Abstract
Background
Although metabolic syndrome (MS) is a typical condition of middle-aged/older person, the association between MS and mortality risk has not been confirmed in people over 65 years. We hypothesized that while in the elderly MS phenotype might lose its value in predicting mortality risk, the two core factors of MS, i.e. insulin resistance (IR) and low-grade systemic inflammation (LGSI) would not.
Methods
1011 community-dwelling older individuals (InCHIANTI study) were included. MS phenotype was defined by NCEP-ATP-III criteria. IR was calculated by HOMA; high-sensitivity C-reactive protein was measured by ELISA. Subjects were divided into four groups based on presence/absence of IR (HOMA ≥2.27) and LGSI (hs-CRP ≥ 3g/L): Group 1: no IR/LGSI (reference); Group 2: LGSI only; Group 3: IR only; Group 4: IR+LGSI. Hazard Ratios (HR) for 9-years cardiovascular (CVD) and total mortality, according to IR/LGSI groups, were estimated in subjects with (n.311) and without MS by Cox model.
Results
31.8% of subjects with MS phenotype had no IR, 45.3% had no LGSI; moreover, 51% of subjects with both IR and LGSI didn’t display the MS phenotype. MS phenotype was not associated with CVD (HR:1.29; 95%C.I.:0.92–1.81) or total (HR:1.07; 95%C.I.:0.86–1.34) mortality risk, whereas the presence of IR plus LGSI was associated with increased CVD (no MS: HR 2.07, 95%CI:1.12–3.72; MS: HR 9.88, 95%CI:2.18–4), and overall (no MS: HR 1.72, 95%CI:1.001–3.17; MS: HR 1.51, 95%CI:1.02–2.28) mortality risk. The presence of IR (HR: 6.90, 95%CI:1.45–32) or LGSI (HR 7.56, 95%CI:1.63–35) was associated with CVD mortality, only among individuals with MS phenotype.
Conclusions
Among community dwelling older individuals, IR and LGSI, but not MS phenotype, was associated with 9-years overall and CVD mortality risk. Since a reduced “overlap” between MS phenotype and its physiopathological core (IR and LGSI) might be present with aging, we suggest that the definition of MS might be more holistic in advanced age, and probably comprise the measurement of IR and LGSI.
Key terms: Insulin Resistance, C Reactive Protein, Mortality, Metabolic Syndrome, Elderly
INTRODUCTION
Metabolic syndrome (MS) is a phenotype characterized by the clustering of some cardiovascular risk factors including impaired glucose tolerance, central obesity, dyslipidemia, and hypertension (1). Although the clinical value of diagnosing MS remains still controversial, the role of MS as a possible predictor of cardiovascular disease (CVD), coronary heart disease (CHD), and total mortality in adult population has been largely demonstrated (2), also by systematic review and meta-analysis (3).
MS is a condition of middle-aged and older people, as its prevalence progressively increases to a maximum of 25–40% among individuals aged over 70 years (4). Nevertheless, the association between MS phenotype and mortality has not been consistently confirmed in people over 65 years. Some studies reported a significant association of MS with total (5–7) or CVD mortality (5;8;9) also in older cohorts, while others found no association (10–13). On the whole, it appears that MS phenotype becomes a weaker predictor of CVD/total mortality in late life, and this concept is supported by studies comparing mortality risk in middle-age versus elderly individuals (12;14).
IR and low-grade systemic inflammation (LGSI), two conditions found very often in people with MS, may account for mortality risk associated with MS. IR is widely considered the physiopathological base of MS, and has been associated with increased CVD/total mortality, both in diabetic and non-diabetic individuals (15–18). LGSI, diagnosed by chronic elevation of C reactive protein (CRP), also seems to play an important role in the development of both IR and MS (19;20). Interestingly, not only LGSI participates to atherosclerosis process (21;22), but has been also associated with CVD/total mortality both in middle-age (23–25) and older populations (26–28).
We hypothesized that while in the elderly MS phenotype might lose its value in predicting CVD/total mortality risk, the two core factors of MS (i.e. IR and/or LGSI) would not. In order to verify this hypothesis, we investigated the combined effect of IR and LGSI on the risk for 9-years CVD/total mortality in older individuals with and without MS enrolled in the InCHIANTI study.
