Abstract
The effects of physical activity on cognition in older adults have been extensively investigated in the last decade. Different interventions such as aerobic, strength, and gross motor training programs have resulted in improvements in cognitive functions. However, the mechanisms underlying the relationship between physical activity and cognition are still poorly understood. Recently, it was shown that acute bouts of exercise resulted in reduced executive control at higher relative exercise intensities. Considering that aging is characterized by a reduction in potential energy ( max − energy cost of walking), which leads to higher relative walking intensity for the same absolute speed, it could be argued that any intervention aimed at reducing the relative intensity of the locomotive task would improve executive control while walking. The objective of the present study was to determine the effects of a short-term (8 weeks) high-intensity strength and aerobic training program on executive functions (single and dual task) in a cohort of healthy older adults. Fifty-one participants were included and 47 (age, 70.7 ± 5.6) completed the study which compared the effects of three interventions: lower body strength + aerobic training (LBS-A), upper body strength + aerobic training (UBS-A), and gross motor activities (GMA). Training sessions were held 3 times every week. Both physical fitness (aerobic, neuromuscular, and body composition) and cognitive functions (RNG) during a dual task were assessed before and after the intervention. Even though the LBS-A and UBS-A interventions increased potential energy to a higher level (Effect size: LBS-A—moderate, UBS-A—small, GMA—trivial), all groups showed equivalent improvement in cognitive function, with inhibition being more sensitive to the intervention. These findings suggest that different exercise programs targeting physical fitness and/or gross motor skills may lead to equivalent improvement in cognition in healthy older adults. Such results call for further investigation of the multiple physiological pathways by which physical exercise can impact cognition in older adults.
Keywords: Energy cost of walking, Peak oxygen uptake, Potential energy, Dual task, Cognition, Mobility
Introduction
The effects of physical activity on cognition in older adults have been extensively investigated in the last decade (see Bherer et al. 2013 for a review). Intervention (Kramer et al. 1999; Renaud et al. 2010b; Langlois et al. 2012) as well as cross sectional (Renaud et al. 2010a; Boucard et al. 2012; Berryman et al. 2013) and longitudinal (Yaffe et al. 2001; Barnes et al. 2003; Larson et al. 2006) studies suggest that higher physical fitness levels are associated with better cognitive functions. Different review articles and meta-analyses of intervention studies also support these results, which tend to confirm the beneficial effects of physical activity on cognitive functions and mental health in older adults (Smith et al. 2010; Colcombe and Kramer 2003; Hillman et al. 2008; Voss et al. 2011; Angevaren et al. 2008; Matta Mello Portugal et al. 2013). Moreover, it seems that physical fitness has a selective enhancing effect on executive functions (Kramer et al. 1999; Colcombe and Kramer 2003; Smiley-Oyen et al. 2008). However, the mechanisms underlying this relationship are still poorly understood. The cardiovascular hypothesis suggests that aerobic fitness, measured by maximal oxygen uptake ( O2 max), is the main physiological mediator, which determines cognitive functions. However, this hypothesis has been questioned (Etnier et al. 2006). Indeed, improvements in cognition were reported independently of aerobic fitness after a physical training intervention (Smiley-Oyen et al. 2008). Among the other effective physical training interventions, it seems that strength training represents a privileged stimulation that could have an additive effect on cognition when compared to aerobic training (Colcombe and Kramer 2003). It was suggested that aerobic and strength training improved cognition through different molecular pathways (BDNF and IGF-1, respectively) known for their effect on neuronal growth, survival, and differentiation (Voss et al. 2011; Cassilhas et al. 2012). Moreover, it was recently suggested that gross motor training involving coordination, balance, and agility activities led to improvements in cognition independently of aerobic fitness (Voelcker-Rehage et al. 2011; Forte et al. 2013). However, to our knowledge, no studies compared the effects of these three interventions (aerobic, strength, and gross motor activities) on cognitive functions.
The link between motor function and cognition in older adults has gained increasing interest over the last few years. The combination of a locomotive and a cognitive task, known as the dual-task paradigm, is commonly used in mobility assessment and fall prevention (Beauchet et al. 2009). It implies that two tasks executed simultaneously will result in an altered performance in one or both tasks in comparison to performance in one task alone (Yogev-Seligmann et al. 2008). In an interesting study, increased risk of falling was reported in older adults who had to stop walking when talking (Lundin-Olsson et al. 1997). Different explanations have been proposed to explain this phenomenon. Among them, the capacity-sharing theory suggests that attentional resources are limited which could explain why performance in one or two attention-demanding tasks executed in parallel could potentially deteriorate (Yogev-Seligmann et al. 2008). Interestingly, aging is related to a reduced max (Hawkins and Wiswell 2003; Fleg et al. 2005) and a greater metabolic energy cost of walking (MECW) (Malatesta et al. 2003). These phenomena were described as a reduction in potential energy, defined as the energy available above what is essential for independent living (Schrack et al. 2010), and could lead to an increase in the relative effort associated with usual gait speed. Recently, a report from our research group suggested that executive control during acute bouts of exercises declined at higher relative physical effort intensities (Labelle et al. 2013). Therefore, one could argue that increasing the potential energy available could represent one mechanism by which physical training interventions lead to better cognitive functions in a dual-task situation. Indeed, the efficiency of high-intensity interval training to increase max has been demonstrated in older adults (Nemoto et al. 2007; Guiraud et al. 2012) and it appears that lower body strength training could represent an effective method to decrease MECW (Romero-Arenas et al. 2013; Karlsen et al. 2009). It was suggested that changes in fiber type distribution, reduced contribution of type 2 fibers, higher rate of force development, improved stretch-shortening cycle, and better intermuscular coordination could at least partially explain the beneficial effects of a strength training program on the metabolic energy cost of locomotion (Ronnestad and Mujika 2013; Perrault 2006; Hortobagyi et al. 2011). Along with these neuromuscular adaptations, changes in mitochondrial function and efficiency could also explain the relationship between strength training and MECW (Perrault 2006).
The objective of the present study was to assess the effects of a short-term high-intensity strength and aerobic training program on executive functions in a cohort of healthy older adults. Our hypothesis was that combined high-intensity training with emphasis on lower body strength would increase peak oxygen uptake and reduce the MECW more than a similar intervention focusing on upper body resistance training or gross motor activities. By increasing the potential energy available, the lower-body training program would reduce the relative intensity of the locomotive task, thereby reducing the attentional load related to walking. Ultimately, these fitness adaptations would allow an individual to allocate more attention to a cognitive task involving executive functions, which should result in better cognitive performance in a dual-task situation.
Methods
Overview
Participants included in this study were asked to complete an 8-week training protocol for a total of 24 training sessions of approximately 60 min each. Participants had to complete a cognitive, physical fitness, and functional capacity assessment both prior to and after the training protocol in order to monitor training adaptations.
Participants available for the entire duration of the study aging between 60 and 85 years old were considered for inclusion. Participants were excluded if they were taking medication known to have an effect on gait and balance (benzodiazepines, neuroleptics, antidepressants), as well as being diagnosed with any significant orthopaedic, neurological, cardiovascular, or respiratory problem. A diagnosis of a progressive somatic or psychiatric disease was also considered as an exclusion criterion. In addition, being under general anesthesia in the 6 months prior to the beginning of the study, restricted mobility (use of walking aid), movement disorders, epilepsy, and major visual or hearing impairments were among other reasons to exclude participants. Potential participants were also excluded if they smoked or had uncontrolled alcohol or drug abuse. Finally, a minimal score of 24 on the Mini Mental State Examination (MMSE) (Folstein et al. 1975) was required to be included in the study. All criteria were assessed during a telephone screening and the first scheduled meeting at the research center. A geriatrician and a neuropsychologist completed, as described elsewhere (Langlois et al. 2012), an evaluation to confirm that all participants met the study’s requirements. Briefly, five main components were investigated by the geriatrician: (1) medical and family medical history, (2) functional capacity (questionnaire on the ability to perform activities of daily living—ADL and instrumental activities of daily living—IADL), (3) medication list, (4) general overview of all physiological systems, (5) physical examination. The neuropsychological battery assessed global cognitive functioning (MMSE), abstract verbal reasoning (similarities of the Weschler Adult Intelligence Scale—WAIS III), processing speed (Digit Symbol Coding subtest of the WAIS III), working memory (Digit Span backward/forward subtests of the WAIS III) and executive functions (inhibition/flexibility conditions of the modified Stroop Color-Word Test). Scores for inhibition and flexibility were computed by subtracting the average of the naming/reading conditions from the inhibition or the flexibility components (Langlois et al. 2012). Using this ratio, smaller difference scores are associated with better executive function abilities. In this study, to obtain a general executive function score, results for inhibition and flexibility were added together. As for the other remaining cognitive tests, higher scores represented better performances. Two questionnaires were also used to assess: (1) sleep quality (Pittsburgh Sleep Quality Index—PSQI) (Buysse et al. 1989) and, (2) the Profile of Mood States (POMS) (Cayrou et al. 2000). Scores were computed as previously reported (Berryman et al. 2013).
