Abstract
Streptococcus pneumoniae is the primary etiological agent of community-acquired pneumonia and a major cause of meningitis and bacteremia. Three conserved pneumococcal proteins—pneumolysin, pneumococcal surface adhesin A (PsaA), and pneumococcal surface protein A (PspA)—are currently being investigated as vaccine candidates. Such protein-based vaccines, if proven effective, could provide a cheaper alternative to conjugate vaccine formulae. Few data from sub-Saharan Africa exist concerning the development of natural antibody to these antigens, however. To investigate the age-specific development of antiprotein immunoglobulin G (IgG) and IgA antibody responses, the sera of 220 persons 2 weeks to 84 years of age from coastal Kenya were assayed using enzyme-linked immunosorbent assays. IgG and IgA antibody responses to each antigen were observed in all age groups. Serum concentrations of IgG and IgA antibody responses to PspA and PdB (a recombinant toxoid derivative of pneumolysin), but not to PsaA, increased significantly with age (P < 0.001). No decline was observed in the sera of the elderly. Anti-protein IgG concentrations were only weakly correlated (0.30 < r < 0.56; P < 0.0001), as were IgA concentrations (0.24 < r < 0.54; P < 0.0001).
In Kenya, as in other developing countries, invasive pneumococcal disease (IPD) is responsible for substantial morbidity and mortality (6, 8). In populations for which incidence data are available, the risk of IPD peaks at the end of the first and beginning of the second year of life, declining sharply thereafter. Disease incidence remains low through puberty and early adulthood, rises gradually through middle age, and increases dramatically for individuals over 65 (15, 18, 23). Recent large-scale efficacy trials have shown that a 7-valent pneumococcal conjugate vaccine is effective against IPD and, to a lesser extent, against pneumonia caused by vaccine types (3, 12, 17). Unfortunately, the price of this vaccine will likely preclude its inclusion in national immunization programs for many countries with the greatest burden of pneumococcal disease. Recent studies also suggest that coverage for such multivalent vaccines may not be universally optimal across the developing world (7, 9). Furthermore, it remains unclear whether serotype replacement will result in significant increases in non-vaccine-type disease in the face of widespread conjugate vaccine use (16). Streptococcus pneumoniae vaccines based on conserved pneumococcal protein antigens such as pneumococcal surface protein A (PspA), pneumolysin, and pneumococcal surface adhesin A (PsaA) are currently under study (4, 5) and, if proven effective, may provide an alternative that is less expensive while affording greater coverage.
Immunity to PspA, pneumolysin, and PsaA in European adults and children with and without pneumococcal disease has been studied extensively (20-22). To the best of our knowledge, the development of naturally acquired immunity to these three antigens in an African population has not yet been described. In the present study, the age-specific pattern of development of antiprotein IgG and IgA in a population in coastal Kenya was investigated. It was hypothesized that IgG and IgA concentrations would peak in the second or third year, maintain a plateau for several decades, and begin to decline slowly in middle age, mirroring the epidemiological pattern in pneumococcal disease that has been observed in several populations (15, 18, 23).
MATERIALS AND METHODS
Study population.
The study population comprised 220 of 800 participants enrolled in a long-term cohort study investigating the natural history of acquired immunity to malaria in Eza Moyo and central Ngerenya in the Kilifi District in coastal Kenya. This locale was part of the study area defined in 1991 for intensive demographic surveillance. At the time of the parent study, the two locations had a total population of about 15,000 persons residing in about 1,003 households. Households were selected randomly from among all the households included in censuses. Selection criteria included stable residence in the study area over the study period and informed consent. There was a deliberate bias towards households with a broad range of representation across the age groups, thus reducing the number of visits required during weekly surveillance. A random age-stratified subsample of the above-described subjects (bled in October 2000) was obtained; only two participants in this subset were from the same household. A total of 20 test sera were assayed in the following age groups: <1, 1, 2, 51 to 60, and >60 years. A total of 15 samples were assayed for the following age classes: 3, 4, 5, 6 to 10, 11 to 20, 21 to 30, 31 to 40, and 41 to 50 years. Age strata were chosen a priori on the basis of projections of how antibody concentrations were likely to change with age.
