Abstract
Purpose
The aim of this study was to analyze the seminal plasma of patients with idiopathic/male factor infertility and healthy controls with proven fertility by NMR spectroscopy, with a hope of establishing difference in biomarker profiles, if any, between the groups.
Methods
A total of 103 subjects visiting the infertility clinic of Manipal University with normozoospermic parameters, oligozoospermia, asthenozoospermia, azoospermia and teratozoospermia were included. Semen characteristics were analysed by standard criteria. Seminal plasma was subjected to NMR spectroscopy at a 700 MHz 1H frequency. The resultant data was analyzed by appropriate software.
Results
The analysis revealed significant differences between the fertile control group and other forms of male infertility. Interestingly, seminal plasma profile of the idiopathic infertility group showed distinct segregation from the control population as well as other infertile groups. The difference in biomarker profiles between the idiopathic infertility and the rest of the groups combined could originate from either the up-regulation or down regulation of a several compounds, including lysine, arginine, tyrosine, citrate, proline and fructose.
Conclusion
Our data suggests the presence of a metabolic reason behind the origin of idiopathic infertility. 1H NMR based metabonomic profiling based on concentration of biomarker lysine has the potential to aid in the detection and diagnosis of idiopathic infertility in an efficient manner.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-014-0282-4) contains supplementary material, which is available to authorized users.
Keywords: Male infertility, Idiopathic infertility, Seminal plasma, NMR, NMR spectroscopy, Metabolite, Semen
Introduction
It is estimated that 10–15 % of couples are infertile, and of those, nearly half will involve male factors [45]. Despite improvements in the diagnosis and treatment, management of male factor infertility still remains a challenging task. The clinical utility of traditional seminal fluid based markers in the prediction of gamete quality and embryo viability is still controversial, often leading to poor success rates when Assisted Reproductive Technology (ART) is employed. In addition, many infertile couples continue to be labeled with the diagnosis of idiopathic infertility or given descriptive diagnoses that do not provide a cause for their defect. For other individuals with a known etiology, effective cures are lacking [11].
There is hence a need for the identification of alternative, rapid, noninvasive methods to diagnose specific etiologies of male factor infertility. The use of novel metabolomic techniques may hold the key to more accurately diagnosing and treating male factor infertility. It is within this context that the search for potential biomarkers in male infertility can begin [21]. A biomarker is a distinctive biological or biologically derived indicator of a process, event, or condition that can be objectively measured, evaluated, and compared.
Metabolomics and metabonomics encompass the comprehensive and simultaneous systematic profiling of multiple metabolite concentrations and their cellular and systemic fluctuations in response to drugs, diet, lifestyle, environment, stimuli and genetic modulations, in order to characterize the beneficial and adverse effects of such interactions [7]. Even though measuring the metabolome is a considerable analytical challenge owing to the absence of methods to amplify metabolites and their labile nature, complexity and heterogeneity, rapid technological advances have enables us to detect a wide range of small molecules.
The metabolome is the final downstream product of the genome and is defined as the total quantitative collection of small molecular weight compounds present in a cell or organism which participate in metabolic reactions required for growth, maintenance and normal function. The human metabolome is believed to be made of nearly 3,000 such small molecule metabolites and is considered to be closer to the phenotype than the transcriptome or proteome as post-transcriptional and post translational modifications are known to exert a strong influence on the metabolic fluxes [11].
The identification and quantification of biomarkers is done through a combination of analytical, biochemical and spectral analysis, thereby establishing the signatures of the metabolites for healthy control population and test subjects with specific illnesses. Differences in the concentration of specific biomarkers are then used in creating unique metabolomics profiles which would help differentiate test subjects from controls. This information is then coupled with bioinformatics to make the analysis as predictive as possible [28, 29, 34].
There has been growing interest in the field of metabolomics which provides useful information about the phenotype an organism through analysis of various biomarkers in body fluids [22, 35, 39, 46, 37, 7]. This technology provides for assessment of metabolic changes within an organism, even when standard clinical chemistry markers are within normal limits [31]. Metabolomics as a diagnostic tool for metabolic classification of individuals offers potential advantages that classical diagnostic approaches do not, based on discovery of a suite of clinically relevant biomarkers. The great asset of this platform is the quantitative, non-invasive analysis of easily accessible body fluids ([20]; Zhang et al., 2012).
Although different body fluids have been popular targets of analysis through omics platform, seminal plasma has not received much attention in this regard. Seminal plasma is the liquid component of semen and composed of secretions from the testis, epididymis and male accessory glands [6]. At pH 7.35–7.50, it has buffering properties, protecting spermatozoa from the acidic environment of the vagina. It contains a high concentration of fructose, which is a major nutrient for spermatozoa during their journey in the female reproductive tract. The average protein concentration of human seminal plasma ranges from 35 to 55 g/l [32].
Human seminal fluid dilutes and transports spermatozoa to the ovum for fertilization and provides a metabolic support to the cells bathed in it. Immediately following ejaculation, a series of reaction occur, initiated by semenogelin (Sg) I and II aggregation to form a gelatinous mass, which is then cleaved by prostate specific antigen (PSA) after 5–20 min, facilitating liquefaction [18, 30]. Subsequently, the further reactions over the next 6–8 h results in hyperactivation and capacitation of the spermatozoa [27].
Disruptions to the biochemical and biophysical reactions following ejaculation are a major cause of infertility in men [43, 15]. Since seminal fluid has important roles in spermatozoon survival and overall fertilization success, its impairment can be directly connected to infertility. It is therefore logical to assume that the characterization of different biomarkers in this liquid component could have valuable implications, both in the diagnosis and treatment of male infertility [32].
Although the study of cellular metabolic products (metabolomics) as potential male fertility biomarkers is currently in its infancy, it is believed that the use of novel metabolomic techniques can enable the more accurate diagnosis and treatment of male factor infertility. With the aim of addressing this issue, this study has been proposed, to analyze the seminal plasma of patients with idiopathic/male factor infertility and healthy controls with proven fertility by Nuclear Magnetic Resonance (NMR) spectroscopy, with a hope of establishing difference in biomarker profiles, if any, between the two groups. Qualitative and quantitative measurement (absolute concentration) of metabolites in seminal plasma will be carried out, and the resultant data subjected to multivariate analysis, to determine the signature biomarkers and possible descriptors of infertility.