Once included, the participants signed a written statement of informed consent. The protocol and procedures had been reviewed and approved by the Research Ethics Board of the Geriatric Hospital where the research took place. In addition, the study was conducted in accordance with recognized ethical standards and national/international laws. Furthermore, upon inclusion, participants were told to avoid any changes in their daily routines and eating habits.
After the first appointment at the research center, participants were randomized into three different interventions: (1) aerobic training combined with strength training of the lower body (LBS-A), (2) aerobic training combined with strength training of the upper body (UBS-A), and (3) gross motor activities (GMA). While LBS-A was considered as the main treatment with regards to the hypothesis for this study, UBS-A served as a control for energy expenditure during the protocol. Briefly, participants in the third group (GMA) were involved in stretching, locomotion, manipulation, and relaxation activities and this group served as a control for social interactions.
Tests and measures
All tests were completed at the research center of the geriatric institution where the study took place. A detailed schedule of the tests and measures is presented in Table 1.
Table 1.
Study overview
| Weeks | Sessions | Tests, Measures and training |
|---|---|---|
| 1 | 1 | 1- Medical and neuropsychological assessment, , treadmill familiarization |
| 2 | 2–3 | 2- Metabolic energy cost of walking and executive functions in a dual task, functional capacity 3- Isokinetic strength assessment |
| 3–4 | 4–7 | 4- Body composition, strength training familiarization 5–6- Strength training familiarization 7- Inertial strength assessment |
| 5–12 | 8–31 | 8 to 30- Training 31- Body composition, inertial strength assessment |
| 13 | 32 | 32- |
| 14 | 33–34 | 33- Metabolic energy cost of walking and executive functions in a dual task, Functional capacity 34- Isokinetic strength assessment |
Physiological assessment
Peak oxygen uptake
was determined during a maximal continuous graded test performed on an ergocycle (Corival Recumbent, Lode B.V., Groningen, The Netherlands) as previously described (Berryman et al. 2013). Initial mechanical power was set at 50 watts for males and 35 watts for females. Power was then increased by 15 watts every 60 s, with a fixed pedaling cadence of 60 to 80 revolutions per minute. Strong standardized verbal encouragements were given throughout the test. Termination criterion was the inability to maintain the required pedaling cadence. The highest (Moxus, AEI Technologies, Naperville, IL, USA) over a 30-s period during the test was considered as (in ml.kg-1.min-1).
Metabolic energy cost of walking
MECW was assessed during a specific experimental session using procedures described in a previous lab report (Berryman et al. 2012). After a familiarization protocol, participants had to perform three 6-min constant speed tests at 2.4, 4, and 5.6 km h−1 in a random order (ABC, BCA, CAB), interspersed by a 3-min standing recovery period. Mean (Moxus, AEI Technologies, Naperville, IL, USA) of the two last minutes of each walking condition was considered as walking metabolic demand and then divided by walking speed to obtain the gross metabolic energy cost of walking (in ml.kg-1.km-1). Caloric unit cost (in kcal.kg-1.km-1) was calculated as described elsewhere (Fletcher et al. 2009). Potential energy was defined as the difference between peak oxygen uptake and gross oxygen uptake at a submaximal walking speed (Schrack et al. 2010).
Cognition
Executive functions in a dual task
Executive functions were assessed with the random number generation task (RNG) (Audiffren et al. 2009). Participants had to randomly produce sequences of digits (using numbers from 1 to 9), at a precise rhythm of one answer per second. This task was considered complete after 100 answers were given. Following the MECW assessment, participants were first familiarized with this task. Afterwards, participants had to complete the task five times: a first time at rest while standing still on the treadmill (single task 1), three times while walking at all three experimental speeds (dual task 1, 2, and 3), and a last time at rest while standing on the treadmill (single task 2). During the dual-task conditions, walking speed sequence and testing conditions were the same as they were during the MECW assessment (three 6-min constant speed tests interspersed by a 3-min standing recovery period). However, no oxygen measurements were recorded. The dual-task condition actually occurred at the fourth minute of every constant speed walking test. Since 100 correct answers were expected at a rhythm of one answer per second, participants walked at least 5 min and 40 s at every speed. Data was analyzed with the RgCalc software (Towse and Neil 1998). A value for six different scores was computed. While the Turning Point Index (TPI—changes between ascending and descending phases), adjacency score (numbers presented in pairs; 3–4) and runs score (consecutive numbers mentioned in an ascending phase) are related to inhibition, the redundancy index (R—redundancy in answers), coupon score (number of answers before giving all possibilities) and the mean repetition gap (MRG—mean of given answers before a repetition occurs) are considered as measures of updating/working memory (Audiffren et al. 2009). Single task performance was obtained by averaging scores of both single-task conditions. A higher score characterizes improvements in TPI and MRG, while for all other indices (adjacency, runs, R, coupon), better performances are related to lower scores.
Functional capacity
After the MECW and executive functions assessment, participants were asked to complete a short battery of five functional capacity tests. The tests consisted of handgrip strength (maximal isometric voluntary contraction) (Abizanda et al. 2012), lower body muscular endurance (30-s chair stand) (Jones et al. 1999) and mobility (timed up and go (Podsiadlo and Richardson 1991), 10-m maximal walking speed (Berryman et al. 2013), and 6-min walk test (Kervio et al. 2003)).
Isokinetic strength assessment
Concentric muscular strength was assessed bilaterally at three joints (knee, ankle, hip) using an isokinetic dynamometer (Biodex III, Biodex Medical Systems, New York, USA) (Milot et al. 2007). In the present report, only the results for the dominant leg, defined as the preferred kicking leg (Hartmann et al. 2009), will be presented. Gravity adjustment was accounted for by using the dynamometer software before each measurement. Maximal strength was assessed for lower limb joints (knee, ankle, and hip—flexors and extensors) while the rate of force development was assessed only for the knee extensors (for details, see Berryman et al. 2013).
Body composition assessment
Body composition was assessed using a standard dual-energy x-ray absorptiometry (DXA—Lunar Prodigy; GE Healthcare, Madison WI, USA) protocol (Nana et al. 2013). Participants were asked to empty their bladder prior to the test, to wear light exercise clothes, and to remove all jewelry and metal objects. Considering that it was not possible to complete all assessments in the morning, participants were not asked to arrive in a fasted state. Calibration was completed each morning according to the manufacturer’s guidelines. The same trained operator completed each scan. Data analyses were done with GE Encore software (enCORE2011, GE Healthcare, version 13.60).
Inertial strength assessment (1 RM)
Functional maximal strength was assessed using the one repetition maximal (1 RM) inertial method as described previously (Verdijk et al. 2009). For participants in the UBS-A group, 1 RM was assessed with the seated press exercise whereas participants in the LBS-A were assessed with the leg press exercise. GMA group was randomly divided into two subgroups; one being assessed on the seated press (GMA-U; n = 8) while the other was tested on the leg press (GMA-L; n = 8). Before the pre intervention testing session, all participants had to complete three familiarization sessions. All exercises were completed on guided devices (Atlantic Inc., Laval, Quebec, Canada). During the first session, loads were rather light and emphasis was on positioning, postural control, and exercise execution. In sessions 2 and 3, loads were gradually increased in order to reach 10–12 RM on the main exercise (leg press or seated press) by the end of session 3. All three sessions occurred within 2 weeks, typically three rest days apart (Monday–Friday). 1 RM post intervention tests were held during the last training session. Since high-intensity was maintained during this session, it was also considered as a training session.
Training intervention
The 2-month intervention involved three training sessions of approximately 60 min weekly for a total of 24 sessions. All sessions were held at the gym facility of the geriatric institution. Typically, training sessions were held on Mondays (day 1), Wednesdays (day 2), and Fridays (day 3). A graduate student in kinesiology supervised all training sessions in a one to four coach/participants ratio. Each session started with a general-to-specific warm-up period followed by main activities. For UBS-A and LBS-A, strength exercises were always completed before aerobic training.
Warm-up
The first 10 min were the same for all groups and consisted of a general warm-up using one of the three available ergometers (recumbent bike, elliptical, or treadmill). Clear instructions were given to participants to select different ergometers from session to session. After the general warm-up, participants in the GMA group were directed to their main activities whereas participants from the UBS-A and LBS-A group executed a more specific warm-up, which consisted of light strength exercises (UBS-A: pushes and pulls using elastic bands, LBS-A: chair stands).
Aerobic training
On days 1 and 3, aerobic training consisted of a high-intensity interval protocol. Briefly, participants had to perform 15-s bouts of cycling on a recumbent ergometer (LifeFitness, Kinequip, St-Hubert, Quebec, Canada) at an intensity corresponding to the maximal aerobic power (MAP) measured during the incremental test. After each high-intensity bout, an active 15-s recovery was prescribed at an intensity corresponding to 60 % of the MAP. Each session involved two sets with each set lasting between 4 and 7 min. Therefore, during each set, participants had to perform between 2 and 3.5 min of cycling at their MAP. Exact procedures regarding volume periodization are presented in Table 2. A recovery period of 5 min was allowed between sets. On day 2, a continuous 20-min cycling protocol was established. Intensity was set at 60 % for the first 4 weeks and at 65 % for the remainder of the program.
Table 2.
