Abstract
Acyloxyacyl hydrolase (AOAH) is an unusual but highly conserved lipase, previously described only in myeloid cells, that removes secondary fatty acyl chains from bacterial lipopolysaccharides (LPS) and may also act on various glycero(phospho)lipids. Deacylation by AOAH greatly reduces the ability of LPS to stimulate cells via CD14-MD-2-Toll-like receptor 4. We report here that renal cortical tubule cells produce AOAH and secrete it into urine, where it can deacylate LPS. In vitro studies revealed that proximal tubule cells secrete pro-AOAH, which can be taken up by bladder cells and processed to the heterodimeric, more enzymatically active, mature form of AOAH. AOAH can then be used by the recipient cells to deacylate LPS. The enzyme produced by proximal tubule epithelium may thus be shared with downstream cells. In addition, mature AOAH is found in the urine. We suggest that cortical tubule cells may produce and secrete AOAH to limit inflammatory responses to gram-negative bacteria throughout the urinary tract.
Gram-negative bacterial urinary tract infections (UTIs) afflict millions of people worldwide. Women are more susceptible to UTI than are men. Symptomatic infections arise when bacteria adhere to epithelial cells that line the urethra, bladder and, in some cases, the kidneys. Uropathogenic Escherichia coli, the etiologic agents of nearly 80% of UTIs, are able to survive and multiply within the urinary tract. They must overcome a battery of host defenses that can kill or eliminate bacteria; these include the low pH, rapid flow, and high osmolarity of the urine, as well as urinary constituents such as defensins, secretory immunoglobulin A (IgA), Tamm-Horsfall protein, uromucoid, and urea. Bacterial multiplication may also be limited by exfoliation of the superficial epithelial cells that line the bladder (14, 15).
Another important host defense is the body's inflammatory reaction. Members of the receptor complex that initiates inflammatory responses to gram-negative bacterial LPS (CD14-MD-2-Toll-like receptor 4 [TLR4]) have been detected in uroepithelial cells in the bladder in vivo (1) and in the T24, J82, and 5637 human bladder cell lines (1, 24, 25). Although human renal epithelium does not seem to produce TLR4 (1), murine renal proximal tubule cells possess TLR4, CD14, and MD-2, and they respond to lipopolysaccharide (LPS) by producing chemokines and other mediators (33, 35). Since mice that lack a functional TLR4 protein do not mount a normal mucosal inflammatory response to uropathogenic E. coli and are unable to clear the bacteria (23, 25, 26), LPS recognition seems to be essential for effective host defense in the urinary tract, at least in mice.
Animals thus have sensitive mechanisms for recognizing, and responding to, LPS within the urinary tract. Much less is known about how harmful responses to LPS are prevented. One potential mechanism for modulating host responses to LPS is acyloxyacyl hydrolase (AOAH), a eucaryotic lipase that removes secondary fatty acyl chains (lauroyl, myristoyl, and palmitoyl) that are substituted to the hydroxyl groups of glucosamine-linked 3-hydroxyacyl residues in lipid A, the bioactive center of LPS (7). Such limited deacylation has been shown to attenuate cytokine and chemokine responses to LPS, in keeping with the important role that acyloxyacyl linkages play in lipid A bioactivity (9, 22, 29) and in the ability of gram-negative bacteria to stimulate inflammation (5, 29). For example, the cytokine responses of T24 bladder cells to E. coli invasion were greatly reduced when the infecting strain lacked one of the secondary acyl chains on its lipid A due to a mutation in the msbB (waaN) gene (1).
Prior to the experiments described here, AOAH had been found in myeloid-lineage cells—neutrophils, monocytes/macrophages, and dendritic cells—which can deacylate both purified LPS and the LPS contained in intact gram-negative bacteria (12, 19, 34). We report here the unexpected finding that AOAH is also produced by renal cortical tubule epithelial cells and that these cells secrete the enzyme into the urine, where it can act on LPS. We also present evidence that the proximal tubule-derived enzyme can be taken up by downstream cells within the urinary tract and used by them to deacylate LPS. Our observations raise the possibility that LPS deacylation plays a role in limiting inflammatory reactions to gram-negative bacteria that enter the urinary tract.
MATERIALS AND METHODS
Chemicals.
Unless otherwise indicated, chemicals were purchased from Sigma Chemical Co., St. Louis, Mo.
Mouse strains.
Specific-pathogen-free mice were housed in the UT-Southwestern Animal Resource Center and fed a standard diet. ICR and C57BL/6 (both from Harlan, Indianapolis, Ind.) and 129/S6EvTac (Taconic, Germantown, N.Y.) mice were used. AOAH-null 129 and C57BL/6 mice were produced as described by Lu et al. (11).
Northern analysis.
A mouse multiple tissue northern blot (BD Biosciences Clontech) was probed with a 32P-radiolabeled 1-kb (Asp718 to HindIII) cDNA fragment of the 5′ coding region of mouse AOAH cDNA (GenBank no. AF018172). The blot was stripped and reprobed first with a 350-bp cDNA probe from the 3′ (AhdI to XbaI) noncoding region of mouse AOAH and then with a 500-bp EcoRI-to-EcoRI fragment of the murine β-actin cDNA (kindly provided by I. Shimomura [28]).
AOAH activity assays.