The secondary use of sera for this project was approved by the institutional review boards of the Harvard School of Public Health and the Kenya Medical Research Institute. It was deemed that the research could potentially benefit the study participants and community from which the samples were drawn and that testing applied no additional risk to individuals or community members.
Serologic methods.
The method of Tharpe et al. (25, 26) was used for serum IgG and IgA assays of all three antigens with some modifications. Flat-bottomed microtiter plates (Immulon II HB; Dynatech Corp., Chantilly, Va.) were coated with PdB, a recombinant toxoid derivative of pneumolysin (5 μg/ml), PsaA (3.5 μg/ml), or recombinant PspA from the Rx1 strain of S. pneumoniae (0.9 μg/ml) in 0.01 M phosphate-buffered saline (PBS) and incubated for 16 h at 4°C (100 μl/well). Plates were washed four times with 0.05% PBS-Tween 20 wash buffer and then blocked with 10% fetal calf serum-PBS for 1 h at 37°C.
In IgG enzyme-linked immunosorbent assays (ELISAs), serum samples were initially diluted 1:400 for PspA and PdB and 1:100 for PsaA in 10% fetal calf serum-PBS. Test sera were assayed in duplicate in five twofold dilutions. Serum from a high-titer donor was used as a reference serum. Sandoglobulin (Sandoz, Cugy, Switzerland) was a high-titer quality control. Plates were incubated for 1 h at 37°C and then washed four times. Horseradish peroxidase-conjugated antihuman Fc Pan IgG monoclonal antibody (clone PH6043; Hybridoma Reagent Laboratory, Baltimore, Md.) was diluted 1:10,000 and pipetted at 100 μl/well. Plates were incubated at 37°C for 2 h and then washed four times. Plates were developed using 3,3′,5,5′-tetramethyl-benzidine (TMB one-component microwell peroxidase substrate; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for 25 min at room temperature. Reaction was stopped with 0.18 M sulfuric acid. Absorbance readings were taken at 450 nm (reference, 620 nm) within 5 min.
In IgA ELISAs, serum samples were diluted 1:50 for all three antigens. Sera from high-titer donors served as the reference serum and quality control. Following a 1-h incubation at 37°C and a wash cycle, bound antibody was labeled with horseradish peroxidase-conjugated mouse antihuman Fd Pan IgA (clone HP6123; Hybridoma Reagent Laboratories) at 1:1,000 dilution for an additional 2 h at 37°C. Subsequent procedures were performed as described for the IgG assay.
PdB was provided by James Paton, Adelaide University, Adelaide, Australia. PsaA and Rx1 PspA antigens were supplied by Aventis Pasteur Ltd. (Toronto, Canada).
Statistical analysis.
ELISA results were analyzed by a four-parameter logistic-log curve fit program (ELISA v. 1.11; Centers for Disease Control and Prevention, Atlanta, Ga.) and expressed in arbitrary units (units per milliliter) as a percentage of the reference serum concentration. The standard was calibrated as containing 100 U of anti-PdB, anti-PsaA, and anti-PspA antibodies/ml. The detection limit was set at 2.50, 0.30, and 0.50 U/ml for anti-PspA, PsaA, and PdB IgG and at 1.00, 1.50, and 1.50 U/ml for anti-PspA, PsaA, and PdB IgA, respectively. Samples with Ig concentrations below this limit were assigned to one-half the detection limit.
STATA software was employed for analysis. To address the non-Gaussian distribution of the antibody data, nonparametric methods were employed in univariate analyses (Spearman correlation, Mann-Whitney test, and Kruskal-Wallis test). For multivariate linear and nonlinear regression analyses, data were log transformed. Linear regression was used to explore the association between log antibody concentration and sex, adjusting for age. As the development of natural immunity with age is expected to be a nonlinear process characterized by an early increase in antibody levels followed by a long plateau, the relationship between age and log serum antibody concentration was modeled using nonlinear least-squares regression. A three-parameter logistic (sigmoidal) model was fit to the data. Only the results for the B2 parameter, the slope, are reported below. A B2 value that is significantly greater than zero indicates a positive association between age and antibody concentration.
RESULTS
Of the study participants, 136 (61.8%) were female. The age groups spanning 21 to 60 years tended to include more females than males. This reflects the sex structure of the original census population of the parent study.