Materials and methods
Subjects
Patients visiting the infertility clinic of Kasturba Medical College and fertile controls, known to have fathered a child within 12 months, participated in the study. The Institutional Ethical Committee’s approval was obtained before performing the experiment. Idiopathic infertility included infertile patients, with normozoospermic semen parameters, who had a history of infertility of at least 2 years and normal female partners i.e., normal reproductive history, normal day 2 follicular stimulating hormone (FSH) and luteinizing hormone (LH) levels, normal ovulation (by follicular ultrasound study), and tubal patency (by hysterosalpingogram). Care was taken to ensure that the samples in the control group and experimental groups were age matched. 103 subjects participated in the study, after provision of a written, informed consent. All subjects were asked to provide semen samples after 3–5 days of ejaculatory abstinence. Semen specimens were produced by masturbation directly into a sterile plastic container, in a room specially provided for this purpose and located adjacent to the laboratory. After liquefaction, semen processing and analysis was performed according to the recommendations of the World Health Organization [49]. A part of the sample was used for routine andrological examination (Supplementary information S1). Seminal plasma was separated for NMR spectroscopy.
Semen analysis
Seminal volume was determined in a graduated tube and sperm concentration was assessed by conventional method using Makler counting chamber (Sefi Medical Instruments, Israel) and expressed in millions/mL. The sperm motility was assessed in at least 100 sperm and expressed as percent of motile sperm (sum of rapid progression plus slow progression sperm). Sperm morphology was assessed by Shorr staining and sperm viability by Eosin-Nigrosin stain.
Nuclear magnetic resonance spectroscopy
The NMR spectra of the samples were acquired at a laboratory situated ~850 km away from the point of collection of samples. Samples were centrifuged at 200 x g for 10 min to separate the cells from the rest of the fluid. The fluid component (supernatant) was stored at −80 °C for NMR spectroscopy, until shipment. The samples were shipped in commercial insulated shipping containers. Maximal care was taken and stringent quality control has been applied while shipping of samples. Small chunks of dry ice were first placed at the bottom of the shipping container, and then the sample box was placed in the centre of shipping box, followed by placement of dry ice over and around the box. Immediately after shipment, the samples were shifted to a −80 °C deep freezer available with the host facility. Just prior to analysis, the samples were thawed at room temperature for 15 min and then diluted 2:1 in D2O containing 50 mg/ml 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS-D4), as a chemical shift and concentration reference. 600 μl of the sample was transferred immediately to a 5-mm NMR tube and inserted into the spectrometer. After allowing for temperature equilibration (to 300 K), the sample was shimmed and data were acquired approximately 15 min post-thaw.
1H NMR spectra
1H NMR spectra of the samples were acquired on a 700 MHz Bruker NMR spectrometer. The acquisition parameters were: A total of 16 transients collected in 32 K data points with a relaxation delay of 4 s between the consecutive transients and a spectral width of 20 ppm. Suppression of the water signal was achieved by continuous irradiation during the relaxation and mixing time. Acquisition time for each spectrum was ~1.14 min. The spectra were phase and baseline corrected by using an automated program provided by Bruker, Biospin. All the spectra were referenced to DSS-D4 as 0.00 ppm.
Multivariate data analyses
The NMR spectra were subjected to multivariate analysis using Simca- P + 12.0. Prior to that, the phase and baseline corrected 1H NMR spectra were divided into bins of 0.04 ppm width integrated. The working region (0.5 to 9.5 ppm) was normalized with respect to the whole spectrum to take care of the dilution effects and additionally, the data was mean centered and pareto scaled. The data matrices so generated were imported to Simca- P + 12.0 and used for multivariate data analyses.
Initially, Principal Component Analysis (PCA) was carried out to check the trend in the data find out the outliers in the sample set. PCA is an unsupervised method, where no class entity is assigned to the sample set a priori; hence the model displays any hidden pattern in the data. The PCA analysis was inadequate to draw any specific conclusion regarding the clustering among the sample classes. Therefore, Orthogonal Partial Least Square-Discriminant Analysis (OPLS-DA) and O2PLS-DA was employed. This algorithm reveals more subtle changes in the occurrence and concentration of specific metabolites by focusing on compounds responsible for the discrimination between two classes (i.e. control and infertile group). Unlike PCA, the bins in the OPLS-DA and O2PLS-DA are assigned a variable importance, with higher numbers corresponding to bins that contributed more substantially to the class separation between control and infertile group. Since OPLS-DA and O2PLS-DA is a supervised method, therefore, class specificity is assigned to the sample set a priori. This further refines the class segregation. The OPLS-DA/O2PLS-DA models can be judged by using two parameters - R2Y and Q2 (cum), the former shows the fraction of total variance explained by the model in the classes and the latter shows the extent of separation of the two classes, or in other words, the predictability of the model. The Q2(cum) is calculated using cross validation methods that uses 1/7th of the total data in each cross validation round. The OPLS-DA/O2PLS-DA model generates the predictive component which explains the class specific variation and the orthogonal component which explains variations not related to the class definition. Here also, the data is visualized using the scores and loadings plot as earlier. OPLS-DA, in addition, provides loadings S-plot which helps to identify the class of sample where certain group of variable is increased or decreased, hence aiding in data interpretation.
Results
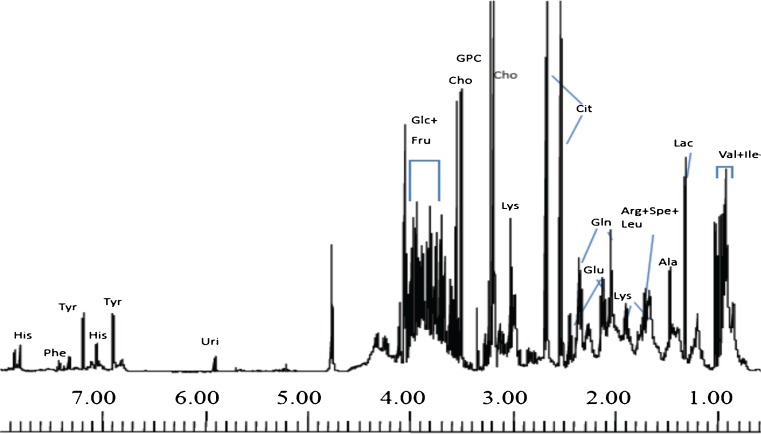
The mean semen characteristics of the various subjects are presented (Table 1). Resonances from a number of low molecular weight metabolites can be identified immediately from previously assigned chemical shift, such as choline, glycerophosphocholine (GPC), citrate, fructose, lactate and resonances from amino acids such as alanine, valine, leucine and isoleucine and is presented in Fig. 1 [22]. In addition, broad bands, characteristic of proteins, have also been observed throughout the entire spectrum, the assignment of which is hindered due to broadening. In view of the abundance of proteins such as semenogelin (Sg), PSA and albumin [3, 52], it is possible that few of the peaks observed in the spectra of the present study originate and correspond to resonances from these proteins.