Anesthetized 3-month-old female C57BL/6 mice were sacrificed by cervical dislocation, and individual tissues were excised, rinsed with saline, weighed, and sonicated (Branson Sonifier 450; VWR, West Chester, Pa.) to complete disruption in 500 μl of ice-cold lysis buffer (0.2% Triton X-100 in phosphate-buffered saline [PBS; pH 7.2], with 0.5 μg of aprotinin/ml, 0.5 μg of leupeptin/ml, and 2.5 mM EDTA). The lysates were assayed for AOAH activity at 37°C as previously described (18) by using a double-labeled (3H-acyl chains/14C-glucosamine) Salmonella enterica serovar Typhimurium LPS as a substrate. Mouse urine was assayed by using the same reaction mixture (18).
In situ hybridization and riboprobes.
A 1-kb fragment of the 5′ coding region of AOAH cDNA (5′-Asp718 to 3′-HindIII) was inserted into pBluescript KS(+) (Stratagene, La Jolla, Calif.). The plasmid was linearized with BglII, and a 650-bp antisense riboprobe, labeled with [35S]UTP, was generated by in vitro transcription from the T7 promoter by using the Ambion MaxiScript kit (Ambion, Austin, Tex.). A 517-bp sense riboprobe was similarly generated by using the T3 promoter according to the manufacturer's instructions. The probes were stored at −80°C and used within 2 days of preparation.
Female ICR mice (Harlan) and AOAH −/− and +/+ 129 and C57BL/6 mice were anesthetized (with ketamine-acepromazine), and tissues were isolated after transcardial perfusion with cold heparin-treated diethyl pyrocarbonate (DEPC)-saline and then with chilled 4% formaldehyde-DEPC-PBS (pH 7.4), freshly prepared from paraformaldehyde. Samples were incubated in 4% formaldehyde for 16 h and then transferred to sterile DEPC-saline. Kidneys were dehydrated and paraffin embedded, and 4-μm sections were placed onto microscope slides treated with Vectabond (Vector Laboratories, Burlingame, Calif.). Slides were stored desiccated at 4°C until use. In situ hybridization was performed as previously described (27), with the riboprobes described above.
Real-time PCR.
Total RNA was isolated from washed urinary bladders and from pooled renal cortex and medulla fractions obtained from wild-type C57BL/6 and 129 mice (RNAqueous Kit; Ambion). A region of the AOAH cDNA was amplified with the primers CCAACTCTCTGGTGTAACTGGATTT and TCTCAAACGATGGTAAATGGATTTT. A TaqMan MGB probe (FAM dye-labeled) ACGAGTGGAATTGAAG and primers were designed and synthesized by Applied Biosystems (Foster City, Calif.). Murine AOAH cDNA was the standard. TaqMan rodent GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control reagents were used to measure GAPDH gene expression. All real-time PCRs were performed with TaqMan one-step RT-PCR master mix reagents kit on the ABI Prism 7700 sequence detection system.
Cell culture.
LLC-PK1 porcine proximal tubule cells (American Type Culture Collection [ATCC], CL-101), T24 human bladder cells (ATCC, HTB-4), and AOAH-transfected and untransfected BHK570 cells (31) were cultured in low-glucose Dulbecco modified Eagle medium (Invitrogen, Carlsbad, Calif.), Vitacell McCoy's 5a medium (ATCC), or DMEM with glutamine and 4.5 g of glucose (Fisher Scientific)/liter, respectively. All cell lines were grown in 2% (wt/vol) PSG (penicillin, streptomycin, and glutamine; Invitrogen), 5% CO2, and 5% (LLC-PK1) or 10% (the others) heat-inactivated fetal calf serum (HyClone, Logan, Utah).
Antibodies.
To produce murine anti-mouse AOAH monoclonal antibodies, we immunized AOAH −/− mice thrice, at monthly intervals, with 100 μg of a plasmid that expressed murine AOAH cDNA from a cytomegalovirus promoter. We then administered ∼109 PFU of an adenovirus vector that produces murine AOAH, prepared as previously described (3). When the mice still did not have high anti-AOAH antibody titers, we expressed the large subunit of murine AOAH in E. coli by using the pET-30 LIC vector (Novagen); inclusion bodies were subjected to preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and bands containing recombinant AOAH were excised and then injected, emulsified in complete or incomplete Freund adjuvant, at monthly intervals (three injections). Although the antibody titers remained low, we fused the spleens from immunized mice with SP2/0-IL-6 myeloma cells and screened the resulting hybridomas to isolate anti-murine AOAH IgG antibodies. The 2F3-2A4 MAb was purified from hybridoma supernatant by using a protein A-Sepharose column and concentrated by using an Amicon Centricon YM-100 filter (Millipore, Bedford, Mass.).
AOAH immunoprecipitation and Western blotting.
Specific immunoprecipitation of 35S-labeled porcine (LLC-PK1) AOAH was performed with rabbit anti-human AOAH and preimmune antibodies as described by Staab et al. (31). In other experiments, C57BL/6 mouse urine samples (3 ml) and lysates of AOAH-transfected and untransfected BHK570 cells were precleared with protein A-Sepharose in immunoprecipitation wash buffer (0.1% Triton X-100, 0.1% SDS, 50 mM Tris-Cl [pH 7.4], 300 mM NaCl, 5 mM EDTA, 0.02% sodium azide, 1 mM phenylmethylsulfonyl fluoride, 2 μg of leupeptin/ml), 30 μg of bovine serum albumin was added, and AOAH was immunoprecipitated with 300 μl of protein A-Sepharose (prebound with mouse anti-murine AOAH MAb 2F3-2A4). Beads were washed four times with IP wash buffer and once with PBS. Then, 20 μl of sample buffer was added, and the samples were immediately heated (100°C, 5 min) and loaded onto a 4 to 20% gradient gel (Bio-Rad, Hercules, Calif.). After SDS-PAGE and overnight transfer to an Immobilon-P membrane (Millipore), the membrane was blocked in 4% dry milk-TTBS (2 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature and probed with primary antibody 2F3-2A4 (4.5 μg/ml) for 1 h at room temperature. After five washes in TTBS, the blot was incubated with peroxidase-conjugated sheep anti-mouse IgG antibody (Amersham) for 1 h at room temperature. The membrane was washed five times as described above, treated with SuperSignal West Pico ECL reagent (Pierce, Rockford, Ill.), and exposed to Kodak film to visualize the bands.
