Abstract
Purpose
Dysplasia of the Fibrous Sheath (DFS) is a primitive flagellar pathology for which a broad spectrum of ultrastructural flagellar abnormalities has been described responsible for a severe to total asthenozoospermia. To this phenotype other morphological abnormalities including cephalic and abnormalities in nuclear structure can be associated that could compromise embryonic development in case of use of Assisted Reproductive Technology (ART). The aim of this study was to evaluate the level of DNA fragmentation and aneuploidy rate in ejaculated spermatozoa of Tunisian men presented with DFS sperm defect associated to high percentage of head abnormalities and to compare the results with those from fertile men.
Methods
Sperm DNA fragmentation was evaluated by the terminal desoxynucleotidyl transferase mediated deoxyuridine triphosphate biotin nick-end labelling (TUNEL) assay. The study of meiotic segregation was performed by Fluorescence in situ hybridization (FISH) for chromosomes X, Y and 18.
Results
The mean DNA fragmentation index was significantly higher in patients compared to the control group. FISH revealed a significantly higher incidence of sperm aneuploidies compared with controls. All patients showed elevated frequencies of sex chromosomes disomy, disomy 18 and diploidy.
Conclusions
In some cases of syndromic teratozoospermia due to sperm tail structural abnormalities, such as DFS, other morphological cephalic abnormalities may be associated. In these cases we have demonstrated impaired sperm nuclear quality which will affect the results in ICSI. Hence the interest of a thorough study of the sperm nucleus in these forms of infertility in order to predict the chances of success in ART.
Keywords: Akinozoospermia, Dysplasia of the fibrous sheath, Aneuploidy, DNA fragmentation
Introduction
First introduced by Chemes et al., [1] the denomination Dysplasia of the Fibrous Sheath (DFS) (including ‘stump tail’ and ‘short tail’ sperm defects) refers to a condition of absent or severely reduced sperm motility associated to major alterations in the fibrous sheath and dysplastic development of the tail during spermatogenesis [1,2]. At light microscopy this isolate defect, involving the majority of ejaculated sperm, show short and irregularly thick tails. Ultrastructural studies revealed a heterogeneous array of sperm tail anomalies caused by a hyperplastic and disorganized fibrous sheath and the alteration of other cytoskeletal and axonemal components of the flagellar [2–4]. Frequent familial incidence of DFS has been reported that suggests a genetic origin of this syndrome [2,3,5].
To date the molecular defect in human DFS is undefined. Several proteins have been identified on isolated human sperm fibrous sheath [6,7], and only two proteins AKAP3 and AKAP4 (members of the A-kinase anchor protein family) involved in organizing the basic structure of the fibrous sheath [8] were described as the most abundant structural protein of the fibrous sheath [9]. It was demonstrated that the absence of these proteins is associated to sperm immotility [10,8,11]. Baccetti et al., [11] have reported in one DFS patient a deletion of the AKAP4/AKAP3 binding regions and have suggested that in some cases the DFS sperm defect could be associated with alterations of AKAP3 and AKAP4 gene sequences.
Since intracytoplasmic sperm injection (ICSI) is the sole treatment for resolving the infertility of patients with sperm tail defects, the study of chromosomal content and DNA fragmentation of their spermatozoa is of great interest. Few studies have analyzed sperm aneuploidy in spermatozoa from patients with sperm tail defects [12], especially with the DFS defect [13–16], in which elevated frequencies of sex chromosomes disomy and diplodies were detected.
In the present paper, we describe the combination of two sperm pathologies in seven infertile patients: DFS associated to sperm head abnormalities particularly microcephalic head and abnormal acrosome. Because these patients are recruited for ICSI treatment, we studied meiotic segregation by fluorescence in situ hybridization (FISH) to evaluate the frequency of chromosomal aneuploidy in sperm nuclei for chromosomes X, Y and 18 and analyzed the level of sperm DNA fragmentation by the terminal desoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL) assay.
Material and methods
Patients and controls
We selected seven infertile men referred to our laboratory over a period of 3 years (2010–2012) for semen analysis after several years of primary infertility (mean duration of 5 years). Their ages ranged from 29 to 43 years with a mean of 36 years. Sperm analysis, evaluated according to the World Health Organization guidelines [17], evoked the DFS sperm defect associated with high percentage of head abnormalities. There was no history of radiotherapy, chemotherapy, orchitis, toxic exposure, trauma, varicocele, testicular torsion, chronic illness, or medication. Hormones concentrations of FSH, LH, Testosterone and the seminal levels of biochemical markers of accessory sex glands were in the normal range. Two patients had the notion of infertility in the family without consanguinity in the respective families. The karyotype and molecular analysis of the Y chromosome microdeletion findings were normal for all patients. A group of fertile patients (n = 20) with normal semen profiles was included as a control group. The local ethics committee approved the present protocol, and all patients and controls gave informed consent for the present study.
