Abstract
Purpose
To elucidate the problems in infertility treatment for women after conservative therapy for endometrial cancer (EC) or atypical complex endometrial hyperplasia (ACEH).
Methods
The clinical outcomes of 21 patients who underwent assisted reproductive technology after conservative therapy (group A) and 42 control women (group B) were retrospectively analyzed.
Results
There was no significant difference in the number of retrieved oocytes, fertilization rate or the number of transferred embryos between the two groups. Women in group A had a significantly thinner endometrium and a reduced implantation rate compared to those for women in group B. There was no significant difference in the cumulative clinical pregnancy and delivery rates between group A and B. The patients in group A required significantly more embryos for achieving a live-birth.
Conclusions
Our results indicate that a thin endometrium after repeated curettage may have a negative effect on endometrial receptivity of patients after conservative treatment for EC/ACEH. Clinicians should reconsider their present protocols and make efforts to minimize the damage to normal endometrium.
Keywords: Endometrial cancer, Pregnancy, ART, Implantation, Endometrial thickness
Introduction
Endometrial cancer (EC) in women under the age of 40 is relatively uncommon, accounting for approximately 5 % of all cases [1]. Atypical complex endometrial hyperplasia (ACEH) is a precancerous lesion and can progress to EC in several years. The occurrence of the disease in premenopausal women is often related to nulliparity, ovulation disorders, and long exposure to unopposed estrogen. Therefore, many patients have no children at the time of the diagnosis and desire fertility preservation. The standard treatment of early-stage EC is hysterectomy with bilateral salpingo-oophorectomy. Hormonal therapy with high-dose progestin administration has only been proven to be effective in carefully selected patients with early-stage EC or ACEH.
So far, there have been an increasing number of successful deliveries after conservative treatment for EC/ACEH [2–4]. We suppose that many patients undergo infertility treatment including assisted reproductive technology (ART), because we speculate most of the patients have ovulation disorders, which could contribute to the occurrence of the disease. We recently reported that the use of ovulation induction drugs for patients with EC/ACEH does not increase the recurrence of the disease and that it has an advantageous effect through the resulting pregnancy on the oncologic outcome [5].
However, as far as we know, there have been few reports comparing the outcome of infertility treatment with patients without EC/ACEH. Elizur et al. [6] compared the ART outcome of 31 controlled ovarian stimulation cycles conducted in 8 patients with that of 3,239 consecutive cycles in their unit. They reported that the endometrial thickness on the day of hCG was significantly thinner in patients with EC/ACEH and that 6 of 8 women achieved live-births probably because of their young age. However, their study was cycle-based, and may have overestimated the patients with a thin endometrium and repeated implantation failure. Additionally, in the last decade, there has been a trend toward minimizing the number of transferring embryos to avoid multiple pregnancies [7], in accordance with an increased success rate of ART in the same time period. Therefore, to analyze the outcome of infertility treatment for women with EC/ACEH over the years, comparison with age- and time-matched controls is preferable.
In the present study, we analyzed the results of ART conducted on patients with and without EC/ACEH. The aim of this study was to elucidate the problems in infertility treatment for women after conservative therapy for EC/ACEH.
Methods
This retrospective study was approved by the Institutional Review Board of our institution. We evaluated patients with EC/ACEH who achieved a complete response after conservative treatment and who underwent ART at the University of Tokyo hospital between December 1999 and January 2012. All the patients underwent total endometrial curettage to determine the initial pathological diagnosis. Only women who were 40 years and under and were diagnosed with grade 1, stage 1a EC or ACEH (according to the 1988 International Federation of Gynecology and Obstetrics classification) were given approval for conservative therapy. The details of the conservative treatment were described previously [5]. Briefly, after 26 weeks of high-dose progestin therapy using oral medroxyprogesterone acetate (600 mg/d) and another 6 months of cyclic oral contraceptive pills, the patients were allowed to attempt pregnancy. We recommended that the patients undergo infertility treatment as soon as possible after achieving a complete response. Women who had no history of infertility treatment started with ovulation induction drugs and timed intercourse or intrauterine insemination. Those who did not conceive after conventional infertility treatment and those who had a history of infertility treatment or other infertility factors were recommended to undergo ART.