Uptake and deacylation of LPS by bladder cells.
T24 cells were grown in six-well culture dishes (2 × 105 cells per well) for 2 days. Media from confluent AOAH-transfected and untransfected BHK 570 cells were cleared by centrifugation and added to washed T24 cells in the presence or absence of 10 mM mannose-6-phosphate (M6P), glucose-6-phosphate (G6P), or d-(+)-mannose. After 5 h at 37°C, the cells were washed three times with PBS and reincubated with 0.8 ml of T24 cell medium that contained 100 ng of E. coli LCD25 [3H]LPS (specific activity, 4,200 dpm/ng) (17). The cells in two wells were immediately lifted and kept at 4°C. The remaining cells were incubated at 37°C in 5% CO2 for 1 h, washed twice with PBS, and reincubated with fresh T24 cell medium for 24 and 48 h. Pelleted cells were washed and then lysed in 0.6 ml of PBS-0.1% Triton X-100. Then, 0.25 ml was removed to a fresh microfuge tube, and the insoluble [3H]LPS was removed by centrifugation after the addition of 0.625% Triton X-100, 1.25 mg of bovine serum albumin/ml, and 1 ml of 100% ethanol, followed by incubation at −20°C for 20 min. The radioactivity in the ethanol-soluble fraction and the original lysate was counted (Packard Tri-Carb 2100TR; Perkin-Elmer, Boston, Mass.) and analyzed to obtain the percentage of 3H disintegrations per minute that was released from the [3H]LPS over time. Protein assays were done on cell lysates by using the Bradford method (Bio-Rad).
AOAH ELISA.
Maxisorb enzyme-linked immunosorbent assay (ELISA) plates (Fisher Scientific) were coated with 0.5 μg of 3C5 (8F8) monoclonal mouse anti-human AOAH antibody/ml in 0.1 M NaHCO3 overnight at 4°C. All wells were washed three times in PBST (0.005% Tween 20 in PBS [pH 7.2]) after each step, and all antibodies were diluted in block buffer (1% milk-PBST). Wells were blocked for 1 h at 37°C, samples and recombinant human AOAH standard were incubated at 4°C overnight or at 37°C for 2 h, secondary antibody (E553, purified polyclonal rabbit anti-human AOAH, final concentration 10 μg/ml) was added for 1 h at 37°C, biotin-conjugated goat anti-rabbit IgG (0.075 μg/ml) (Zymed, San Francisco, Calif.) was added for 1 h at 37°C, 2 μg of alkaline phosphatase-conjugated streptavidin (Jackson Laboratories)/ml was incubated for 30 min at 37°C, and the plate was developed with alkaline phosphatase substrate (5 mM Sigma 104 and 0.1 M Sigma alkaline buffer 221 in water). The optical densities were read at 405 nm (MRX Revelation; Dynex Technologies, Chantilly, Va.).
RESULTS
AOAH is produced in the kidney.
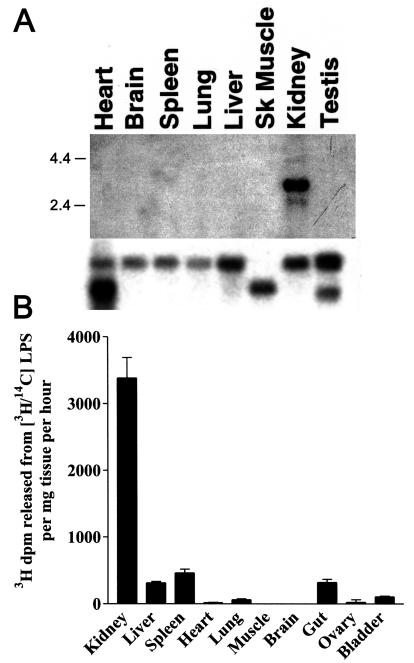
We first determined the abundance of AOAH mRNA in different murine tissues by using a 1-kb, 32P-labeled fragment of AOAH cDNA to probe a membrane that contained polyadenylated RNAs extracted from eight tissues. We found intense hybridization to kidney mRNA (Fig. 1A). An identical result was obtained when we used a different (nonoverlapping) AOAH cDNA fragment as probe (data not shown). In a survey of tissue lysates, AOAH activity was also greatest in the kidney (Fig. 1B). Analysis of the ethanol-soluble 3H-labeled lipids by using thin-layer chromatography confirmed that the renal enzyme releases only secondary acyl chains from radiolabeled LPS (7) (data not shown).
FIG. 1.
AOAH expression in murine tissues. (A) Northern analysis of multiple tissue RNAs from C57BL/6 mice (Clontech) with labeled AOAH cDNA as probe (top). The blot was stripped and reprobed to detect β-actin (bottom). The migration positions of size markers (in kilobase pairs) are shown. (B) AOAH activity in lysates of freshly harvested C57BL/6 mouse tissues. Measurements were performed in duplicate. The bars show means plus one standard deviation of data from three independent experiments.