Table 1.
Semen characteristics of fertile controls and infertile subjects subjected to NMR spectroscopic analysis
| Group | N | Age (Yrs) | Seminal volume (ml) | Sperm count (million/ml) | Total sperm motility (%) | Sperm with normal morphology (%) |
|---|---|---|---|---|---|---|
| Control | 6 | 36.17 ± 1.60 | 2.67 ± 0.40 | 64.50 ± 9.94 | 71.50 ± 5.74 | 35.5 ± 3.01 |
| Idiopathic infertility | 17 | 36.24 ± 0.99 | 2.12 ± 0.18 | 60.41 ± 5.37 | 66.00 ± 1.97 | 35.41 ± 1.77 |
| Oligozoospermia | 20 | 34.53 ± 1.18 | 3.82 ± 0.66 | 9.64 ± 0.99 | 41.50 ± 4.26 | 15.60 ± 1.85 |
| Asthenozoospermia | 20 | 37.20 ± 0.97 | 3.00 ± 0.27 | 45.85 ± 4.99 | 42.95 ± 3.29 | 20.25 ± 1.84 |
| Teratozoospermia | 20 | 38.15 ± 1.41 | 3.80 ± 0.32 | 55.90 ± 0.544 | 53.3 ± 4.16 | 17.00 ± 0.88 |
| Azoospermia | 20 | 32.85 ± 0.93 | 2.78 ± 1.30 | NA* | NA* | NA* |
NA* - Not applicable
Fig. 1.
Assigned 700 MHz 1- dimensional NMR spectrum of seminal plasma of a healthy fertile individual. The aliphatic and aromatic part of the spectrum is shown. Assignments are done based on 2- dimensional NMR spectroscopy and literature survey. Keys: Val: Valine, Leu: Leucine, Ile: Isoleucine, Lac: Lactate, Ala: Alanine, Arg: Arginine, Spe: Spermidine, Leu: Leucine, Lys: Lysine, Glu: Glutamate, Gln: Glutamine, Cit: Citrate, Cho: Choline, GPC: Glycerophosphocholine, Glc: Glucose, Fru: Fructose, Uri: Uridine, Tyr: Tyrosine, His: Histidine, Phe: Phenylalanine
PCA scores plot revealed significant differences between the control population and idiopathic infertility and oligozoospermic patients. However, no or little difference was observed when the other infertile groups viz. azoospermic, teratozoospermic and asthenozoospermic patients. In addition, the idiopathic infertile patient group was also compared with all other infertile groups using PCA. No significant segregation was observed (Supplementary information, S2A and S2B).
To identify more subtle variations, supervised analyses such as O2PLS-DA and OPLS-DA were performed. Although on the technical front, O2PLS-DA is similar to OPLS-DA, the former is able to handle more than two classes of samples, thereby resulting in more than one predictive component. This places the generic OPLS algorithm at an advantage over machine learning tools such as Support Vector Machine (SVM). Although the SVM methods and Artificial neural networks (ANN) have been used in combination with PCA, both these methods do not provide quite the interpretative simplicity of projective ‘latent- space’ methods such as PCA and PLS [50, 17].
In the present case, we resorted to O2PLS-DA to investigate the overall changes in the metabolic profile among different infertile groups. To achieve this, O2PLS-DA modeling was performed on all the infertile individuals with selected NMR variables (VIP > 1.0 from another O2PLS_DA model involving all variables). This resulted in fairly significant model and three predictive components could be computed (R2Y = 0.34, Q2 (cum) = 0.22). The 1st (most significant) component showed clustering of the idiopathic infertility group from all other infertile individuals (Supplementary information S2C). The second component grouped oligozoospermia and asthenozoospermia together and clustered them from azoospermic, teratozoospermic and idiopathic infertile groups (Supplementary information S2D). The third component, on the other hand, grouped azoospermia and teratozoospermia together while clustering the other three groups together (Supplementary information S2D). These results suggested the presence of significant differences among categories of infertility. In order to further understand these differences with the control samples, a pair wise OPLS-DA approach was taken.