Aerobic training prescription
| Week | Volume Sets × reps × time |
|---|---|
| 5 and 9 | 2 × 10 × 15 s |
| 6 and 10 | 2 × 12 × 15 s |
| 7 and 11 | 2 × 14 × 15 s |
| 8 and 12 | 2 × 8 × 15 s |
High-intensity bouts at maximal aerobic power (MAP)
Active recovery between bouts (15 s at 60 % MAP)
Passive recovery between sets (5 min)
Strength training
Strength training was similar in terms of volume and intensity for both UBS-A and LBS-A groups. During each training session, participants had to complete four rounds of a two- to three-station circuit that started with exercises for strength development (4–8 RM) followed by exercises planned for strength endurance development (12–20 RM). In a circuit round, rest periods corresponded to the time it took to go from one station to the other (approximately 30 s). Before starting another circuit round, a rest period of 2 min was allowed. For the LBS-A group, the leg press and body weight plantar flexion exercises were prescribed on days 1 and 3 (i.e., each performed for a total of 16 days). The leg extension and leg flexion exercises were executed on alternate days (i.e., each performed for a total of 12 days) whereas the floor hip extension exercise was on day 2 only (i.e., performed for a total of 8 days). For the UBS-A group, two different training sessions were prescribed on alternate days: day 1 (12 sessions) consisted of seated chest presses and shoulder lateral/frontal abductions and day 2 (12 sessions) consisted of wrist flexions, seated horizontal rowing, and shoulder external rotations. Training prescription for all exercises was made in accordance to the ACSM guidelines for strength development in older adults (ACSM 1998). Details regarding training volume and intensity are presented in Table 3.
Table 3.
Strength training prescription
| Lower body | ||
|---|---|---|
| Days 1 and 3 | ||
| 1- Leg press 4 sets of 4–6 RM (Guided device) |
2- Leg extension or flexion (Alternate days) 4 sets of 6–8 RM (Guided device) |
3- Standing plantar flexion Weeks 1–4: bilateral Weeks 5–8: unilateral 4 sets of 20 repetitions (Body weight) |
| Day 2 | ||
| 1- Leg extension or flexion (Alternate days) 4 sets of 6–8 RM (Guided device) |
2- Unilateral hip extension 4 sets of 12 repetitions (Body weight) |
|
| Upper body | ||
| Day 1 | ||
| 1- Seated chest press 4 sets of 4–6 RM (Guided device) |
2- Shoulder frontal/lateral abductions 4 sets of 20 RM (Free weights) |
3- Wrist flexion 4 sets of 12 RM (Free weights) |
| Day 2 | ||
| 1- Horizontal rowing 4 sets of 4–6 RM (Guided device) |
2- Shoulder external rotations 4 sets of 12 repetitions (Elastic bands) |
|
Rest between stations: 30 s approximately
Rest between circuit rounds: 2 min
RM repetitions maximum
GMA training
During the first 2 weeks, stretching activities were prescribed in order to improve overall body flexibility. After joint mobilization exercises, different static stretching exercises were maintained for a duration of 20–30 s. These exercises were done in a variety of positions: standing or seated on a chair or on a yoga mat. To complete these first six sessions, time was spent doing relaxation exercises focusing on different patterns to slow down breathing. At this point, participants were in a supine position on a yoga mat. For the next 3 weeks, training sessions started with some locomotion exercises in which participants had to walk through obstacles and carry different objects (balls) to a given goal. The remainder of the sessions was dedicated to stretching and relaxation exercises as executed in the first six sessions. During the last 3 weeks, all training sessions started with 15 min of ball manipulation. Aside from classic juggling lessons, other games such as throwing a ball to a fixed target (basket) were presented to participants. These final nine sessions ended with a recall on previous exercises: locomotion, stretching, and relaxation exercises.
Statistical analysis
Standard statistical methods were used for the calculation of means and standard deviations. Normal Gaussian distribution of the data was verified by the Shapiro–Wilk test and homogeneity of the variance by the Levene test. Baseline differences were assessed with one-way ANOVAs for all variables showing a normal distribution and homogeneity of the variance. Otherwise, between group differences were assessed with the Kruskal–Wallis test. Differences between groups for the categorical variables from the medical/cognitive domain were assessed with a Chi2 test. Training-related effects were analyzed using two-way ANOVAs (time × group) with repeated measures on the time factor. The magnitude of the observed differences on the time factor was assessed for each group by Hedges’ g (g) (Dupuy et al. 2012). As proposed by Cohen (Cohen 1988), the magnitude of the effect was considered small (0.2 < ES ≤ 0.5), moderate (0.5 < ES ≤ 0.8), or large (ES > 0.8). When an interaction was found, relative differences ((post-training − pre-training)/pre-training × 100) were compared between groups using one-way ANOVAs. If the one-way ANOVA was significant, post hoc analyses using the Bonferroni test were completed. Pearson’s correlations coefficients were computed to verify the association between changes in fitness (potential energy) and dual-task performance. We considered a correlation over 0.90 as very high, between 0.70 and 0.89 as high and between 0.50 and 0.69 as moderate (Munro 1997). Significance level was set at p < 0.05 for all analyses. Statistical tests were conducted with the IBM SPSS statistics software, version 20.
Results
Participation
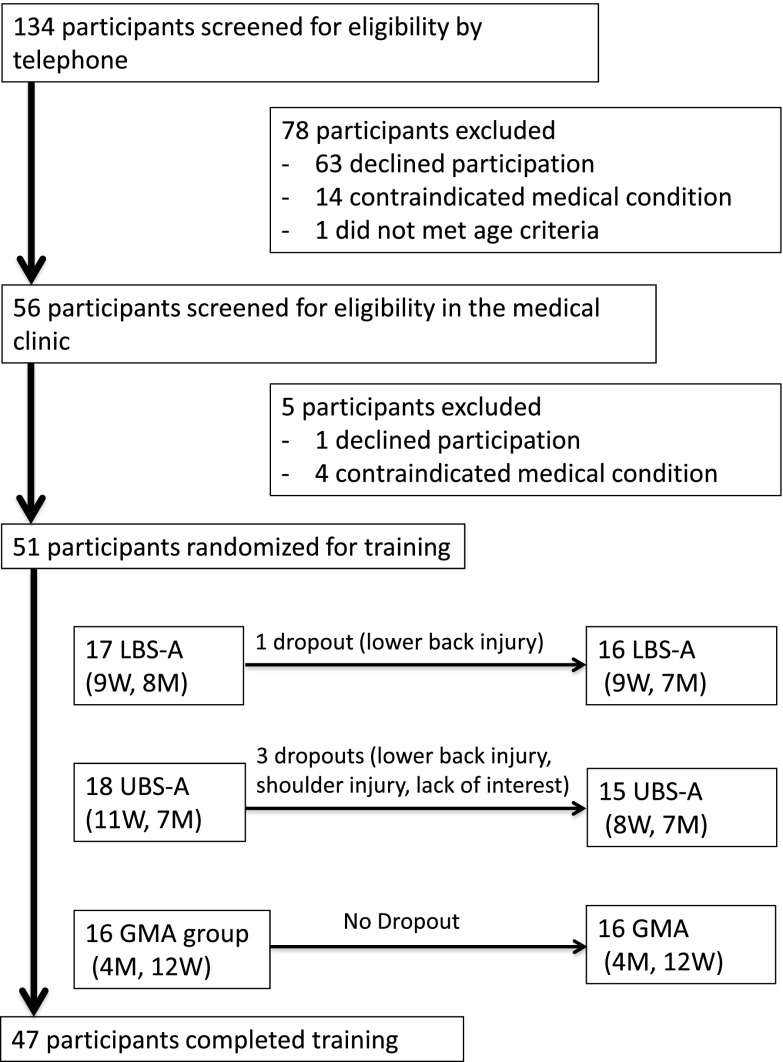
As described in Fig. 1, 51 participants were randomized into one of the three experimental groups. After inclusion in the study, one participant from the LBS-A group and three participants from the UBS-A group decided to stop, thus 47 participants completed the intervention. In three cases, previous injuries (2 lower back – 1 shoulder) came back after involvement in strength straining. The other dropout was related to a lack of interest. Participants’ medical and cognitive characteristics at enrolment are presented in Table 4. Differences between groups were significant (p < 0.05) for sleep quality, executive functions, and arthritis. As shown in Tables 5, 6, 7, and 8, participants in the GMA group tended to be less physically fit in all assessed domains (body composition, aerobic capacity, strength, and functional capacity).
Fig. 1.
Flow chart
Table 4.