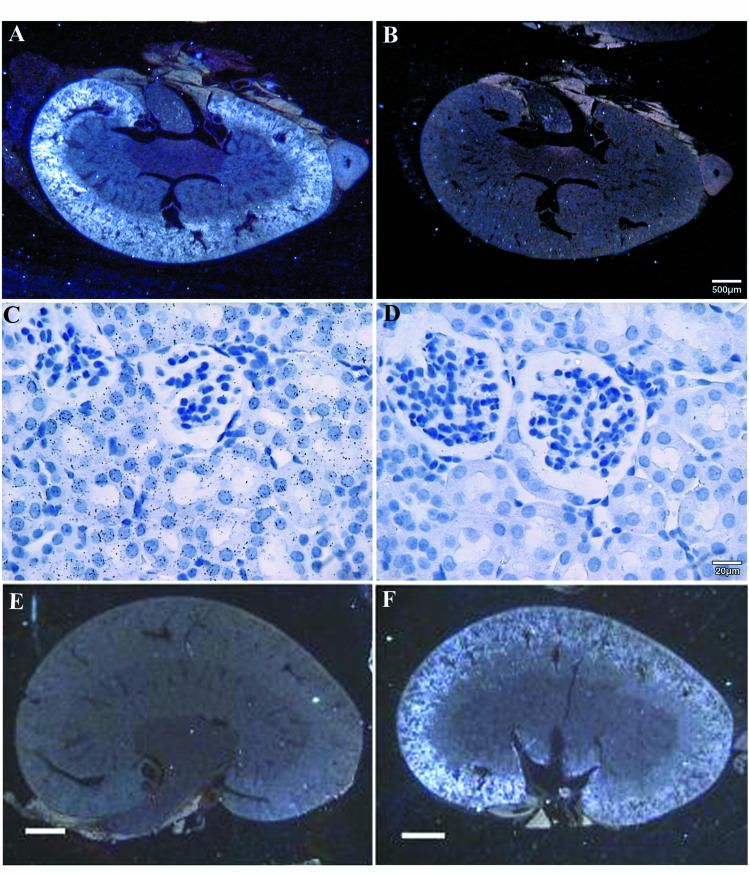
In situ hybridization with an antisense AOAH probe localized AOAH mRNA to the renal cortex (Fig. 2A, and F). High power views revealed that the silver grains overlie proximal tubule cells and not glomeruli (Fig. 2C and D). These data suggest that AOAH is produced within proximal tubules. No hybridization was apparent in kidney sections from AOAH −/− mice (11) (Fig. 2E).
FIG. 2.
Localization of AOAH mRNA in murine kidney by in situ hybridization. Panels: A, C, E, and F, antisense probe; B and D, sense probe. Panels A to D are from ICR (outbred) mice, whereas panels E and F are from AOAH −/− (E) and +/+ (F) 129 mice. The antisense probe hybridized to the renal cortex (panels A and F) and was found over cortical tubules but not glomeruli (panel C). The bars indicate 500 μm (A and B), 20 μm (C and D), or 1 mm (E and F). The experiment shown in panels A to D was repeated three times; each experiment used kidney from a different mouse. The results shown in panels E and F were confirmed with kidneys from AOAH +/+ and AOAH −/− C57BL/6 mice.
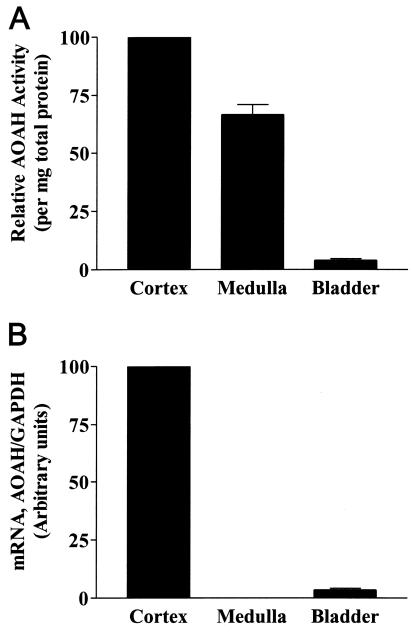
We next assayed freshly isolated renal cortex, renal medulla, and urinary bladder for AOAH activity and for the presence of AOAH mRNA. Whereas all of these tissues had AOAH activity (Fig. 3A), AOAH mRNA was found in the renal cortex and, in much lower amounts, in the bladder (Fig. 3B). The high level of activity in the medulla probably reflects the AOAH contained within urine itself, whereas the activity found in washed bladder may have come from AOAH that bound to the surfaces of, or was taken up by, bladder epithelial cells. Alternatively, bladder cells or blood leukocytes may have contributed the small amount of AOAH detected in the bladder.
FIG. 3.
AOAH is synthesized in the renal cortex. (A) AOAH activity in lysates of renal cortex, medulla, and washed bladder. Each bar shows the mean and standard error of three or more measurements. The mean activity in seven bladder samples was 2.5% of the activity in total kidney lysates. (B) Real-time PCR analysis of AOAH and GAPDH mRNA in renal cortex, medulla, and bladder. This experiment was performed in duplicate and repeated twice, using tissues from different mice, with similar results.
Proximal tubule cells secrete pro-AOAH.