Twenty-one patients who were diagnosed with EC/ACEH and hoped to preserve fertility underwent ART after achieving a complete response (group A). Controlled ovarian stimulation (COS) protocol for each patient was determined based on ovarian reserve. The COS and oocyte retrieval protocols were described previously [8]. The insemination method (i.e. conventional in vitro fertilization [IVF] or intracytoplasmic sperm injection [ICSI]) was determined based on the semen quality of the husband. Embryo transfer was conducted 3 days after oocyte retrieval. Embryo quality was evaluated according to Veeck et al. [9]. On day 3, embryos with more than 6 cells and minor fragmentation (grade 1 or 2) were classified as good-quality embryos. In patients with a high risk of ovarian hyperstimulation syndrome, embryo transfer was canceled, and all good-quality embryos were cryopreserved for future transfers.
Two control women were selected for each patient with EC/ACEH without notice on the outcome of ART (group B). Both of two controls were within 2 years of age as the patient. One underwent the first ART treatment just before the patient, and the other did just after the patient. Each patient underwent ART within 2 months as her controls.
The parameters in the first treatment cycle—COS protocol, hMG dose, estradiol level and endometrial thickness on the day of hCG administration, number of follicles and retrieved oocytes, insemination method, fertilization and embryo development, number of transferred embryos, endometrial thickness at the first transfer, clinical pregnancy rate and implantation rate—were compared between the 2 groups. When they did not conceive after fresh embryo transfer cycles, they underwent frozen embryo transfer or additional oocyte retrieval. The cumulative clinical pregnancy and live-birth rates including subsequent treatment cycles were also compared between the 2 groups.
Excel Statistics Ver.6.0 (Esumi Co Ltd, Tokyo, Japan) was used as a statistical software program. The chi-square and Fisher’s exact probability tests were used to investigate the distribution of categorical variables. The Wilcoxon rank-sum tests were applied to compare the continuous variables. Statistical significance was defined as p < 0.05.
Results
The characteristics of the patients in group A and B are shown in Table 1. There was no significant difference in age, ratio of nulligravida and nulliparous women, or duration of infertility between the 2 groups. Four of 21 patients in group A were obese (body mass index [BMI] ≥ 30 kg/m2), whereas 3 of 42 patients in group B were slightly obese (25 kg/m2 ≤ BMI <30 kg/m2) and none were obese. A slightly increased BMI was observed in group A compared with group B although this was not significantly different.
Table 1.
Characteristics of the patients in the study groups
| Group A (n = 21) | Group B (n = 42) | p | |
|---|---|---|---|
| Agea | 30–39, 34 | 29–41, 34 | 0.75 |
| Nulligravida women | 18/21 | 32/42 | 0.52 |
| Nulliparous women | 20/21 | 38/42 | 0.66 |
| Duration of infertility (month) | 3–69, 41 | 4–120, 38 | 0.57 |
| BMI | 18.6–32.9, 21.3 | 15.9–29.4, 20.6 | 0.11 |
| Ovulation factor (%) | 66.7 | 14.3 | <0.01 |
| Tubal factor (%) | 23.8 | 35.7 | 0.40 |
| Male factor (%) | 4.8 | 52.4 | <0.01 |
| Unexplained infertility (%) | 23.8 | 19.0 | 0.75 |
aValues are gives as min-max, median unless otherwise indicated
In group A, significantly more patients had ovulation disorders and less had male infertility compared with group B. There was no difference in the ratio of women with tubal or unexplained infertility.
The outcome of the first ART treatment (first oocyte retrieval or first embryo transfer cycle) is summarized in Table 2. Seventeen of 21 women (81 %) in group A and 35 of 42 women (83.3 %) in group B underwent COS using a long protocol. Basal FSH and LH levels, total hMG dose, and estradiol levels on the day of hCG were comparable between the 2 groups. However, endometrial thickness of women in group A on the day of hCG was significantly thinner compared to that of women in group B.
Table 2.