Semen analysis
For each patient at least two consecutive semen analyses were performed within a period of 3 months. Semen samples were collected by masturbation after 3 days of sexual abstinence and examined after liquefaction for 30 min at 37 °C. The basic semen parameters were evaluated according to the 2010 World Health Organization guidelines [17]. Sperm morphology was assessed according to the David’s classification [18]. Eosin-nigrosin viability test was performed by dissolving 1 g eosin with 1 g of fresh sperm and 3 g nigrosin. A few minutes after staining, the samples were observed at light microscope and stained sperm head (dead spermatozoa) and unstained sperm head (viable spermatozoa) were scored. A minimum of 100 spermatozoa were counted.
Semen preparation
After semen analysis, the sperm sample was washed twice in phosphate-buffered saline (PBS, pH = 7.4) (Sigma, St Louis, MO) and centrifuged at 400 g for 5 min. The pellet was then resuspended in a fixative solution of methanol/acetic acid (Merck, Darmstadt, Germany) for at least 30 min at 4 °C. Spermatozoa were then spread on a slide for FISH analysis and DNA fragmentation.
Analysis of aneuploidy
The sperm nuclei were decondensed for 2 min by using a solution of NaOH 1 N, washed with distilled water, dehydrated through an ethanol series (70 %, 90 %, 100 %) and air dried. After dehydration, FISH was performed with X, Y, 18 centromeric probes (Vysis-Abott, Rungis, France) denaturized at 72 °C for 7 min. After hybridization at 37 °C for 2 h, the slides were washed in 1X standard saline citrate (1X SSC) and counterstained with 6-diamino-2-phenylindole solution, and spermatozoa were analyzed with an Axioplan epifluorescence microscope (Leica, Wetzlar, Germany). Observation and interpretation criteria were based on the number of spots for X, Y, and 18 chromosomes on the sperm nuclei. For each patient, a minimum of 1000 sperm nuclei were counted. Only intact spermatozoa bearing a similar degree of decondensation and clear hybridization signals were scored; disrupted or overlapping spermatozoa were excluded from analysis.
Detection of DNA fragmentation
The presence of DNA strand breaks in spermatozoa was evaluated by the terminal desoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL) assay using the ApopTag Apoptosis Detection Kit (QBiogene, Paris, France). The procedure was carried out according to the manufacturer’s instruction. Briefly, for cell permeabilization, the slides were first incubated in phosphate buffer saline (PBS) with a solution of 1 % Triton X100 (Sigma) and then equilibrated with the equilibration buffer at room temperature for 10 s and incubated in a dark moist chamber at 37 °C for 1 h with the terminal desoxynucleotidyl transferase (TdT) solution to allow DNA elongation. After stopping the enzyme reaction, the slides were washed twice in PBS and the DNA elongation was revealed by incubating the cells with anti-digoxigenin antibody coupled to peroxidase for 30 min in a dark moist chamber. The peroxidase was revealed with diaminobenzidine (DAB). The sperm nuclei were counterstained with Harris’s hematoxylin (RAL, Martillac, France) and finally mounted using Faramount mounting (Dako, Carpinteria, CA, USA).
The slides were observed under a microscope (Zeiss, Oberkochen, Germany) equipped with a 100X magnification lens. Spermatozoa with fragmented DNA had brown-colored nuclei, and the other cells with intact DNA were blue-gray (counter coloration with Harris’s haematoxylin). On each slide, approximately 500 cells were counted, and the DNA fragmentation index (DFI) was calculated.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences, version 15 (SPSS, Chicago, IL, USA). The comparisons between the controls and patients were calculated using Student’s t-test. Spearman’s correlation coefficients were also calculated. A significant statistical difference was accepted when P <0.05.
Results
Semen characteristics and detailed morphology under light microscopy
The different characteristics of sperm examination of patients are reported in Table 1. According to the 2010 World Health Organization guidelines [17], semen analysis showed a mean normal volume of 2.5 ± 0.5 ml, a mean concentration of 17.47 ± 20.88 × 106/ml (range 0.26 × 106 to 48 × 106) and a mean total progressive motility of 1.42 ± 3.77 %. In fact, a total absence of motility was observed in 6 patients, whereas in patient 7 only 10 % of progressive motility was registered. Eosin-nigrosin staining revealed that on average 78.00 ± 9.32 % of sperm cells were viable. The light microscopy showed for all patients an absolute monomorphic teratozoospermia (100 % atypical forms) with a predominance of short thick tails (58.42 ± 18.97 %) and sperm head abnormalities particularly microcephalic head (50.14 ± 15.2 %) and abnormal acrosome (65 ± 13.89 %) (Fig. 1). The multiple anomalies index was increased compared to the control group (2.33 ± 0.38 versus 1.41 ± 0.14), reflecting the high incidence of morphological abnormalities in DFS patients.
Table 1.
