Abstract
The fibulin family of extracellular matrix/matricellular proteins is comprised of long fibulins (fibulins-1, -2, -6) and short fibulins (fibulins-3, -4, -5, -7) and is involved in protein-protein interaction with the components of basement membrane and extracellular matrix proteins. Fibulins-1, -2, -3, -4, and -5 bind the monomeric form of elastin (tropoelastin) in vitro and fibulins-2, -3, -4, and -5 are shown to be involved in various aspects of elastic fiber development in vivo. In particular, fibulins-4 and -5 are critical molecules for elastic fiber assembly and play a non-redundant role during elastic fiber formation. Despite manifestation of systemic elastic fiber defects in all elastogenic tissues, fibulin-5 null (Fbln5−/−) mice have a normal lifespan. In contrast, fibulin-4 null (Fbln4−/−) mice die during the perinatal period due to rupture of aortic aneurysms, indicating differential functions of fibulin-4 and fibulin-5 in normal development. In this review, we will update biochemical characterization of fibulin-4 and fibulin-5 and discuss their roles in elastogenesis and outside of elastogenesis based on knowledge obtained from loss-of-function studies in mouse and in human patients with FBLN4 or FBLN5 mutations. Finally, we will evaluate therapeutic options for matrix-related diseases.
Keywords: elastic fibers, integrin, collagen fibers, aortic aneurysm, cutis laxa, ECM
1. Introduction
Since the discovery of prototype fibulin-1 as a 100 kDa integrin-binding protein in 1989 (Argraves et al., 1989), the fibulin family has expanded to include seven members over the last 20 years (reviewed in (Argraves et al., 2003, Timpl et al., 2003, Yanagisawa and Davis, 2010)). Fibulins are characterized by the presence of repeated calcium binding EGF-like motifs and a C-terminal fibulin domain. Much progress has been made to determine biochemical properties of fibulins as well as elucidate the biological roles of fibulins using loss-of-function studies in vivo and human genetic and pathological studies. In particular, fibulins-3, -4, and -5 have been established as elastogenic short fibulins (as opposed to long fibulins that include fibulins-1, 2- and -6) and shown to play a crucial role in various aspects of elastic fiber development in vivo (reviewed in (Wagenseil and Mecham, 2007, Yanagisawa and Davis, 2010)). Although fibulin-2 was shown to interact with tropoelastin most potently in vitro (Sasaki et al., 1999), inactivation of fibulin-2 in vivo did not cause appreciable phenotypes until animals were bred on a Fbln5-null background to unmask the role of fibulin-2 in the formation of internal elastic lamina of the aortic wall (Chapman et al., 2010, Sicot et al., 2008). Fibulin-3, on the other hand, was shown to bind weakly to tropoelastin in vitro; however, a loss of fibulin-3 in mice exhibited a unique defect of elastic fibers in a tissue-restricted manner, i.e. disrupted elastic fibers in fascia connective tissues of the body wall (Kobayashi et al., 2007, McLaughlin et al., 2007).
Fibulins-4 and -5 are thus far the most critical molecules for aiding assembly of elastic fibers. Fibulin-5 exhibits higher binding affinity to tropoelastin compared to fibulin-4 (Choudhury et al., 2009, Kobayashi et al., 2007), and in vitro elastogenic assays using human foreskin fibroblasts showed that addition of fibulin-5 induced robust elastin-positive fibrous structures (Hirai et al., 2007b). We also observed that fibulin-5 potently induced elastic fibers compared to fibulin-4 (unpublished observation). Conversely, the knockdown of either fibulin-4 or fibulin-5 abolished elastin fiber formation in human gingival fibroblasts and dermal fibroblasts (Yamauchi et al., 2010). Fibulin-5 is expressed at much higher levels than fibulin-4 in major elastogenic organs, including the aorta, lung, and skin (Kobayashi et al., 2007). It is interesting, however, that fibulin-4-null (Fbln4−/−) mice exhibit a remarkably more severe phenotype and die between late gestation and the perinatal period (Horiguchi et al., 2009, Huang et al., 2010, McLaughlin et al., 2006). Fibulin-5-null (Fbln5−/−) mice, in contrast, live through adulthood with progressively worsening elastic fiber defects that involve all elastogenic tissues (Nakamura et al., 2002, Yanagisawa et al., 2002). These in vivo data indicate that either fibulin-4 and fibulin-5 are involved in different aspects of elastic fiber formation that cannot be compensated for, or that fibulin-4 has additional roles that affect embryonic development. We will review biochemical properties of fibulin-4 and fibulin-5 and discuss how these molecules are involved in elastogenesis, as well as describe differential roles of fibulin-4 and fibulin-5 beyond elastogenic functions.
2. Fibulin-4 and fibulin-5 during elastic fiber assembly
Elastogenesis involves multiple protein-protein interactions that are precisely regulated in a spatiotemporal manner (reviewed in (Wagenseil and Mecham, 2007, Yanagisawa and Davis, 2010)). Both fibulin-4 and fibulin-5 have a modular structure that interacts with various molecules implicated in each step of elastogenesis in vitro (Fig. 1) (Giltay et al., 1999, Horiguchi et al., 2009, Liu et al., 2004). Elastin is first secreted as a monomer, called tropoelastin, from elastogenic cells, including dermal fibroblasts, vascular smooth muscle cells (SMCs), and lung alveolar cells. Tropoelastin is shown to form self-aggregates and undergo phase separation, called coacervation (reviewed in (Yeo et al., 2011)). It is not clear whether coacervation indeed occurs in vivo or if it requires binding of tropoelastin to the cell surface; however, coacervation is suggested to render efficient crosslinking to elastin (Keeley et al., 2002). Fibulin-5 binds full-length tropoelastin, potentiates coacervation efficiency, and lowers coacervation temperature in a dose-dependent manner (Hirai et al., 2007b, Wachi et al., 2008). Fibulin-4 also binds tropoelastin in a Ca2+-dependent manner (McLaughlin et al., 2006). Using elastin-like polypeptides, fibulin-5 and (to a lesser extent) fibulin-4 were shown to inhibit maturation of coacervation without affecting the coacervation temperature (Cirulis et al., 2008). These results suggest that coacervation can be compromised and the maturation phase of coacervation may be dysregulated in the absence of fibulin-5 or fibulin-4. Interestingly, oxidization of tropoelastin has been shown to facilitate coacervation, but decreases binding to fibulin-4 and fibulin-5 and results in markedly reduced cross-linking and formation of elastin-positive aggregates instead of intact elastic fibers (Akhtar et al., 2010). These notions are consistent with the in vivo observation that large elastin aggregates are accumulated in the skin of Fbln5−/− mice (Choi et al., 2009).