MATERIALS AND METHODS
This study is part of the “Invecchiare in Chianti” (Aging in the Chianti area, InCHIANTI) study, a prospective population-based study of older persons, designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (INRCA, Florence, Italy). The study included participants randomly selected from the residents in two towns of the Chianti area. A detailed description of sampling procedure and data collection method has been previously published (29). Briefly, in 1998, 1270 persons ≥65 years were randomly selected from the population registries. Additional subjects (n=29) were randomly selected to obtain a sample of 30 men and 30 women aged ≥ 90 years old. Of the initial 1299 subjects, 39 were not eligible. Clinical visits and assessments were performed in the study clinic and were preceded by an interview conducted at the participants’ homes. Trained interviewers administered structured questionnaires on dietary intakes, household composition, social networks, economical status, education, and general information on health and functional status. The INRCA Ethical Committee ratified the entire study protocol. The analyses presented here are based on data from 1011 individuals aged over 64 in which metabolic parameters and inflammatory mediators had been measured at baseline visit.
Clinical chemistry parameters
All parameters were measured on fresh serum/plasma drawn after 12 hours overnight fasting, after the patient has been sedentary in sitting/supine position for 15 minutes.
Plasma lipids, fasting glucose and insulin, and high sensitivity C reactive protein (hs-CRP) were measured as previously described(30). Cut off value for LGSI was defined at hs-CRP value ≥ 3.0 mg/L, as reported in literature (31). Insulin resistance was calculated according to the Homeostasis Model Assessment (HOMA) as follows: fasting insulin (U/L) x fasting glucose (mmol/L)/22.5.
Insulin Resistance/Low Grade Systemic Inflammation (IR/LGSI) classification
Subjects were divided into four groups based on HOMA and hs-CRP values:
1: HOMA <2.27 (median value) and hs-CRP <3 mg/L = no IR, no LGSI (reference)
2: HOMA <2.27 and hs-CRP ≥3 mg/L = no IR, LGSI
3: HOMA ≥2.27 and hs-CRP <3 mg/L = IR, no LGSI
4: HOMA ≥2.27 and hs-CRP ≥3 mg/L = IR and LGSI
MS was defined by criteria of the 2005 NCEP-ATPIII-AHA/NHLBI statement (32) in the presence of ≥3 of following criteria:
Waist circumference ≥102 cm in men or ≥88 cm in women
Triglycerides ≥150 mg/dL or hypertriglyceridemia treatment
HDL-C <40 mg/dL in men or <50 mg/dL in women or low HDL-C treatment
Blood pressure ≥130/85 mmHg or hypertension treatment
Fasting glucose ≥100 mg/dL or hyperglycemia treatment
Mortality Follow-up
Participants were evaluated for the 3-year (2001 to 2003), 6-year (2004 to 2006) and 9-year follow-up visits (2007 to 2009). Mortality data of the original InChianti cohort were collected using data from the Mortality General Registry maintained by the Tuscany Region, and the death certificates that are deposited immediately after death at the Registry office of the municipality of residence. Cardiovascular mortality, based on underlying cause of death, was defined as any cardiovascular mortality and coded using the International Classification of Diseases, 9th Revision (ICD-9 codes: 390–459).
Other Measures
The presence of specific medical conditions was established using standardized criteria combining self-reported history, medical records, and clinical examination. The following diseases were considered: coronary heart disease (CHD- acute myocardial infarction, chronic heart failure, cardiac arrest, acute and chronic ischemic heart disease), peripheral arterial disease (PAD), stroke, hypertension, and diabetes. Prevalent cardiovascular disease (CVD) was defined as the presence of one of the following: CHD, PAD, and stroke. The ankle-brachial index (ABI) was measured in all subjects by using a Doppler stethoscope (Parks model41-A; Parks-Medical Electronics, Inc; Aloha, Oregon). Besides clinical information, the diagnosis of PAD was made If ABI value <0.9
Statistical analysis
Continuous variables were expressed as mean (SD) or median (interquartile range) when necessary. Means were compared by ANOVA with Bonferroni post-hoc test for multiple comparison, while medians were compared by Mann-Whitney test. Correlations between continuous variables were tested by Pearson’s correlation. Proportion were compared by the χ2 test. Hazard Ratios (HR) for all-cause/CVD mortality, according to IR/LGSI groups, were separately estimated in subjects with (n.311) and without MS (n.700) by Cox proportional hazard regression analysis. The IR/LGSI group 1 (no IR, no LGSI) was considered as reference category. The assumption of proportionality of all variables introduced in the models was assessed through the analysis of Schoenfeld residuals. The Cox models were adjusted for other factors associated with mortality including age, gender, smoking habit, total cholesterol (TC), BMI, and diabetes. For CVD mortality, CHD and previous stroke were also included into the model. Analyses were performed by SPSS for Windows statistical package, version 13.0.