Sperm analysis results for the DFS patients and control group
| Sperm parameter | Patient number (n = 7) | Control group (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean ± SD | Mean ± SD | |
| Volume (ml) | 2.5 | 2 | 2.5 | 2.5 | 2 | 2.5 | 3.5 | 2.5 ± 0.5 | 3.20 ± 1.43 |
| Sperm concentration (X106/ml) | 0.26 | 8.6 | 47 | 3.1 | 48 | 3.16 | 12.2 | 17.47 ± 20.88 | 139 ± 59.95 |
| Viability (%) | 74 | 88 | 92 | 70 | 81 | 67 | 74 | 78.00 ± 9.32 | 83.00 ± 4.24 |
| Progressive motility (%) | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1.42 ± 3.77 | 55.51 ± 3.49 |
| Abnormal forms (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 ± 0.00 | 54.33 ± 10.65 |
| Abnormal acrosome (%) | 63 | 70 | 81 | 75 | 50 | 43 | 73 | 65 ± 13.89 | 15.67 ± 9.57 |
| Microcephalic head (%) | 50 | 62 | 68 | 53 | 51 | 47 | 20 | 50.14 ± 15.2 | 14.86 ± 12.37 |
| Irregular head (%) | 37 | 21 | 37 | 44 | 11 | 26 | 10 | 26.57 ± 13.35 | 8.00 ± 5.71 |
| Tapered head (%) | 13 | 4 | 6 | 4 | 24 | 19 | 27 | 21.71 ± 9.46 | 11.19 ± 11.94 |
| Bent tail (%) | 23 | 4 | 17 | 12 | 13 | 10 | 3 | 11.71 ± 7.01 | 9.00 ± 3.34 |
| Short tail (%) | 72 | 61 | 77 | 70 | 37 | 65 | 27 | 58.42 ± 18.97 | 1.86 ± 2.17 |
| Multiple anomalies index | 2.83 | 1.91 | 2.49 | 2.71 | 2.41 | 2.21 | 1.81 | 2.33 ± 0.38 | 1.41 ± 0.14 |
Fig. 1.
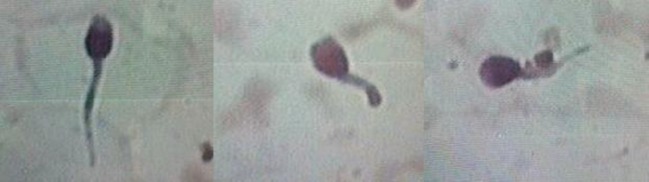
Observation at light microscopy of spermatozoa with short and irregularly thick tails
We estimated a frequency of DFS sperm defect associated with head abnormalities to be 0.17 %. In fact, over 3 years (2010–2011 and 2012) among 4195 infertile men consulting in our laboratory, 7 patients presented with this particular phenotype.
Analysis of meiotic segregation
The results of triple color FISH were reported in Table 2 and illustrated in Fig. 2. On average 1000 sperm nuclei were scored for each patient. The mean frequencies of X-bearing and Y-bearing sperm in DFS patients were 40.87 and 41.48 % respectively. The mean total sperm aneuploidy rate (combined diploidy, disomy and nullisomy of analyzed chromosomes) was 17.65 ± 2.17 % significantly higher compared to that of the control group (1.48 ± 0.22 %). Summarizing FISH results, we observed that the incidence of disomy X (2.97 ± 0.87 %), disomy Y (3.18 ± 0.67 %) and disomy XY (3 ± 0.26 %) was significantly higher (p <0.05) compared to the controls (respectively; 0.24 ± 0.07 %, 0.28 ± 0.08 % and 0.55 ± 0.17 %). Total rate of sex chromosomes disomy (9.16 ± 1.51 %) was then significantly higher (p < 0.05) than in the control group (1.08 ± 0.24 %). The diploidy rate (2.63 ± 0.55 %) was significantly increased (p = 0.001) compared with that of the controls (0.13 ± 0.08 %), as well as for the frequency of disomy of chromosome 18 (2.05 ± 0.56 % vs 0.16 ± 0.08 %).
Table 2.