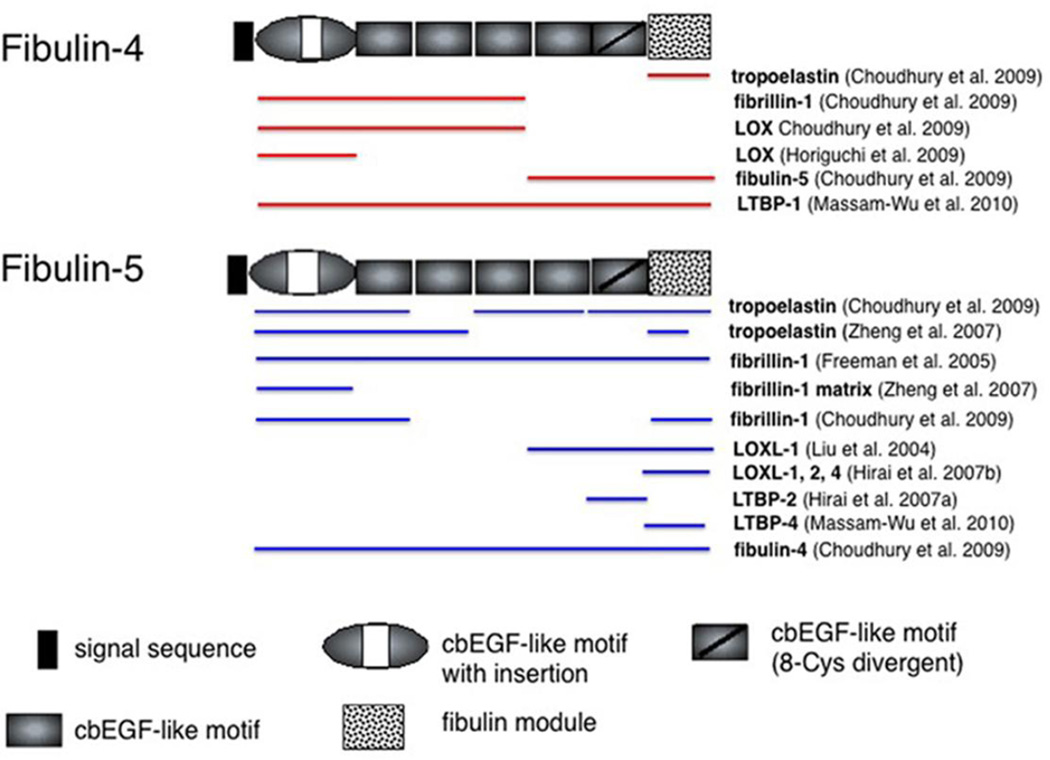
Fig. 1. Schematic presentation of fibulins-4 and -5.
Fibulin-4 and fibulin-5 are shown with known interacting proteins involved in elastic fiber assembly. Red and blue lines indicate interacting domain(s) for fibulin-4 and fibulin-5, respectively, determined by solid-phase binding assays, BIAcore, or Co-IP. The line encompassing an entire sequence indicates that the binding domain(s) have not been determined.
Microfibrils, which are mainly composed of fibrillin-1 and fibrillin-2, serve as scaffolds for deposition of tropoelastin (Rock et al., 2004, Trask et al., 2000). Fibrillin-1 null mice (Fbn1−/−) showed a neonatal lethal phenotype due to ascending aortic rupture with disorganized elastic fibers (Carta et al., 2006). Knocking out Fbn2 on a Fbn1-null background further exacerbated the aortic defects with poorly organized elastin and embryonic lethality, suggesting functional redundancy between fibrillin-1 and fibrillin-2 in vivo (Carta et al., 2006). Fibrillin-1 microfibrils are dependent on the fibronectin matrix for deposition, and knockdown of fibronectin completely abolishes the formation of fibrillin-1 microfibrils (Kinsey et al., 2008, Sabatier et al., 2009). Since neither fibulin-4 nor fibulin-5 bind fibronectin in vitro, it is unlikely that these molecules affect initial formation of a microfibrillar scaffold (El-Hallous et al., 2007, Kobayashi et al., 2007).
Fibulin-5 was shown to bind N-terminal fibrillin-1 fragments and co-localize with microfibrils (Freeman et al., 2005, Zheng et al., 2007). It was further demonstrated that fibulin-5 binding to fibrillin-1 markedly potentiates binding between fibrillin-1 fragments and tropoelastin, acting as an adaptor to form a ternary complex (El-Hallous et al., 2007). In other experiments, fibulin-5 was shown to bind tropoelastin or fibrillin-1 without influencing the overall complex formation and it was suggested that fibulin-5 facilitates targeting and deposition of tropoelastin onto microfibrils (Choudhury et al., 2009). Although the precise sequence of events during tropoelastin deposition onto microfibrils is not completely determined, these data collectively suggest that fibulin-5 binds tropoelastin to navigate onto a fibrillin-1 dominant microfibrillar scaffold while simultaneously mediating proper coacervation prior to cross-linking. However, fibulin-4 seems to play relatively a minor role in this process compared to fibulin-5 (Fig. 2).
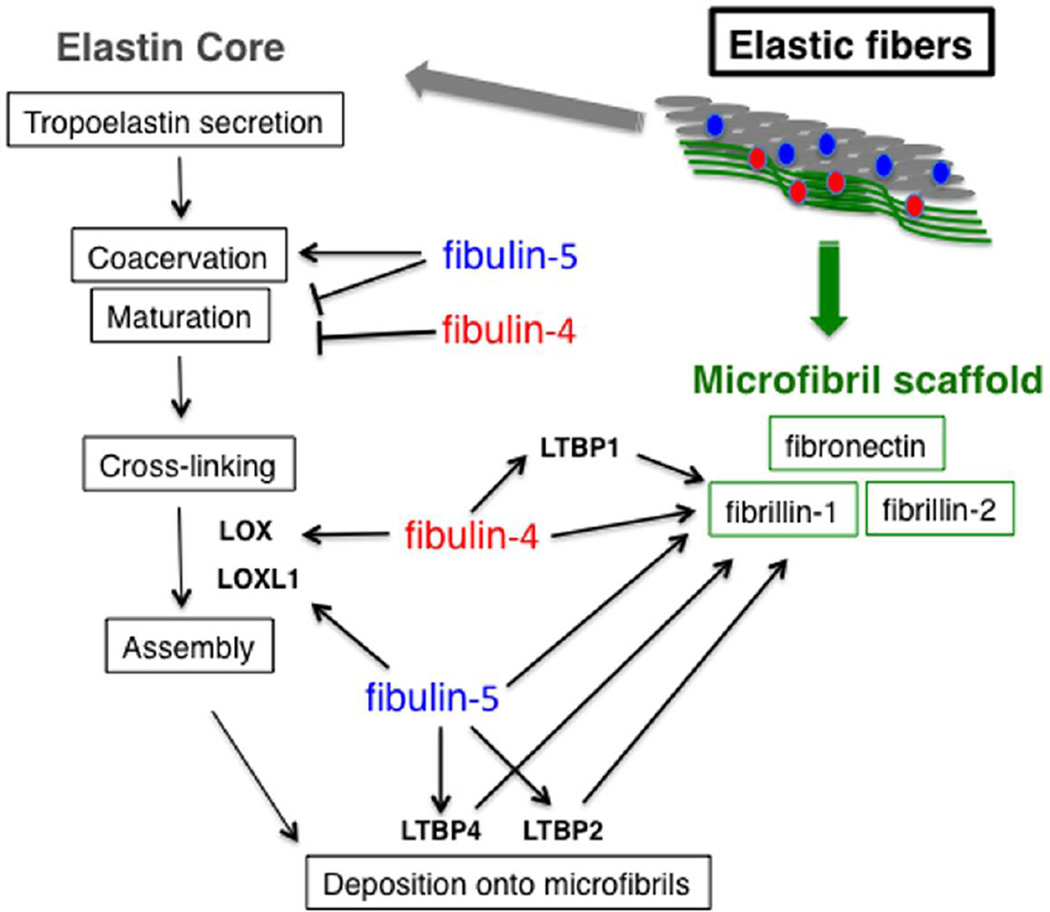
Fig. 2. Proposed functions of fibulins-4 and -5 during elastogenesis.
