Abstract
The term sarcopenia refers to the loss of muscle mass that occurs with ageing. On the basis of study results showing that muscle mass is only moderately related to functional outcomes, international working groups have proposed that loss of muscle strength or physical function should also be included in the definition. Irrespective of how sarcopenia is defined, both low muscle mass and poor muscle strength are clearly highly prevalent and important risk factors for disability and potentially mortality in individuals as they age. Many chronic diseases, in addition to ageing, could also accelerate decrease of muscle mass and strength, and this effect could be a main underlying mechanism by which chronic diseases cause physical disability. In this Review, we address both age-related and disease-related muscle loss, with a focus on diabetes and obesity but including other disease states, and potential common mechanisms and treatments. Development of treatments for age-related and disease-related muscle loss might improve active life expectancy in older people, and lead to substantial health-care savings and improved quality of life.
Introduction
Ageing is accompanied by major changes in body composition that can negatively affect functional status in older adults, including a progressive decrease in muscle mass, strength, and quality, accompanied by an increase in fat mass.1,2 Changes in skeletal muscle are especially important because muscle is essential for locomotion. Loss of muscle mass has commonly been termed sarcopenia, which is rooted in the Greek words meaning loss of flesh.3
Sarcopenia is distinct from muscle wasting, which more broadly refers to involuntary loss of body mass (both muscle mass and fat).4 Baumgartner and colleagues5 described sarcopenia as a skeletal mass index (dual energy x-ray absorptiometry-derived appendicular skeletal mass divided by body height squared in metres) that is two or more standard deviations below the reference values for young adults. Janssen and colleagues6 later proposed to convert absolute skeletal muscle mass (kg) to percentage of weight (muscle mass/body mass × 100), and described sarcopenia as a percentage of skeletal muscle mass that was more than one standard deviation below reference values for young adults based on bioelectric impedance analysis. Various other criteria for sarcopenia have also been proposed.7
Dynapenia is a term used specifically to define loss of muscle strength.8 The European Working Group on Sarcopenia in Older People recommends use of low muscle mass combined with low muscle function (either strength or physical performance) as criteria for the diagnosis of sarcopenia.9 An international committee proposed a definition of sarcopenia on the basis of a decline in muscle mass and walking speed.10 No standardly used definition exists for assessment of sarcopenia, and consensus definitions based on critical analyses of large databases are being developed.
Irrespective of operational definitions used to define sarcopenia, high prevalence of low muscle mass and poor muscle strength with ageing is very clear. Sarcopenia might affect up to half of people aged 80 years and older, but, without a standard definition, no real estimates of prevalence and incidence are possible.11,12 Age-related muscle loss is a strong risk factor for disability, hospitalisation, and death in older adults.10,13 The contribution of chronic diseases such as diabetes and obesity, the prevalence of which also increase with ageing, to age-related muscle loss is unclear; arguably, definitions of sarcopenia that have been developed cannot necessarily differentiate the effects of ageing on muscle loss independent of other age-associated disease conditions that are prevalent in older individuals. Notwithstanding uncertainties, it has been estimated that a 10% reduction in age-related muscle loss would result in savings of US$1·1 billion per year in health-care costs in the USA.14 Thus, reduction of the burden of muscle loss in ageing potentially has broad but substantial public health benefits. In addition to ageing, many chronic diseases might cause accelerated decline of muscle mass and strength and, through this mechanism, increase the risk of physical disability. In this Review we address both age-related and disease-related muscle loss, with a focus on diabetes and obesity, in addition to potential common mechanisms and treatments.
Age-related muscle loss
Lean muscle mass generally contributes up to about 50% of total bodyweight in young adults, but decreases with age to be about 25% of total bodyweight by age 75–80 years.15 Declining muscle mass in the lower extremities with ageing is most significant to mobility status, and the cross-sectional area of the vastus lateralis (quadriceps) muscle decreases by up to 40% between the ages of 20 and 80 years.16 Decreases in muscle function have been thought to be largely due to parallel changes in muscle mass.17 However, it has become clear that decreases in muscle strength exceed what is expected on the basis of the decline in muscle mass during ageing,18 especially after the age of 60–70 years.19 The progressive mismatch between mass and strength probably occurs because of a deterioration of muscle quality.20 Results of studies have also suggested that muscle strength might be more important than muscle mass as a determinant of functional limitations and mobility status in older age.13
Many factors contributing to age-related loss of muscle mass and strength have been suggested, with physical inactivity probably being the most important.3 A disruption could occur in several positive regulators (eg, the interlinked protein kinase B [Akt] and mammalian target of rapamycin [mTOR] pathways) of muscle hypertrophy.21 However, the true mechanisms are unclear and probably include primary muscle factors such as mitochondrial dysfunction, oxidative stress, a pro-inflammatory state, or metabolic inefficiencies; non-muscle factors such as loss of motor neurones, alteration of the neuromuscular plaque, or imbalance between denervation and reinnervation; and hormonal changes (eg, insulin, testosterone, oestrogen, GH, insulin-like growth factor 1 [IGF-1], vitamin D, parathyroid hormone).17,21
As a result of skeletal muscle loss, the basal metabolic rate decreases by about 30% between the ages of 20 and 70 years.22 Lower energy expenditure with ageing is due to not only decreased basal metabolic rate but also probable decreased intensity and duration of physical activity, and decreased postprandial energy expenditure due to decreased fat oxidation. However, caloric intake does not necessarily decrease over the lifespan.23 Instead, inadequate dietary protein during even a short period can result in loss of muscle mass even in the setting of adequate energy intake, especially in the presence of a pro-inflammatory state.24
At the cell and tissue level, age-associated muscle loss is characterised by preferential type II myofibre atrophy, fibre necrosis and fibre-type grouping, expanded motor units, increased intramyocellular lipids, increased collagen, impaired neurological modulation of contraction, enhanced reactive oxygen species, reduced mitochondrial function and biogenesis, increased mitochondrial apoptosis, and altered satellite cell function.16,25 Intrinsic contractility is also reduced in the intact fibres in older adults.26 An important process that characterises ageing muscle is fat infiltration, which occurs both at a macroscopic level between muscle groups, and at a microscopic level between and inside myocytes. Evidence exists that the amount of intramyocellular lipid deposition is correlated with the percentage fat mass used as a proxy measure of adiposity.27 However, the causal link from adiposity to intramyocellular lipid deposition is unclear, and results of studies have suggested that it might be related to reduced oxidative capacity of mitochondria and stagnation of unused fuel. This theory is consistent with the age-related changes in mitochondrial function and biogenesis that have been consistently described in human beings and rodents.28
To retain their anatomical integrity and function, muscles need continuous repair and maintenance, and some evidence exists that the repair mechanism is dysfunctional in older individuals. For example, in rodent studies, older (aged 19–25 months) compared with younger (aged 3–8 months) mice show impaired muscle regenerative capacity due to reduced satellite cell proliferation and differentiation, and this deficit can be substantially reduced in parabiotic experiments when older animals are exposed, by cross-transfusion of blood, to the circulation of a genetically identical younger animal.29-31 There is also some evidence that defects in repair are related to the tendency of ageing satellite cells to acquire an adipocytic phenotype.