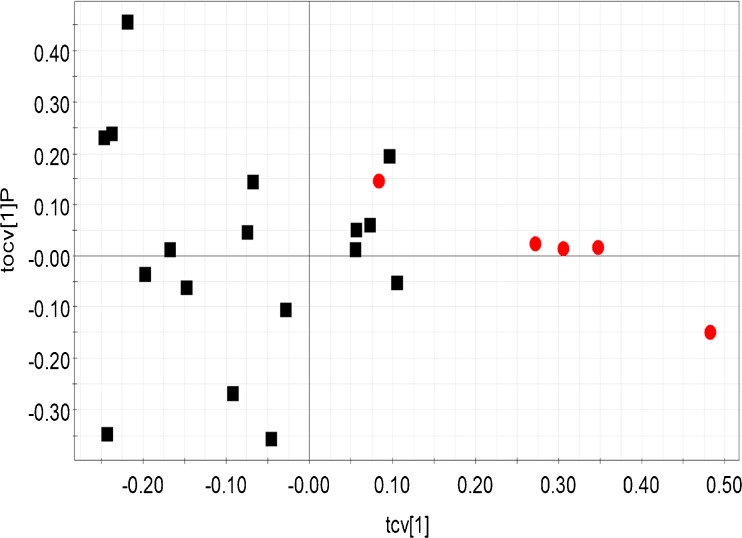
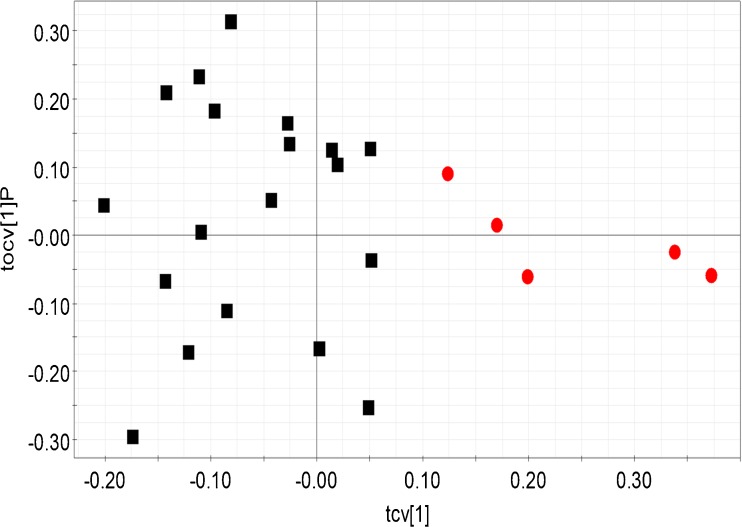
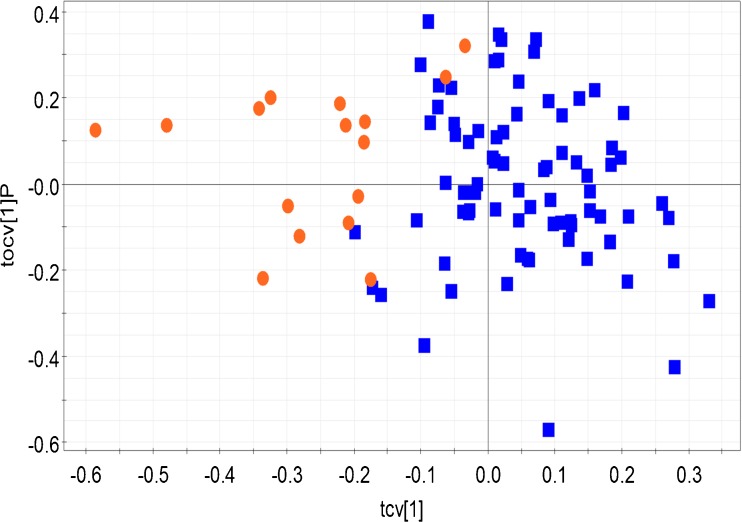
The OPLS-DA analysis revealed significant biochemical differences between the fertile control group and other forms of male infertility – idiopathic infertility, oligozoospermia (Figs. 2 and 3). Idiopathic infertile class and oligozoospermic patients were significantly segregated from the control population (Q2 (cum) = 0.61 and 0.68 respectively). Table 2 provides the R2Y and Q2 (cum) of all the built OPLS-DA models. When the control population and azoospermic groups were considered, comparatively lesser difference was seen (Table 2). Comparison of the control group with either asthenozoospermia or teratozoospermia failed to reveal any significant difference in biomarker profiles. Interestingly, when the idiopathic group was compared against all other forms of infertility, distinct clustering was observed, highlighting distinct differences in profiles between the groups (Fig. 4). The bins affecting the clustering of the relevant models are provided in Table 3 along with their loadings and Variable Importance on Projection (VIP) values. 2D correlation spectra of specific samples were acquired and analyzed in order to identify the metabolites related to the bins. These metabolites are tabulated in Table 4.
Fig. 2.
Cross validated scores plot constructed from the OPLS-DA model of seminal plasma 1H NMR profile of fertile control population (red dots) and idiopathic infertile groups (black squares). Model details: R2Y = 0.73 and Q2 (cum) = 0.61. Each point (dots or square) represents the seminal plasma 1H NMR spectral profile from a single individual
Fig. 3.
Cross validated scores plot constructed from the OPLS-DA model of seminal plasma 1H NMR profile of fertile control population (red dots) and oligozoospermic infertile groups (black squares). Model details: R2Y = 0.89 and Q2 (cum) = 0.68. Each point (dots or square) represents the seminal plasma 1H NMR spectral profile from a single individual
Table 2.
The R2Y and Q2 (cum) statistics for the models
| Model | R2Y | Q2(cum) |
|---|---|---|
| Control and idiopathic infertility | 0.73 | 0.61 |
| Control and oligozoospermia | 0.89 | 0.68 |
| Control and asthenozoospermia | 0.71 | 0.00 |
| Control and teratozoospermia | 0.44 | −0.31 |
| Control and azoospermia | 0.39 | 0.22 |
| Idiopathic infertility & all other infertile groups | 0.60 | 0.50 |
Fig. 4.
Cross validated scores plot constructed from the OPLS-DA model of seminal plasma 1H NMR profile of idiopathic infertility patients (orange dots) and all other infertile groups (blue squares). Model details: R2Y = 0.60 and Q2 (cum) = 0.50. Each point (dots or square) represents the seminal plasma 1H NMR spectral profile from a single individual
Table 3.
Significantly perturbed bins along with the loadings and Variable Importance on Projection (VIP) values
| Model * | Bin | Loading | VIP | |
|---|---|---|---|---|
| W [1] | P (corr) | |||
| Control and idiopathic infertility | 3.76 | 0.25 | 0.92 | 3.62 |
| 0.96 | 0.25 | 0.92 | 3.62 | |
| 1 | 0.24 | 0.96 | 3.50 | |
| 1.72 | 0.24 | 0.95 | 3.48 | |
| 3.96 | 0.21 | 0.95 | 3.08 | |
| Control and Oligozoospermia | 3.24 | 0.37 | 0.71 | 5.48 |
| 3.8 | −0.27 | −0.7 | 4.01 | |
| 3.72 | −0.27 | −0.71 | 3.98 | |
| Control and azoospermia | 3.24 | 0.41 | 0.75 | 5.96 |
| 3.8 | −0.31 | −0.87 | 4.49 | |
| 3.72 | −0.29 | −0.67 | 4.27 | |
| 4.04 | −0.21 | −0.74 | 3.01 | |
| Idiopathic infertility and other infertile groups | 3.12 | −0.25 | −0.61 | 3.68 |
| 0.96 | 0.28 | 0.82 | 4.15 | |
| 1.72 | 0.25 | 0.82 | 3.72 | |
| 1.48 | 0.20 | 0.86 | 2.98 | |
* Only those models which showed significant clustering were considered)
Table 4.