Elastic fibers are divided into elastin core (gray) and microfibril scaffold (green). Each step of elastogenesis is shown on the left and molecular interactions among components of elastic fibers and potential functions of fibulins are indicated by arrows.
Cross-linking of elastin is the next critical step for generating insoluble polymerized elastin. This process is mediated by the lysyl oxidase enzyme family, which includes lysyl oxidase (LOX) and lysyl oxidase like (LOXL)-1 (reviewed in (Lucero and Kagan, 2006)). LOX and LOXL-1 are shown to be involved in elastic fiber formation in vivo and Lox knockout mice exhibit perinatal lethality due to rupture of aortic aneurysm with failure to cross-link both collagen and elastin (Hornstra et al., 2003, Maki et al., 2005). Interestingly, Loxl1 knockout mice live into adulthood but exhibit defective elastic fiber remodeling, manifested as development of pelvic organ prolapse following vaginal delivery and progressive pulmonary emphysema (Liu et al., 2004). In vitro binding analysis showed that LOX strongly binds fibulin-4, whereas LOX binding to fibulin-5 was only observed by solid-phase binding assays but not by BIAcore (Choudhury et al., 2009). Co-immunoprecipitation (Co-IP) assays also confirmed the binding between fibulin-4 and LOX, which was mediated between the N-terminal region of fibulin-4 and propeptide of LOX. It was further shown that fibulin-4 markedly increases tropoelastin binding to LOX (Hirai et al., 2007b). Fibulin-5, in contrast, binds LOXL-1 in vitro through its C-terminal domain and is suggested to tether LOXL-1 onto elastic fibers (Liu et al., 2004) and/or activate LOXL-1 by facilitating cleavage of preproLOXL-1 into an active LOXL-1 (Choi et al., 2009). These findings indicate that the preferential binding between LOX and fibulin-4, and between LOXL-1 and fibulin-5, together facilitates crosslinking of elastic fibers in vivo. The fact that LOX is also essential for collagen crosslinking, along with the striking phenotypic similarity between Lox−/− and Fbln4−/− mice, raises the question of whether the fibulin-4-LOX interaction may also be biologically relevant outside of elastogenesis. However, LOX activation and localization have not yet been assessed in Fbln4−/− mice, and this question remains to be investigated.
Cell surface binding of fibulin-5 to subsets of integrin receptors, including αVβ1, αVβ3, α9β1 and α5β1, have been demonstrated in vitro (Lomas et al., 2007, Nakamura et al., 2002). Since fibulin-5 contains a RGD integrin-binding motif, it was initially hypothesized that fibulin-5 bridges the gap between the cell surface and elastic fibers during the final step of elastic fiber assembly. Solid phase binding assays using immobilized αVβ3 integrin showed that fibulin-5 binding to αVβ3 was weak unless reduction and alkylation was introduced to unmask the RGD site in fibulin-5 (Kobayashi et al., 2007). In addition, mice containing alleles in which RGD was mutated to RGE to disrupt integrin binding showed normal elastic fibers in the aorta, lungs, skin and vaginal wall. This finding suggested that RGD-mediated integrin binding was not required for elastic fiber assembly in vivo (Budatha et al., 2011). Recently, it has been shown that all short fibulins (fibulins-3, -4, and -5) adhere to human fibroblasts and SMCs, possibly mediated by cell surface heparan sulfate in a calcium-dependent manner (Djokic et al., 2013). It is not clear, however, whether fibulin-5 binding to the cell surface is a necessary step for elastogenesis in vivo, or induces specific intracellular signaling pathways.
Several molecules have been reported to interact with fibulin-4 or fibulin-5 and affect the formation of elastic fibers in vitro. Latent TGFβ binding protein-2 (LTBP-2), which belongs to the fibrillin/LTBP family but lacks binding to the latency-associated propeptide-TGFβ complex (small latency complex or SLC), interacts with fibulin-5 and facilitates fibrillin-1 microfibril-dependent elastin deposition (Hirai et al., 2007a). When LTBP-2 is knocked down, elastin preferentially deposits onto fibrillin-2 microfibrils. Another study showed that LTBP-2 binds fibulin-5 and inhibits tropoelastin-fibulin-5 interaction (Sideek et al., 2013). LTBP-1 binds strongly to fibulin-4 and forms a ternary complex involving LTBP-1, fibrillin-1 and fibulin-4 (Massam-Wu et al., 2010). LTBP-4, which can bind the SLC, also binds fibulin-5 and induces linear deposition of fibulin-5 onto microfibrils and promotes elastic fiber assembly (Noda et al., 2013).
In addition to the different biochemical properties of fibulin-4 and fibulin-5, distinct tissue distribution, localization within the tissue, expression levels, and temporal expression patterns may account for the differential roles of fibulin-4 and fibulin-5 in elastogenesis. It is also important to identify interacting proteins and examine their tissue distribution to understand whether the mechanism of elastogenesis differs among tissues in vivo. Finally, primary structural defects caused by the loss of elastic fibers need to be distinguished from secondary defects resulting from abnormal tissue architecture, such as increased mechanical stress and stress-induced signaling pathways, in order to obtain a full understanding of pathophysiology resulting from the loss of fibulin-4 and fibulin-5 in vivo.
3. Functions of fibulin-4 outside of elastogenesis
3.1. Fibulin-4 and aortic development
Since Fbln4−/− mice die early in life, it has been challenging to identify extra-elastogenic functions of fibulin-4 from in vivo phenotypes. We previously reported that Fbln4−/− aortic SMCs were less differentiated as judged by the reduced expression of SMC-specific terminal differentiation markers and persistent expression of embryonic SMC markers (Huang et al., 2010). In addition, Fbln4−/− cells exhibited an increase in phosphorylated Histone H3 under serum-starved conditions, mimicking the embryonic SMC phenotype. Interestingly, these immature and proliferative phenotypes are strikingly similar to Eln−/− SMCs as well as cells from patients with deletion of the elastin gene (supravalvular aortic stenosis and Williams-Beuren Syndrome (Li et al., 1998, Urban et al., 2002)). Since tropoelastin mRNA levels are comparable between Fbln4−/− and wild-type aortas (Horiguchi et al., 2009) and soluble tropoelastin protein levels are even higher in the mutants (Le et al., 2014), it is possible that the absence of fibulin-4 in SMCs may influence elastin-dependent intracellular signaling mediated by a putative elastin receptor (Mochizuki et al., 2002). Alternatively, fibulin-4 deficient SMCs may acquire a hyperproliferative SMC phenotype secondary to the disorganized elastin matrix in the aortic wall. It is also possible that a fibulin-4-containing vascular microenvironment is necessary to support maintenance of a differentiated SMC phenotype in vivo.