Frequencies of disomies, diploidies and nullisomies for 18, X and Y chromosomes in patients carriers of a combination of DFS associated to head abnormalities
| Patient number | Disomy frequency (%) | Diploidy frequency (%) | Nullisomy frequency (%) | ||||
|---|---|---|---|---|---|---|---|
| 1818 | XX | YY | XY | 1818XX, 1818YY, 1818XY | X,Y | 18 | |
| 1 | 2.22 | 3.45 | 2.59 | 3.23 | 2.76 | 0.85 | 1.00 |
| 2 | 2.01 | 1.66 | 2.58 | 2.67 | 2.58 | 0.85 | 2.00 |
| 3 | 2.46 | 2.38 | 3.26 | 2.61 | 2.86 | 1.86 | 1.87 |
| 4 | 1.44 | 2.31 | 2.50 | 3.08 | 2.41 | 1.35 | 1.72 |
| 5 | 2.55 | 4.04 | 3.24 | 3.08 | 3.15 | 1.00 | 1.39 |
| 6 | 2.57 | 3.26 | 4.16 | 3.29 | 3.15 | 1.98 | 1.85 |
| 7 | 1.15 | 3.74 | 3.93 | 3.07 | 2.68 | 1.15 | 1.53 |
| Mean ± SD | 2.05 ± 0.56 | 2.97 ± 0.87 | 3.18 ± 0.67 | 3.00 ± 0.26 | 2.63 ± 0.55 | 1.51 ± 0.69 | 1.62 ± 0.34 |
| Control group Mean ± SD | 0.16 ± 0.08 | 0.24 ± 0.07 | 0.28 ± 0.08 | 0.55 ± 0.17 | 0.13 ± 0.08 | 0.13 ± 0.04 | 0.04 ± 0.02 |
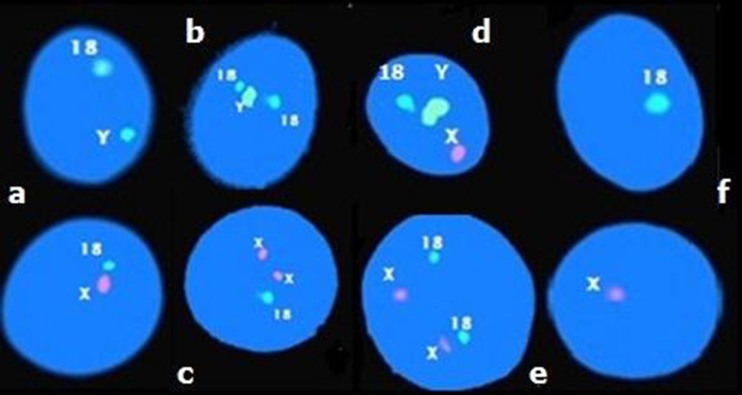
Fig. 2.
Fluorescence in situ hybridization with probes for chromosomes X (red), Y (green) and 18 (blue) on sperm of one DFS patient showing (a) haploid spermatozoa X18/Y18; (b) disomy 18; (c) disomy X; (d) disomy XY; (e) diploidy XX1818; (f) nullisomy 18/0 and 0/X
Our results also showed that for DFS patients no statistically significant difference was observed between the mean values of XY disomy (3.00 ± 0.26 %) compared to X disomy (2.97 ± 0.87 %) or Y disomy (3.18 ± 0.67 %) which suggested that both meiotic I and II divisions were affected.
Correlation between aneuploidy and semen parameters
No correlations were observed between sperm concentration, total aneuploidy rate and sex chromosomes disomy rate. However significant correlations were observed between impaired mobility and total aneuploidy rate (r = 0.408, p <0.001) and sex chromosomes disomy rate (r = 0.612, p <0.001). Also no significant correlations were observed between microcephalic head, abnormal acrosome, short flagella, total aneuploidy rate and sex chromosomes disomy rate. Especially we found that diploidy was correlated to microcephalic heads (r = 0.108, p = 0.001) and short flagella (r = 0.216, p = 0.001). Finally the multiple anomalies index correlated significantly to the disomy XY (r = 0,288, p = 0,046) and diploidy (r = 0,199, p = 0,046).
Analysis of DNA fragmentation
Our patients with DFS sperm defect associated with high percentage of head abnormalities showed a significant higher percentage of DNA damage. The mean percentage of DNA fragmentation assessed by TUNEL assay in the patients group was 32.66 ± 4.61 %. However, this value in the control group was only 10.41 ± 3.77 %.
Discussion
Dysplasia of the fibrous sheath (DFS) refers to a condition of severely reduced or absent sperm motility, associated to serious distortions of the fibrous sheath constituents with dysplastic development of the tail during spermatogenesis [1,2]. DFS sperm defect is therefore diagnosed by the presence of short, thick and irregular sperm tail during routine light microscopic examination of a semen sample. The objectives of this study were to study sperm aneuploidy and analyze sperm DNA fragmentation in 7 infertile patients described as having DFS sperm defect associated to high percentage of head abnormalities in particular microcephalic heads and abnormal acrosomes. To our knowledge this is the first study that described such particular association of two sperm phenotypes of presumably genetic origin. We reported an estimation of the frequency of this condition to be 0.17 % among the infertile population reflecting the rarity of this phenotype. In the literature, few individual case reports have been published, there were 15 reported cases of DFS/stump tails/short tails [4,13,15]. Bacceti et al., [14] reported a large series of 12 patients with DFS sperm defects while Mitchell et al., [43] investigated ICSI outcomes for 21 patients with distinct ultrastructural flagellar abnormalities, 12 of them presented with “short tail” syndrome. Rawe et al., [19] and Moretti et al., [20] have reported respectively one and two cases of DFS sperm defect associated with acephalic sperm and abnormal head-tail attachment.