IgG antibody responses to PdB, PsaA, and PspA were detectable in nearly 100% of individuals in all age groups. There was no relation between the amount of IgG reactive to the three protein antigens and sex either overall (Mann-Whitney z = −0.06, −0.17, and −1.4; P > 0.05) or in linear regression analyses adjusted for age (t = −1.19, 0.31, and −0.43; P > 0.05).
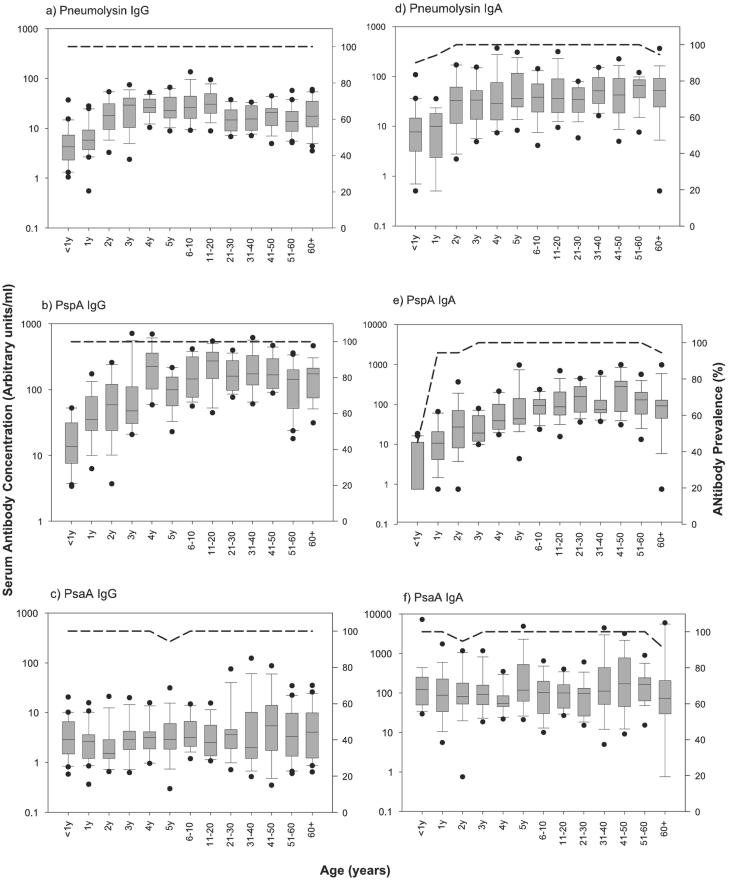
For PdB and PspA, IgG concentrations increased rapidly with age in early childhood and leveled off in adolescence and early adulthood (Fig. 1). Statistically, this relationship was manifest as a significant, positive value for the slope of B2, the sigmoidal curve, and a good fit between the sigmoidal curve and data (high R2). Anti-PdB IgG concentrations increased nearly fourfold between the first and third years of life and reached adult levels at about 4 years of age (R2 = 0.9345, B2 = 1.19; P < 0.001). Anti-PspA IgG concentration increased 13-fold from the age range of 0 to 11 months to the age of 4 years and began to stabilize in the age range of 6 to 10 years (R2 = 0.9701; B2 = 0.60, P < 0.001). In contrast, the specific immune response against PsaA did not change significantly with age (Fig. 1) (R2 = 0.4849, B2 = 0.12; P = 0.49).
FIG. 1.
Age-specific pattern of development of serum anti-Pdb (a and d), antipneumococcal surface adhesin (PsaA) (b and e), and antipneumococcal surface protein (PspA) (c and f) IgG and IgA. Serum antibody concentrations are displayed as box-and-whisker plots representing the 25th, 50th, and 75th percentiles of the data. Error bars indicate the 10th and 90th percentiles. Filled circles represent outliers (data points outside this range). Dashed lines (right-hand y axes) indicate the proportions of subjects with detectable antibodies.
IgG concentrations were all significantly correlated (P < 0.0001). Spearman's correlation coefficients for PdB-PspA, PdB-PsaA, and PspA-PsaA IgG were 0.56, 0.30, and 0.37, respectively. No indication of immunological senescence in the form of a continuous decline in antibody concentrations in persons approaching middle and old age was observed for any of the three antigens. Antibody levels, however, did decrease somewhat between the 11-to-20 age group and 21-to-30 age group for PdB, stabilizing thereafter.