Vascular SMC-specific Fbln4 knockout mice (Fbln4SMKO) and hypomorphic Fbln4 mice (Fbln4R/R) were generated, in which Fbln4 levels in the aorta were reduced to approximately 6–8% and 40%, respectively (Hanada et al., 2007, Horiguchi et al., 2009, Huang et al., 2010). These mice develop ascending aortic aneurysms and tortuous descending aorta with disorganized elastic fibers and accumulation of collagen fibers. It is interesting to note that endothelial cell (EC)-specific knockout Fbln4 mice do not develop an aneurysmal phenotype; however, EC-SMC double knockout mice exhibit an exacerbated aneurysmal phenotype with the lesion extending over the aortic arch and involving the descending thoracic aorta (Huang et al., 2010), our unpublished observation). In addition, Fbln4R/R mice which maintain as much as 40% of Fbln4 in the aorta still exhibit an aneurysm phenotype similar to Fbln4SMKO, indicating that fibulin-4 derived from ECs or other cell types may play additional roles in protecting the aortic wall from aneurysm formation during the embryonic and postnatal period.
3.2. Fibulin-4 and angiotensin-TGFβ pathway
It was reported that TGFβ-mediated signaling was elevated in the aortas of Fbln4R/R mice and in patients carrying mutations in the FBLN4 gene, as indicated by an increase in phosphorylated (p-) Smad2/3 levels (Hanada et al., 2007, Renard et al., 2010). We have reported that local angiontensin-converting enzyme (ACE) was increased in the aneurysmal wall, resulting in an increase of mature angiontensin II and p-ERK1/2 in Fbln4SMKO mice (Huang et al., 2013). Angiotensin II is a positive regulator of TGFβ signaling by inducing transcription of TGFβ, and Fbln4SMKO aortas showed increased p-Smad 2/3, but not Smad1/5/8. Interestingly, ACE levels were comparable between isolated wild-type and Fbln4SMKO SMCs, indicating that additional mechanical stress on the vessel wall (i.e. pressure or flow), disruption of cell-matrix connections, or contribution from other cell types may be required for upregulation of ACE in vivo. In both Fbln4R/R and Fbln4SMKO mouse models, angiotensin II receptor blockade (ARB) effectively prevented the development of aortic aneurysms. It was suggested that the protective effect of ARB was due to reduction of systemic blood pressure in Fbln4R/R (Moltzer et al., 2011) and blocking of angiotensin II-mediated signaling in Fbln4SMKO (Huang et al., 2013), respectively. It is noteworthy that an ACE inhibitor also effectively prevented aneurysms in Fbln4SMKO mice, which shows a distinct difference from a Marfan mouse model (Fbn1C1039G/+) in which an ARB was more effective than an ACE inhibitor in preventing aortic root aneurysms (Habashi et al., 2011, Habashi et al., 2006). However, it remains unknown whether TGFβ upregulation is a direct consequence of loss of fibulin-4 or a secondary event due to upregulation of angiotensin II in the aortic wall.
Another unanswered question is whether TGFβ plays a causative role in the pathogenesis of aortic aneurysms in Fbln4-mutant mice similar to what has been suggested in Marfan syndrome and related diseases (Dietz et al., 1991, Loeys et al., 2005, Neptune et al., 2003). Fibrillin-1 tethers the large latency complex (LLC) comprised of covalently linked LTBP (predominantly LTBP-1) and SLC via direct binding, aiding in sequestration of latent TGFβ in the ECM (Isogai et al., 2003). Immuno-EM studies indicated that fibulin-4 is localized on microfibrils (Kobayashi et al., 2007), and one study suggested that fibulin-4 stabilizes LLC on microfibrils by directly binding to LTBP-1 and fibrillin-1 and forming a ternary complex (Massam-Wu et al., 2010). Conversely, LTBPs, fibulin-2, and fibulin-4 were found to compete for binding to the third EGF-like domain (EGF3) of fibrillin-1 in in vitro binding assays (Ono et al., 2009). These biochemical studies are informative but limited in their ability to directly assess the effect of loss of fibulin-4 on TGFβ bioavailability in vivo. Therefore, additional tools such as a TGFβ reporter mouse will be useful to monitor active TGFβ levels during aneurysm formation. In addition, genetic ablation of TGFβ signaling components or treating Fbln4−/− mice with a TGFβ neutralizing antibody should prove useful in addressing the contribution(s) of TGFβ to the pathology of Fbln4-mutant mice.
4. Non-elastogenic functions of fibulin-5: Protease regulation mediated by the integrin binding domain of fibulin-5
Systemic elastic fiber defects in Fbln5−/− mice are already present during early postnatal life, thus the role of fibulin-5 has been suggested to be predominantly developmental. However, deterioration of elastic fibers is observed in Fbln5−/− mice as they age, especially in the lung and vaginal tissues, resulting in emphysema and pelvic organ prolapse, respectively (Drewes et al., 2007, Yanagisawa et al., 2002). Pelvic organ prolapse is characterized by abnormal protrusion of female pelvic organs (i.e. uterus, bladder and vagina) and this phenotype is not observed until later in life in Fbln5−/− mice. This observation led to the hypothesis that abnormal elastic fibers alone may not be sufficient to induce pelvic organ prolapse, and suggested additional, non-elastogenic functions of fibulin-5.
Our group documented that both MMP-9 and MMP-2 were upregulated in vaginal tissues of adult Fbln5−/− mice and MMP-9 activity was detected after puberty and preceded the onset of pelvic organ prolapse (Budatha et al., 2011). Since MMP-9 is not elevated in other elastogenic tissues, such as the aorta and skin, it is likely that fibulin-5 suppresses protease activity in tissues that undergo continuous remodeling of elastic fibers. Indeed, using primary vaginal stromal cells, Fbln5−/− cells exhibited higher MMP-9 activity in response to fibronectin and this increase was inhibited by recombinant fibulin-5. Interestingly, Fbln5RGE/RGE cells, which produce fibulin-5 containing a mutation in the RGD motif that disrupts integrin binding, also exhibited high MMP-9 in response to fibronectin. In both cases, MMP-9 activity was inhibited by a β1 integrin blocking antibody, indicating that fibulin-5 inhibits fibronectin-induced β1 integrin-mediated elevation of MMP-9. This is consistent with an earlier report in which fibulin-5 was shown to bind α5β1 fibronectin receptor and blocked fibronectin-mediated activation of β1 integrin, including stress fiber formation and focal adhesions, and this inhibition was reversed by adding β1 integrin-activating antibody (Lomas et al., 2007).