The potential mechanisms described have been identified in the context of ageing, but several lines of evidence suggest that some are also targeted by diseases characterised by accelerated decline of muscle mass and strength with ageing. Understanding of the extent to which age-related and disease-related muscle loss share common mechanisms could help to indicate new potential targets for intervention.
Muscle loss in endocrine diseases
Diabetes
The global prevalence of diabetes is projected to rise exponentially during the next few decades, with the greatest burden in older individuals (aged >65 years).32,33 Up to 70% of adults with diabetes have difficulty carrying out routine physical tasks, with lower extremity mobility limitations particularly evident, and diabetes is a potent risk factor for most geriatric syndromes.34 Although comorbidities—such as cardiovascular disease and obesity—probably contribute to physical disability in diabetes, evidence is emerging that part of the mobility reduction process in older individuals with diabetes is mediated by a direct effect of diabetes on skeletal muscle.34 For example, studies have suggested that impaired muscle function potentially mediates the association of diabetes with impaired gait and low walking speed in older adults (aged 65 and older).35 In both cross-sectional and longitudinal studies, accelerated loss of muscle mass and strength is recorded in individuals with diabetes, is greater with longer diabetes duration or higher HbA1c, and is attenuated by use of insulin sensitisers.36-38 Longer duration of diabetes is also associated with proportionally lower quadriceps strength in older adults (aged 50 and older).39
High fasting and post-challenge concentrations of both glucose and insulin also independently associate with muscle loss in individuals without diabetes, suggesting that dysglycaemia or insulin resistance, or both, could be risk factors for accelerated muscle loss.40 Notably, relatively severe hyperglycaemia and insulin resistance have also been linked to slower walking speed.41
Diabetes and insulin resistance are more common in older than in younger individuals, and are associated with frailty—a geriatric condition of physiological vulnerability to stressors, associated with adverse outcomes such as disability and mortality.42,43 Hyperglycaemia is associated with the development of frailty and incident mobility limitations, potentially mediated by loss of muscle.44
Insulin resistance results in decreased stimulation of protein synthesis pathways and increased activation of protein degradation pathways that might ultimately lead to muscle loss in type 2 diabetes (figure 1). Insulin is a powerful anabolic signal and substantially stimulates muscle protein synthesis in young people but not in older people. The age-related insulin resistance of muscle protein synthesis might be overcome by supraphysiological insulin concentrations.45
Figure 1. Pathways of accelerated muscle loss in type 2 diabetes.
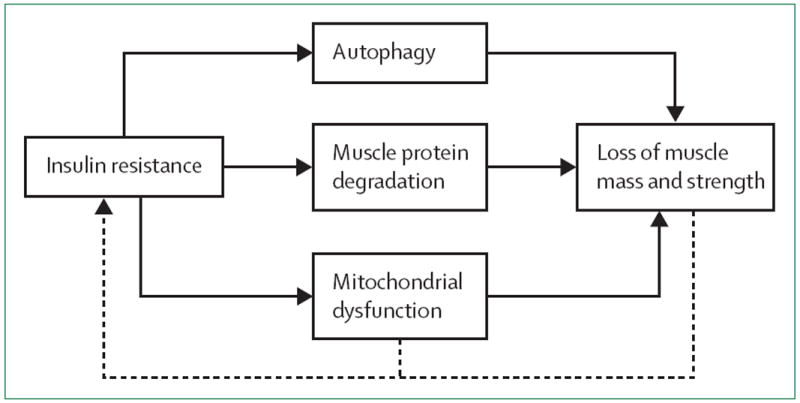
The presence of insulin resistance in type 2 diabetes leads to autophagy, muscle protein degradation (ie, via the ubiquitin-proteasome proteolytic pathway), and mitochondrial dysfunction (solid line). These processes ultimately lead to loss of muscle mass or muscle strength, or both. A cycle is created in which loss of muscle mass and muscle strength lead to decreased surface area for glucose transport and potential exacerbation of insulin resistance (dotted line). The progression of mitochondrial dysfunction might also worsen insulin resistance (dotted line). Increasing severity of insulin resistance then stimulates pathways resulting in accelerated loss of muscle, and the cycle starts again.44-51
The physiological intracellular insulin signalling cascade activates the mTOR pathway and inhibits autophagy, including lysosomal degradation of proteins and organelles. These effects of insulin are dysfunctional in the presence of insulin resistance and might contribute to accelerated muscle loss in diabetes.46 The balance between muscle hypertrophy and atrophy is also altered in diabetes. In insulin resistance, insulin or IGF-1 signalling is suppressed, leading to downregulation of the phosphatidylinositol 3 kinase/Akt pathway and decreased protein synthesis, as well as to forkhead box protein O1 phosphorylation. Phosphorylated forkhead box protein O1 stimulates expression of the E3 enzymes atrogin-1 and muscle ring finger-1 via increased activation of the ubiquitin-proteasome proteolytic pathway. Increased expression of these E3 enzymes in insulin resistant individuals contributes to muscle protein degradation, a mechanism not shared by age-related sarcopenia.47 Myofibre size in skeletal muscle is also reduced in people with type 2 diabetes.53
Skeletal muscle, mitochondrial function, and bio-energetic capacity could also be impaired in diabetes. Some studies showed that muscle mitochondria are smaller and have less defined internal membranes (with presence of vacuoles) in patients with obesity or type 2 diabetes versus in patients of healthy weight. Low mitochondrial size correlates with low glucose disposal rate and insulin sensitivity.48,49 However, some studies did not detect any significant effect of diabetes on muscle mitochondria.54 Thiazolidinediones are drugs that improve insulin sensitivity but also suppress proteolysis pathways and stimulate mitochondrial biogenesis,47 in part via induction of peroxisome proliferator-activated receptor gamma coactivator 1 α (PGC-1α). PGC-1α is a transcriptional coactivator that has reduced gene expression in muscles of patients with type 2 diabetes,55 and might have a role in preventing muscle atrophy.53,56
Diabetes is also characterised by reduced mitochondrial electron transport chain activity,50 which results in energetic inefficiency. Whether muscle mitochondrial dysfunction in type 2 diabetes is the primary cause of insulin resistance or vice versa is unclear.51 Nonetheless, skeletal muscle ATP production increases in response to exogenous insulin in people without diabetes, but this increment is reduced in individuals with diabetes and is related to impaired insulin response.52 In-vivo mitochondrial function (measured with phosphorus-31 magnetic resonance spectroscopy) is also lower in muscle of patients with type 2 diabetes compared with age-matched and BMI-matched controls.57 Many of the changes in skeletal muscle mitochondrial function recorded in individuals with diabetes are similar to those also noted in ageing.16