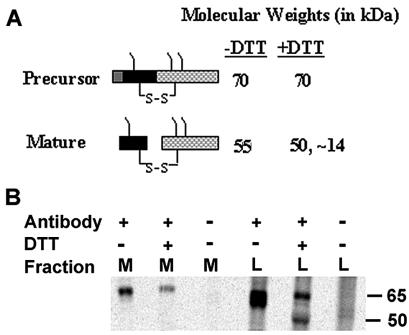
We next wanted to determine how proximal tubule cells produce AOAH in vitro. In cultured fibroblasts, recombinant human AOAH is synthesized as a precursor (pro-AOAH, Mr = 70,000) that is proteolytically processed to form the mature enzyme, a heterodimer of Mr ∼55,000 in which glycosylated large and small subunit peptides are disulfide linked (Fig. 4A). Treatment with dithiothreitol (DTT) cleaves the two subunits, which then migrate at apparent molecular weights of ∼50,000 and ∼14,000 as demonstrated by SDS-PAGE (31). Previous studies found that the mature enzyme is 10- to 20-fold more active toward LPS in vitro than is pro-AOAH (31). To study the biosynthesis of AOAH by renal proximal tubule cells, we used a porcine proximal tubule cell line, LLC-PK1 (21). AOAH was immunoprecipitated from lysates and culture medium of LLC-PK1 cells that had been allowed to incorporate [35S]methionine and [35S]cysteine for 5 h. The cell lysates contained both precursor and mature enzyme, whereas only the precursor was found in the medium (Fig. 4B). As expected, treatment with DTT did not change the size of the precursor, whereas it decreased the apparent Mr of mature AOAH to ∼50,000 (only the large subunit is shown). Similar results were obtained by immunoprecipitation and Western blot analysis of media and lysate fractions of LLC-PK1 cells (data not shown). Proximal tubule cells thus release AOAH precursor (pro-AOAH) into their growth medium in vitro.
FIG. 4.
Proximal tubule cells secrete pro-AOAH. (A) Diagram of AOAH structure, showing the conversion of the precursor (pro-AOAH) into mature AOAH. (B) Production of 35S-AOAH by porcine proximal tubule cells in vitro. Labeled AOAH was immunoprecipitated as described in Methods and studied by SDS-PAGE and autoradiography. M, medium; L, lysate. Antibody +, anti-AOAH; antibody −, preimmune IgG. Only pro-AOAH is seen in the media, whereas the lysate contains both pro-AOAH and mature AOAH.
AOAH is found in voided urine.
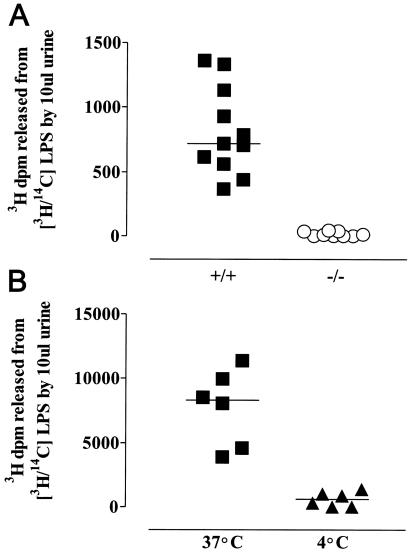
Using the standard AOAH reaction mixture, which contains detergent, we found LPS-deacylating activity in normal mouse urine (Fig. 5A). Urine from 129 or C57BL/6 AOAH-null mice was not active (Fig. 5A), indicating that the deacylating activity detected in wild-type urine is due to AOAH. Analysis of the reaction products by thin-layer chromatography confirmed that only the secondary, nonhydroxylated fatty acids were removed from the LPS by the enzyme in urine (data not shown). AOAH activity was also found in freshly voided murine urine that was tested without adding detergent (Fig. 5B).
FIG. 5.
AOAH activity in mouse urine. (A) LPS deacylation by urine from AOAH +/+ and −/− mice. Urine (10 μl) was added to 490 μl of AOAH reaction mixture containing 1 μg of [3H/14C]LPS and incubated at 37°C for 18 h before the addition of ethanol and further steps as described in Materials and Methods. (B) LPS deacylation by murine urine. Fresh urine (10 μl) was incubated with [3H/14C]LPS (0.5 μg) at 4 and 37°C for 18 h. AOAH reaction mixture was then added to provide protein for coprecipitation of intact LPS, followed by ethanol. The remaining steps are described in Materials and Methods. Each datum point represents a different mouse.
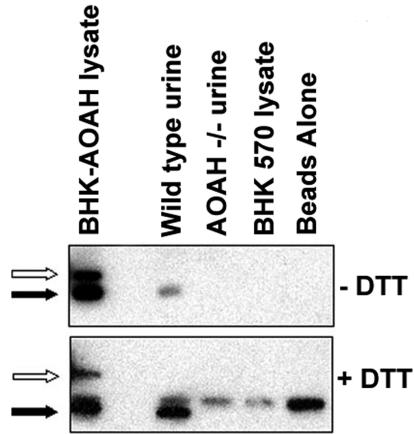
To determine whether the AOAH in the urine is the precursor or the mature form, we collected urine from both wild-type and AOAH-null mice, precipitated AOAH with a murine anti-mAOAH monoclonal antibody, and performed a Western blot (see Materials and Methods). We found the mature (Mr ∼55,000) form of AOAH (Fig. 6), suggesting that the precursor may be converted to the mature form during the enzyme's transit through the urinary tract. Alternatively, mature AOAH may be secreted by proximal tubules in vivo.
FIG. 6.
Mature AOAH is found in urine. Equal volumes of wild-type and AOAH-null urine were immunoprecipitated with an anti-murine AOAH monoclonal antibody and assayed by Western blot as described in Materials and Methods. Lysates of BHK cells transfected with AOAH were used as the positive control. The results are representative of three experiments with similar results. Note in the upper panel that the BHK-AOAH cell lysate contains both pro-AOAH (open arrow) and mature AOAH (solid arrow). Wild-type urine only has mature AOAH. After treatment with DTT (lower panel), mature AOAH migrates with an apparent Mr of 50 kDa (solid arrow). The band at an apparent Mr of 55 kDa in the lower panel is the heavy chain of the murine monoclonal antibody used for immunoprecipitation.