Assignment of significantly perturbed metabolites from different OPLS-DA models
| Model | Metabolites decreased in control | Metabolites increased in control |
|---|---|---|
| Control and azoospermia | Guanidoacetate (3.8 ppm, S), Fructose (3.82 ppm, 4.04 ppm, M), Unassigned (3.72 ppm, M) | Unassigned (3.24 ppm, M) |
| Control and idiopathic infertility | - | Unassigned (3.76 ppm), Valine (0.96 ppm, d, 1.01 ppm, d), 2- hydroxyisovalerate (0.98 ppm, d), Lysine (1.74 ppm, m), Hippurate (3.96 ppm, d), Fructose (3.98 ppm, M) |
| Control and oligozoospermia | Guanidoacetate (3.8 ppm,S), Fructose (3.82 ppm, 4.04 ppm, M), Unassigned (3.72 ppm, M) | Unassigned (3.24 ppm, M) |
| Model | Metabolites increased in idiopathic infertility group | Metabolites decreased in idiopathic infertility group |
| Idiopathic infertility and other infertile groups | Malonate (3.12 ppm, d) | Valine (0.96 ppm, d,), 2- hydroxyisovalerate (0.98 ppm, d), Alanine (1.48 ppm, d) |
Control group, as implicated earlier was distinct from the azoospermic patients. It was revealed that this clustering was due to decreased guanidoacetate and fructose in the patient population. In addition, one unassigned peak was decreased in the azoospermic patients (3.24 ppm) while another was increased (3.72 ppm). It was revealed that the control population was distinct from similarly perturbed metabolite levels. However, the idiopathic infertile groups were also distinct from the control population due to increased level of malonate and decreased level of valine, 2- hydroxyisovalerate, lysine, hiipurate and fructose. It turned out that valine and 2- hydroxyisovalerate along with alanine were also decreased in the idiopathic infertile groups when compared to other infertile groups (Table 4).
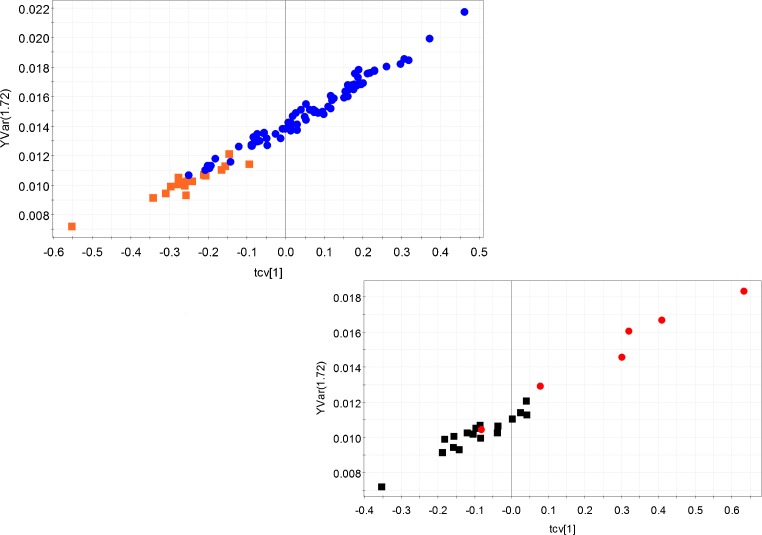
As Table 1 suggests, the different sperm characteristics such as sperm count, motility etc. are not much different between the idiopathic infertility and the control groups. Therefore, the diagnosis of idiopathic infertility is a challenge. We chose the lysine multiplet peak (1.74 ppm) in order to build a predictive model for idiopathic infertility and built two separate OPLS models - one comprising of the idiopathic infertile groups and all other infertile groups, another with the idiopathic infertile group along with the controls. Both the models showed significantly good predictive ability. The statistical parameters are tabulated in Table 5. The cross validated scores plots of the models are also demonstrated (Fig. 5a and b).
Table 5.
Statistics of OPLS model based on lysine multiplet at 1.74 ppm built to predict the idiopathic infertility
| Model | R2Y | Q2(cum) |
|---|---|---|
| Idiopathic infertility and control | 0.99 | 0.97 |
| Idiopathic infertility and other infertile groups | 0.98 | 0.98 |
Fig. 5.
The correlation of cross validated scores of two OPLS models with normalized peak intensity of lysine at 1.74 ppm. (a). idiopathic infertility (orange dots) and all other infertile groups (blue squares). Model detail: R2Y = 0.99 and Q2(cum) = 0.97, Equation of the regression line: normalized intensity of lysine (1.74 ppm) = 0.015 * cross validated scores of OPLS model + 0.014. (b). Idiopathic infertility (black squares) and fertile control population (red dots). Model detail: R2Y = 0.98 and Q2 (cum) = 0.98. Equation of the regression line: normalized intensity of lysine (1.74 ppm) = 0.012 * cross validated scores of OPLS model + 0.011
Discussion
NMR spectroscopy is one of the most common and widely used spectroscopic techniques in the field of metabolomics approach which is based on non-destructive examination of intact tissue and biological fluids. NMR is intrinsically rich in information, offering structural and quantitative information simultaneously, with metabolite identification capabilities of even the most complex of mixtures, without the need of separation or sample preparation. Although NMR is considered to be relatively insensitive and involves high cost, it can measure many molecules simultaneously and provide and detailed structural information of the molecule(s) under study [40].
There is growing interest in the application of high-resolution 1H NMR spectroscopy to human seminal fluid. The potential of 31P-NMR spectroscopy as sensitive tool for assessment of testicular metabolic integrity and differentiation of normal testicles from those with markedly decreased spermatogenesis was highlighted over a decade ago [10]. The likelihood that 1H NMR spectroscopy may provide some quantitative markers which may be used for examining infertility was subsequently established [16]. More recently, the application of 1H-NMR to testicular tissue sections has been found to yield a unique metabolic signature (based upon phosphocholine tissue concentrations) for spermatogenesis, which could aid in the non-invasive diagnosis of sperm in men with non-obstructive azoospermia [1]. Attempts have also been made to test the effects of an injectable contraceptive on the distribution of amino acids [9]. The metabolomic approach as applied to the study of seminal plasma has also highlighted an association of oxidative stress and spermatogenic abnormalities [11].