The biological significance of fibulin-5 binding to β1 integrin has also been reported in mouse pancreas tumor studies. In Fbln5−/− mice, pancreatic tumor growth and tumor angiogenesis was suppressed compared with wild-type mice and this was due to an increase in the level of reactive oxygen species (ROS), which resulted in elevated DNA damage and apoptosis of tumor endothelial cells. The ROS production was shown to be dependent on β1 integrin, and treatment with an antioxidant was sufficient to downregulate ROS levels and restore tumor growth in Fbln5-mutant mice (Schluterman et al., 2010). In a separate study using lung cancer cell lines, fibulin-5 was shown to suppress MMP-7 expression and activity in an RGD-dependent manner, and this suppression was correlated with increased p-ERK levels (Yue et al., 2009). Interestingly, fibulin-5 was shown to be hypermethylated and the expression was downregulated in primary tumors and lung cancer cell lines, contributing to an increase in cancer cell invasion (Yue et al., 2009). More recently, fibulin-5 was shown to be required for urokinase-type plasminogen activator (uPA)-mediated cell migration (Kapustin et al., 2012). Interaction between uPA and fibulin-5 resulted in generation of plasmin, which cleaves the N-terminal cbEGF-like domain of fibulin-5 containing the integrin-binding motif and disrupts fibulin-5-β1 integrin binding, releasing the inhibitory effects of fibulin-5 on β1 integrin-mediated cell migration (Kapustin et al., 2012). Taken together, these studies point to the role of fibulin-5 in regulating protease activity by interacting with β1 integrin as an endogenous competitive ligand. It seems that fibulin-5 modulates the microenvironment and cellular functions by acting as a negative regulator in a context-dependent manner.
5. Fibulins-4 and -5 in human diseases
Mutations in both FBLN4 and FBLN5 have been identified in patients with cutis laxa (OMIM 219100, OMIM 614434, OMIM 614437). Consistent with data from knockout mouse models, FBLN4 mutations lead to a broader, more severe range of phenotypes than FBLN5 mutations, and the phenotypic abnormalities observed in patients lend further support for the role of these proteins in elastic fiber assembly as well as provide insight for future research directions. The majority of currently identified FBLN4 mutations occur in the cbEGF-like domains (Al-Hassnan et al., 2012, Erickson et al., 2011, Iascone et al., 2012, Kappanayil et al., 2012, Sawyer et al., 2013), and are predicted to either disrupt disulfide bond formation and proper folding of the domain or disrupt calcium binding (Dasouki et al., 2007, Hucthagowder et al., 2006, Renard et al., 2010). Additionally, one report showed decreased secretion of fibulin-4 into the ECM along with alterations in TGFβ1 signaling in FBLN4 mutant skin fibroblasts and tissues, consistent with observations in Fbln4 deficient mice (Renard et al., 2010).
The most prominent and consistent defects resulting from FBLN4 mutations are ascending aortic aneurysms, arterial tortuosity along with other arterial defects, and cutis laxa, and are consistent with phenotypes observed in Fbln4−/− mice (Hoyer et al., 2009, Sawyer et al., 2013). Multiple case studies found respiratory problems including diaphragm abnormalities, which are consistent with diaphragm herniation and rupture found in Fbln4 deficient mice (Erickson et al., 2011, Horiguchi et al., 2009, Hucthagowder et al., 2006, Iascone et al., 2012). Additional defects identified in multiple patients with FBLN4 mutations include bradycardia, joint laxity, hypotonia, and craniofacial dysmorphism including features similar to Loeys-Dietz (Loeys et al., 2005) or Arterial Tortuosity Syndrome (Coucke et al., 2006). Multiple FBLN4 patients were also reported to have skeletal abnormalities including bone fractures, rib/long bone defects, or decreased bone mineral density (Dasouki et al., 2007, Erickson et al., 2011, Hoyer et al., 2009, Hucthagowder et al., 2006, Sawyer et al., 2013), but these findings have not been further investigated. Conditional knockouts of fibulin-4 in skeletal tissues will help clarify which skeletal abnormalities specifically result from fibulin-4 loss, along with their associated mechanisms. Overall, although FBLN4 mutations are relatively rare, the diverse array of defects has led some to suggest the possibility of as-yet unidentified roles for fibulin-4, and studies are currently underway to address these issues.
In contrast to FBLN4 mutations, patients with FBLN5 mutations presented mainly with cutis laxa and showed disruption of elastic fibers in skin and aorta without evidence of aortic aneurysms, consistent with data obtained from Fbln5−/− mice. Two mutations in FBLN5 (S227P and C217R) were separately identified in multiple cases of cutis laxa (Callewaert et al., 2013, Claus et al., 2008, Elahi et al., 2006, Hu et al., 2006, Loeys et al., 2002). Both mutations are found in highly conserved residues in the fourth cbEGF-like domain and are predicted to lead to protein misfolding based on functional studies showing decreased secretion and matrix deposition. Additionally, these mutants showed decreased affinity for tropoelastin (Hu et al., 2006). Jones et al. used multiple biophysical techniques to further demonstrate that these two mutations introduce changes in fibulin-5 that are likely to be pathogenic, including increased dimerization of S227P, and a likely reduction in protein stability of C217R mutants (Jones et al., 2010).
In addition to cutis laxa, several reports have been published of FBLN5 variants associated with age-related macular degeneration (AMD) (Jones et al., 2010, Lotery et al., 2006, Stone et al., 2004). Expression of fibulin-5 mutants in COS-7 cells showed four out of nine mutants associated with AMD led to decreased fibulin-5 secretion (Lotery et al., 2006). Interestingly, five heterozygous FBLN5 mutations were reported in patients with Charcot-Marie-Tooth disease, three of which were novel and two of which were previously identified as variants in AMD patients (Auer-Grumbach et al., 2011, Safka Brozkova et al., 2013). Although the mutations segregated with the disease, functional studies were not performed, and the reported phenotypes were complex and variable. Therefore, further study is needed to identify the specific pathologic mechanisms involved.
6. New therapeutic approaches toward matrix-related diseases
Several promising strategies have been proposed to prevent or ameliorate progression of matrix-related defects in animal mouse models. Pharmacological intervention using ARB was first demonstrated in Fbn1C1039G/+ mice based on the increased bioavailability of TGFβ and improvement of the lung emphysematous phenotype by anti-TGFβ neutralizing antibodies (Neptune et al., 2003). The rationale for selecting an ARB to treat aneurysms in the Fbn1C1039G/+ mouse was to downregulate TGFβ levels by blocking its upstream regulator, angiotensin II (Habashi et al., 2006). This strategy solidified the notion that disruption of fibrillin-1 not only affects structural integrity but also alters intracellular signaling, which shed light on a novel role of ECM in tissue development and homeostasis. Our study and others also support the effectiveness of ARB in prevention of aneurysms in Fbln4 mutant mice, but the mechanism of action seems to be different from that of the Marfan mouse model as discussed in the previous section.