With the exception of the <1 year age class, in which the prevalence of anti-PspA IgA was 45%, prevalence of detectable IgA was nearly 100% for all antigens and all age classes. No significant difference in IgA concentrations between males and females was found for PdB and PsaA (Mann-Whitney z = −0.32 and −0.17 [P > 0.05]; linear regression adjusted for age, t = −0.60 and 0.13 [P > 0.05]). Females had a median anti-PspA IgA concentration 32% higher than males (Mann-Whitney z = −2.00, P = 0.05), but this association was not significant in a linear regression that was adjusted for age (t = −0.74; P = 0.459).
Anti-Pdb and PspA IgA concentrations increased markedly in the first few years of life, reaching a plateau in adolescence and early adulthood (Fig. 1). Anti-PdB IgA increased nearly fourfold between the first and third years of life, peaked in the fifth year of life, and plateaued thereafter (R2 = 0.9090, B2 = 0.83; P < 0.001). Anti-PspA IgA concentrations rose 28-fold from the first to the fifth year of age and then stabilized (R2 = 0.9238, B2 = 0.72; P < 0.001). As seen with the IgG data, anti-PsaA IgA concentrations did not vary with age (R2 = 0.0000, B2 = −0.20; P = 1.000).
There was no evidence of a decline in antibody levels in middle-aged or elderly persons for any of the three antigens. Spearman's correlation coefficients for PdB-PspA, PdB-PsaA, and PspA-PsaA IgA were 0.54, 0.26, and 0.24 (P < 0.0001), respectively. IgG-IgA Spearman correlation coefficients for PdB, PspA, and PsaA were 0.42, 0.63, and 0.40 (P < 0.0001), respectively.
DISCUSSION
As reported previously (21, 24), IgG and IgA responses to PsaA, PspA, and pneumolysin are stimulated in nature and the ability to respond to these antigens develops early in life. The pattern of correlation of IgG and IgA with age differs for the three proteins. The prevalence of detectable anti-Pdb (pneumolysin toxoid derivative), anti-PsaA, and anti-PspA antibodies is nearly 100% for all age classes save the age class of <1 year for PspA IgA. PdB IgG and IgA levels rise gradually from birth to the fourth and fifth years of life, respectively, and remain relatively stable thereafter. Anti-PspA IgG and IgA concentrations increase from birth to about the age ranges of 6 to 10 and 11 to 20 and then stabilize. Given that PspA is a surface-expressed protein, the slow development with age is unexpected. One explanation is that antibodies raised against PspA of different clades are not equally cross-reactive with the RX1 PspA antigen (clade 2, family 1) used in the ELISA. Another explanation for the slow accumulation of antibodies with age is that there is differential access to PspA epitopes on the surface of pneumococcus due to differences in thickness of capsule or phase of the bacteria.
Antibody to PsaA is evident in the sera in early infancy. PsaA-specific antibody concentrations are comparable in all age groups, with levels in the youngest children being of a magnitude similar to that of adult levels. This finding is consistent with data for Finnish children presented by Rapola et al. (21), where PsaA antibodies were shown to reach adult levels at ages as early as 6 months. Antibody levels in children >2 years of age were not observed to exceed adult levels in this data set, however. This observation, in addition to that of the higher level of antibody prevalence observed for PspA and Pdb, may be related to differences in the intensities of pneumococcal transmission in the different populations. More intense transmission is likely to be associated with an earlier age of acquisition, repeated antigenic stimulation, and a greater proportion of the population with high antibody levels.
The absence of a steady decline in protein-specific antibody concentrations in older age groups suggests that waning protein antibody levels may not be responsible for increased disease risk in the elderly that is consistently observed in many populations (15, 23). It may be the case that while naturally acquired antibodies persist in sera, avidity and opsonophagocytic activity decrease in comparison to the results seen with younger adults (13). It should also be noted, however, that the demographic pyramid of the population in Kenya is such that persons over 60 were hard to find and those aged 70 years or more were rare. Given that few people of this age know the year of their birth and that social status is strongly linked to age, it is possible that some individuals overstated their ages and were placed erroneously in the age group of >60 years.