In the present paper all of the studied patients presented an absolute teratozoospermia (100 % atypical forms). In addition to a general observation of the typical morphologic characteristics of DFS sperm defect, we observed a predominance of sperm head abnormalities particularly microcephalic head and abnormal acrosome. Most patients had oligozoospermia and total absence of progressive motility which is in accordance with data reported in the literature showing that a severe oligo-astheno-teratozoospermia was consistently associated with DFS sperm defect [4,20].
Despite a normal blood karyotype, our patients have a significantly increased frequency of chromosomal abnormalities in their sperm compared to the control group. In fact we noted a significant increased incidence of sex chromosomes disomies, of diploidy and of 18 disomy. In dysplasia of fibrous sheath sperm, the most frequently studied genetic sperm tail defect, the alterations in sex chromosomes disomies and diploidy have been recorded by different groups. Baccetti et al., [14] analyzed spermatozoa from a group of 12 DFS patients and showed that the mean frequency of chromosome 18 disomy was in the normal range, whereas the mean frequencies of sex chromosomes disomies and of diploidies were double those of the control group. Rives et al., [15] demonstrated also in two DFS patients an increased rate of sex chromosomes aneuploidy, as well as an elevated rate of diploidy and a frequency of 18 disomy close to the controls. In another study, Moretti and Collodel [16] confirmed the higher sex chromosomes disomies and diploidies in case of DFS, in agreement with our results and the other reports. Contrary to these observations, Viville et al., [13] analyzed spermatozoa from only one patient with “short tail” syndrome using chromosome X, Y, and 1 specific probes and they found that aneuploidy/diploidy rate was comparable with that in fertile controls. In a recent study [20] FISH on chromosomes 18, X, and Y was performed to analyze spermatozoa from two patients presented with DFS sperm defect associated to a second abnormality; the presence of acephalic spermatozoa. FISH results revealed an increased frequency of sex chromosomes disomy and diploidy in one patient, whereas the second patient showed a slight increase in diploidy compared to the control group [20].
Association between morphological sperm deformities and sperm chromosomal abnormalities has been investigated extensively but the results were controversial. Most of the studies showed that teratozoospermia, like other forms of abnormal semen profiles (asthenozoospermia, oligozoospermia), is a marker of elevated sperm aneuploidy [21–28]. Indeed, sperm of isolated teratozoospermic men have shown higher rates of chromosomal abnormalities than that of fertile controls [24,26,28]. However, in a recent study Perrin et al., [29] demonstrate that sperm morphology is not as good predictor of chromosomal content. In fact, they found a very important inter-individual variability in patients with teratozoospermia [29].
FISH studies on some infertile men suggested that certain types of predominant morphological sperm abnormalities, such as large-headed multi-flagellar spermatozoa [13,30,28,31] and round-headed spermatozoa or globozoospermia [32,33] are associated with a very significantly increased frequency of aneuploidy. Therefore sperm head deformities appear to be related to defects of their chromosome contents. Rives et al., [15] demonstrate that systematic sperm flagella abnormalities detected by light microscopy or by electron microscopy may be associated with an increased rate of sex chromosome aneuploidy, as well as an elevated rate of diploidy. However in our study we did not find a significant correlation between the different specific morphological abnormalities (microcephalic head, abnormal acrosome, short flagella) and aneuploidy, with the exception of a significant correlation between diploidy and microcephalic heads and short flagella. Diploidy frequency was significantly higher compared to that of the control group. It has been suggested that diploidy is the most constant and frequent chromosome abnormality detected in spermatozoa from infertile males with meiotic disorders or low sperm count [34]. Rives et al., [15] suggested that diploidy was the most common chromosome abnormality found in spermatozoa from males with systematic morphological sperm abnormalities.
Alterations of microtubules or their motors can interfere with the normal attachment and orientation of the bivalents on the metaphase plate. The chromosomes may be unable to migrate to the poles at anaphase and any severe meiotic disorder can affect the anaphase I checkpoint, giving rise to the production of diploid spermatozoa [15].
Another observation of this study was that for our patients no statistically significant difference was observed between the mean value of XY disomy compared to X disomy or Y disomy, which suggested that both meiotic I (MI) and II (MII) divisions were affected by incomplete partition of homologous chromosomes during meiosis I and of sister chromatids during meiosis II. However FISH analysis performed in one DFS patient reported by Baccetti et al., [11] highlighted an increased frequency of XY disomy, indicating a segregation anomaly at the first meiotic division contrary to Rives et al., [15] who reported elevated frequency of disomic XX and YY spermatozoa demonstrating that nondisjunctions occur preferentially at the second meiotic division for sex chromosomes. Flagella and centrosome components are of the same origin and composed by microtubules and their motors. Alteration of microtubules (perturbation of axonemal complex, absence of axonemal complex central structure and their motors) may disturb the spindle assembly during the first and second meiotic division leading to meiotic nondisjunctions and spermatozoa aneuploidy. Furthermore, disturbances of these components probably occur at variable levels (MI or MII) in dysplasia of fibrous sheath, resulting in an increased rate of aneuploidy for sex chromosomes [15].