Obesity
Obesity is defined as abnormal or extensive fat accumulation that negatively affects health. The BMI cut-points used to define obesity were derived from results of studies that explored the relationship between BMI and mortality and detected a steep increase in all-cause mortality at BMIs higher than 30 kg/m2. However, loss of height and lean body mass and increasing fat mass that occur with ageing uncouple the relationship of BMI and obesity, and attenuate associations with mortality. Loss of height results in a higher BMI, or overestimation of fatness, whereas a decrease in lean body mass underestimates fatness.58 Whether these criteria for obesity are appropriate for older individuals, though, is unclear,59 with some authors suggesting that increased BMI (ie, in the overweight range) might not necessarily be associated with an increased risk of mortality in elderly people.60 In healthy young and older individuals, bone and muscle tend to be correlated with bodyweight, probably because gravity and inertial forces during movement stimulate mechano-receptors in both bone and muscle that modulate the production of growth factors.61 However, results of studies of body composition have shown that this adaptive mechanism might be impaired in older individuals who are obese. As a result, obese individuals might have relatively low muscle strength in view of their body size and have an increased risk of disability.62 Fat infiltration of muscle (both intramuscular and intermuscular) is further related to poor lower extremity physical performance.63 There might also be cross-talk between muscle and fat where contracting skeletal muscles release myokines that exert endocrine effects on visceral fat.64
Age-related loss of muscle mass is typically offset by gains in fat mass. As a result, bodyweight in men and women might be quite stable in middle age, or slightly increased even though the relative amount of body fat actually increases in comparison with lean tissue—an important step in the development of sarcopenic obesity.65 After the age of 70 years, fat-free mass and fat mass tend to decrease in parallel.21
Other criteria for sarcopenic obesity have been put forward. Baumgartner and colleagues66 first described sarcopenic obesity as a skeletal mass index that was less than two standard deviations below the sex-specific reference for a young, healthy population, with a percentage of body fat greater than 27% in men and 38% in women (roughly a BMI of 27 kg/m2). An alternative description by Davison and colleagues67 included criteria of body fat in the upper two quintiles and muscle mass in the lower two quintiles using bioelectric impedance. Other criteria for sarcopenic obesity have also been put forward,68 but are so heterogeneous that a systematic literature review that compared eight different definitions projected prevalences of sarcopenic obesity in older US adults ranging from 4·4–84% in men and 3·6–94% in women.69 Generally, irrespective of definition, the prevalence increased with each decade of age and was lower in non-Hispanic black people than in white people. The absence of a standardised definition for sarcopenic obesity and different body composition indices and proposed cut-offs represents a major clinical and research limitation.
Sarcopenia and obesity can co-occur, and synergistically are associated with worse functional decline and outcomes than is either condition alone.70 Findings from few studies suggest that obesity alone might contribute more to lower physical function than does sarcopenia alone, but this probably depends on the degree of muscle loss.71 Baumgartner and colleagues66 showed that both men and women older than 60 years with sarcopenic obesity had a significantly increased risk of having three or more physical disabilities compared with non-obese individuals after adjustment for age, and this association was stronger than with either sarcopenia or obesity alone.66 Results of further studies showed that sarcopenic obesity might predict onset of instrumental activities of daily disability in older adults72—an algorithm to screen for sarcopenic obesity clinically has been proposed.58
Many of the proposed mechanisms for sarcopenic obesity overlap with those proposed for age-related sarcopenia.58 Net contractile mass might be smaller than estimated because of myosteatosis, namely infiltration of skeletal muscle with fat and connective tissue. Abnormal protein synthesis rates and anabolic resistance to exercise are especially evident in sarcopenic obesity.73 It has been argued that, in the presence of obesity, muscles function in the higher range of their capacity spectrum, which might be less energy efficient and, in the long run, lead to accumulated damage.
The development of sarcopenic obesity might be related to several processes (figure 2). In obesity, senescent, pro-inflammatory cells in fat might contribute to development of sarcopenia.74 The increased presence of enhanced reactive oxygen species and chronic inflammation related to an increased fatty-acid load could also result in mitochondrial damage in skeletal muscle.75 A high-fat diet might result in reduced expression of PGC-1α (a driver of mitochondrial biogenesis), and of genes necessary for mitochondrial oxidative phosphorylation, establishment of oxidative myofibres, and vascularisation.76 However, further research is needed to better understand the mechanisms underlying sarcopenic obesity.
Figure 2. Development of sarcopenic obesity.
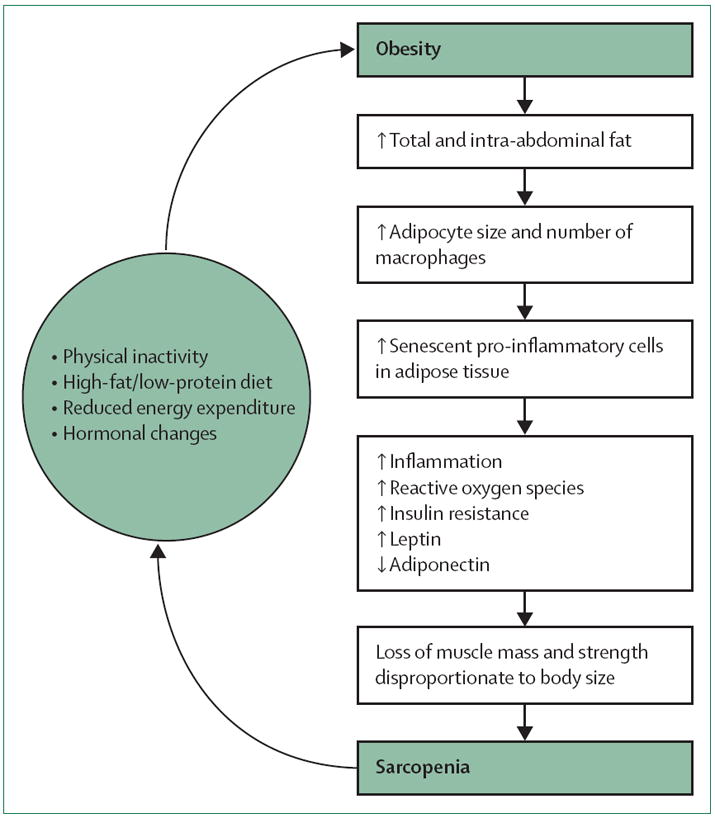