A new strategy to target microRNAs (miRs), particularly miR-29, was demonstrated in treatment of angiontensin II-induced aneurysms, elastase-induced abdominal aneurysm, and aneurysms in a Marfan mouse model (Boon et al., 2011, Maegdefessel et al., 2012, Merk et al., 2012). MicroRNAs are well-conserved small noncoding RNAs that regulate protein expression by degrading target mRNA or inhibiting translation (reviewed in (Small and Olson, 2011)). MicroRNA-29 is consisted of three family members (miR-29a, miR-29b, and miR-29c). MicroRNA-29b in particular is increased in mouse and human aneurysms, including Fbn1C1039G/+, Fbln4R/R, and angiotensin II-treated mice, and patients with bicuspid or tricuspid aortic valves with thoracic aortic aneurysms. MicroRNA-29b regulates a variety of genes encoding ECM proteins, including elastin, fibrillin-1, collagen type 1 α chain (Col1a1), and Col5a1 (Boon et al., 2011). Others reported that miR-29b also regulates anti-apoptotic genes such as Bcl-2 and Mcl-1 (Mott et al., 2007). Administration of locked nucleic acid-mediated antisense oligonucleotide for miR-29b was shown to prevent the increase in aortic diameter and upregulate target mRNA levels in the aortas of angiotensin II-treated mice and Fbn1C1039G/+ mice (Merk et al., 2012). These data indicate that deterioration of ECM by suppressing ECM genes and anti-apoptotic genes is a critical aspect of aneurysm formation in vivo. In a separate experiment using an elastase-induced aortic aneurysm model, miR-29b was shown to be effective in restoration of target gene expression and stabilization of the vessel wall by increasing fibrosis and decreasing MMP-9 levels (Maegdefessel et al., 2012). Interestingly, it was shown that losartan effectively reduced the expression of miR29-b, resulting in a decrease of caspase-3 and caspase-9, and an increase of Bcl-2, Mcl-2, Eln and Col3a1. Taken together, the combination of anti-miR-29b and losartan may work synergistically to prevent aneurysms in vivo.
The modeling of patients’ SMCs has been accomplished by generating induced pluripotent stem (iPS) combined with in vitro differentiation into SMCs using fibroblasts from patients with supravalvular aortic stenosis (SVAS). SVAS is caused by deletion in the elastin gene (ELN) and is characterized by hyperproliferation of SMCs and narrowing of the ascending aorta (Ge et al., 2012, Kinnear et al., 2013). A mouse model of SVAS was established by deleting the Eln gene in vivo, and primary Eln-null SMCs recapitulate human disease phenotypes (Li et al., 1998). The established iPS-derived SMCs showed decreased SMC markers and increased migration and proliferation, which were reversed by addition of elastin or elastin binding protein ligand 2, or by driving RhoA activity. It is interesting that the abnormal phenotypes of SMCs within the aortic wall can be reproduced in vitro, indicating that the effect of loss of elastin does not only affect vessel integrity but also induces cell-autonomous effects that can be potentially corrected by pharmacological interventions. Since primary Fbln4-null SMCs exhibit hyperproliferation and reduction of SMC marker proteins in vitro, it may be useful to establish stable iPS-derived SMCs for characterizing abnormal SMC phenotype and identifying intracellular signaling pathways underlying the disease. Furthermore, iPS-derived SMCs may be useful for engineering of synthetic vessels to examine mechanical properties of the diseased vessels (Hibino et al., 2012).
7. Concluding remarks
Considerable progress has been made in the last decade in determining the biochemical properties of fibulins and their biological functions during development and disease progression. Furthermore, it is clear that fibulins-4 and -5 possess extra-elastogenic functions that have yet to be discovered. It is therefore critical to distinguish the effects caused by a primary loss of proteins from a structural consequence of the loss of protein to understand the overall functions in vivo. Moreover, information on the effect of overexpression of these proteins on tissue homeostasis as well as the possibility of one or more fibulins serving as biomarkers for pathological conditions may be important. Finally, the idea that each fibulin has a specific cell surface receptor is an interesting avenue to pursue to understand matrix-cell interactions in various in vivo settings.
Highlights.
Fibulins-4 and -5 bind elastin and are essential for development of elastic fibers.
Mutations in FBLN4 or FBLN5 gene cause autosomal recessive cutis laxa.
Smooth muscle cell-derived fibulin-4 is crucial for preventing ascending aortic aneurysm.
Fibulin-5 regulates protease activities in an integrin dependent manner.
Anti-miR29-b therapy may be useful for subsets of ECM-related diseases.
Acknowledgement
The work was supported by grants from National Institutes of Health (R01HL106305 and R01HD06482401 to HY) and an NIH institutional Training in Cardiovascular Research grant (5T32HL007360-34 to CLP). HY is an Established Investigator of the American Heart Association.
Abbreviation
- TGFβ
transforming growth factor beta
- cb-EGF
calcium binding epidermal growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akhtar K, Broekelmann TJ, Miao M, Keeley FW, Starcher BC, Pierce RA, Mecham RP, Adair-Kirk TL. Oxidative and nitrosative modifications of tropoelastin prevent elastic fiber assembly in vitro. The Journal of biological chemistry. 2010;285:37396–37404. doi: 10.1074/jbc.M110.126789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hassnan ZN, Almesned AR, Tulbah S, Hakami A, Al-Omrani A, Al Sehly A, Mohammed S, Majid S, Meyer B, Al-Fayyadh M. Recessively inherited severe aortic aneurysm caused by mutated EFEMP2. The American journal of cardiology. 2012;109:1677–1680. doi: 10.1016/j.amjcard.2012.01.394. [DOI] [PubMed] [Google Scholar]
- Argraves WS, Dickerson K, Burgess WH, Ruoslahti E. Fibulin, a novel protein that interacts with the fibronectin receptor beta subunit cytoplasmic domain. Cell. 1989;58:623–629. doi: 10.1016/0092-8674(89)90097-4. [DOI] [PubMed] [Google Scholar]
- Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO reports. 2003;4:1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer-Grumbach M, Weger M, Fink-Puches R, Papic L, Frohlich E, Auer-Grumbach P, El Shabrawi-Caelen L, Schabhuttl M, Windpassinger C, Senderek J, Budka H, Trajanoski S, Janecke AR, Haas A, Metze D, Pieber TR, Guelly C. Fibulin-5 mutations link inherited neuropathies, age-related macular degeneration and hyperelastic skin. Brain : a journal of neurology. 2011;134:1839–1852. doi: 10.1093/brain/awr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circulation research. 2011;109:1115–1119. doi: 10.1161/CIRCRESAHA.111.255737. [DOI] [PubMed] [Google Scholar]