Hormonal profiles in the study groups and outcomes in the first treatment cycle
| Group A(n = 21) | Group B (n = 42) | p | |
|---|---|---|---|
| COS protocol long/short/Antagonist | 17/1/3 | 35/1/6 | 0.88 |
| Basal FSH(mIU/ml) a | 3.9–16.4, 7.9 | 1–18.3, 7.4 | 0.73 |
| Basal LH (mIU/ml) | 2.4–23.4, 4.6 | 1.3–13.6, 3.9 | 0.46 |
| hMG dose (IU) | 750–4,500, 1,650 | 900–8,250, 1,950 | 0.60 |
| E2 on the day of hCG (pg/ml) | 219–7,301, 1,143 | 300–10,212, 1,322 | 0.49 |
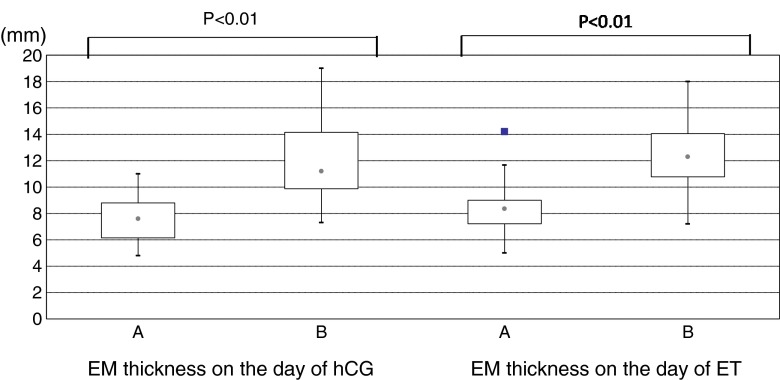
| EM thickness on the day of hCG (mm) | 4.8–11, 7.6 | 7.3–19, 11.2 | <0.01 |
| No. of follilces | 1–30, 7 | 2–27, 9 | 0.54 |
| No. of retrieved oocytes | 1–39, 6 | 1–25, 9 | 0.63 |
| Insemination method Conventional/ICSI |
17/4 | 21/21 | 0.03 |
| Fertilization rate | 63.5 % (n = 219) | 60.3 % (n = 403) | 0.44 |
| Good-quality embryo/fertilized oocyte | 30.2 % (n = 139) | 35.3 % (n = 243) | 0.30 |
| No. of transferred embryos at first ET | 1–3, 2 | 1–3, 2 | 0.58 |
| EM thickness on the day of ET | 5–14.2, 8.4 | 7.2–18, 12.5 | <0.01 |
| Clinical pregnancy/first ET | 9.5 % (n = 21) | 35.7 % (n = 42) | 0.036 |
| Implantation rate at first ET | 4.9 % (n = 41) | 21.0 % (n = 81) | 0.031 |
aValues are gives as min-max, median unless otherwise indicated
The number of follicles and retrieved oocytes was not significantly different. Although significantly more patients in group B required ICSI, the fertilization rate and embryo preimplantation development were comparable. However, women in group A had a significantly thinner endometrium and a lower clinical pregnancy and implantation rates compared to those for women in group B. Box-plots of endometrial thickness on the day of hCG administration and embryo transfer in the study groups are shown in Fig. 1.
Fig. 1.
Box plots showing distribution of endometrial thickness on the day of hCG administration and embryo transfer in the two study groups
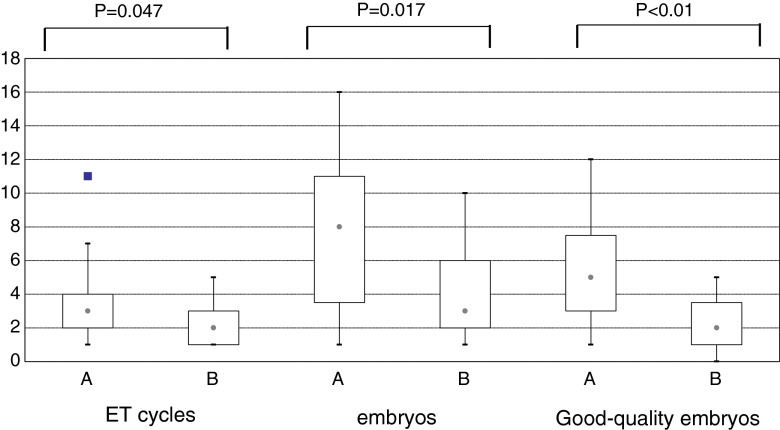
Cumulative ART outcomes in the study groups are summarized in Table 3. Conclusively, 14 of 21 patients conceived in group A, and 13 women delivered healthy babies. The cumulative clinical pregnancy rate and delivery rate were 66.7 and 61.9 %, respectively, which was comparable with the patients in group B. However, the patients in group A required significantly more embryos and number of transfer cycles for achieving live-births. Box-plots of the number of embryo transfer cycles, embryos and good-quality embryos for achieving deliveries in the study groups are shown in Fig. 2.
Table 3.