As human immunodeficiency virus (HIV)-infected persons have impaired responses to at least one pneumococcal protein antigen (1), the age-specific pattern of antibody concentration may have been confounded by HIV status. No HIV testing was performed, however, as permission for the test was not obtained during the consent process of the parent study. Estimates of HIV prevalence in Kilifi over 2000 and 2001 are 6.7% for children admitted to hospital and 8.8% for women attending prenatal clinics (11). As the level of HIV prevalence is relatively low, the magnitude of potential confounding is likely to be limited.
The age-specific patterns of antibody development are a function of the underlying immunogenicity of the antigen, the accessibility of epitopes, the pattern of exposure to pneumococcus, maturation of the host immune response over time, serum half-life of the antibodies, and the boosting potential of carriage. Given that carriage prevalence is known to decrease in adulthood (10, 14), the observed data led to the question of how antibody levels are maintained as exposure decreases. There are several possible explanations, however. Protein-specific antibody could be long lived. Cross-reactive antigens could provide adequate boosting of the antibody response. The reported decrease in carriage rates may be partly an artifact of nasopharyngeal sampling processes which do not have the sensitivity to detect carriage episodes of short duration (i.e., less than 1 month). Such shorter carriage events might nonetheless be adequate to boost antibody levels.
The appearance of anti-PdB, anti-PspA, and anti-PsaA antibodies in early life suggests a role for these immune responses in the prevention of IPD. While vaccine-induced anticapsular antibodies have been shown to provide strong protection against IPD in immunocompetent children aged 2 to 5 years or older (2, 19), naturally acquired anticapsular antibodies are not detectable in the sera until the age of 2 years 9 months (27), after the decline in IPD risk that typically occurs between 12 and 24 months of age (15, 18, 23). Anti-PspA and anti-Pdb antibodies, on the other hand, appear in the serum of children with a time course more consistent with the development of immunity to IPD.
Overall, PspA, PsaA, and PdB were found to be immunogenic early in life. The development of IgG and IgA reactive against PspA and PdB was strongly age dependent, while anti-PsaA antibodies were present at adult levels in early infancy. Substantial waning of antibody levels was not observed in the elderly, suggesting that another mechanism is involved in the increase in disease susceptibility in old age.
Acknowledgments
This study was supported by the National Institutes of Health (grant AI148935 to M.L.), Howard Hughes Medical Institute (C.F.L.), and the Wellcome Trust of Great Britain (fellowship 061089 to J.A.G.S.).
This paper is published with the permission of the Director of the Kenya Medical Research Institute.
Editor: J. N. Weiser
REFERENCES
- 1.Amdahl, B. M., J. B. Rubins, C. L. Daley, C. F. Gilks, P. C. Hopewell, and E. N. Janoff. 1995. Impaired natural immunity to pneumolysin during human immunodeficiency virus infection in the United States and Africa. Am. J. Respir. Crit. Care Med. 152:2000-2004. [DOI] [PubMed] [Google Scholar]
- 2.Austrian, R., R. M. Douglas, G. Schiffman, A. M. Coetzee, H. J. Koornhof, S. Hayden-Smith, and R. D. Reid. 1976. Prevention of pneumococcal pneumonia by vaccination. Trans. Assoc. Am. Physicians 89:184-194. [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety, and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., S. K. Hollingshead, G. S. Nabors, J. C. Paton, and A. Brooks-Walter. 2000. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine 19:S87-S95. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 6.Fedson, D., and J. Scott. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17:S11-S18. [DOI] [PubMed] [Google Scholar]
- 7.Gordon, S. B., S. Kanyanda, A. L. Walsh, K. Goddard, M. Chaponda, V. Atkinson, W. Mulwafu, E. M. Molyneux, E. E. Zijlstra, M. E. Molyneux, and S. M. Graham. 2003. Poor potential coverage for 7-valent pneumococcal conjugate vaccine, Malawi. Emerg Infect. Dis. 9:747-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwood, B. 1999. The epidemiology of pneumococcal infection in children in the developing world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 10.Hendley, J. O., M. A. Sande, P. M. Stewart, and J. M. Gwaltney, Jr. 1975. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J. Infect. Dis. 32:55-61. [DOI] [PubMed] [Google Scholar]
- 11.Kenya Ministry of Health. 2001. AIDS in Kenya: background, projections, impact, interventions and policy, sixth ed. National AIDS and STD Control Programme. Kenya Ministry of Health, Nairobi, Kenya.