In addition to the high levels of chromosomal abnormalities, our patients with DFS sperm defect associated to high percentage of head abnormalities showed a significantly higher level of DNA fragmentation compared to the controls. To our knowledge, the present study provides the first report of a TUNEL analysis specifically performed on spermatozoa of patients with DFS sperm defect. This observation is in agreement with other reports in the literature which indicated an increased rate of sperm DNA fragmentation in infertile men with abnormal sperm parameters than in fertile men [35–37]. Several studies have attempted to correlate DNA fragmentation rate with sperm morphological abnormalities. Brahem et al., [38] found that DNA fragmentation was correlated to the incidence of sperm head abnormalities especially with macrocephalic and amorphous heads. These correlations suggest that sperm head defects may be in part due to a reduction of sperm DNA integrity. Whereas, Mehdi et al., [37] reported a highest percentage of DFI found among the patients having a high percentage of microcephalic spermatozoa and anomalies of the acrosome. Thus, the possibility of a causal link between DNA integrity and spermatozoa morphology should be suspected. Muratori et al., [39] showed that the extent of sperm DNA fragmentation was positively correlated to abnormal morphology and associated with defects of the sperm tail, while Brahem et al., [38] reported a significant correlation between DNA fragmentation and total abnormal tails especially short tails. The chances of conception decrease drastically when DNA fragmentation index (DFI) is >30 % [40]. For our patients DNA fragmentation was observed, on average, in 32.66 ± 4.61 % of spermatozoa using the TUNEL technique. The presence of such high percentage of spermatozoa with DNA damage may have a negative effect on the outcome of ART. Indeed, for patients with sperm flagellar defects ICSI could be the only tool able to bypass their reproductive problem. Nevertheless, the use of their spermatozoa may pose two different but related problems: 1) genetic risk including chromosomic and genic risk for the offspring and 2) fertilization problems with uncertain rate of pregnancy [15]. However, in the literature good fertilization rate, pregnancies, and live births were reported by few reports for DFS disorder. As reviewed by Garza and Patrizio [41] a successful pregnancy after ICSI with the birth of a healthy baby was reported by Olmedo et al., [42] for one DFS patient. Chemes and Rawe [4] described ICSI results for 12 patients presented with DFS. The mean overall fertilization rate was 63 %, 10 pregnancies were achieved (83 %), two miscarriages (20 %) and eight deliveries (67 %), and a total of 14 healthy babies born. Mitchell et al., [43] reported ICSI outcomes for 21 patients with distinct ultrastructural flagellar abnormalities, 12 of them presented with “short tail” syndrome . Only for 14 out of 21 patients fertilization was achieved with a mean rate of 62 %, pregnancy rate of 57 % (12 pregnancies), and live-birth rate of 43 % (9 deliveries).
To date there are no reports on gene anomalies in patients with DFS except partial deletions in the Akap3 and Akap4 genes in one patient reported by Baccetti et al., [11]. It is possible that DFS is a multigenic disease caused by alterations in several different gene products. Although the sample is small, we suggest a possible genetic origin of the described composed defect, as the observed sperm alterations remained stable during the 3 months in which sperm analysis was repeated two times, and particularly because we observed a common sperm phenotype that was predominant in all patients reported in this study.
Conclusion
In conclusion, as far as is known this is the first study reporting FISH and TUNEL sperm analysis performed in infertile patients with systematic sperm defect DFS associated to head abnormalities. We have demonstrated an increased level of sperm DNA fragmentation and chromosomal aneuploidy with elevated frequency of sex chromosomes disomy, of disimy 18 and of diploidy. Despite the small sample our results confirm the importance of morphologic sperm evaluation, study of sperm chromosomal aneuploidy and DNA fragmentation before ART. Indeed, an impaired sperm nuclear quality will affect the results in assisted fertilization in ICSI. Hence, the interest of a thorough study of the sperm nucleus in these forms of infertility in order to predict the chances of success in ART.
Footnotes
Capsule Impaired sperm nuclear quality was observed in patients with DFS sperm defect associated to high percentage of head abnormalities which will affect the results in ICSI.