Cumulative ART outcomes in the study groups
| Group A (n = 21) | Group B (n = 42) | p | |
|---|---|---|---|
| Cumulative clinical pregnancy rate | 66.7 % | 66.7 % | 1.00 |
| Cumulative delivery rate | 61.9 % | 59.5 % | 0.86 |
| No. of oocyte retrieval cycles for delivery a | 1–6, 2 | 1–3, 1 | 0.14 |
| No. of ET cycles for delivery | 1–11, 3 | 1–5, 2 | 0.047 |
| No. of embryos for delivery | 1–16, 8 | 1–10, 3 | 0.017 |
| No. of good-quality embryos for delivery | 1–12, 5 | 0–5, 2 | <0.01 |
aValues are gives as min-max, median unless otherwise indicated
Fig. 2.
Box plots showing distribution of the number of embryo transfer cycles, embryos and good-quality embryos for achieving deliveries in the two study groups
Discussion
To the best of our knowledge, this is the first report to investigate the ART outcome of patients with EC/ACEH after conservative treatment. In the present study, we showed that patients with EC/ACEH achieved a comparable cumulative pregnancy and delivery rate; however, they required significantly more embryos for a live-birth compared with control women.
The numbers in this study are small, but this is reflective of the incidence of women under 40 who have undergone conservative treatment for EC/ACEH, achieved complete response and subsequently pursue ART.
The cumulative pregnancy and delivery rates were similar between the 2 groups because the drop-out rate of the patients with EC/ACEH was lower compared to that of the control patients. After conservative therapy, the patients were always at risk for recurrence of the disease and resulting hysterectomy. Actually, 5 of 8 patients with no live-births in group A suffered from recurrence (data not shown). Therefore, close follow-up is required in intervening cycles to avoid unnecessary exposure to estrogen. For this reason, oral contraceptives pills were administered to women in group A just after the onset of menstruation when serum samples were not indicative of pregnancy. Their endometrial tissues were checked for recurrence at regular intervals in intervening cycles. Hysterectomy was recommended for those who gave up childbearing. Therefore, the patients with EC/AECH continued ART treatment in our institution.
We observed that two-thirds (14/21) of the patients with EC/ACEH had ovulation disorders; this was significantly lesser in the control group. Many but not all of the patients with EC/ACEH had polycystic ovarian syndrome (PCOS) or other ovulation disorders. However, there was no significant difference of BMI between the two groups. In 21 patients with EC/ACEH, only 4 were obese (BMI ≥ 30 kg/m2). The result is not surprising because the majority of PCOS patients in Asian countries are not obese, unlike those in Western countries, as previously reported [10].
In group B, significantly more women had male infertility and underwent ICSI. It indicates that many of younger infertile couples with no ovulation disorders undergo ART due to severe male factors. The use of ICSI may have a disadvantageous effect in embryo development [11] compared with conventional IVF.
The results in the first treatment cycle showed that there was no statistical significance in hMG dose, E2 value on the day of hCG, number of retrieved oocytes, fertilization rate, or preimplantation embryo development, indicating that fertility-preserving management with medroxyprogesterone has no adverse effect on ovarian reserve or oocyte quality. On the other hand, the endometrial thickness of the women with EC/ACEH was significantly thinner and the implantation rate was lower compared to those for women in the control group. Endometrial thickness has been reported to be one of the most important factors for success in ART. Zhang et al. [12] reported a significant difference in the pregnancy rate in patients with an endometrial thickness above and below 9 mm, whereas Kovacs et al. [13] and Richter et al. [14] showed a gradual increase in the implantation rate with increasing endometrial thickness. In the present study, none of the patients whose endometrial thickness was <7 mm achieved a live-birth (data not shown).
The thin endometrium of the patients with EC/ACEH is obviously the results of repeated endometrial curettage in the treatment and examination of the disease. The initial purpose of endometrial curettage is complete examination and removal of malignant cells, so most of clinicians perform curettage thoroughly in all directions. However, this procedure can cause irreversible mechanical damage to the normal endometrial cells and lead to unresponsive endometrium [15]. Recently, several investigators demonstrated the efficacy of hysteroscopic resection of early stage EC combined with hormonal therapy [16, 17]. Mazzon et al. reported four cases of live-births in six patients. Though they do not refer to endometrial thickness after conservative treatment, hysteroscopic resection can minimize the specimen and does not seem to give unnecessary damage to normal endometrial cells. In addition, a meta-analysis study shows hysteroscopic management for early stage EC is not related to the increased risk of peritoneal dissemination [18]. So far there has been no report comparing endometrial thickness and reproductive outcomes after hysteroscopic surgery with conventional curettage treatment. However, this new method can become an alternative in the viewpoint of fertility-preserving therapy.