Medical/cognitive characteristics of participants
| General | LBS-A | UBS-A | GMA |
|---|---|---|---|
| Gender (number of females/males) | 9/7 | 8/7 | 12/4 |
| Age (years) | 69.8 (3.9) | 69.5 (6.1) | 72.7 (6.3) |
| Education (years) | 15.3 (3.4) | 14.9 (2.4) | 14.1 (3.0) |
| POMSGlobal (score) | 8.1 (21.1) | 10.3 (17.9) | 33.7 (39.9) |
| PSQIGlobal—score/21 | 4.9 (3.8)a0.028 | 4.1 (2.5)a0.002 | 7.7 (3.3) |
| Number of daily medications | 3.8 (3.4) | 2.9 (2.9) | 4.8 (2.3) |
| Cognition | |||
| MMSE—score/30 | 29.0 (1.1) | 28.6 (0.9) | 28.9 (1.2) |
| Verbal reasoning—score/32 | 22.8 (6.2) | 20.2 (4.2) | 20.4 (5.5) |
| Processing speed—score/132 | 62.6 (14.9) | 52.6 (9.6) | 59.1 (14.9) |
| Working memory—score/30 | 17.9 (4.3) | 16.3 (4.4) | 15.1 (3.3) |
| Executive functions—s | 66.8 (10.6)a0.004 | 73.2 (14.7) | 90.9 (26.5) |
| Cardiovascular diseases | |||
| Hypertension | 3 | 5 | 8 |
| Diabetes | 0 | 3 | 1 |
| Dyslipidemia | 3 | 5 | 5 |
| Angina | 1 | 0 | 0 |
| Infarctus | 0 | 1 | 0 |
| Arrhythmia | 1 | 1 | 1 |
| Valvular disease | 0 | 1 | 2 |
| Pulmonary diseases | |||
| COPD | 0 | 0 | 1 |
| Asthma | 1 | 1 | 1 |
| Musculoskeletal disorders | |||
| Arthritis | 6 | 12b0.029 | 14b0.003 |
| Osteoporosis | 4 | 3 | 5 |
| History of fractures | 2 | 5 | 5 |
| Other musculoskeletal problems | 8 | 9 | 8 |
| History of falls | 2 | 2 | 1 |
| History of depression | 1 | 2 | 1 |
| Sedentary lifestyle | 1 | 2 | 2 |
Data are reported as mean (SD) or number of participants
a p value, different from GMA at baseline
b p value, different from LBS-A at baseline
Table 5.
Anthropometric characteristics
| LBS-A (n = 16) | UBS-A (n = 14) | GMA (n = 16) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| BMI (kg/m2) | 24.4 (2.4) | 24.1 (2.3) | 26.1 (4.0) | 26.0 (3.8) | 26.7 (4.6) | 26.5 (4.5) |
| Waist (cm) | 86.8 (7.9) | 87.9 (8.2) | 92.8 (12.3) | 93.2 (12.4) | 91.1 (9.7) | 92.4 (8.9) |
| FMa<0.001* (%) | 31.6 (4.8)b0.014 | 30.6 (4.6) | 32.6 (7.6)b0.048 | 32.0 (7.8) | 39.0 (8.4) | 39.0 (8.8) |
| FFMa0.01** (%) | 65.8 (4.9)b0.029 | 66.8 (4.7) | 64.4 (7.1) | 65.2 (7.4) | 59.1 (8.5) | 58.7 (8.2) |
| BMD (g/cm2) | 1.07 (0.11) | 1.08 (0.11) | 1.15 (0.16) | 1.14 (0.15) | 1.12 (0.13) | 1.11 (0.11) |
Data are reported as mean (SD)
BMI body mass index, FM fat mass, FFM fat-free mass, BMD bone mineral density
a p value, time effect
b p value, different from GMA at baseline
Interaction: *p = 0.009, **p = 0.003
Table 6.
Aerobic performance
| LBS-A (n = 16) Except MECW (n = 14) |
UBS-A (n = 15) | GMA (n = 16) Except MECW (n = 15) |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
|
a,b<0.001* (ml kg−1 min−1) |
26.6 (4.1)c 0.001 | 29.0 (4.3) | 25.4 (6.0)c0.012 | 28.3 (5.8) | 20.1 (4.3) | 19.9 (4.5) |
| MECW (kcal kg−1 km−1) | ||||||
| 2.4 km h−1 b<0.001 | 1.37 (0.17) | 1.30 (0.13) | 1.36 (0.22) | 1.33 (0.21) | 1.41 (0.19) | 1.27 (0.18) |
| 4 km h−1 b0.005** | 0.98 (0.13) | 0.95 (0.12) | 0.98 (0.14) | 0.98 (0.16) | 1.00 (0.10) | 0.93 (0.06) |
| 5.6 km h−1 b0.012 | 0.95 (0.11) | 0.93 (0.11) | 0.98 (0.14) | 0.95 (0.13) | 0.95 (0.09) | 0.90 (0.08) |
Data are reported as mean (SD)
MECW metabolic energy cost of walking
aPeak oxygen uptake
b p value, time effect
c p value, different from GMA at baseline
Interaction: *p < 0.001, **p = 0.031
Table 7.
Neuromuscular performance
| LBS-A (n = 16) Except KEXT and KFLEX (n = 15) |
UBS-A (n = 15) Except HEXT and HFLEX (n = 14) |
GMA (n = 16) Except ADF, HEXT, and HFLEX (n = 14) Except APF (n = 15) |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Isokinetic strength (Nm kg−1) | ||||||
| APF 180°/s a0.004 | 2.16 (0.42) b0.001 | 2.05 (0.38) | 2.00 (0.51)b0.013 | 1.82 (0.54) | 1.49 (0.59) | 1.39 (0.47) |
| ADF 30°/s a<0.001 | 0.37 (0.06)b0.008 | 0.33 (0.08) | 0.33 (0.08) | 0.31 (0.07) | 0.30 (0.07) | 0.27 (0.08) |
| KEXT 60°/s | 1.77 (0.38)b0.012 | 1.81 (0.36) | 1.74 (0.59)b0.019 | 1.78 (0.60) | 1.22 (0.51) | 1.28 (0.49) |
| KFLEX 60°/s | 0.86 (0.21) | 0.94 (0.25) | 0.91 (0.28)b0.021 | 0.86 (0.24) | 0.65 (0.27) | 0.71 (0.30) |
| HEXT 120°/s | 1.70 (0.30) | 1.73 (0.49) | 1.64 (0.50) | 1.52 (0.55) | 1.48 (0.53) | 1.41 (0.59) |
| HFLEX 120°/s* | 1.33 (0.37)b0.029 | 1.40 (0.33) | 1.38 (0.55)b0.039 | 1.04 (0.35) | 0.87 (0.37) | 0.95 (0.38) |
| MIVC** | 2.09 (0.33) | 2.30 (0.47) | 2.05 (0.61) | 2.02 (0.64) | 1.64 (0.61) | 1.58 (0.48) |
| Rate of force development (Nm s−1 kg−1) | ||||||
| Maximum | 8.23 (3.36) | 10.07 (5.78) | 7.75 (4.11) | 7.87 (3.28) | 7.33 (5.90) | 7.22 (4.98) |
Data are reported as mean (SD)
APF ankle plantar flexors, ADF ankle dorsi flexors, KEXT knee extensors, KFLEX knee flexors, HEXT hip extensors, HFLEX hip flexors, MIVC maximal isometric voluntary contraction
a p value, time effect
b p value, different from GMA at baseline
Interaction: *p < 0.001, **p = 0.027
Table 8.
Functional capacity
| LBS-A (n = 16) | UBS-A (n = 15) | GMA (n = 16) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Gripa0.001 (kg) | 43.0 (18.7) | 44.7 (20.6) | 44.9 (18.1) | 49.4 (18.6) | 37.7 (13.5) | 38.6 (15.4) |
| TUG (s) | 5.1 (0.6)b0.003 | 4.9 (0.6) | 5.4 (0.9)b0.046 | 5.3 (0.7) | 6.2 (1.0) | 6.1 (1.1) |
| CHSTa0.009 (repetitions) | 19.7 (5.3)b0.001 | 20.1 (6.2) | 16.3 (5.0) | 17.3 (4.1) | 13.3 (3.0) | 15.0 (4.0) |
| 10MWTa<0.001 (s) | 4.5 (0.6)b0.002 | 4.3 (0.7) | 4.7 (0.8)b0.02 | 4.4 (0.8) | 5.5 (0.8) | 5.2 (0.9) |
| 6MWTa0.001 (m) | 636 (48)b0.002 | 650 (51) | 586 (79) | 616 (72) | 548 (75) | 559 (83) |
Data are reported as mean (SD)
TUG timed up and go, CHST chair stands, 10MWT 10-m maximal walking test, 6MWT 6-min walk test
a p value, time effect
b p value, different from GMA at baseline
Compliance to the training program was very high. Participants who completed the protocol in its entirety attended on average 96.9 % (±4 %) of all training sessions. No significant differences were found between groups.
Because of personal beliefs, one participant from the UBS-A group refused to complete the body composition assessment. Measurement errors resulted in some data being removed from the analysis. For the MECW, data from three participants (two LBS-A, one GMA) was not considered because of air leakage in the facemask that was not detected during the evaluation period. Data for one participant from the GMA-L group was removed from the 1 RM analysis because of a significant difference in positioning while performing pre- and post-strength assessment, which resulted in an outlier value after the intervention. Finally, because some participants experienced specific joint pain while performing the isokinetic strength assessment, some data was removed from the analysis (knee extension, one LBS-A; knee flexion, one LBS-A and one GMA; ankle dorsiflexors, two GMA; ankle plantar flexors, one GMA; hip extensors and flexors, two GMA and one UBS-A).