The use of proton NMR spectroscopy to investigate the lipid composition of human spermatozoa and seminal fluids with specific reference to changes after cryopreservation, revealed the release or activation of both phospholipase A(2) and sphingomyelinase in human spermatozoa due to the freezing/thawing cycle [37]. The utility of 1H NMR spectroscopy to highlight changes in the seminal plasma of infertile men with spinal cord injury (SCI) due to neurological dysfunction of the prostate has been demonstrated [3, 4]. The presence of higher peptidase activity in infertile men with SCI and that uridine is likely to be an essential precursor to metabolites required for capacitation and is a potential marker for the prognosis of functional fertility recovery in patients with SCI has now been established [24, 23].
In the context of phytochemical research, Withania somnifera therapy has been found to repair the disturbed concentrations of lactate, alanine, citrate, GPC, histidine, and phenylalanine in seminal plasma thereby aiding in recovery of semen quality post treatment [14]. Supporting the notion that diabetes effected changes in the metabolome of the testicle itself, the accumulation of advanced glycation end products accumulate in the reproductive tract of men with diabetes [25], and metabolic profile changes in the testes of mice with streptozotocin-induced type 1 diabetes mellitus has been reported [26].
In ART, metabolomics has identified an association between ART outcomes and levels of ROS in follicular fluid (FF) and embryo culture media [11]. This approach has also been applied to the study of human embryos [8, 40, 42] and oocytes [44]. More recently, the development of a screening technology using Raman and near-infrared spectroscopy was used to detect biomarkers in spent culture medium, with differences in algorithms generated for positive compared to negative in vitro fertilization outcomes [38, 41]. A few studies using the single embryo transfer (SET) model on day 2 and 3 showed higher mean viability scores for embryos that resulted in a pregnancy with fetal heart activity, compared with those that did not [40, 47]. Interestingly, metabolic profiling of embryo culture media was shown to be independent of embryo morphology. However, large scale, prospective studies are necessary to relate the metabolic profile of the medium to the developmental potential of human oocyte and embryo.
As can be seen from literature presented above, the field of metabolomics and its potential to contribute to identification of male fertility-related biomarkers is great. Analysis of small molecular weight metabolites is one of the ways of assessing reproductive efficiency. For example, the level of fructose has been implicated as a marker of seminal vesicle function for different classes of male infertility patients [2]. Levels of true corrected seminal fructose have been suggested as a better marker of seminal vesicle function [12]. Although, there are debates over the level of fructose and infertility [2], our analysis suggests that fructose level in the azoospermic and oligozoospermic males are indeed low compared to healthy fertile males confirming the earlier observations [48]. Fructose is believed to be the energy source for the spermatozoa and it is therefore possible that scarcity in it would lead to incorrect generation of spermatozoa. This could be the possible reason for oligozoospermia or azoospermia in the relevant group of patients.
More importantly, our study suggests the presence of a metabolic reason behind the origin of idiopathic infertility. This is stressed upon by the fact that seminal plasma profile of this group showed distinct segregation from the control population as well as other infertile groups. When compared to the control populations, the idiopathic infertile group showed lower levels of valine, 2- hydroxyisovalerate, lysine, hippurate and fructose. In addition, when compared to the other infertile groups, low levels of valine, 2- hydroxyisovalerate and lysine have also been observed. Among different characteristics, the sperm motility was slightly, albeit statistically significantly, lowered in the idiopathic infertile group than the control. Fructose was implicated as one of the factors affecting the sperm motility earlier [33]. In addition, our data suggests that idiopathic infertile patients may be characterized by the low concentration of valine, which, in extension might be indicative of valine degradation. Lysine is another such amino acid which was found to be lower in the idiopathic infertile group. Our data suggests that the level of lysine could be of diagnostic use for idiopathic infertility. Microdeletion analysis of Y chromosome has become a standard genetic marker for the idiopathic male infertility [5]. However, this report indicates that lysine concentration in the seminal plasma may be a good indicator of idiopathic infertility. A very good regressive relation of the lysine concentration and t- scores of NMR profile in the seminal plasma of idiopathic infertility and other infertile groups (normalized concentration of lysine = 0.01494*t- scores of seminal plasma NMR profile + 0.0141, R2Y = 0.98) along with similarly good regressive relation in idiopathic infertile group and control population (normalized conc of lysine = 0.01219*t- scores of seminal plasma NMR profile + 0.0114, R2Y = 0.97) suggests that 1H NMR based metabonomic profiling has the potential to detect idiopathic infertility in an efficient manner.
In this study, the presence of a difference in biochemical profiles, between different forms of male infertility, with a special emphasis on idiopathic infertility, has been established. Results of the present study agree and complement another recent study where 1H NMR spectroscopic studies on human seminal plasma using probative discriminant function analysis has been attempted and alanine, citrate, GPC, tyrosine and phenylalanine for determination of infertility has been proposed [13]. Although certain diagnostic criterion based on genetic tests has been proposed earlier for detection of idiopathic infertility, [5, 51] our method shows promise towards a more robust and efficient diagnostic technique.
The importance of male factor in fertility issues has only been recognized lately. As a first step forward, the present study has provided a rationale for search of a new biochemical marker for evaluating the pathogenesis of male infertility. This is relevant considering the fact that estimation of biochemical parameters is seldom done in daily routine investigations of the seminal plasma. In the context of unexplained infertility, metabolomic analysis may be useful in establishing a cause for the same, thereby aiding in diagnosis and therapeutic intervention.
The discovery of noninvasive, highly sensitive and specific biomarkers by NMR spectroscopy would eliminate the need for invasive testing in the infertile man and also allowing an expanded and more specific classification of male factor infertility [19, 36]. It is possible that the observed differences in biomarker profiles of patients with different forms of infertility by 1H-NMR spectroscopy could highlight the presence of clinically relevant information. At this juncture, albeit when clinical applications for use of NMR spectroscopy have not yet been clearly defined, we hope that attempts to achieve a complete assignment of the 1H NMR metabolic profile for seminal fluid may enhance the information obtainable from metabolomic studies and aid as a valuable tool useful in the diagnosis and management of male infertility.
Electronic supplementary material
(DOC 28 kb)
(JPEG 355 kb)
(JPEG 364 kb)
(JPEG 358 kb)
(JPEG 374 kb)
Acknowledgments
The guidance by Dr. Satish Kumar Adiga, Professor and Clinical Embryologist, Division of Reproductive Medicine, Department of OBG, Kasturba Medical College, Manipal, India is gratefully acknowledged by the authors. The help of National Facility for High Field NMR in TIFR is greatly acknowledged. AS acknowledges the Council of Scientific and Industrial Research, Government of India, for providing SPM Fellowship.