References
- 1.Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48(4):664–9. doi: 10.1016/s0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 2.Chemes HE, Olmedo SB, Carrere C, Oses R, Carizza C, Leisner M, et al. Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod. 1998;13(9):2521–6. doi: 10.1093/humrep/13.9.2521. [DOI] [PubMed] [Google Scholar]
- 3.Chemes HE. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21(6):799–808. [PubMed] [Google Scholar]
- 4.Chemes EH, Rawe YV. Sperm pathology: a step beyond descriptive morphology. origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update. 2003;9(5):405–28. doi: 10.1093/humupd/dmg034. [DOI] [PubMed] [Google Scholar]
- 5.Baccetti B, Capitani S, Collodel G, Di Cairano G, Gambera L, Moretti E, et al. Genetic sperm defects and consanguinity. Hum Reprod. 2001;16(7):1365–71. doi: 10.1093/humrep/16.7.1365. [DOI] [PubMed] [Google Scholar]
- 6.Jassim A, Gillott DJ, al-Zuhdi Y, Gray A, Foxon R, Bottazzo GF. Isolation and biochemical characterization of the human sperm tail fibrous sheath. Hum Reprod. 1992;7(1):86–94. doi: 10.1093/oxfordjournals.humrep.a137566. [DOI] [PubMed] [Google Scholar]
- 7.Francavilla S, Cordeschi G, Pelliccione F, Bocchio M, Francavilla F. Isolated teratozoospermia: a cause of male sterility in the era of ICSI? Front Biosci. 2007;12:69–88. doi: 10.2741/2049. [DOI] [PubMed] [Google Scholar]
- 8.Brown PR, Miki K, Harper DB, Eddy EM. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol Reprod. 2003;68(6):2241–8. doi: 10.1095/biolreprod.102.013466. [DOI] [PubMed] [Google Scholar]
- 9.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61(1):103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 10.Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248(2):331–42. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- 11.Baccetti B, Collodel G, Estenoz M, Manca D, Moretti E, Piomboni P. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum Reprod. 2005;20(10):2790–4. doi: 10.1093/humrep/dei126. [DOI] [PubMed] [Google Scholar]
- 12.Collodel G, Moretti E. Sperm morphology and aneuploidies: defects of supposed genetic origin. Andrologia. 2006;38(6):208–15. doi: 10.1111/j.1439-0272.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 13.Viville S, Mollard R, Bach ML, Falquet C, Gerlinger P, Warter S. Do morphological anomalies reflect chromosomal aneuploidies? Hum Reprod. 2000;15(12):2563–6. doi: 10.1093/humrep/15.12.2563. [DOI] [PubMed] [Google Scholar]
- 14.Baccetti B, Collodel G, Gambera L, Moretti E, Serafini F, Piomboni P. Fluorescence in situ hybridization and molecular studies in infertile men with dysplasia of the fibrous sheath. Fertil Steril. 2005;84(1):123–9. doi: 10.1016/j.fertnstert.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 15.Rives N, Mousset-Simeon N, Mazurier S, Mace B. Primary flagellar abnormality is associated with an increased rate of spermatozoa aneuploidy. J Androl. 2005;26(1):61–9. [PubMed] [Google Scholar]
- 16.Moretti E, Collodel G. Three cases of genetic defects affecting sperm tail: a FISH study. J Submicrosc Cytol Pathol. 2006;38(2–3):137–41. [PubMed] [Google Scholar]
- 17.Laboratory manual for the examination and processing of human semen. 5. New York: Cambridge University Press; 2010. [Google Scholar]
- 18.Eustache F, Auger J. Inter-individual variability in the morphological assessment of human sperm: effect of the level of experience and the use of standard methods. Hum Reprod. 2003;18(5):1018–22. doi: 10.1093/humrep/deg197. [DOI] [PubMed] [Google Scholar]
- 19.Rawe VY, Terada Y, Nakamura S, Chillik CF, Olmedo SB, Chemes HE. A pathology of the sperm centriole responsible for defective sperm aster formation, syngamy and cleavage. Hum Reprod. 2002;17(9):2344–9. doi: 10.1093/humrep/17.9.2344. [DOI] [PubMed] [Google Scholar]
- 20.Moretti E, Geminiani M, Terzuoli G, Renieri T, Pascarelli N, Collodel G. Two cases of sperm immotility: a mosaic of flagellar alterations related to dysplasia of the fibrous sheath and abnormalities of head-neck attachment. Fertil Steril. 2011;95(5):e19–23. doi: 10.1016/j.fertnstert.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Calogero AE, De Palma A, Grazioso C, Barone N, Romeo R, Rappazzo G, et al. Aneuploidy rate in spermatozoa of selected men with abnormal semen parameters. Hum Reprod. 2001;16(6):1172–9. doi: 10.1093/humrep/16.6.1172. [DOI] [PubMed] [Google Scholar]
- 22.Gole LA, Wong PF, Ng PL, Wang XQ, Ng SC, Bongso A. Does sperm morphology play a significant role in increased sex chromosomal disomy? a comparison between patients with teratozoospermia and OAT by FISH. J Androl. 2001;22(5):759–63. [PubMed] [Google Scholar]