Bladder cells take up pro-AOAH.
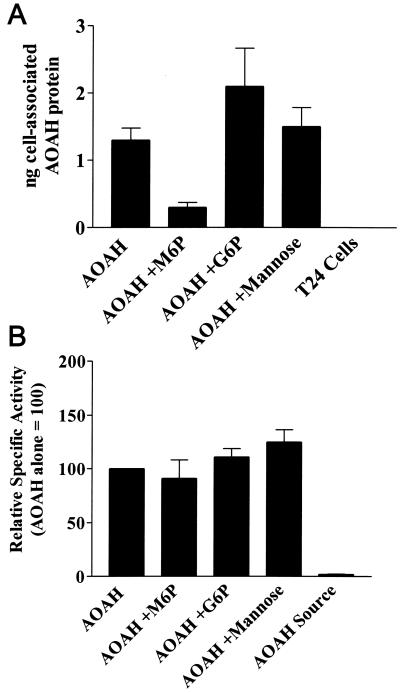
We next sought to determine whether secreted AOAH can be taken up by cells that do not produce the enzyme. When medium containing pro-AOAH (from confluent AOAH-transfected BHK 570 cells) was added to cultures of T24 human bladder cells, the T24 cells bound the enzyme (Fig. 7A). Binding could be blocked by adding M6P but not G6P or mannose (Fig. 7A), suggesting strongly that the binding of AOAH, a heavily N-glycosylated protein (6, 31), is M6P receptor dependent. Since the specific activity of the enzyme (3H-labeled fatty acids released from [3H/14C]LPS per ng of AOAH protein) was ∼50-fold higher in the T24 cell lysates than in the BHK medium (Fig. 7B), it is likely that pro-AOAH is processed to mature AOAH by the T24 cells (31), either on the cell surface or after being internalized.
FIG. 7.
Bladder cells take up AOAH precursor. (A) Uptake of pro-AOAH by T24 cells after incubation with pro-AOAH-containing medium for 5 h in the presence or absence of 10 mM M6P, G6P, or mannose. Washed cells were lysed and AOAH protein was assayed by ELISA. Each bar shows the mean and standard error of data from four independent experiments. (B) AOAH specific activity (activity/nanograms of protein) in T24 cell lysates. Compared to the pro-AOAH added in the medium (AOAH Source), cell-associated AOAH had a much greater specific activity, reflecting its activation by the T24 cells. M6P inhibited AOAH binding (A) but did not prevent the activation of the cell-associated AOAH (B).
Bladder cells that have taken up AOAH can deacylate LPS.
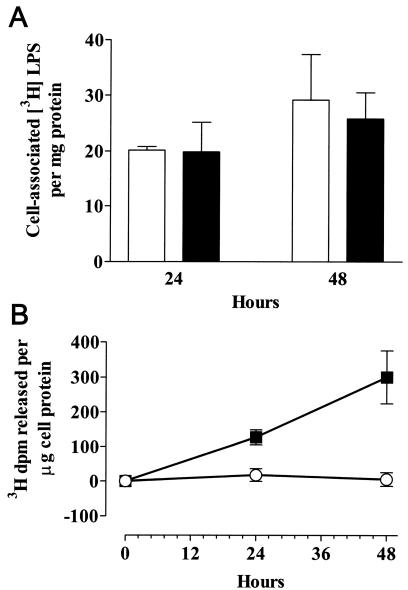
Finally, we wanted to know whether bladder cells that take up AOAH can use the enzyme to deacylate LPS. T24 cells were incubated with medium from confluent AOAH-transfected or untransfected BHK 570 cells for 5 h, washed, and then reincubated in medium that contained 125 ng of [3H]LPS/ml. At 24 and 48 h, cells were washed and then lysed to measure the cell-associated 3H radioactivity and the fraction of the 3H that was ethanol soluble (i.e., released from the LPS backbone). As shown in Fig. 8, acquisition of AOAH allowed the cells to deacylate a significant fraction of the LPS that became cell associated over time, whereas control cells were unable to deacylate cell-associated LPS.
FIG. 8.
AOAH confers LPS-deacylating activity to bladder cells. T24 cells were allowed to take up AOAH for 5 h, washed, and then incubated with [3H]LPS (125 ng/ml) for the times indicated. Control cells were incubated with medium that did not contain AOAH. Whereas control and AOAH-containing cells took up similar amounts of [3H]LPS (A), only the AOAH-containing cells removed 3H-labeled fatty acids from the LPS backbone (B). The data represent combined results of three separate experiments; the error bars represent one standard error of the mean. Solid squares and bars, T24 cells with AOAH; open circles and bars, control T24 cells.
DISCUSSION
Several lines of evidence suggest that the inflammatory response to gram-negative bacterial invasion of the urinary tract can be triggered when host innate immune defenses sense the presence of bacterial LPS. CD14 and TLR4, critical cellular elements for LPS recognition, are present on murine bladder and proximal tubule cells. In addition, the neutrophilic leukocytosis that occurs in response to experimental urinary tract infection is significantly diminished in mice that cannot respond to LPS due to a mutation in the cytosolic domain of TLR4 (10, 25). Although inflammatory responses seem to diminish as bacteria are cleared from the urine and the provoking stimulus is thus removed, LPS-inactivating molecules should help animals damp these responses and prevent their potentially harmful consequences.