Obesity triggers a cascade of events including increased adipocyte size and number of macrophages, more pro-inflammatory senescent cells in adipose tissue, increased inflammatory markers, reactive oxygen species, insulin resistance, and leptin along with lowered adiponectin, and, ultimately, loss of muscle mass and strength disproportionate to relatively greater body size. The skeletal muscle, or engine, is insufficient to transport the obese individual and leads to the development of sarcopenia. Sarcopenia, in turn, is associated with physical inactivity, reduced energy expenditure, and possibly other processes that increase obesity. Either sarcopenia or obesity could be the initial step in the development of sarcopenic obesity, creating a vicious cycle.57,71-74
Hypogonadism in men
Several hormones are dysregulated with ageing, and androgen effects on body composition including muscle mass are well documented.77 In men, total testosterone concentrations decrease by 1% per year and bioavailable testosterone by 2% per year from the age of 30 years. Dihydroepiandrosterone sulphate is a precursor to testosterone, with concentrations that also decrease with ageing—in men aged 70–80 years, dihydroepiandrosterone sulphate concentrations are only about 20% of their peak concentrations at the age of 20 years.78
One disease-related model of hypogonadism in older men is the use of androgen-deprivation therapy for prostate cancer. After 6 months of treatment, androgen-deprived older men have decreased appendicular skeletal muscle, decreased lean tissue, and increased body fat compared with untreated controls.79 Results of randomised trials have also shown that supraphysiological doses of testosterone in men result in increased fat-free mass, muscle size, and strength.80
Findings from studies have shown an association of low free testosterone concentrations with limited mobility in older men.81 Results of a randomised controlled trial of older men with mobility limitations and hypogonadism showed improvements in muscle strength and stair-climbing power in the androgen-treated group.82 However, not all studies have had positive outcomes on functional status with testosterone treatment.83 Additionally, testosterone replacement is not without potential risks, including increased respiratory disorders (eg, cough, shortness of breath, asthma or chronic obstructive pulmonary disease (COPD) exacerbation, sleep apnoea) and skin and subcutaneous tissue disorders (eg, application site reactions, itching, erythema, foot ulcers, and increased hair growth).84 Perhaps most importantly, adverse cardiovascular outcomes with testosterone replacement have also been shown, but further study is needed in this area.84
Testosterone increases muscle protein synthesis, probably through increased use of intracellular aminoacids in skeletal muscle. Additionally, enhanced expression of androgen receptors occurs in response to supraphysiological amounts of testosterone, which might also contribute to increased muscle hypertrophy.85 Another possible pathway might involve augmentation of the GH axis, creating an anabolic state.86 Dual therapy with testosterone and GH in older men who have normal testosterone concentrations results in improvements in lean body mass, compared with placebo or either treatment alone.87 However, despite significant increases in lean mass after treatment with testosterone and GH, tests of muscle performance do not necessarily show substantial improvements after 8 weeks.88 Hypogonadal states are also associated with increased truncal obesity, which might contribute to high concentrations of cytokines that further contribute to sarcopenia.89 Overall, although the potential benefits of androgen therapy in sarcopenia have not been fully explored, there is no definitive evidence that androgen stimulation in men increases functional status.
Growth hormone deficiency
GH is a pituitary hormone that regulates development and coordinates the postnatal growth of several target tissues, including skeletal muscle. GH secretion occurs in a pulsatile manner, with a major surge at the onset of slow-wave sleep, and a lesser surge a few hours after meals. The secretion of GH is highest at puberty. It has anabolic and lipolytic effects mediated by IGF-1, which is predominantly produced in the liver.90 After the age of 30 years, GH secretion steadily declines at a rate of about 1% per year.91,92 Increased adiposity and circulating concentrations of free fatty acids inhibit GH production and decrease plasma concentrations of IGF-1. This decrease in IGF-1 is associated with decreased muscle size and strength, decreased protein synthesis, and increased cell death,93 which in turn leads to increased visceral fat and decreased lean body mass.94
GH treatment in adults with hypopituitarism and associated GH deficiency can improve body composition and increase lean body mass.95 A systematic review noted that individuals treated with GH had a significant decrease in fat mass (−2·08 kg, 95% CI −1·35 to −2·80 kg) and a significant increase in lean body mass (+2·13 kg, 95% CI 1·32 to 2·94 kg). However, no overall change was noted in bodyweight comparing treated participants with those taking placebo or doing exercise. Participants who received GH therapy were also more likely to have side-effects including soft tissue oedema, arthralgias, carpal tunnel syndrome, and gynaecomastia, and to develop fasting hyperglycaemia or diabetes,96 which limits its use in the clinical setting.
Hyperthyroidism
Hyperthyroidism can also be associated with muscle loss and decreased physical function. Hyperthyroidism is associated with increased muscle protein breakdown and resulting increased aminoacid release.97 Muscle mass can be reduced by up to 20% and muscle strength by 40% in patients with severe thyrotoxicosis. With normalisation of thyroid concentrations, muscle mass and strength are re-established, but this can take up to 9 months to occur.98
Hypercortisolism
Hypercortisolism can present clinically with proximal muscle weakness due to endogenous glucocorticoid excess. Glucocorticoids inhibit protein synthesis and stimulate protein degradation in skeletal muscles. IGF-1 is decreased and myostatin is increased.99 Together, these changes result in protein catabolism, muscle atrophy, and, ultimately, weakness. More than half of patients with Cushing’s syndrome (from exogenous or endogenous glucocorticoid excess) can develop muscle weakness.100 Treatment for Cushing’s syndrome that lowers cortisol concentrations might lead to improvement of muscle function.
