [Google Scholar]
- 29.Sette, A., S. Buus, S. Colon, C. Miles, and H. W. Grey. 1988. I-Ad-binding peptides derived from unrelated protein antigens share a common structural motif. J. Immunol. 141:45-48. [PubMed] [Google Scholar]
- 30.Shulman, M., C. D. Wilde, and G. Kohler. 1978. A better cell line for making hybridomas secreting specific antibodies. Nature 276:269-270. [DOI] [PubMed] [Google Scholar]
- 31.Skjot, R. L. V., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer, J. S., J. H. Freed, and R. T. Kubo. 1993. Expression and function of mixed isotype class II molecules in normal mice. J. Immunol. 151:6822-6832. [PubMed] [Google Scholar]
- 35.Spencer, J. S., M. A. M. Marques, M. C. B. S. Lima, A. P. Junqueira-Kipnis, B. C. Gregory, R. W. Truman, and P. J. Brennan. 2002. Antigenic specificity of the Mycobacterium leprae homologue of ESAT-6. Infect. Immun. 70:1010-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley, S. A., S. Raghavan, W. W. Hwang, and J. S. Cox. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. USA 100:13001-13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Truman, R. W., and J. L. Krahenbuhl. 2001. Viable M. leprae as a research reagent. Int. J. Lepr. Other Mycobact. Dis. 69:1-12. [PubMed] [Google Scholar]
- 39.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP-10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Pittius, N. C. G., J. Gamieldien, W. Hide, G. D. Brown, R. J Siezen, and A. D. Beyers. 2001. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2:44.1-44.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vekemans, J., C. Lienhardt, J. S. Sillah, J. G. Wheeler, G. P. Lahai, M. T. Doherty, T. Corrah, P. Andersen, K. P. W. J. McAdam, and A. Marchant. 2001. Tuberculosis contacts but not patients have higher gamma interferon responses to ESAT-6 than do community controls in The Gambia. Infect. Immun. 69:6554-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vissa, V. D., and P. J. Brennan. 2002. Impact of the Mycobacterium leprae genome sequence on leprosy research, p. 85-118. In Genomics of GC rich gram-positive bacteria. Caister Academic Press, Wymondham, United Kingdom.
- 43.Voller, A., D. E. Bidwell, and A. Bartlett. 1979. The enzyme-linked immunoassay (ELISA). Dynatech Laboratories, Inc., Alexandria, Va.
- 44.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White, J., M. Blackman, J. Bill, J. Kappler, P. Marrack, D. Gold, and W. Born. 1989. Two better cell lines for making hybridomas expressing specific T cell receptors. J. Immunol. 143:1822-1825. [PubMed] [Google Scholar]
- 46.World Health Organization. 2003. Leprosy elimination campaigns: impact on case detection. Wkly. Epidemiol. Rec. 78:9-16. [PubMed] [Google Scholar]
- 47.World Health Organization. 2002. Leprosy elimination campaigns. Wkly. Epidemiol. Rec. 77:17-20. [PubMed] [Google Scholar]
- 48.World Health Organization. 2002. Leprosy. Global situation. Wkly. Epidemiol. Rec. 77:1-8. [PubMed] [Google Scholar]
- 49.Yokoyama, W. M. 1997. Production of monoclonal antibodies, p. 2.5.1-2.5.17. In J. E. Coligan, A. M. Druisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y.