Body composition
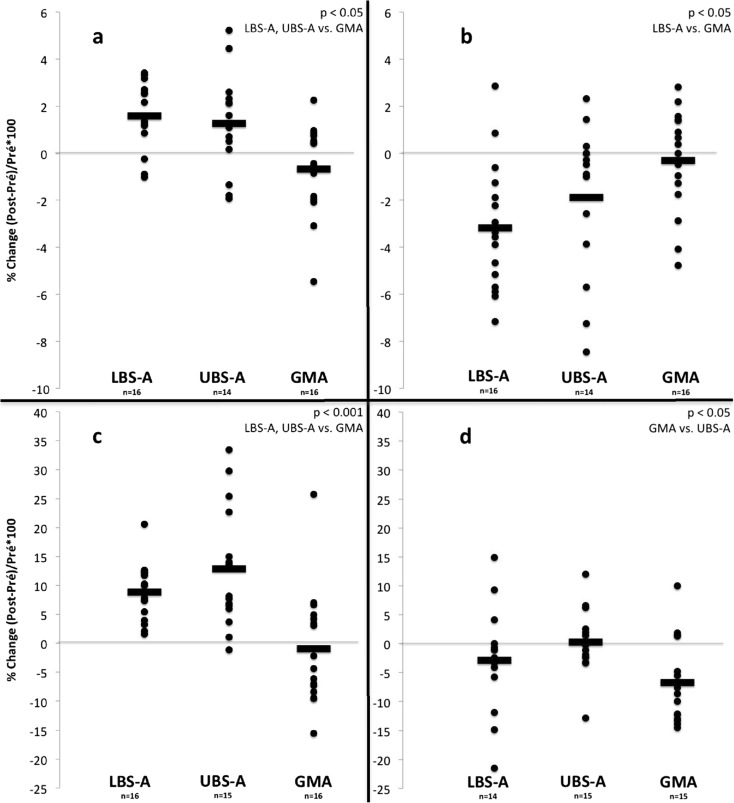
A two-way ANOVA with repeated measures revealed an effect of time (p < 0.05) and a time by group interaction (p < 0.05) for both fat and fat-free mass (Table 5). A one-way ANOVA with Bonferroni post hoc tests showed that the observed pre–post relative changes (see Fig. 2a, b) were significantly different between groups for fat-free mass (LBS-A and UBS-A vs. GMA; p < 0.05) and for fat mass (LBS-A vs. GMA; p < 0.05). The magnitude of the pre–post difference for fat and fat-free mass was considered small for LBS-A (g = −0.20 and 0.20, respectively), and trivial for both UBS-A (g = −0.07 and 0.11, respectively) and GMA (g = 0.00 and −0.05, respectively).
Fig. 2.
Relative changes for: a fat-free mass b fat mass c d MECW at 4 km h−1
Aerobic capacity
Aerobic capacity data is presented in Table 6 and Fig. 2. For , an effect of time (p < 0.001) and a time by group interaction (p < 0.001) were found. The observed pre–post relative changes (see Fig. 2c) were significantly different between groups (LBS-A and UBS-A vs. GMA; p < 0.001). The magnitude of the pre–post difference was considered moderate (g = 0.51) for the LBS-A group whereas it was small (g = 0.47) and trivial (g = −0.04) for the UBS-A and GMA groups.
A time effect (p < 0.05) was found for the MECW at all walking speeds while a time by group interaction (p < 0.05) was found only at 4 km h−1. The observed pre–post relative changes at 4 km h−1 (see Fig. 2d) were significantly different between groups (GMA vs. UBS-A; p < 0.05). The magnitude of the pre–post difference was considered moderate to trivial for the LBS-A group (g = −0.44, −0.24, and −0.16 at 2.4, 4, and 5.6 km h−1, respectively) whereas it was rather trivial to small for the UBS-A group (g = −0.10, 0.02, and −0.21 at 2.4, 4, and 5.6 km h−1, respectively). For the GMA group, differences were considered moderate (g = −0.68, −0.76, and −0.54 at 2.4, 4, and 5.6 km h−1, respectively).
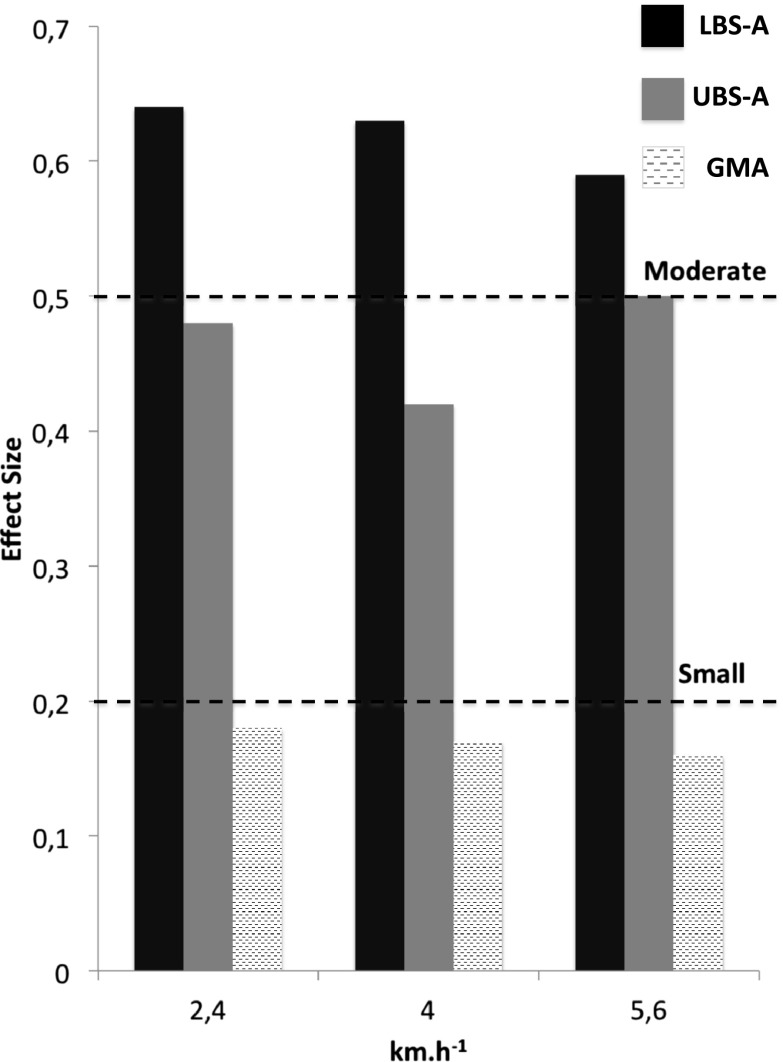
For potential energy, an effect of time (p < 0.001) and a time by group interaction (p < 0.05) were observed for all walking conditions. The observed pre–post changes were significantly different between groups (LBS-A and UBS-A vs. GMA; p < 0.05) at 2.4 and 4 km h−1. At 5.6 km h−1, a significant difference (p < 0.05) was observed only between UBS-A and GMA, while there was a tendency towards significance for LBS-A vs. GMA (p = 0.085). The magnitude of the pre–post difference (see Fig. 3) was considered moderate for the LBS-A group (g = 0.64, 0.63, and 0.59 at 2.4, 4, and 5.6 km h−1, respectively) whereas it was rather small for the UBS-A group (g = 0.48, 0.42, and 0.50 at 2.4, 4, and 5.6 km h−1, respectively). For the GMA group, differences were considered trivial (g = 0.18, 0.17, and 0.16 at 2.4, 4, and 5.6 km h−1, respectively).
Fig. 3.
Effect sizes for potential energy at different walking speed
Neuromuscular parameters
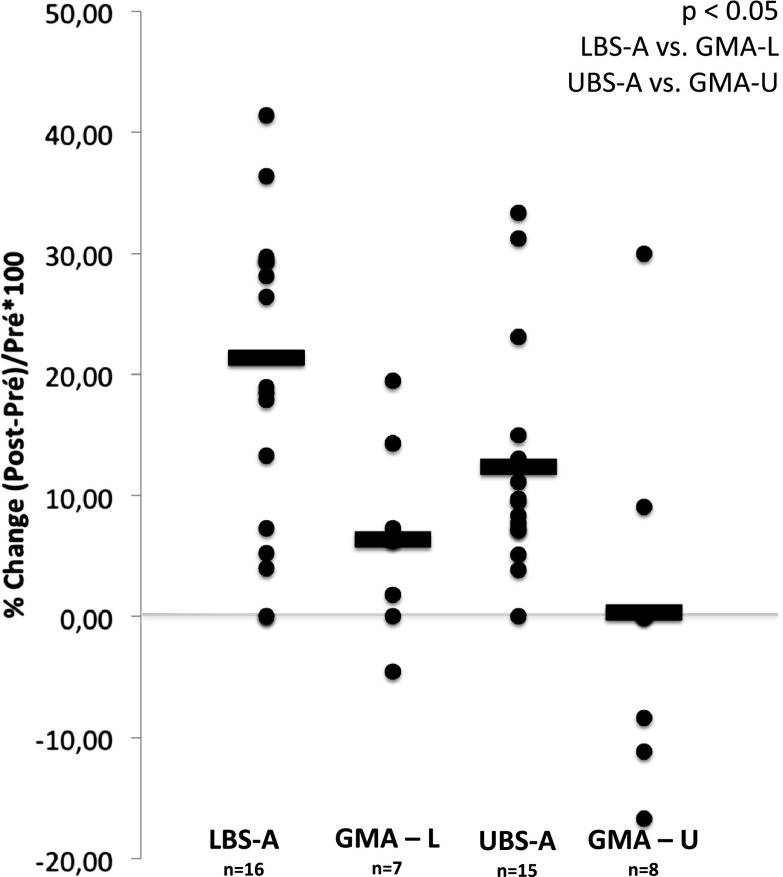
An effect of time (p < 0.001) and a time by group interaction (p < 0.05) for both 1 RM leg press and seated press were found. For leg press, the magnitude of the pre–post difference was considered small for the LBS-A group (g = 0.40) while it was trivial for the GMA-L group (g = 0.11). For seated press, effect sizes were small for the UBS-A group (g = 0.21) and trivial for the GMA-U group (g = 0.00). Relative pre–post changes are presented in Fig. 4 for all groups.