Vitamin D deficiency
Strong observational evidence exists that vitamin D deficiency causes muscle weakness with muscle fibre atrophy, impairment of muscle quality, and increased intramuscular fat.101 Older adults are more vulnerable to vitamin D deficiency than are younger adults. Vitamin D supplementation might also have wide-ranging beneficial effects in reducing falls and improving muscle strength, but evidence is inconclusive; large randomised controlled trials are addressing this hypothesis.102
Osteoporosis
Osteoporosis is also associated with the loss of muscle function and mobility limitations, although the direction of this association is unclear.103 Muscle biopsy specimens from patients with osteoporosis show atrophy of type II muscle fibres. The degree of fibre atrophy is proportional to the degree of bone mineral density loss.104
Muscle loss in other disease states
Muscle loss might also occur in other disease states, including rheumatoid arthritis, peripheral arterial disease, COPD, congestive heart failure, advanced kidney disease, cirrhosis, cancer, and HIV, which we describe briefly here; however, other disease states might also be associated with muscle loss. Cachexia is a separate condition in which the prominent clinical feature is weight loss, and muscle wasting is associated with severe illness that occurs much more rapidly than does age-related or disease-related muscle loss.105
Muscle mass and strength are commonly decreased in patients with rheumatoid arthritis. Several factors are likely to contribute to muscle loss, including the presence of pro-inflammatory cytokines in rheumatoid arthritis. Additionally, reduced protein synthesis in myocytes, physical activity limitations, insulin resistance, and inadequate protein ingestion could occur.106
Peripheral arterial disease can also be associated with reduced muscle power and poor physical function.107 The severity of peripheral arterial disease might correlate with the degree of both muscle strength and muscle mass.108 Repeated episodes of ischaemia-reperfusion might be a common pathway associated with muscle loss in many medical conditions—including peripheral arterial disease, congestive heart failure, and COPD—that cause damage necessitating frequent regeneration, and exhaust the repairing capacity of resident satellite cells. Chronic or intermittent hypoxaemia could also affect mitochondrial function in a similar way to age-related muscle loss, leading to oxidative stress, reduced energy availability, and decreased protein synthesis. Hypoxia and persistent damage can also induce a pro-inflammatory state that leads to muscle loss.109
Uraemia in chronic kidney disease also leads to accelerated muscle protein breakdown, and involves mechanisms similar to other catabolic conditions such as cancer cachexia, starvation, insulin deficiency, and sepsis via the ubiquitin-proteasome proteolytic pathway.110 Notably, expression of the ubiquitin ligases atrogin-1 and MuRF-1 in muscle increases dramatically in catabolic states. Atrogin-1 and MuRF-1 could serve as a biomarker for rates of proteolysis and muscle loss.111 Vitamin D supplementation in patients who are deficient might also improve muscle strength and functional status in those with chronic kidney disease.112
In patients with advanced liver cirrhosis, skeletal muscle protein synthesis is also reduced, and muscle protein breakdown is increased, leading to reduced muscle mass. Poor nutrition, hormonal and metabolic abnormalities, and inflammatory factors could also contribute to muscle loss in cirrhosis.113 Patients with cirrhosis also have significantly reduced exercise capacity and muscle strength that might adversely affect clinical outcomes.114
Cancer could result in muscle loss that develops slowly compared with critical illness. Tumour-induced inflammation can lead to increased production of tumour necrosis factor alpha, interleukin 6, interferon gamma, and other cytokines, which results in increased protein catabolism, decreased protein anabolism, insulin resistance, and lipolysis.115 Although decreased caloric intake and anorexia associated with cancer might contribute to muscle loss, this is not the only factor; aggressive caloric supplementation alone cannot reverse the skeletal muscle loss.116 Both aerobic and resistance exercise might improve upper and lower body muscle strength in patients with cancer.117
HIV is also associated with increased circulating concentrations of pro-inflammatory cytokines that can alter protein balance similarly to cancer.109 Additionally, individuals with HIV can have reduced testosterone concentrations.118 Patients with HIV have lower muscle strength than do healthy individuals; however, resistance exercise can safely increase the strength of older patients with HIV to equal that of counterparts.119 Oxandrolone is an anabolic steroid treatment that has been used to treat muscle loss in diseases such as HIV, and also in patients with other catabolic disorders such as neuromuscular diseases, or in individuals who have had severe trauma, burn injury, or infections, with notable improvements in muscle function and faster recovery.120
Treatments for age-related and disease-related muscle loss
Exercise
Ageing and chronic disease have many common mechanisms that could contribute, in an additive or synergistic way, to the development of muscle loss (figure 3). Consequently, exercise and dietary interventions have been explored in both ageing and diseases such as diabetes and obesity to preserve muscle mass and strength.
Figure 3. Potential mechanisms of age-related and disease-related muscle loss.
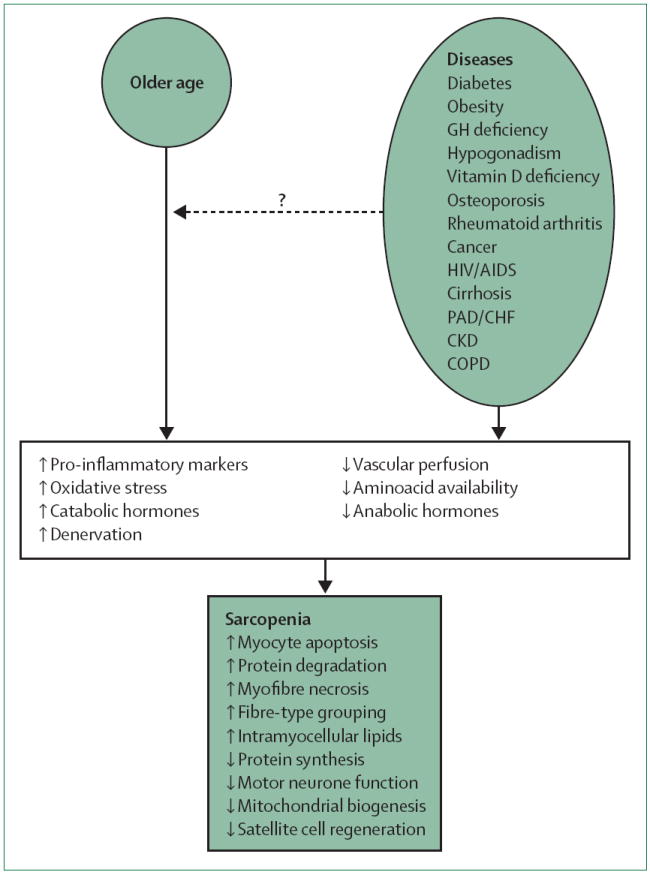
An almost-unexplored hypothesis is that chronic diseases, many of which increase in prevalence with age, contribute to the age-related decrease in muscle mass and strength observed in many older individuals (dashed arrow). The presence of either old age or a specific disease, or both, has been linked with increasing pro-inflammatory cytokines, oxidative stress, catabolic hormones, and denervation; and decreasing vascular perfusion, aminoacid bioavailability, and anabolic hormones. These mechanisms could affect skeletal muscle characteristics that have been associated with sarcopenia, including increased myocyte apoptosis, protein degradation, myofibre necrosis, fibre-type grouping, and intramyocellular lipids; or decreased protein synthesis, motor neurone function, mitochondrial biogenesis, and satellite cell regeneration. GH=growth hormone. PAD=peripheral arterial disease. CHF=congestive heart failure. CKD=chronic kidney disease. COPD=chronic obstructive pulmonary disease.
Resistance exercise stimulates protein synthesis and results in increased muscle mass and strength.121 The increase in muscle strength occurs almost immediately, after a few days of exercise, and much before a noticeable increase in muscle mass, suggesting that the primary effect of exercise might not be on hypertrophy but rather on muscle quality. The combination of progressive resistance training and aerobic exercise results in maximum benefits to weight loss, skeletal muscle mass and strength gain, and improvements in insulin resistance in trials of older individuals aged 60–80 years with obesity.122 The highest muscle gains of up to about 1 kg after 6 months were observed in the resistance exercise group.122 Resistance exercise is effective and safe to prevent muscle loss even in old (mean age 87 years) and frail individuals, potentially by also decreasing skeletal muscle apoptosis and improving mitochondrial function.123 Methodological considerations for the design of future clinical trials targeting mitochondrial dysfunction to treat sarcopenia have been proposed.124
Villareal and colleagues125 showed that 6 months of weekly behavioural therapy for weight loss (of no more than 10%) combined with exercise three times per week (each session lasting 90 min) can improve function and frailty in older obese individuals (aged >65 years). Further, resistance training can improve body composition independent of weight loss. In a hallmark study, Fiatarone and colleagues123 showed that an 8 week resistance training programme could significantly increase muscle mass in frail men and women (mean age 87 years) living in nursing home care.