AOAH has been highly conserved during animal evolution (30). Prior to these studies, it had been found only in the myeloid cells (neutrophils, monocytes/macrophages, and dendritic cells) that comprise the front-line innate defense versus bacteria and fungi. We now report that AOAH is produced by renal proximal tubule cells and secreted into urine. The enzyme is thus present throughout the fluid compartment in which bacteria move as they ascend the urinary tract. Since the urine enzyme can deacylate LPS ex vivo, it may act on LPS shed from gram-negative bacteria as they grow in vivo. In addition, since proximal tubule cells can respond to, and internalize, gram-negative bacteria (2, 4), they might (like macrophages (8) and dendritic cells (11) also use AOAH to inactivate the LPS found in the bacterial cell wall.
Recent studies have revealed that bacterial adherence and colonization of bladder epithelium are maximal when bacteria express type I pili (13, 16). The innate immune response to E. coli may be triggered in the bladder by type I pili in a way that is dependent upon intact LPS, since polymyxin B and detoxified LPS inhibit interleukin-6 production in cultured bladder epithelial cells that have been invaded by gram-negative uropathogens (25). To investigate the possibility that bladder cells might take up and use exogenous AOAH, we used an in vitro model system in which cultured bladder cells were incubated with either pro-AOAH produced by a proximal tubule cell line or recombinant pro-AOAH from transfected BHK 570 cells. We found that bladder cells can bind pro-AOAH, convert it into the mature, more active form, and use it to deacylate LPS. These data are thus consistent with the idea that AOAH, secreted into the urine by cortical tubule cells, might serve to allow downstream cells (in collecting ducts, renal pelvis, or bladder) to deacylate LPS.
In contrast to the in vitro setting, where the immature, less-active form of AOAH was secreted by proximal tubule cells, we found that voided murine urine contains the mature, highly active form of AOAH. Although the low pH of urine may create an environment which favors proteolytic cleavage of pro-AOAH as it descends through the urinary tract, we were unable to show that urine from AOAH −/− mice can cleave pro-AOAH in vitro (data not shown). Alternatively, proximal tubules may secrete the mature form in vivo or epithelial cells might process the precursor and return the mature enzyme to the urine.
We have also found high levels of AOAH activity in human renal cortex and in first-passage primary cortical cells from both murine and human kidneys (data not shown). In contrast, AOAH activity could not be detected in numerous murine medullary cell lines (IMCD3 or TAL, thick ascending limb cell) or in a human proximal tubule cell line, A498, that has often been used for studies of LPS-induced renal inflammation. As has been shown for other proximal tubule cell enzymes (32), the production of AOAH may be lost as proximal tubule cells differentiate into long-term cell lines. Studies that address the ability of proximal tubule cells to respond to LPS or gram-negative bacteria should now consider the possibility that native proximal tubule epithelium can also inactivate LPS via enzymatic deacylation.
We thus propose that AOAH acts on LPS that is present in the urinary tract, which is thought to be transiently inhabited by gut-derived bacteria throughout life. Although we have focused here on this role, it is important to note that the enzyme is a lipase that can also act on numerous glycerolipids and glycerophospholipids in vitro (20). It is thus conceivable that it could have other function(s) within the urinary tract. Further definition of AOAH's role will be important for understanding how animals defend the urinary tract from bacterial infection while preventing inflammation-induced injury.
Acknowledgments
This study was supported by NIH grant AI18188.
We thank Patricia Preisig and Christopher Lu (Nephrology Division, Department of Internal Medicine, UT-Southwestern) for much-needed advice, Xiang-Hong Li for expertly managing our mouse colony, Wayne Lai (Dept Pathology, UT-Southwestern) for helping us prepare monoclonal antibodies to murine AOAH and Jeffrey Stark for preparing tissue sections.
Editor: D. L. Burns
REFERENCES
- 1.Bäckhed, F., M. Söderhäll, P. Ekmann, S. Normark, and A. Richter-Dahlfors. 2001. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell. Microbiol. 3:153-158. [DOI] [PubMed] [Google Scholar]
- 2.Chippendale, G. R., J. W. Warren, A. L. Trifillis, and H. L. T. Mobley. 1994. Internalization of Proteus mirabilis by human renal epithelial cells. Infect. Immun. 62:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulthard, M. G., J. Swindle, R. S. Munford, R. D. Gerard, and R. S. Meidell. 1996. Adenovirus-mediated transfer of a gene encoding acyloxyacyl hydrolase (AOAH) into mice increases tissue and plasma AOAH activity. Infect. Immun. 64:1510-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg, M. S., B. Newman, S. J. Utsalo, A. L. Trifillis, J. R. Hebel, and J. W. Warren. 1994. Internalization of Escherichia coli into human kidney epithelial cells: comparison of fecal and pyelonephritis-associated strains. J. Infect. Dis. 169:831-838. [DOI] [PubMed] [Google Scholar]
- 5.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 6.Hagen, F. S., F. J. Grant, J. L. Kuijper, C. A. Slaughter, C. R. Moomaw, K. Orth, P. J. O'Hara, and R. S. Munford. 1991. Expression and characterization of recombinant human acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides. Biochemistry 30:8415-8423. [DOI] [PubMed] [Google Scholar]