Fig. 4.
Relative changes for the inertial maximal strength test (1 RM)
Results (means ± SD) for the isokinetic strength assessment are presented in Table 7. An effect of time for the ankle plantar flexors (p < 0.05) and dorsiflexors (p < 0.001) was found. Effect sizes were considered trivial to small for the ankle plantar flexors (g = −0.25, −0.33, and −0.16 for LBS-A, UBS-A, and GMA, respectively) while they were small to moderate for the ankle dorsiflexors (g = −0.54, −0.30, and −0.35 for LBS-A, UBS-A, and GMA, respectively).
A time by group interaction for the hip flexors (p < 0.001) and MIVC (p < 0.05) was observed. The observed pre–post relative changes were significantly different between groups for the hip flexors (LBS-A and GMA vs. UBS-A; p < 0.05) but not for the MIVC (p = 0.169). The magnitude of the pre–post difference was considered trivial to moderate for the hip flexors (g = 0.19, −0.58, and 0.18 for LBS-A, UBS-A, and GMA, respectively) while effect size were trivial to small for MIVC (g = 0.46, −0.04, and −0.11 for LBS-A, UBS-A, and GMA, respectively).
Functional capacity
Results for all functional capacity tests are presented in Table 8. The statistical analysis revealed a time effect for grip strength, chair stands, 10MWT, and the 6MWT (p < 0.05), but no time by group interaction in any of the parameters. Effect sizes were trivial to small for grip strength (g = 0.08, 0.23, and 0.05 for LBS-A, UBS-A, and GMA, respectively), chair stands (g = 0.07, 0.20, and 0.41 for LBS-A, UBS-A, and GMA, respectively) and the 6MWT (g = 0.26, 0.37, and 0.12 for LBS-A, UBS-A, and GMA, respectively). Small effect sizes were reported for the 10MWT (g = −0.27, −0.30, and −0.28 for LBS-A, UBS-A, and GMA, respectively).
Executive functions
Table 9 presents performance in the RNG task at rest (single task). An effect of time (p < 0.05) for two indices of inhibition (TPI and adjacency) was found. The magnitude of the pre–post difference was considered small for TPI (g = 0.39, 0.23, and 0.34 for LBS-A, UBS-A, and GMA, respectively) and for adjacency (g = −0.43, −0.31, and −0.44 for LBS-A, UBS-A, and GMA, respectively).
Table 9.
Random number generation performance (single task)
| LBS-A (n = 16) | UBS-A (n = 15) | GMA (n = 16) | ||||
|---|---|---|---|---|---|---|
| Rest | Pre | Post | Pre | Post | Pre | Post |
| Inhibition | ||||||
| TPI a0.003 | 81.7 (12.2) | 86.7 (12.1) | 74.2 (17.3) | 78.3 (13.2) | 80.2 (17.9) | 86.3 (11.5) |
| Runs | 1.04 (0.42) | 1.07 (0.29) | 1.40 (0.67) | 1.31 (0.55) | 1.06 (0.56) | 0.99 (0.41) |
| Adjacencya<0.001 | 35.6 (9.0) | 31.1 (10.2) | 43.3 (13.8) | 39.0 (12.9) | 37.8 (13.7) | 32.0 (9.8) |
| Working memory | ||||||
| R | 1.72 (1.35) | 1.52 (0.78) | 1.55 (0.73) | 1.91 (0.91) | 1.48 (0.95) | 1.71 (0.80) |
| Coupon | 17.7 (4.0) | 17.2 (2.5) | 17.8 (3.4) | 18.7 (4.9) | 16.6 (3.1) | 17.0 (3.5) |
| MRG | 7.6 (0.6) | 7.7 (0.4) | 7.9 (0.5) | 7.7 (0.5) | 7.8 (0.5) | 7.6 (0.4) |
Data are reported as mean (SD)
For runs, adjacency, R, and coupon scores, lower scores represent better performances
a p value, time effect
Table 10 presents performance in the RNG task while walking at different speeds (dual task). At 2.4 km h−1, an effect of time (p < 0.05) was found for two indices of inhibition (TPI and adjacency) and for two indices of working memory (R and MRG) without any time by group interaction. Effect sizes were small for TPI (g = 0.29, 0.20, and 0.32 for LBS-A, UBS-A, and GMA, respectively), small to moderate for adjacency (g = −0.29, −0.28, and −0.69 for LBS-A, UBS-A, and GMA, respectively), trivial to moderate for R (g = 0.18, 0.43, and 0.50 for LBS-A, UBS-A, and GMA, respectively) and for MRG (g = −0.62, −0.07, and −0.42 for LBS-A, UBS-A, and GMA, respectively).
Table 10.
Random ,number generation performance (dual task)
| LBS-A (n = 16) | UBS-A (n = 15) | GMA (n = 16) | ||||
|---|---|---|---|---|---|---|
| 2.4 km h−1 | Pre | Post | Pre | Post | Pre | Post |
| Inhibition | ||||||
| TPI a0.03 | 81.3 (9.9) | 85.1 (13.6) | 71.3 (18.3) | 75.6 (21.6) | 80.2 (20.4) | 86.3 (11.8) |
| Runs | 1.15 (0.46) | 1.11 (0.46) | 1.78 (1.08) | 1.54 (1.33) | 1.22 (0.81) | 1.05 (0.40) |
| Adjacencya<0.001 | 36.9 (10.9) | 33.6 (10.6) | 45.5 (14.1) | 40.9 (16.0) | 37.5 (14.4) | 28.3 (9.2) |
| Working memory | ||||||
| R a0.004 | 1.30 (0.93) | 1.47 (0.79) | 1.52 (0.93) | 2.23 (1.80) | 1.62 (1.11) | 2.32 (1.46) |
| Coupon | 19.9 (19.7) | 16.6 (3.0) | 17.9 (3.5) | 19.5 (8.3) | 16.9 (4.4) | 20.4 (8.6) |
| MRG a0.016 | 8.2 (0.6) | 7.7 (0.8) | 7.8 (0.8) | 7.7 (1.0) | 7.8 (0.8) | 7.5 (0.6) |
| 4 km h−1 | ||||||
| Inhibition | ||||||
| TPI | 82.8 (13.4) | 84.2 (15.9) | 73.4 (18.5) | 71.1 (19.4) | 80.1 (17.4) | 86.7 (13.5) |
| Runs | 1.06 (0.49) | 1.13 (0.49) | 1.67 (0.72) | 1.46 (0.80) | 1.15 (0.77) | 0.81 (0.42) |
| Adjacencya0.028 | 35.9 (11.8) | 33.8 (11.0) | 45.6 (14.2) | 42.3 (15.3) | 37.6 (15.8) | 32.4 (9.6) |
| Working memory | ||||||
| R* | 1.77 (0.93) | 1.40 (0.75) | 1.65 (1.06) | 2.01 (1.23) | 1.62 (0.75) | 2.23 (1.43) |
| Coupon | 17.4 (4.3) | 16.2 (2.5) | 19.5 (7.5) | 19.5 (7.7) | 17.5 (3.3) | 20.1 (5.1) |
| MRG | 7.6 (0.7) | 7.8 (0,6) | 7.7 (0.6) | 7.6 (0.6) | 7.6 (0.7) | 7.4 (0.6) |
| 5.6 km h−1 | ||||||
| Inhibition | ||||||
| TPIa0.047 | 80.6 (11.6) | 82.8 (14.7) | 71.5 (20.5) | 76.1 (20.9) | 79.4 (21.0) | 85.8 (13.2) |
| Runs | 1.23 (0.47) | 1.14 (0.39) | 1.52 (0.77) | 1.41 (0.91) | 1.55 (1.71) | 0.82 (0.36) |
| Adjacencya0.007 | 37.2 (9.4) | 33.0 (11.0) | 45.9 (14.3) | 39.8 (14.8) | 35.1 (15.9) | 32.6 (11.4) |
| Working memory | ||||||
| Ra0.007 | 1.48 (0.88) | 1.81 (0.99) | 1.64 (1.08) | 2.47 (1.78) | 1.58 (1.02) | 1.81 (0.81) |
| Coupon | 19.0 (4.5) | 17.5 (4.9) | 17.7 (6.6) | 22.6 (18.2) | 17.0 (4.8) | 19.2 (5.2) |
| MRGa<0.001** | 7.8 (0.6) | 7.7 (0.7) | 7.8 (0.6) | 7.3 (0.8) | 7.9 (0.6) | 7.3 (0.5) |
Data are reported as mean (SD)
For runs, adjacency, R, and coupon scores, lower scores represent better performances
a p value, time effect
Interaction: *p = 0.049, **p = 0.05
At 4 km h−1, an effect of time (p < 0.05) for adjacency and a time by group interaction for R (p < 0.05) were observed. The observed pre–post changes on R were almost significantly different between groups (LBS-A vs. GMA; p = 0.054). Effect sizes were trivial to small for adjacency (g = −0.18, −0.21, and −0.35 for LBS-A, UBS-A, and GMA, respectively) and moderate for R (g = −0.42, 0.30, and 0.48 for LBS-A, UBS-A, and GMA, respectively).