In summary, after conservative treatment in patients with EC/ACEH, a thinner endometrium and a reduced implantation rate were observed compared with control patients. Additionally, implantation failure in the patients with EC/ACEH was not conclusive, and the cumulative pregnancy/delivery rate was comparable with that of the control women. When treating patients with EC/ACEH, the high recurrence rate of the disease, unless they conceive after a complete response, should be considered. Therefore, the introduction of double embryo transfer or other management protocols suitable for those with implantation failure may be necessary along with efforts to minimize the damage to normal endometrium necessary for implantation.
Footnotes
Capsule A thin endometrium after repeated curettage may have a negative effect on endometrial receptivity of patients undergoing ART after conservative treatment for EC/AECH.
References
- 1.Pellerin GP, Finan MA. Endometrial cancer in women 45 years of age or younger: a clinicopathological analysis. Am J Obstet Gynecol. 2005;193:1640–1644. doi: 10.1016/j.ajog.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Park JY, Seong SJ, Kim TJ, Kim JW, Kim SM, Bae DS, et al. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol. 2013;121:136–142. doi: 10.1097/aog.0b013e31827a0643. [DOI] [PubMed] [Google Scholar]
- 3.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266.e1–266.12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Dursun P, Erkanli S, Guzel AB, Gultekin M, Tarhan NC, Altundag O, et al. A Turkish Gynecologic Oncology Group study of fertility-sparing treatment for early-stage endometrial cancer. Int J Gynaecol Obstet. 2012;119:270–273. doi: 10.1016/j.ijgo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Ichinose M, Fujimoto A, Osuga Y, Minaguchi T, Kawana K, Yano T, et al. The influence of infertility treatment on the prognosis of endometrial cancer and atypical complex endometrial hyperplasia. Int J Gynecol Cancer. 2013;23:288–293. doi: 10.1097/IGC.0b013e31827c18a1. [DOI] [PubMed] [Google Scholar]
- 6.Elizur SE, Beiner ME, Korach J, Weiser A, Ben-Baruch G, Dor J. Outcome of in vitro fertilization treatment in infertile women conservatively treated for endometrial adenocarcinoma. Fertil Steril. 2007;88:1562–1567. doi: 10.1016/j.fertnstert.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 7.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945. doi: 10.1136/bmj.c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto A, Fujiwara T, Oishi H, Hirata T, Yano T, Taketani Y. Predictive factors of successful pregnancy after assisted reproductive technology in women aged 40 years and older. Reprod Med Biol. 2009;8:145–149. doi: 10.1007/s12522-009-0023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veeck L. In: An atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. Veeck L, editor. New York: Parthenon; 1999. pp. 46–51. [Google Scholar]
- 10.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 11.Dumoulin JC, Coonen E, Bras M, van Wissen LC, Ignoul-Vanvuchelen R, Bergers-Jansen JM, et al. Comparison of in-vitro development of embryos originating from either conventional in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 2000;15:402–409. doi: 10.1093/humrep/15.2.402. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Chen CH, Confino E, Barnes R, Milad M, Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2005;83:336–340. doi: 10.1016/j.fertnstert.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Richter KS, Bugge KR, Bromer JG, Levy MJ. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril. 2007;87:53–59. doi: 10.1016/j.fertnstert.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18:2337–2341. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 15.Shufaro Y, Simon A, Laufer N, Fatum M. Thin unresponsive endometrium–a possible complication of surgical curettage compromising ART outcome. J Assist Reprod Genet. 2008;25:421–425. doi: 10.1007/s10815-008-9245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurelli G, Di Vagno G, Scaffa C, Losito S, Del Giudice M, Greggi S. Conservative treatment of early endometrial cancer: preliminary results of a pilot study. Gynecol Oncol. 2011;120:43–46. doi: 10.1016/j.ygyno.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Mazzon I, Corrado G, Masciullo V, Morricone D, Ferrandina G, Scambia G. Conservative surgical management of stage IA endometrial carcinoma for fertility preservation. Fertil Steril. 2010;93:1286–1289. doi: 10.1016/j.fertnstert.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Chang YN, Zhang Y, Wang YJ, Wang LP, Duan H. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957–961. doi: 10.1016/j.fertnstert.2011.07.1146. [DOI] [PubMed] [Google Scholar]