Additionally, increases in muscle mass are accompanied by increased resting metabolic rate, and induce positive changes in muscle fibres. In as little as 12 weeks, resistance training for 2 or 3 days a week can lead to muscle hypertrophy.126 Resistance training increases muscle fibre size to similar degrees in old and young untrained adults.127 In frail adults older than 85 years, 3 months of resistance training selectively increased type II fibres, which are preferentially lost with ageing.128
In parallel, increased contractile activity promotes oxidative fibre type transformation, PGC-1α expression, mitochondrial biogenesis, and muscle protein synthesis, and could lead to reduced inflammation and oxidative stress.56 Satellite cells in muscle might also be activated by exercise.129
Additionally, findings from several studies have shown the benefits of exercise (aerobic and resistance) for improvement of glucose metabolism and insulin sensitivity in people with type 2 diabetes.130 Further, in overweight and obese individuals with diabetes, an energy-restricted high-protein diet combined with resistance training achieved greater weight loss and more favourable changes in body composition than did either intervention alone after 16 weeks.131 After exercise, the ability of insulin to stimulate GLUT4-mediated glucose transport is substantially improved.132 Exercise might also stimulate translocation of GLUT4 in skeletal muscle through a mechanism distinct from insulin,133 perhaps through direct effects on the insulin-signalling cascade.
Diet
Contrary to the clear beneficial effects of exercise on muscle health, whether changes in diet are effective to prevent sarcopenia has not been definitively shown in adequately sized randomised controlled trials. The timing of protein supplementation either before or immediately after exercise could have benefits on muscle protein synthesis compared with delayed protein ingestion, probably indicating greater delivery of aminoacids to actively exercising muscle.134 Even without the stimulus of resistance exercise, a high-protein diet might also promote muscle anabolism. In preliminary studies of older men and women with sarcopenia, oral supplementation with 16 g per day of essential aminoacids was associated with increased lean mass at 6 months and a further increase at 18 months, along with improved insulin sensitivity.135
Dietary treatments for sarcopenic obesity might include a smaller energy deficit (200–750 kcal reduction) than usual weight-loss programmes. Moderate weight loss of about 5% in older women was found to improve insulin resistance, fat distribution, and muscle lipid infiltration, and preserve thigh muscle mass, with only a slight decrease in appendicular lean mass.136 Some authors have proposed that, to counter the effects of sarcopenic obesity, healthy older adults with adequate renal function should have a dietary protein intake that reaches or even exceeds the recommended daily allowance to prevent muscle protein catabolism during weight loss.137 Increased protein intake might maintain muscle mass during calorie-restricted diets to a greater extent than does usual protein intake. Ageing does not necessarily impair the anabolic response to a protein-rich meal.138 High-quality protein sources such as lean meat, fish, non-fat dairy products, and soy might be preferable.23 However, effects of protein supplementation, especially at high levels, need to be better investigated over the long term. Supplementation with high-protein meal replacements, or specifically with essential or branched-chain aminoacids, or both, might be beneficial but needs further study. Conversely, low protein intake has been associated with decreased muscle strength in individuals with high concentrations of inflammatory markers.24
Conclusions and future directions
Despite the progress made in characterisation of sarcopenia and its complications, there is no cure for age-related or disease-related muscle loss. Clearly exercise helps to maintain muscle mass and strength, but probably does not affect the biological process that ultimately leads to sarcopenia. Thus, the most effective strategy to treat these conditions is an open question. Novel therapies are being investigated. Several ongoing clinical trials might offer evidence for potential therapies to prevent or reverse loss of muscle mass and strength in the future. The lifestyle interventions and independence for elders (LIFE) study139 is a phase 3 multicentre randomised controlled trial designed to give further evidence about whether lifestyle modification interventions are effective and practical for preventing major mobility disability in older adults, and will provide key findings. Results of clinical trials investigating use of anti-inflammatory therapies for prevention of other outcomes such as arterial stiffening, and that also assess changes in muscle mass and strength as secondary outcomes, can provide important insights. Further studies investigating the effects of testosterone therapy with or without exercise on muscle function are almost complete. Trials investigating the effects of vitamin D on muscle fibre type and other parameters of muscle function and physical performance are underway and proposed. However, these are only a few examples, and many other promising studies investigating novel therapies to prevent loss of muscle mass and strength are ongoing.
Search strategy and selection criteria.
We searched Medline and PubMed using the search terms “sarcopenia”, “muscle loss”, “muscle atrophy”, “muscle wasting”, “muscle mass”, and “muscle strength” in combination with the terms “diabetes”, “obesity”, “androgen deficiency”, “hypogonadism”, “growth hormone deficiency”, “IGF-1”, “hyperthyroidism”, “Cushing’s”, “osteoporosis”, “vitamin D”, “rheumatoid arthritis”, “peripheral arterial disease”, “congestive heart failure”, “COPD”, “HIV”, “cancer”, “liver cirrhosis”, or “chronic kidney disease” on Nov 19, 2013, for articles in any language. We did not limit by date but largely selected publications from the past 10 years, and did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we deemed relevant. Review articles and book chapters are cited to provide readers with more details and more references than this Review has room for. Our reference list was modified on the basis of comments from peer reviewers.
Acknowledgments
The authors are supported by the US National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK093583, T32-DK062707), the Johns Hopkins Older Americans Independence Center (P30-AG021334), and the intramural research programme of the US National Institute on Aging.
Footnotes
Contributors
RRK contributed to the structure of the Review, selection of references, and preparation of the Review and figures. MC contributed to the selection of references and preparation of the Review. LF contributed to the structure of the Review, selection of references, and preparation of the Review and figures.
Declaration of interests
We declare tbhat we have no competing interests.
Contributor Information
Rita Rastogi Kalyani, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, The Johns Hopkins University, Baltimore, MD, USA.
Mark Corriere, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, The Johns Hopkins University, Baltimore, MD, USA.
Luigi Ferrucci, Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA.