At 5.6 km h−1, an effect of time (p < 0.05) was found for two indices of inhibition (TPI and adjacency) and for two indices of working memory (R and MRG). Moreover, a time by group interaction for MRG was observed. A one-way ANOVA with Bonferroni post hoc tests showed that the observed pre–post relative changes on MRG were almost significantly different between groups (LBS-A vs. GMA; p = 0.053). Effect sizes were trivial to small for TPI (g = 0.16, 0.21, and 0.33 for LBS-A, UBS-A, and GMA, respectively) and for adjacency (g = −0.38, −0.40, and −0.17 for LBS-A, UBS-A, and GMA, respectively), small for R (g = 0.33, 0.48, and 0.23 for LBS-A, UBS-A, and GMA, respectively) and trivial to large for MRG (g = −0.09, −0.57, and −1.12 for LBS-A, UBS-A, and GMA, respectively).
Correlational analysis
In order to verify the association between changes in fitness (potential energy) and dual-task performance, a correlational analysis was conducted. No correlations were found between pre–post changes in potential energy and pre–post changes in any of the RNG indices during the dual task.
Discussion
The objective of the present study was to determine the effects of a short-term high-intensity strength and aerobic training program on executive functions in a cohort of healthy older adults. Our hypothesis was that combined high-intensity training with emphasis on lower body strength would increase peak oxygen uptake and reduce the MECW more than a similar intervention focusing on upper body resistance training. By increasing the potential energy available, the lower-body training program would reduce the relative intensity of the locomotive task, thereby reducing the attentional load associated with walking. Ultimately, these fitness adaptations would allow an individual to allocate more attention to a cognitive task involving executive functions, which should result in better cognitive performance in a dual-task context.
Results suggest that the intervention produced gains in aerobic and inertial strength as well as body composition and functional capacity improvements. From a cognition perspective, inhibition scores were improved after the intervention both in a single- and a dual-task context. During the dual-task condition, results also suggest that these inhibition improvements were observed while walking at 2.4 and 5.6 km h−1 altered working memory. Contrary to our hypothesis, these cognitive changes were not specific to the lower-body strength and aerobic training program. Indeed, increasing the potential energy (higher and reduced MECW) does not represent a preferential method to improve cognitive functions either in a single- or a dual-task condition. Rather, it appears that RNG performance was improved similarly in all three groups, particularly on inhibition indices (TPI and/or adjacency) during both experimental conditions (single and dual task). This specific effect of physical training on inhibition has been previously reported. Recently, in a cross-sectional study, higher physical activity levels were associated with better inhibition performance while this effect was not significant for working memory (Boucard et al. 2012).
These results find some support in recent reports. In a group of healthy older adults (69.8 ± 3.4 years old), the RNG (single) task was used to assess the effects of two training modalities on executive functions. After 3 months of training twice a week, the authors reported that both progressive resistance training and multicomponent training (coordination, balance, agility, stretching, and relaxation) were similarly efficient to improve performance on inhibition indices (Forte et al. 2013). Interestingly, no references were made to any indices of working memory. However, it was suggested that mechanisms to explain these cognitive gains differed between the two modes of exercise. Whereas strength gains tended to mediate cognitive improvements, it was speculated that motor tasks involving coordination and perceptual adaptations were sufficient to increase cognitive performance. Similarly, a 12-month training intervention revealed that both cardiovascular and coordination training were effective in increasing cognitive performance in a group of older adults aged between 62 and 79 years old (Voelcker-Rehage et al. 2011). Both interventions resulted in a decrease of the activation of the prefrontal cortex, which was interpreted as better information processing. However, it appears that mechanisms underlying these performances were specific to the intervention. Whereas cardiovascular training was associated with increased activation of the sensorimotor network, the coordination training resulted in elevated activation of the visual–spatial network.
Intriguingly, working memory scores were altered in dual-task conditions (at 2.4 and 5.6 km h−1). Although purely speculative, it could be argued that improvements in inhibition led participants to produce digit sequences more randomly, which could increase stress on working memory. It is also suggested that this effect was only observed at 2.4 and 5.6 km h−1 because of the particular demands of walking at these speeds (maintain balance and higher relative intensity, respectively). At 4 km h−1, a time by group interaction was found in favor of the LBS-A group (R—working memory). It could be argued that this group took advantage of the higher improvements in potential energy at this comfortable walking speed. Clearly, more research is needed to confirm these hypotheses.
Taken together, these results suggest that the cardiovascular hypothesis alone is insufficient to explain the mechanisms supporting the physical fitness/cognition relationship. Some previous reports demonstrate that improvements in cardiovascular fitness are not systematically related to better cognitive performance (Smiley-Oyen et al. 2008; Etnier et al. 2006). Moreover, different interventions such as strength training (through the IGF-1 molecular pathway) and coordination/balance programs were also effective to improve cognition in older adults (Cassilhas et al. 2007; Forte et al. 2013; Voelcker-Rehage et al. 2011). Likewise, a recent cross-sectional analysis from our lab suggests that participants with better cognitive flexibility performances were characterized not only by greater cardiovascular health as measured by but also by better performances in neuromuscular and general mobility tests (Berryman et al. 2013). Nonetheless, results from both cross-sectional and intervention studies demonstrated that higher cardiovascular fitness was related to better cognitive performance in older adults (Renaud et al. 2010a, b; Kramer et al. 1999). Aerobic training, related to the BDNF molecular pathway (Cassilhas et al. 2012), has shown associations to increases in hippocampal volume and greater serum levels of BDNF (Erickson et al. 2011). Considering all these mechanisms, one could argue that multiple pathways could lead older adults to better cognitive functions; the appropriate intervention being prescribed based upon the individual’s strengths and weaknesses as well as considering his adaptability to the training program chosen.
It has to be acknowledged that this study had some limitations. The dual-task paradigm implies simultaneously performing two tasks which should result in performance decrements in one or both tasks if attentional resources were exceeded (Yogev-Seligmann et al. 2008). In the dual-task model presented in this study, cognitive performance was assessed with the RNG task while MECW represented the main variable regarding the locomotive task. Since participants had to wear a facemask during the MECW assessment, it was suggested that completion of the RNG task be done afterwards to facilitate the evaluator comprehension of the number sequences. This methodological issue could have led to biased data interpretation. Indeed, gait speed and variability are two common variables known to be altered in a dual-task condition (Montero-Odasso et al. 2012). Since speed was kept constant on the treadmill during the dual task, it is still possible that gait variability, or any other kinematic variable, was modified during the dual task, which could have an effect on the MECW (Holt et al. 1995). Moreover, since participants were not instructed to prioritize one task over another, each individual’s focus and performance on each task probably differed based on personal postural control and self-awareness ability (Yogev-Seligmann et al. 2012). However, considering the main objective of this study, which was to assess the effect of an increase in potential energy on cognitive performance in single and dual task, this bias does not represent a major limitation.
Since no time × group interactions were found in the single-task condition, one could argue that a learning effect could explain the observed results. This phenomenon is probably not involved in this study since it was reported that the RNG task is not influenced by the practice effect (Towse and Valentine 1997; Audiffren et al. 2009; Jahanshahi et al. 2006). However, it has to be mentioned that this was recently challenged. Indeed, a practice effect was reported for adjacency but not for other inhibition scores (TPI and runs) (Forte et al. 2013). Taken together, these observations do not cast doubt on the results of the present study. The absence of a time × group interaction in RNG performances could also be explained by differences at baseline for the executive function scores as measured with the Stroop task, which could have led the LBS-A group to a ceiling effect. However, this phenomenon is rather unlikely since no differences between groups were found at baseline for both single- and dual-task RNG performances, which was the main outcome with regards to cognitive performance. Finally, one could argue that the small sample size of this study could explain why no differences were found between groups with regards to the main hypothesis. However, it has to be mentioned that studies with similar sample size revealed significant cognitive improvements after a physical training intervention (Baker et al. 2010; Fabre et al. 2002). Therefore, it is rather unlikely that the results presented in this article are related to a lack of statistical power.