- 7.Hall, C. L., and R. S. Munford. 1983. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc. Natl. Acad. Sci. USA 80:6671-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz, S. S., Y. Weinrauch, R. S. Munford, P. Elsbach, and J. Weiss. 1999. Deacylation of lipopolysaccharide in whole Escherichia coli during destruction by cellular and extracellular components of a rabbit inflammatory peritoneal exudate. J. Biol. Chem. 274:36579-36584. [DOI] [PubMed] [Google Scholar]
- 9.Kitchens, R. L., R. J. Ulevitch, and R. S. Munford. 1992. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J. Exp. Med. 1760:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder, H., I. Engberg, I. M. Baltzer, K. Jann, and C. Svanborg-Eden. 1988. Induction of inflammation by Escherichia coli on the mucosal level: requirement for adherence and endotoxin. Infect. Immun. 56:1309-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, M., M. Zhang, R. L. Kitchens, S. Fosmire, A. Takashima, and R. S. Munford. 2003. Stimulus-dependent deacylation of bacterial lipopolysaccharide by dendritic cells. J. Exp. Med. 197:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luchi, M., and R. S. Munford. 1993. Binding, internalization, and deacylation of bacterial lipopolysaccharides by human neutrophils. J. Immunol. 151:959-969. [PubMed] [Google Scholar]
- 13.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulvey, M. A. 2002. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 4:257-271. [DOI] [PubMed] [Google Scholar]
- 15.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 16.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munford, R. S., L. C. DeVeaux, J. E. Cronan, Jr., and P. D. Rick. 1992. Biosynthetic radiolabeling of bacterial lipopolysaccharide to high specific activity. J. Immunol. Methods 148:115-120. [DOI] [PubMed] [Google Scholar]
- 18.Munford, R. S., and A. L. Erwin. 1992. Eucaryotic lipopolysaccharide deacylating enzyme. Methods Enzymol. 209:485-492. [DOI] [PubMed] [Google Scholar]
- 19.Munford, R. S., and C. L. Hall. 1985. Uptake and deacylation of bacterial lipopolysaccharides by macrophages from normal and endotoxin-hyporesponsive mice. Infect. Immun. 48:464-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munford, R. S., and J. P. Hunter. 1992. Acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides, has phospholipase, lysophospholipase, diacylglycerollipase, and acyltransferase activities in vitro. J. Biol. Chem. 267:10116-10121. [PubMed] [Google Scholar]
- 21.Nielsen, R., H. Birn, S. K. Moestrup, M. Nielsen, P. Verroust, and E. I. Christensen. 1998. Characterization of a kidney proximal tubule cell line, LLC-PK1, expressing endocytotic active megalin. J. Am. Soc. Nephrol. 9:1767-1776. [DOI] [PubMed] [Google Scholar]
- 22.Pohlman, T. H., R. S. Munford, and J. M. Harlan. 1987. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J. Exp. Med. 165:1393-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schilling, J. D., S. M. Martin, C. S. Hung, R. G. Lorenz, and S. J. Hultgren. 2003. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 100:4203-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilling, J. D., S. M. Martin, D. A. Hunstad, K. P. Patel, M. A. Mulvey, S. S. Justice, R. G. Lorenz, and S. J. Hultgren. 2003. CD14- and toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect. Immun. 71:1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schilling, J. D., M. A. Mulvey, C. D. Vincent, R. G. Lorenz, and S. J. Hultgren. 2001. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166:1148-1155. [DOI] [PubMed] [Google Scholar]
- 26.Shahin, R. D., I. Engberg, L. Hagberg, and C. Svanborg-Eden. 1987. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J. Immunol. 138:3475-3480. [PubMed] [Google Scholar]
- 27.Shelton, J. M., M. H. Lee, J. A. Richardson, and S. B. Patel. 2000. Microsomal triglyceride transfer protein expression during mouse development. J. Lipid Res. 41:532-537. [PubMed] [Google Scholar]
- 28.Shimomura, I., Y. Bashmakov, and J. D. Horton. 1999. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 274:30028-30032. [DOI] [PubMed] [Google Scholar]
- 29.Somerville, J. E., Jr., L. Cassiano, B. Bainbridge, M. D. Cunningham, and R. P. Darveau. 1996. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Investig. 97:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staab, J. F., S. Fosmire, M. Zhang, A. W. Varley, and R. S. Munford. 1999. Distinctive structural features are shared by human, lapine, and murine acyloxyacyl hydrolases. J. Endotoxin Res. 5:205-208. [Google Scholar]
- 31.Staab, J. F., D. L. Ginkel, G. B. Rosenberg, and R. S. Munford. 1994. A saposin-like domain influences the intracellular localization, stability, and catalytic activity of human acyloxyacyl hydrolase. J. Biol. Chem. 269:23736-23742. [PubMed] [Google Scholar]
- 32.Trifillis, A. L., A. L. Regec, and B. F. Trump. 1985. Isolation, culture and characterization of human renal tubular cells. J. Urol. 133:324-329. [DOI] [PubMed] [Google Scholar]
- 33.Tsuboi, N., Y. Yoshikai, S. Matsuo, T. Kikuchi, K. Iwami, Y. Nagai, O. Takeuchi, S. Akira, and T. Matsuguchi. 2002. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J. Immunol. 169:2026-2033. [DOI] [PubMed] [Google Scholar]
- 34.Weinrauch, Y., S. S. Katz, R. S. Munford, P. Elsbach, and J. Weiss. 1999. Deacylation of purified LPS by cellular and extracellular components of a sterile rabbit peritoneal inflammatory exudate. Infect. Immun. 67:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfs, T. G., W. A. Buurman, A. van Schadewijk, B. de Vries, M. A. Daemen, and V. P. S. van Hiemstra. 2002. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J. Immunol. 168:1286-1293. [DOI] [PubMed] [Google Scholar]