- 12.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, N. Pierce, and Vaccine Trialists Group. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 13.LeMaoult, J., P. Szabo, and M. E. Weksler. 1997. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol. Rev. 160:115-126. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Evans, N., T. J. O'Dempsey, I. Baldeh, O. Secka, E. Demba, J. E. Todd, T. F. McArdle, W. S. Banya, and B. M. Greenwood. 1996. Nasopharyngeal carriage of pneumococci in Gambian children and in their families. Pediatr. Infect. Dis. J. 15:866-871. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen, S. V., and J. Henrichsen. 1996. Incidence of invasive pneumococcal disease and distribution of capsular types of pneumococci in Denmark, 1989-1994. Epidemiol Infect. 117:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien, K. L., and R. Dagan. 2003. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine 21:1815-1825. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien, K. L., L. H. Moulton, R. Reid, R. Weatherholtz, J. Oski, L. Brown, G. Kumar, A. Parkinson, D. Hu, J. Hackell, I. Chang, R. Kohberger, G. Siber, and M. Santosham. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355-361. [DOI] [PubMed] [Google Scholar]
- 18.O'Dempsey, T. J., T. F. McArdle, N. Lloyd-Evans, I. Baldeh, B. E. Lawrence, O. Secka, and B. M. Greenwood. 1996. Pneumococcal disease among children in a rural area of west Africa. Pediatr. Infect. Dis. J. 15:431-437. [DOI] [PubMed] [Google Scholar]
- 19.Paton, J. C., I. R. Toogood, R. A. Cockington, and D. Hansman. 1986. Antibody response to pneumococcal vaccine in children aged 5 to 15 years. Am. J. Dis. Child. 140:135-138. [DOI] [PubMed] [Google Scholar]
- 20.Rapola, S., V. Jantti, M. Eerola, P. H. Makela, H. Kayhty, and T. Kilpi. 2003. Anti-PsaA and the risk of pneumococcal AOM and carriage. Vaccine 21:3608-3613. [DOI] [PubMed] [Google Scholar]
- 21.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 22.Rapola, S., T. Kilpi, M. Lahdenkari, P. H. Makela, and H. Kayhty. 2001. Antibody response to the pneumococcal proteins pneumococcal surface adhesin A and pneumolysin in children with acute otitis media. Pediatr. Infect. Dis. J. 20:482-487. [DOI] [PubMed] [Google Scholar]
- 23.Robinson, K. A., W. Baughman, G. Rothrock, N. L. Barrett, M. Pass, C. Lexau, B. Damaske, K. Stefonek, B. Barnes, J. Patterson, E. R. Zell, A. Schuchat, and C. G. Whitney. 2001. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA 285:1729-1735. [DOI] [PubMed] [Google Scholar]
- 24.Samukawa, T., N. Yamanaka, S. Hollingshead, K. Klingman, and H. Faden. 2000. Immune responses to specific antigens of Streptococcus pneumoniae and Moraxella catarrhalis in the respiratory tract. Infect. Immun. 68:1569-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tharpe, J. A., and H. Russell. 1996. Purification and seroreactivity of pneumococcal surface adhesin A (PsaA). Clin. Diagn. Lab. Immunol. 3:227-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tharpe, J. A., H. Russell, M. Leinonen, B. D. Plikaytis, R. F. Breiman, G. M. Carlone, E. W. Ades, and J. S. Sampson. 1998. Comparison of a pneumococcal common protein (PsaA) antibody ELISA and a PsaA immune complex ELISA for detection of pneumococcal serum antibody. Pathobiology 66:77-83. [DOI] [PubMed] [Google Scholar]
- 27.Virolainen, A., J. Jero, P. Chattopadhyay, P. Karma, J. Eskola, and M. Leinonen. 1996. Comparison of serum antibodies to pneumolysin with those to pneumococcal capsular polysaccharides in children with acute otitis media. Pediatr. Infect. Dis. J. 15:128-133. [DOI] [PubMed] [Google Scholar]