References
- 1.Candow DG, Chilibeck PD. Diff erences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A Biol Sci Med Sci. 2005;60:148–56. doi: 10.1093/gerona/60.2.148. [DOI] [PubMed] [Google Scholar]
- 2.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(suppl):990–91. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 4.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–99. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Kupelian V, Visser M, et al. Health ABC Study Investigators. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–09. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on defi nition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus defi nition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpato S, Bianchi L, Cherubini A, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP defi nition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/Gerona/glt149. published online Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infi ltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 15.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 16.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 17.Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36:174–82. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 19.Baum K, Hildebrandt U, Edel K, et al. Comparison of skeletal muscle strength between cardiac patients and age-matched healthy controls. Int J Med Sci. 2009;6:184–91. doi: 10.7150/ijms.6.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore AZ, Caturegli G, Metter EJ, et al. Diff erence in Muscle Quality over the Adult Life Span and Biological Correlates in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014 doi: 10.1111/jgs.12653. published online Jan 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013 doi: 10.1155/2013/204164. 204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau D, Cho LM, Jani P, St Jeor ST. Individualizing recommendations for weight management in the elderly. Curr Opin Clin Nutr Metab Care. 2008;11:27–31. doi: 10.1097/MCO.0b013e3282f31744. [DOI] [PubMed] [Google Scholar]
- 23.Benton MJ, Whyte MD, Dyal BW. Sarcopenic obesity: strategies for management. Am J Nurs. 2011;111:38–44. doi: 10.1097/01.NAJ.0000408184.21770.98. quiz 45–46. [DOI] [PubMed] [Google Scholar]
- 24.Bartali B, Frongillo EA, Stipanuk MH, et al. Protein intake and muscle strength in older persons: does infl ammation matter? J Am Geriatr Soc. 2012;60:480–84. doi: 10.1111/j.1532-5415.2011.03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson L. Morphological and functional characteristics of the ageing skeletal muscle in man. A cross-sectional study. Acta Physiol Scand Suppl. 1978;457:1–36. [PubMed] [Google Scholar]
- 26.Larsson L, Li X, Frontera WR. Eff ects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272:C638–49. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 27.Dubé J, Goodpaster BH. Assessment of intramuscular triglycerides: contribution to metabolic abnormalities. Curr Opin Clin Nutr Metab Care. 2006;9:553–59. doi: 10.1097/01.mco.0000241664.38385.12. [DOI] [PubMed] [Google Scholar]
- 28.Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 2010;65:119–28. doi: 10.1093/gerona/glp179. [DOI] [PubMed] [Google Scholar]
- 29.Verdijk LB, Gleeson BG, Jonkers RA, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64:332–39. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–43. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–64. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 32.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–94. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care. 2010;33:1055–60. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpato S, Bianchi L, Lauretani F, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–79. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SW, Goodpaster BH, Lee JS, et al. Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–97. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SW, Goodpaster BH, Strotmeyer ES, et al. Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–12. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 38.Lee CG, Boyko EJ, Barrett-Connor E, et al. Osteoporotic Fractures in Men (MrOS) Study Research Group. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–86. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999–2002. J Am Geriatr Soc. 2013;61:769–75. doi: 10.1111/jgs.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barzilay JI, Cotsonis GA, Walston J, et al. Health ABC Study. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged >or=70 years. Diabetes Care. 2009;32:736–38. doi: 10.2337/dc08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo CK, Lin LY, Yu YH, Wu KH, Kuo HK. Inverse association between insulin resistance and gait speed in nondiabetic older men: results from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2002. BMC Geriatr. 2009;9:49. doi: 10.1186/1471-2318-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–66. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 43.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 44.Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc. 2012;60:1701–07. doi: 10.1111/j.1532-5415.2012.04099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia. 2009;52:1889–98. doi: 10.1007/s00125-009-1430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaushik S, Singh R, Cuervo AM. Autophagic pathways and metabolic stress. Diabetes Obes Metab. 2010;12(suppl 2):4–14. doi: 10.1111/j.1463-1326.2010.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–68. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 48.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 49.Jerusalem F, Engel AG, Peterson HA. Human muscle fiber fine structure: morphometric data on controls. Neurology. 1975;25:127–34. doi: 10.1212/wnl.25.2.127. [DOI] [PubMed] [Google Scholar]
- 50.Mogensen M, Sahlin K, Fernström M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–99. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 51.Phielix E, Mensink M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav. 2008;94:252–58. doi: 10.1016/j.physbeh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Eff ect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–65. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–96. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its infl uences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299:E145–61. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–20. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 58.Waters DL, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27:401–21. doi: 10.1016/j.cger.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Ritz P. Editorial: Obesity in the elderly: should we be using new diagnostic criteria? J Nutr Health Aging. 2009;13:168–69. doi: 10.1007/s12603-009-0052-7. [DOI] [PubMed] [Google Scholar]
- 60.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 61.Russo CR, Ricca M, Ferrucci L. True osteoporosis and frailty-related osteopenia: two diff erent clinical entities. J Am Geriatr Soc. 2000;48:1738–39. doi: 10.1111/j.1532-5415.2000.tb03895.x. [DOI] [PubMed] [Google Scholar]
- 62.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298:2020–27. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 63.Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res. 2012;2012 doi: 10.1155/2012/172957. 172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle-fat cross talk. J Physiol. 2009;587:5559–68. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–78. doi: 10.1093/ajcn/82.4.872. quiz 915–16. [DOI] [PubMed] [Google Scholar]
- 66.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 67.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–09. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 68.Kohara K. Sarcopenic obesity in aging population: current status and future directions for research. Endocrine. 2014;45:15–25. doi: 10.1007/s12020-013-9992-0. [DOI] [PubMed] [Google Scholar]
- 69.Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with diff erent research defi nitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61:974–80. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 70.Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes (Lond) 2009;33:635–44. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal Study. Obesity (Silver Spring) 2009;17:2082–88. doi: 10.1038/oby.2009.109. [DOI] [PubMed] [Google Scholar]
- 72.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 73.Nilsson MI, Dobson JP, Greene NP, et al. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J. 2013;27:3905–16. doi: 10.1096/fj.12-224006. [DOI] [PubMed] [Google Scholar]
- 74.Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–84. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–81. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–33. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 77.Maggio M, Ceda GP, Lauretani F, et al. Gonadal status and physical performance in older men. Aging Male. 2011;14:42–47. doi: 10.3109/13685538.2010.518179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 79.Boxer RS, Kenny AM, Dowsett R, Taxel P. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male. 2005;8:207–12. doi: 10.1080/13685530500361226. [DOI] [PubMed] [Google Scholar]