- Budatha M, Roshanravan S, Zheng Q, Weislander C, Chapman SL, Davis EC, Starcher B, Word RA, Yanagisawa H. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. The Journal of clinical investigation. 2011;121:2048–2059. doi: 10.1172/JCI45636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert B, Su CT, Van Damme T, Vlummens P, Malfait F, Vanakker O, Schulz B, Mac Neal M, Davis EC, Lee JG, Salhi A, Unger S, Heimdal K, De Almeida S, Kornak U, Gaspar H, Bresson JL, Prescott K, Gosendi ME, Mansour S, Pierard GE, Madan-Khetarpal S, Sciurba FC, Symoens S, Coucke PJ, Van Maldergem L, Urban Z, De Paepe A. Comprehensive clinical and molecular analysis of 12 families with type 1 recessive cutis laxa. Hum Mutat. 2013;34:111–121. doi: 10.1002/humu.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. The Journal of biological chemistry. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SL, Sicot FX, Davis EC, Huang J, Sasaki T, Chu ML, Yanagisawa H. Fibulin-2 and fibulin-5 cooperatively function to form the internal elastic lamina and protect from vascular injury. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:68–74. doi: 10.1161/ATVBAHA.109.196725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Bergdahl A, Zheng Q, Starcher B, Yanagisawa H, Davis EC. Analysis of dermal elastic fibers in the absence of fibulin-5 reveals potential roles for fibulin-5 in elastic fiber assembly. Matrix biology : journal of the International Society for Matrix Biology. 2009;28:211–220. doi: 10.1016/j.matbio.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, McGovern A, Ridley C, Cain SA, Baldwin A, Wang MC, Guo C, Mironov A, Jr, Drymoussi Z, Trump D, Shuttleworth A, Baldock C, Kielty CM. Differential regulation of elastic fiber formation by fibulin-4 and-5. The Journal of biological chemistry. 2009;284:24553–24567. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulis JT, Bellingham CM, Davis EC, Hubmacher D, Reinhardt DP, Mecham RP, Keeley FW. Fibrillins, fibulins, and matrix-associated glycoprotein modulate the kinetics and morphology of in vitro self-assembly of a recombinant elastin-like polypeptide. Biochemistry. 2008;47:12601–12613. doi: 10.1021/bi8005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus S, Fischer J, Megarbane H, Megarbane A, Jobard F, Debret R, Peyrol S, Saker S, Devillers M, Sommer P, Damour O. A p.C217R mutation in fibulin-5 from cutis laxa patients is associated with incomplete extracellular matrix formation in a skin equivalent model. The Journal of investigative dermatology. 2008;128:1442–1450. doi: 10.1038/sj.jid.5701211. [DOI] [PubMed] [Google Scholar]
- Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, Fox JE, Mancini GM, Kambouris M, Gardella R, Facchetti F, Willems PJ, Forsyth R, Dietz HC, Barlati S, Colombi M, Loeys B, De Paepe A. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nature genetics. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. American journal of medical genetics. Part A. 2007;143A:2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Djokic J, Fagotto-Kaufmann C, Bartels R, Nelea V, Reinhardt DP. Fibulin-3, -4, and-5 are highly susceptible to proteolysis, interact with cells and heparin, and form multimers. The Journal of biological chemistry. 2013;288:22821–22835. doi: 10.1074/jbc.M112.439158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. The American journal of pathology. 2007;170:578–589. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, Batge B, Davis EC, Reinhardt DP. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. The Journal of biological chemistry. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- Elahi E, Kalhor R, Banihosseini SS, Torabi N, Pour-Jafari H, Houshmand M, Amini SS, Ramezani A, Loeys B. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. The Journal of investigative dermatology. 2006;126:1506–1509. doi: 10.1038/sj.jid.5700247. [DOI] [PubMed] [Google Scholar]
- Erickson LK, Opitz J, Zhou HH. Lethal Osteogenesis Imperfecta-Like Condition with Cutis Laxa and Arterial Tortuosity in MZ Twins due to a Homozygous Fibulin-4 Mutation. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2011 doi: 10.2350/11-03-1010-CR.1. [DOI] [PubMed] [Google Scholar]
- Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, Shuttleworth A, Kielty CM. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. The Biochemical journal. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Ren Y, Bartulos O, Lee MY, Yue Z, Kim KY, Li W, Amos PJ, Bozkulak EC, Iyer A, Zheng W, Zhao H, Martin KA, Kotton DN, Tellides G, Park IH, Yue L, Qyang Y. Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation. 2012;126:1695–1704. doi: 10.1161/CIRCULATIONAHA.112.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay R, Timpl R, Kostka G. Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix biology : journal of the International Society for Matrix Biology. 1999;18:469–480. doi: 10.1016/s0945-053x(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circulation research. 2007;100:738–746. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- Hibino N, Duncan DR, Nalbandian A, Yi T, Qyang Y, Shinoka T, Breuer CK. Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts. The Journal of thoracic and cardiovascular surgery. 2012;143:696–703. doi: 10.1016/j.jtcvs.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. The EMBO journal. 2007a;26:3283–3295. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. The Journal of cell biology. 2007b;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. The Journal of biological chemistry. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Hoyer J, Kraus C, Hammersen G, Geppert JP, Rauch A. Lethal cutis laxa with contractural arachnodactyly, overgrowth and soft tissue bleeding due to a novel homozygous fibulin-4 gene mutation. Clinical genetics. 2009;76:276–281. doi: 10.1111/j.1399-0004.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- Hu Q, Loeys BL, Coucke PJ, De Paepe A, Mecham RP, Choi J, Davis EC, Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15:3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circulation research. 2010;106:583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yamashiro Y, Papke CL, Ikeda Y, Lin Y, Patel M, Inagami T, Le VP, Wagenseil JE, Yanagisawa H. Angiotensin-converting enzyme-induced activation of local angiotensin signaling is required for ascending aortic aneurysms in fibulin-4-deficient mice. Science translational medicine. 2013;5:183ra158, 181–111. doi: 10.1126/scitranslmed.3005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78:1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iascone M, Sana ME, Pezzoli L, Bianchi P, Marchetti D, Fasolini G, Sadou Y, Locatelli A, Fabiani F, Mangili G, Ferrazzi P. Extensive arterial tortuosity and severe aortic dilation in a newborn with an EFEMP2 mutation. Circulation. 2012;126:2764–2768. doi: 10.1161/CIRCULATIONAHA.112.119883. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. The Journal of biological chemistry. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Jones RP, Ridley C, Jowitt TA, Wang MC, Howard M, Bobola N, Wang T, Bishop PN, Kielty CM, Baldock C, Lotery AJ, Trump D. Structural effects of fibulin 5 missense mutations associated with age-related macular degeneration and cutis laxa. Investigative ophthalmology & visual science. 2010;51:2356–2362. doi: 10.1167/iovs.09-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappanayil M, Nampoothiri S, Kannan R, Renard M, Coucke P, Malfait F, Menon S, Ravindran HK, Kurup R, Faiyaz-Ul-Haque M, Kumar K, De Paepe A. Characterization of a distinct lethal arteriopathy syndrome in twenty-two infants associated with an identical, novel mutation in FBLN4 gene, confirms fibulin-4 as a critical determinant of human vascular elastogenesis. Orphanet journal of rare diseases. 2012;7:61. doi: 10.1186/1750-1172-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapustin A, Stepanova V, Aniol N, Cines DB, Poliakov A, Yarovoi S, Lebedeva T, Wait R, Ryzhakov G, Parfyonova Y, Gursky Y, Yanagisawa H, Minashkin M, Beabealashvilli R, Vorotnikov A, Bobik A, Tkachuk V. Fibulin-5 binds urokinase-type plasminogen activator and mediates urokinase-stimulated beta1-integrin-dependent cell migration. The Biochemical journal. 2012;443:491–503. doi: 10.1042/BJ20110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley FW, Bellingham CM, Woodhouse KA. Elastin as a self-organizing biomaterial: use of recombinantly expressed human elastin polypeptides as a model for investigations of structure and self-assembly of elastin. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2002;357:185–189. doi: 10.1098/rstb.2001.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear C, Chang WY, Khattak S, Hinek A, Thompson T, de Carvalho Rodrigues D, Kennedy K, Mahmut N, Pasceri P, Stanford WL, Ellis J, Mital S. Modeling and rescue of the vascular phenotype of Williams-Beuren syndrome in patient induced pluripotent stem cells. Stem cells translational medicine. 