Conclusion
The objective of the present study was to determine the effects of a short-term high-intensity strength and aerobic training program on executive functions in a cohort of healthy older adults. Results revealed that this intervention produced the expected gains on aerobic fitness, inertial strength, and body composition. From a cognitive perspective, inhibition was improved after the intervention both in a single- and a dual-task context. However, contrary to our hypothesis, these cognitive changes were not specific to the lower-body strength and aerobic training program. Indeed, greater potential energy available was not preferentially associated with better cognition, suggesting that interventions targeting flexibility, locomotion, manipulation, and relaxation lead to improvement in cognitive functions in healthy older adults and this improvement is equivalent to the one observed after an aerobic and strength physical fitness program. However, it appears, based on previous reports, that mechanisms underlying these adaptations are specific to the intervention. These findings tend to suggest that multiple pathways could lead older adults to improve cognition through different exercise programs targeting physical fitness and/or general motor abilities. Such observations could help clinicians to plan appropriate interventions based on each individual’s strengths and weaknesses.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR #209441). LB is supported by the Canadian Research Chair Program. NB received a doctoral scholarship from the Quebec Network for Research on Aging (QNRA).
References
- Abizanda P, Navarro JL, Garcia-Tomas MI, Lopez-Jimenez E, Martinez-Sanchez E, Paterna G. Validity and usefulness of hand-held dynamometry for measuring muscle strength in community-dwelling older persons. Arch Gerontol Geriatr. 2012;54(1):21–27. doi: 10.1016/j.archger.2011.02.006. [DOI] [PubMed] [Google Scholar]
- ACSM American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30(6):992–1008. doi: 10.1097/00005768-199806000-00033. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;3:CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- Audiffren M, Tomporowski PD, Zagrodnik J. Acute aerobic exercise and information processing: modulation of executive control in a Random Number Generation task. Acta Psychol (Amst) 2009;132(1):85–95. doi: 10.1016/j.actpsy.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51(4):459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Dubost V, Allali G, Kressig RW, Bridenbaugh S, Berrut G, Assal F, Herrmann FR. Stops walking when talking: a predictor of falls in older adults? Eur J Neurol. 2009;16(7):786–795. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- Berryman N, Gayda M, Nigam A, Juneau M, Bherer L, Bosquet L. Comparison of the metabolic energy cost of overground and treadmill walking in older adults. Eur J Appl Physiol. 2012;112(5):1613–1620. doi: 10.1007/s00421-011-2102-1. [DOI] [PubMed] [Google Scholar]
- Berryman N, Bherer L, Nadeau S, Lauziere S, Lehr L, Bobeuf F, Kergoat MJ, Vu TT, Bosquet L. Executive functions, physical fitness and mobility in well-functioning older adults. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard GK, Albinet CT, Bugaiska A, Bouquet CA, Clarys D, Audiffren M. Impact of physical activity on executive functions in aging: a selective effect on inhibition among old adults. J Sport Exerc Psychol. 2012;34(6):808–827. doi: 10.1123/jsep.34.6.808. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39(8):1401–1407. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Lee KS, Fernandes J, Oliveira MG, Tufik S, Meeusen R, de Mello MT. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Cayrou S, Dickes P, Dolbeault S, Gauvain-Piquard A, Desclaux B. French validation of the Profile of Mood States (POMS) Psychooncology. 2000;9(5):S52–S52. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Dupuy O, Lussier M, Fraser S, Bherer L, Audiffren M, Bosquet L. Effect of overreaching on cognitive performance and related cardiac autonomic control. Scand J Med Sci Sports. 2012 doi: 10.1111/j.1600-0838.2012.01465.x. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52(1):119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Fabre C, Chamari K, Mucci P, Masse-Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. 2002;23(6):415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Fletcher JR, Esau SP, Macintosh BR. Economy of running: beyond the measurement of oxygen uptake. J Appl Physiol. 2009;107(6):1918–1922. doi: 10.1152/japplphysiol.00307.2009. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forte R, Boreham CA, Leite JC, De Vito G, Brennan L, Gibney ER, Pesce C. Enhancing cognitive functioning in the elderly: multicomponent vs resistance training. Clin Interv Aging. 2013;8:19–27. doi: 10.2147/CIA.S36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012;42(7):587–605. doi: 10.2165/11631910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Knols R, Murer K, de Bruin ED. Reproducibility of an isokinetic strength-testing protocol of the knee and ankle in older adults. Gerontology. 2009;55(3):259–268. doi: 10.1159/000172832. [DOI] [PubMed] [Google Scholar]
- Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33(12):877–888. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Holt KJ, Jeng SF, Rr RR, Hamill J. Energetic cost and stability during human walking at the preferred stride velocity. J Mot Behav. 1995;27(2):164–178. doi: 10.1080/00222895.1995.9941708. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66(5):541–547. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Saleem T, Ho AK, Dirnberger G, Fuller R. Random number generation as an index of controlled processing. Neuropsychology. 2006;20(4):391–399. doi: 10.1037/0894-4105.20.4.391. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- Karlsen T, Helgerud J, Stoylen A, Lauritsen N, Hoff J. Maximal strength training restores walking mechanical efficiency in heart patients. Int J Sports Med. 2009;30(5):337–342. doi: 10.1055/s-0028-1105946. [DOI] [PubMed] [Google Scholar]
- Kervio G, Carre F, Ville NS. Reliability and intensity of the six-minute walk test in healthy elderly subjects. Med Sci Sports Exerc. 2003;35(1):169–174. doi: 10.1097/00005768-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Labelle V, Bosquet L, Mekary S, Bherer L. Decline in executive control during acute bouts of exercise as a function of exercise intensity and fitness level. Brain Cogn. 2013;81(1):10–17. doi: 10.1016/j.bandc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Langlois F, Vu TT, Chasse K, Dupuis G, Kergoat MJ, Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci. 2012 doi: 10.1093/geronb/gbs069. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349(9052):617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C, Caillaud C. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol. 2003;95(6):2248–2256. doi: 10.1152/japplphysiol.01106.2002. [DOI] [PubMed] [Google Scholar]
- Matta Mello Portugal E, Cevada T, Sobral Monteiro-Junior R, Teixeira Guimaraes T, da Cruz Rubini E, Lattari E, Blois C, Camaz Deslandes A. Neuroscience of exercise: from neurobiology mechanisms to mental health. Neuropsychobiology. 2013;68(1):1–14. doi: 10.1159/000350946. [DOI] [PubMed] [Google Scholar]
- Milot MH, Nadeau S, Gravel D. Muscular utilization of the plantarflexors, hip flexors and extensors in persons with hemiparesis walking at self-selected and maximal speeds. J Electromyogr Kinesiol. 2007;17(2):184–193. doi: 10.1016/j.jelekin.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro B. Statistical methods for health care research. 3. New York: Lippincott; 1997. [Google Scholar]
- Nana A, Slater GJ, Hopkins WG, Burke LM. Effects of exercise sessions on DXA measurements of body composition in active people. Med Sci Sports Exerc. 2013;45(1):178–185. doi: 10.1249/MSS.0b013e31826c9cfd. [DOI] [PubMed] [Google Scholar]
- Nemoto K, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc. 2007;82(7):803–811. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- Perrault H. Efficiency of movement in health and chronic disease. Clin Invest Med. 2006;29(2):117–121. [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Renaud M, Bherer L, Maquestiaux F. A high level of physical fitness is associated with more efficient response preparation in older adults. J Gerontol B Psychol Sci Soc Sci. 2010;65(3):317–322. doi: 10.1093/geronb/gbq004. [DOI] [PubMed] [Google Scholar]
- Renaud M, Maquestiaux F, Joncas S, Kergoat MJ, Bherer L. The effect of three months of aerobic training on response preparation in older adults. Front Aging Neurosci. 2010;2:148. doi: 10.3389/fnagi.2010.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, Perez-Gomez J, Luque AJ, Lopez-Roman FJ, Alcaraz PE. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334–340. doi: 10.1016/j.exger.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Ronnestad BR, Mujika I. Optimizing strength training for running and cycling endurance performance: a review. Scand J Med Sci Sports. 2013 doi: 10.1111/sms.12104. [DOI] [PubMed] [Google Scholar]
- Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(Suppl 2):S329–S336. doi: 10.1111/j.1532-5415.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the "selective improvement" and "cardiovascular fitness" hypotheses. Ann Behav Med. 2008;36(3):280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towse JN, Neil D. Analyzing human random generation behavior: a review of methods used and a computer program for describing performance. Behav Res Methods Instrum Comput. 1998;30(4):583–591. doi: 10.3758/BF03209475. [DOI] [Google Scholar]
- Towse JN, Valentine JD. Random generation of numbers: a search for underlying processes. Eur J Cogn Psychol. 1997;9(4):381–400. doi: 10.1080/713752566. [DOI] [Google Scholar]
- Verdijk LB, van Loon L, Meijer K, Savelberg HH. One-repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci. 2009;27(1):59–68. doi: 10.1080/02640410802428089. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Godde B, Staudinger UM. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front Hum Neurosci. 2011;5:26. doi: 10.3389/fnhum.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. J Appl Physiol. 2011;111(5):1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord. 2012;27(6):765–770. doi: 10.1002/mds.24963. [DOI] [PubMed] [Google Scholar]