- 80.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 81.Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95:2790–99. doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–99. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 84.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kadi F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol. 2008;154:522–28. doi: 10.1038/bjp.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bondanelli M, Ambrosio MR, Margutti A, Franceschetti P, Zatelli MC, degli Uberti EC. Activation of the somatotropic axis by testosterone in adult men: evidence for a role of hypothalamic growth hormone-releasing hormone. Neuroendocrinology. 2003;77:380–87. doi: 10.1159/000071310. [DOI] [PubMed] [Google Scholar]
- 87.Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–84. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 88.Schroeder ET, He J, Yarasheski KE, et al. Value of measuring muscle performance to assess changes in lean mass with testosterone and growth hormone supplementation. Eur J Appl Physiol. 2012;112:1123–31. doi: 10.1007/s00421-011-2077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mudali S, Dobs AS. Effects of testosterone on body composition of the aging male. Mech Ageing Dev. 2004;125:297–304. doi: 10.1016/j.mad.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–80. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 91.McIntire KL, Hoffman AR. The endocrine system and sarcopenia: potential therapeutic benefits. Curr Aging Sci. 2011;4:298–305. doi: 10.2174/1874609811104030298. [DOI] [PubMed] [Google Scholar]
- 92.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–28. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 93.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–10. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 94.Nass R, Thorner MO. Impact of the GH-cortisol ratio on the age-dependent changes in body composition. Growth Horm IGF Res. 2002;12:147–61. doi: 10.1016/s1096-6374(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 95.Salomon F, Cuneo RC, Hesp R, Sönksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med. 1989;321:1797–803. doi: 10.1056/NEJM198912283212605. [DOI] [PubMed] [Google Scholar]
- 96.Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–15. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 97.Riis AL, Jørgensen JO, Gjedde S, et al. Whole body and forearm substrate metabolism in hyperthyroidism: evidence of increased basal muscle protein breakdown. Am J Physiol Endocrinol Metab. 2005;288:E1067–73. doi: 10.1152/ajpendo.00253.2004. [DOI] [PubMed] [Google Scholar]
- 98.Nørrelund H, Hove KY, Brems-Dalgaard E, et al. Muscle mass and function in thyrotoxic patients before and during medical treatment. Clin Endocrinol (Oxf) 1999;51:693–99. doi: 10.1046/j.1365-2265.1999.00861.x. [DOI] [PubMed] [Google Scholar]
- 99.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 100.Gupta A, Gupta Y. Glucocorticoid-induced myopathy: pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013;17:913–16. doi: 10.4103/2230-8210.117215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–94. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 102.Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews DC. Vitamin D treatment for the prevention of falls in older adults: systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1299–310. doi: 10.1111/j.1532-5415.2010.02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferrucci L, Baroni M, Ranchelli A, et al. Interaction between bone and muscle in older persons with mobility limitations. Curr Pharm Des. 2013 doi: 10.2174/13816128113196660690. published online Sept 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terracciano C, Celi M, Lecce D, et al. Differential features of muscle fiber atrophy in osteoporosis and osteoarthritis. Osteoporos Int. 2013;24:1095–100. doi: 10.1007/s00198-012-1990-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rolland Y, Abellan van Kan G, Gillette-Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care. 2011;14:15–21. doi: 10.1097/MCO.0b013e328340c2c2. [DOI] [PubMed] [Google Scholar]
- 106.de Oliveira Nunes Teixeira V, Filippin LI, Viacava PR, de Oliveira PG, Xavier RM. Muscle wasting in collagen-induced arthritis and disuse atrophy. Exp Biol Med (Maywood) 2013;238:1421–30. doi: 10.1177/1535370213505961. [DOI] [PubMed] [Google Scholar]
- 107.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–10. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 108.Abbatecola AM, Chiodini P, Gallo C, et al. Health ABC study. Pulse wave velocity is associated with muscle mass decline: Health ABC study. Age (Dordr) 2012;34:469–78. doi: 10.1007/s11357-011-9238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buford TW, Anton SD, Judge AR, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9:369–83. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–19. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 111.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004;287:E591–601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- 112.Taskapan H, Baysal O, Karahan D, Durmus B, Altay Z, Ulutas O. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin Nephrol. 2011;76:110–16. doi: 10.5414/cn107160. [DOI] [PubMed] [Google Scholar]
- 113.Tessari P. Protein metabolism in liver cirrhosis: from albumin to muscle myofibrils. Curr Opin Clin Nutr Metab Care. 2003;6:79–85. doi: 10.1097/00075197-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 114.Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18:146–51. doi: 10.1002/lt.22472. [DOI] [PubMed] [Google Scholar]
- 115.Dodson S, Baracos VE, Jatoi A, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265–79. doi: 10.1146/annurev-med-061509-131248. [DOI] [PubMed] [Google Scholar]
- 116.Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–59. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 117.Stene GB, Helbostad JL, Balstad TR, Riphagen II, Kaasa S, Oldervoll LM. Effect of physical exercise on muscle mass and strength in cancer patients during treatment—a systematic review. Crit Rev Oncol Hematol. 2013;88:573–93. doi: 10.1016/j.critrevonc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Grinspoon S, Corcoran C, Lee K, et al. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab. 1996;81:4051–58. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- 119.Souza PM, Jacob-Filho W, Santarém JM, Zomignan AA, Burattini MN. Effect of progressive resistance exercise on strength evolution of elderly patients living with HIV compared to healthy controls. Clinics (Sao Paulo) 2011;66:261–66. doi: 10.1590/S1807-59322011000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;214:489–502. doi: 10.1016/j.jamcollsurg.2011.12.038. discussion 502–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cauza E, Strehblow C, Metz-Schimmerl S, et al. Effects of progressive strength training on muscle mass in type 2 diabetes mellitus patients determined by computed tomography. Wien Med Wochenschr. 2009;159:141–47. doi: 10.1007/s10354-009-0641-4. [DOI] [PubMed] [Google Scholar]
- 122.Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169:122–31. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 123.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 124.Marzetti E, Calvani R, Cesari M, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288–301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–66. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 126.Hikida RS, Staron RS, Hagerman FC, et al. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol A Biol Sci Med Sci. 2000;55:B347–54. doi: 10.1093/gerona/55.7.b347. [DOI] [PubMed] [Google Scholar]
- 127.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 2006;101:531–44. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 128.Kryger AI, Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports. 2007;17:422–30. doi: 10.1111/j.1600-0838.2006.00575.x. [DOI] [PubMed] [Google Scholar]
- 129.Thornell LE. Sarcopenic obesity: satellite cells in the aging muscle. Curr Opin Clin Nutr Metab Care. 2011;14:22–27. doi: 10.1097/MCO.0b013e3283412260. [DOI] [PubMed] [Google Scholar]
- 130.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 131.Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care. 2010;33:969–76. doi: 10.2337/dc09-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frøsig C, Richter EA. Improved insulin sensitivity after exercise: focus on insulin signaling. Obesity (Silver Spring) 2009;17(suppl 3):15–20. doi: 10.1038/oby.2009.383. [DOI] [PubMed] [Google Scholar]
- 133.Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci USA. 1995;92:5817–21. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tipton KD, Rasmussen BB, Miller SL, et al. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001;281:E197–206. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- 135.Solerte SB, Gazzaruso C, Bonacasa R, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 136.Mazzali G, Di Francesco V, Zoico E, et al. Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. Am J Clin Nutr. 2006;84:1193–99. doi: 10.1093/ajcn/84.5.1193. [DOI] [PubMed] [Google Scholar]
- 137.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–56. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 139.Marsh AP, Lovato LC, Glynn NW, et al. LIFE Study Research Group. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–58. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]