Conflicts of interest
The authors report no financial or commercial conflicts of interest.
Footnotes
Soumita Ghosh and Arjun Sengupta these authors contributed equally to this work.
Capsule NMR spectroscopy on seminal plasma of fertile and infertile men revealed significant differences in biomarker profile between idiopathic and other forms of infertility.
References
- 1.Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod. 2010;25:847–852. doi: 10.1093/humrep/dep475. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed Z, Khan MS, Khan MA, Haq A, Rahman J. Seminal fructose in various classes of infertile patients. Pak J Physiol. 2010;6:36–38. [Google Scholar]
- 3.Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171:2230–2232. doi: 10.1097/01.ju.0000125241.77517.10. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrino AP, Rodrigues MA, Matsuo T, Schuquel IT, Costa WF, Santilli JC. Evaluation of seminal citrate level by 1H nuclear magnetic resonance spectroscopy in men with spinal cord injury. Spinal Cord. 2009;47:878–881. doi: 10.1038/sc.2009.62. [DOI] [PubMed] [Google Scholar]
- 5.Ambasudhan R, Singh K, Agarwal JK, Singh SK, Khanna A, Sah RK, et al. Idiopathic cases of male infertility from a region in India show low incidence of Y-chromosome microdeletion. J Biosci. 2003;28:605–612. doi: 10.1007/BF02703336. [DOI] [PubMed] [Google Scholar]
- 6.Batruch I, Lecker I, Kagedan D, Smith CR, Mullen BJ, Grober E, et al. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J Proteome Res. 2011;10:941–953. doi: 10.1021/pr100745u. [DOI] [PubMed] [Google Scholar]
- 7.Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 8.Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod. 2008;14:679–690. doi: 10.1093/molehr/gan066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhury K, Sharma U, Jagannathan NR, Guha SK. Effect of a new injectable male contraceptive on the seminal plasma amino acids studied by proton NMR spectroscopy. Contraception. 2002;66:199–204. doi: 10.1016/S0010-7824(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 10.Chew WM, Hricak H, McClure RD, Wendland MF. In vivo human testicular function assessed with P-31 MR spectroscopy. Radiology. 1990;177:743–747. doi: 10.1148/radiology.177.3.2243981. [DOI] [PubMed] [Google Scholar]
- 11.Deepinder F, Chowdary HT, Agarwal A. Role of metabolomic analysis of biomarkers in the management of male infertility. Expert Rev Mol Diagn. 2007;7:351–358. doi: 10.1586/14737159.7.4.351. [DOI] [PubMed] [Google Scholar]
- 12.Gonzales GF, Villena A. True corrected seminal fructose level: a better marker of seminal vesicle function in infertile male. Int J Androl. 2001;24:255–260. doi: 10.1046/j.1365-2605.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Mahdi AA, Ahmad MK, Shukla KK, Jaiswer SP, Shankhwar SN. 1H NMR spectroscopic studies on human seminal plasma: a probative discriminant function analysis classification model. J Pharm Biomed Anal. 2011;54:106–113. doi: 10.1016/j.jpba.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Mahdi AA, Shukla KK, Ahmad MK, Bansal N, Sankhwar P, et al. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: a proton NMR study at 800 MHz. J Ethnopharmacol. 2013;149:208–214. doi: 10.1016/j.jep.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Hafez B, Goff L, Hafez S. Recent advances in andrology research: physiopathology and clinical application to fertility and infertility. Arch Androl. 1997;39:173–195. doi: 10.3109/01485019708987916. [DOI] [PubMed] [Google Scholar]
- 16.Hamamah S, Seguin F, Barthelemy C, Akoka S, Le Pape A, Lansac J, et al. 1H nuclear magnetic resonance studies of seminal plasma from fertile and infertile men. J Reprod Fertil. 1993;97:51–55. doi: 10.1530/jrf.0.0970051. [DOI] [PubMed] [Google Scholar]
- 17.Hung Y, Huang M. A multi-class IC package type classifier based on kernel-based nonlinear LS-SVM method. J Comput Intell Electron Syst. 2014;7:472–480. doi: 10.1080/18756891.2014.892265. [DOI] [Google Scholar]
- 18.Jonsson M, Linse S, Frohm B, Lundwall A, Malm J. Semenogelins I and II bind zinc and regulate the activity of prostate-specific antigen. Biochem J. 2005;387:447–453. doi: 10.1042/BJ20041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovac JR, Lipshultz LI. The significance of insulin-like factor 3 as a marker of intratesticular testosterone. Fertil Steril. 2013;99:66–67. doi: 10.1016/j.fertnstert.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Kussmann M, Raymond F, Affolter M. Omics-driven biomarker discovery in nutrition and health. J Biotechnol. 2006;124:758–787. doi: 10.1016/j.jbiotec.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Lamb DJ. A look towards the future: advances in andrology expected to revolutionize the diagnosis and treatment of the infertile male. In: Lipshultz L, Howards S, Niederberger C, editors. Infertility in the male. Cambridge: Cambridge University Press; 2009. pp. 642–653. [Google Scholar]
- 22.Lynch MJ, Masters J, Pryor JP, Lindon JC, Spraul M, Foxall PJ, et al. Ultra high field NMR spectroscopic studies on human seminal fluid, seminal vesicle and prostatic secretions. J Pharm Biomed Anal. 1994;12:5–19. doi: 10.1016/0731-7085(94)80004-9. [DOI] [PubMed] [Google Scholar]
- 23.Maher AD, Cloarec O, Patki P, Craggs M, Holmes E, Lindon JC, et al. Dynamic biochemical information recovery in spontaneous human seminal fluid reactions via 1H NMR kinetic statistical total correlation spectroscopy. Anal Chem. 2009;81:288–295. doi: 10.1021/ac801993m. [DOI] [PubMed] [Google Scholar]
- 24.Maher AD, Patki P, Lindon JC, Want EJ, Holmes E, Craggs M, et al. Seminal oligouridinosis: low uridine secretion as a biomarker for infertility in spinal neurotrauma. Clin Chem. 2008;54:2063–2066. doi: 10.1373/clinchem.2008.112219. [DOI] [PubMed] [Google Scholar]