2013;2:2–15. doi: 10.5966/sctm.2012-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. Journal of cell science. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, Sasaki T. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. The Journal of biological chemistry. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- Le VP, Yamashiro Y, Yanagisawa H, Wagenseil JE. Measuring, Reversing, and Modeling the Mechanical Changes Due to the Absence of Fibulin-4 in Mouse Arteries. Biomech Model Mechanobiol. 2014 doi: 10.1007/s10237-014-0556-x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- Liu x, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nature genetics. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11:2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nature genetics. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- Lomas AC, Mellody KT, Freeman LJ, Bax DV, Shuttleworth CA, Kielty CM. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. The Biochemical journal. 2007;405:417–428. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Baas D, Ridley C, Jones RP, Klaver CC, Stone E, Nakamura T, Luff A, Griffiths H, Wang T, Bergen AA, Trump D. Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum Mutat. 2006;27:568–574. doi: 10.1002/humu.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cellular and molecular life sciences : CMLS. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. The Journal of clinical investigation. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. The American journal of pathology. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Kielty CM. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. Journal of cell science. 2010;123:3006–3018. doi: 10.1242/jcs.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Bakall B, Choi J, Liu Z, Sasaki T, Davis EC, Marmorstein AD, Marmorstein LY. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum Mol Genet. 2007;16:3059–3070. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, Tsao PS, Iosef C, Berry GJ, Mohr FW, Spin JM, Alvira CM, Robbins RC, Fischbein MP. miR-29b participates in early aneurysm development in Marfan syndrome. Circulation research. 2012;110:312–324. doi: 10.1161/CIRCRESAHA.111.253740. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. The Journal of biological chemistry. 2002;277:44854–44863. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- Moltzer E, te Riet L, Swagemakers SM, van Heijningen PM, Vermeij M, van Veghel R, Bouhuizen AM, van Esch JH, Lankhorst S, Ramnath NW, de Waard MC, Duncker DJ, van der Spek PJ, Rouwet EV, Danser AH, Essers J. Impaired vascular contractility and aortic wall degeneration in fibulin-4 deficient mice: effect of angiotensin II type 1 (AT1) receptor blockade. PloS one. 2011;6:e23411. doi: 10.1371/journal.pone.0023411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nature genetics. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, Sakai LY, Koli K, Naitoh M, von Melchner H, Suzuki S, Rifkin DB, Nakamura T. Latent TGF-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2852–2857. doi: 10.1073/pnas.1215779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bachinger HP, Sasaki T, Lee-Arteaga S, Zilberberg L, Rifkin DB, Ramirez F, Chu ML, Sakai LY. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. The Journal of biological chemistry. 2009;284:16872–16881. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Holm T, Veith R, Callewaert BL, Ades LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A, Trapane P, Earing MG, Coucke PJ, Sakai LY, Dietz HC, De Paepe AM, Loeys BL. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. European journal of human genetics : EJHG. 2010;18:895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock MJ, Cain SA, Freeman LJ, Morgan A, Mellody K, Marson A, Shuttleworth CA, Weiss AS, Kielty CM. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin-fibrillin-1 cross-link. The Journal of biological chemistry. 2004;279:23748–23758. doi: 10.1074/jbc.M400212200. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Molecular biology of the cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safka Brozkova D, Lassuthova P, Neupauerova J, Krutova M, Haberlova J, Stejskal D, Seeman P. Czech family confirms the link between FBLN5 and Charcot-Marie-Tooth type 1 neuropathy. Brain : a journal of neurology. 2013;136:e232. doi: 10.1093/brain/aws333. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Miosge N, Abrams WR, Rosenbloom J, Timpl R. Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS letters. 1999;460:280–284. doi: 10.1016/s0014-5793(99)01362-9. [DOI] [PubMed] [Google Scholar]
- Sawyer SL, Dicke F, Kirton A, Rajapkse T, Rebeyka IM, McInnes B, Parboosingh JS, Bernier FP. Longer term survival of a child with autosomal recessive cutis laxa due to a mutation in FBLN4. American journal of medical genetics. Part A. 2013;161A:1148–1153. doi: 10.1002/ajmg.a.35827. [DOI] [PubMed] [Google Scholar]
- Schluterman MK, Chapman SL, Korpanty G, Ozumi K, Fukai T, Yanagisawa H, Brekken RA. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Disease models & mechanisms. 2010;3:333–342. doi: 10.1242/dmm.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicot FX, Tsuda T, Markova D, Klement JF, Arita M, Zhang RZ, Pan TC, Mecham RP, Birk DE, Chu ML. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol Cell Biol. 2008;28:1061–1067. doi: 10.1128/MCB.01876-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideek MA, Menz C, Parsi MK, Gibson MA. LTBP-2 competes with tropoelastin for binding to fibulin-5 and heparin, and is a negative modulator of elastinogenesis. Matrix biology : journal of the International Society for Matrix Biology. 2013 doi: 10.1016/j.matbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, Eastman CG, Casavant TL, Sheffield VC. Missense variations in the fibulin 5 gene and age-related macular degeneration. The New England journal of medicine. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nature reviews. Molecular cell biology. 2003;4:479–489. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- Trask TM, Trask BC, Ritty TM, Abrams WR, Rosenbloom J, Mecham RP. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. The Journal of biological chemistry. 2000;275:24400–24406. doi: 10.1074/jbc.M003665200. [DOI] [PubMed] [Google Scholar]
- Urban Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi H, Nonaka R, Sato F, Shibata-Sato K, Ishida M, Iketani S, Maeda I, Okamoto K, Urban Z, Onoue S, Seyama Y. Characterization of the molecular interaction between tropoelastin and DANCE/fibulin-5. Journal of biochemistry. 2008;143:633–639. doi: 10.1093/jb/mvn014. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth defects research. Part C, Embryo today : reviews. 2007;81:229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Tsuruga E, Nakashima K, Sawa Y, Ishikawa H. Fibulin-4 and -5, but not Fibulin-2, are Associated with Tropoelastin Deposition in Elastin-Producing Cell Culture. Acta histochemica et cytochemica. 2010;43:131–138. doi: 10.1267/ahc.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int J Biochem Cell Biol. 2010;42:1084–1093. doi: 10.1016/j.biocel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- Yeo GC, Keeley FW, Weiss AS. Coacervation of tropoelastin. Advances in colloid and interface science. 2011;167:94–103. doi: 10.1016/j.cis.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Yue W, Sun Q, Landreneau R, Wu C, Siegfried JM, Yu J, Zhang L. Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer research. 2009;69:6339–6346. doi: 10.1158/0008-5472.CAN-09-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Davis EC, Richardson JA, Starcher BC, Li T, Gerard RD, Yanagisawa H. Molecular analysis of fibulin-5 function during de novo synthesis of elastic fibers. Mol Cell Biol. 2007;27:1083–1095. doi: 10.1128/MCB.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]