- 23.Harkonen K, Suominen J, Lahdetie J. Aneuploidy in spermatozoa of infertile men with teratozoospermia. Int J Androl. 2001;24(4):197–205. doi: 10.1046/j.1365-2605.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 24.Templado C, Hoang T, Greene C, Rademaker A, Chernos J, Martin R. Aneuploid spermatozoa in infertile men: teratozoospermia. Mol Reprod Dev. 2002;61(2):200–4. doi: 10.1002/mrd.1148. [DOI] [PubMed] [Google Scholar]
- 25.Lewis-Jones I, Aziz N, Seshadri S, Douglas A, Howard P. Sperm chromosomal abnormalities are linked to sperm morphologic deformities. Fertil Steril. 2003;79(1):212–5. doi: 10.1016/S0015-0282(02)04411-4. [DOI] [PubMed] [Google Scholar]
- 26.Tang SS, Gao H, Zhao Y, Ma S. Aneuploidy and DNA fragmentation in morphologically abnormal sperm. Int J Androl. 2010;33(1):e163–79. doi: 10.1111/j.1365-2605.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- 27.Brahem S, Elghezal H, Ghedir H, Landolsi H, Amara A, Ibala S, et al. Cytogenetic and molecular aspects of absolute teratozoospermia: comparison between polymorphic and monomorphic forms. Urology. 2011;78(6):1313–9. doi: 10.1016/j.urology.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 28.Mehdi M, Gmidene A, Brahem S, Guerin JF, Elghezal H, Saad A. Aneuploidy rate in spermatozoa of selected men with severe teratozoospermia. Andrologia. 2012;44(Suppl 1):139–43. doi: 10.1111/j.1439-0272.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- 29.Perrin A, Louanjli N, Ziane Y, Louanjli T, Le Roy C, Gueganic N, et al. Study of aneuploidy and DNA fragmentation in gametes of patients with severe teratozoospermia. Reprod BioMed Online. 2011;22(2):148–54. doi: 10.1016/j.rbmo.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Mateu E, Rodrigo L, Prados N, Gil-Salom M, Remohi J, Pellicer A, et al. High incidence of chromosomal abnormalities in large-headed and multiple-tailed spermatozoa. J Androl. 2006;27(1):6–10. doi: 10.2164/jandrol.05033. [DOI] [PubMed] [Google Scholar]
- 31.Brahem S, Mehdi M, Elghezal H, Saad A. Study of aneuploidy rate and sperm DNA fragmentation in large-headed, multiple-tailed spermatozoa. Andrologia. 2012;44(2):130–5. doi: 10.1111/j.1439-0272.2010.01115.x. [DOI] [PubMed] [Google Scholar]
- 32.Martin RH, Greene C, Rademaker AW. Sperm chromosome aneuploidy analysis in a man with globozoospermia. Fertil Steril. 2003;79(Suppl 3):1662–4. doi: 10.1016/S0015-0282(03)00401-1. [DOI] [PubMed] [Google Scholar]
- 33.Brahem S, Mehdi M, Elghezal H, Saad A. Analysis of sperm aneuploidies and DNA fragmentation in patients with globozoospermia or with abnormal acrosomes. Urology. 2011;77(6):1343–8. doi: 10.1016/j.urology.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Egozcue S, Blanco J, Vidal F, Egozcue J. Diploid sperm and the origin of triploidy. Hum Reprod. 2002;17(1):5–7. doi: 10.1093/humrep/17.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril. 1998;69(3):528–32. doi: 10.1016/S0015-0282(97)00536-0. [DOI] [PubMed] [Google Scholar]
- 36.Aitken RJ, De Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod BioMed Online. 2007;14(6):727–33. doi: 10.1016/S1472-6483(10)60676-1. [DOI] [PubMed] [Google Scholar]
- 37.Mehdi M, Khantouche L, Ajina M, Saad A. Detection of DNA fragmentation in human spermatozoa: correlation with semen parameters. Andrologia. 2009;41(6):383–6. doi: 10.1111/j.1439-0272.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- 38.Brahem S, Mehdi M, Elghezal H, Saad A. Detection of DNA fragmentation and meiotic segregation in human with isolated teratozoospermia. J Assist Reprod Genet. 2011;28(1):41–8. doi: 10.1007/s10815-010-9482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muratori M, Piomboni P, Baldi E, Filimberti E, Pecchioli P, Moretti E, et al. Functional and ultrastructural features of DNA-fragmented human sperm. J Androl. 2000;21(6):903–12. [PubMed] [Google Scholar]
- 40.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 41.Garza SAD, Patrizio P. Reproductive outcomes in patients with male infertility because of Klinefelter’s syndrome, Kartagener’s syndrome, round-head sperm, dysplasia fibrous sheath, and ‘stump’ tail sperm: an updated literature review. Curr Opin Obstet Gynecol. 2013;25(3):229–46. doi: 10.1097/GCO.0b013e32835faae5. [DOI] [PubMed] [Google Scholar]
- 42.Olmedo SB, Nodar F, Chillik C, Chemes HE. Successful intracytoplasmic sperm injection with spermatozoa from a patient with dysplasia of the fibrous sheath and chronic respiratory disease. Hum Reprod. 1997;12(7):1497–9. doi: 10.1093/humrep/12.7.1497. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell V, Rives N, Albert M, Peers MC, Selva J, Clavier B, et al. Outcome of ICSI with ejaculated spermatozoa in a series of men with distinct ultrastructural flagellar abnormalities. Hum Reprod. 2006;21(8):2065–74. doi: 10.1093/humrep/del130. [DOI] [PubMed] [Google Scholar]