- 25.Mallidis C, Agbaje IM, Rogers DA, Glenn JV, Pringle R, Atkinson AB, et al. Advanced glycation end products accumulate in the reproductive tract of men with diabetes. Int J Androl. 2009;32:295–305. doi: 10.1111/j.1365-2605.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 26.Mallidis C, Green BD, Rogers D, Agbaje IM, Hollis J, Migaud M, et al. Metabolic profile changes in the testes of mice with streptozotocin-induced type 1 diabetes mellitus. Int J Androl. 2009;32:156–165. doi: 10.1111/j.1365-2605.2007.00829.x. [DOI] [PubMed] [Google Scholar]
- 27.Marieb EN, Hoehn K. Human anatomy and physiology. 7. Menlo Park, CA: Benjamin/Cummings; 2007. [Google Scholar]
- 28.Mendes P. Emerging bioinformatics for the metabolome. Brief Bioinform. 2002;3:134–145. doi: 10.1093/bib/3.2.134. [DOI] [PubMed] [Google Scholar]
- 29.Nobeli I, Thornton JM. A bioinformatician’s view of the metabolome. Bioessays. 2006;28:534–545. doi: 10.1002/bies.20414. [DOI] [PubMed] [Google Scholar]
- 30.Pampalakis G, Sotiropoulou G. Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim Biophys Acta. 2007;1776:22–31. doi: 10.1016/j.bbcan.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas–liquid partition chromatography. Proc Natl Acad Sci U S A. 1971;68:2374–2376. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponglowhapan S, Essen- Gustavsson B, Linde Forsberg C. Influence of glucose and fructose in the extender during long term storage of chilled canine semen. Theriogentology. 2004;62:1498–1517. doi: 10.1016/j.theriogenology.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Rhee SY, Dickerson J, Xu D. Bioinformatics and its applications in plant biology. Annu Rev Plant Biol. 2006;57:335–360. doi: 10.1146/annurev.arplant.56.032604.144103. [DOI] [PubMed] [Google Scholar]
- 35.Rohr G, Eggert-Kruse W, Kalbitzer HR. NMR spectroscopy in andrology: research uses and possible clinical applications. Int J Androl. 1995;18:12–19. doi: 10.1111/j.1365-2605.1995.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 36.Roth MY, Lin K, Bay K, Amory JK, Anawalt BD, Matsumoto AM, et al. Serum insulin-like factor 3 is highly correlated with intratesticular testosterone in normal men with acute, experimental gonadotropin deficiency stimulated with low-dose human chorionic gonadotropin: a randomized, controlled trial. Fertil Steril. 2013;99:132–139. doi: 10.1016/j.fertnstert.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiller J, Arnhold J, Glander HJ, Arnold K. Lipid analysis of human spermatozoa and seminal plasma by MALDI-TOF mass spectrometry and NMR spectroscopy - effects of freezing and thawing. Chem Phys Lipids. 2000;106:145–156. doi: 10.1016/S0009-3084(00)00148-1. [DOI] [PubMed] [Google Scholar]
- 38.Scott R, Seli E, Miller K, Sakkas D, Scott K, Burns DH. Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study. Fertil Steril. 2008;90:77–83. doi: 10.1016/j.fertnstert.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 39.Segalen J, de Certaines JD, Le Calvé M, Colleu D, Bansard JY, Rio M. 1H nuclear magnetic resonance of human seminal plasma in in vitro fertilization attempts: use of automatic spectrum analysis. J Reprod Fertil. 1995;103:181–187. doi: 10.1530/jrf.0.1030181. [DOI] [PubMed] [Google Scholar]
- 40.Seli E, Botros L, Sakkas D, Burns DH. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2008;90:2183–2189. doi: 10.1016/j.fertnstert.2008.07.1739. [DOI] [PubMed] [Google Scholar]
- 41.Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88:1350–1357. doi: 10.1016/j.fertnstert.2007.07.1390. [DOI] [PubMed] [Google Scholar]
- 42.Seli E, Vergouw CG, Morita H, Botros L, Roos P, Lambalk CB, et al. Noninvasive metabolomic profiling as an adjunct to morphology for noninvasive embryo assessment in women undergoing single embryo transfer. Fertil Steril. 2010;94:535–542. doi: 10.1016/j.fertnstert.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 43.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/S0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 44.Singh R, Sinclair KD. Metabolomics: approaches to assessing oocyte and embryo quality. Theriogenology. 2007;68:S56–S62. doi: 10.1016/j.theriogenology.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Thonneau P, Spira A. Prevalence of infertility: international data and problems of measurement. Eur J Obstet Gynecol Reprod Biol. 1991;38:43–52. doi: 10.1016/0028-2243(91)90206-Z. [DOI] [PubMed] [Google Scholar]
- 46.Tomlins AM, Foxall PJ, Lynch MJ, Parkinson J, Everett JR, Nicholson JK. High resolution 1H NMR spectroscopic studies on dynamic biochemical processes in incubated human seminal fluid samples. Biochim Biophys Acta. 1998;1379:367–380. doi: 10.1016/S0304-4165(97)00116-5. [DOI] [PubMed] [Google Scholar]
- 47.Vergouw CG, Botros LL, Roos P, Lens JW, Schats R, Hompes PG, et al. Metabolomic profiling by near-infrared spectroscopy as a tool to assess embryo viability: a novel, non-invasive method for embryo selection. Hum Reprod. 2008;23:1499–1504. doi: 10.1093/humrep/den111. [DOI] [PubMed] [Google Scholar]
- 48.WHO laboratory manual for the examination of human semen and semen cervical mucus interaction. 2. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- 49.WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 50.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metabolomics. 2013;1:92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yatsenko AN, Roy A, Chen R, Ma L, Murthy LJ, Yan W, et al. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15:3411–3419. doi: 10.1093/hmg/ddl417. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida K, Yamasaki T, Yoshiike M, Takano S, Sato I, Iwamoto T. Quantification of seminal plasma motility inhibitor/semenogelin in human seminal plasma. J Androl. 2003;24:878–884. doi: 10.1002/j.1939-4640.2003.tb03139.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 28 kb)
(JPEG 355 kb)
(JPEG 364 kb)
(JPEG 358 kb)
(JPEG 374 kb)