RESULTS
TABLE 1 reported the general characteristic of the sample according to the presence or absence of MS phenotype as defined by NCEP-ATP III criteria. Subjects with MS were younger and more often females; the prevalence of diabetes, CHD, and peripheral arterial disease was higher in MS individuals compared with those without MS.
TABLE 1.
characteristics of 1011 community-dwelling older individuals from the InChianti study according to the absence (n° 700) or presence (n° 311) of metabolic syndrome (updated NCEP-ATP-III criteria).
| Controls (n. 700) |
Metabolic Syndrome (n. 311) |
P | |
|---|---|---|---|
| AGE (yrs) | 76.1±8 | 75±6 | 0.005 |
| GENDER (F%) | 53.7 | 65.2 | 0.001 |
|
| |||
| ATP III MS CRITERIA (%) | |||
| - INCREASED WAIST | 26.5 | 79.8 | 0.001 |
| - HIGH TRIGLYCERIDES | 9.2 | 61.1 | 0.001 |
| - LOW HDL-C | 9.3 | 54.7 | 0.001 |
| - HYPERTENSION | 90.8 | 98.1 | 0.001 |
| - HYPERGLYCEMIA | 12.3 | 57.4 | 0.001 |
|
| |||
| FASTING GLUCOSE (mg/dL) | 89.1±18 | 109.0±35 | 0.001 |
| FASTING INSULIN (U/L) | 9.44 (6.60–13.0) | 11.5 (8.5–16.3) | 0.001 |
| HOMA score | 2.05 (1.44–2.94) | 3.01 (2.02–4.55) | 0.001 |
| Hs-CRP (mg/L) | 2.42 (1.22–5.47) | 3.36 (1.82–6.48) | 0.001 |
|
| |||
| IR/LGSI Group: | |||
| 1. NO IR, NO LGSI | 240 (34.2%) | 48 (15.4%) | |
| 2. LGSI, NO IR | 165 (23.6%) | 51 (16.4%) | |
| 3. IR, NO LGSI | 172 (24.6%) | 93 (29.9%) | |
| 4. BOTH IR AND LGSI | 123 (17.6%) | 119 (38.3%) | 0.001 |
|
| |||
| TOT. CHOL. (mg/dL) | 215±36 | 221±38 | 0.04 |
| TRIGLYCERIDES (mg/dL) | 105±45 | 175±86 | 0.001 |
| LDL-C (mg/dL) | 135±34 | 138.1±35 | 0.08 |
| HDL-C (mg/dL) | 58±15 | 47±10 | 0.001 |
| BMI (kg/m2) | 26.2±3 | 29.9±4 | 0.001 |
| SBP (mm/Hg) | 148.5±19.5 | 155±19 | 0.001 |
| DBP (mm/Hg) | 83.5±8.3 | 85±8.4 | 0.01 |
|
| |||
| DIABETES (%) | 8.0 | 20.7 | 0.001 |
| CHD (%) | 6.6 | 10.0 | 0.05 |
| STROKE (%) | 6.5 | 6.9 | 0.48 |
| ABI < 0.9 (%) | 8.8 | 17.0 | 0.001 |
|
| |||
| SMOKING (%) | |||
| - NEVER | 58.2 | 63.0 | |
| - FORMER | 30.2 | 27.6 | |
| - CURRENT | 11.6 | 9.4 | 0.08 |
BMI: body mass index; ABI: ankle brachial index; CHD: coronary heart disease IR: insulin resistance; LGSI: low grade systemic inflammation
For CRP, insulin, HOMA, ABI: median (interquartile range)
Participants with MS
The principal characteristics of 311 older subjects with MS diagnosis, according to IR/LGSI classification are described in TABLE 2. Among individuals with MS, those with IR and LGSI (group 4) were the most frequent (38.1%), followed by those with isolated IR (group 2: 29.9%). Individuals with IR and LGSI were younger, more often affected by hyperglycemia/diabetes, and were characterized by higher values of waist circumference, BMI, and plasma triglycerides.
TABLE 2.
characteristics of 311 older subjects with metabolic syndrome (updated NCEP-ATP III criteria) according to presence or absence of insulin resistance and low grade systemic inflammation ( IR / LGSI group ) [one way ANOVA, Bonferroni post-hoc test].
| GROUP 1 CONTROLS |
GROUP 2 LGSI ONLY |
GROUP 3 IR ONLY |
GROUP 4 IR AND LGSI |
||
|---|---|---|---|---|---|
| N° (%) | 48 (15.4%) | 51 (16.4%) | 93 (29.9%) | 119 (38.3%) | |
| AGE | 77±7.5*° | 77±7.7*° | 74±6.1 | 75±6.5 | 0.005 |
| GENDER (F%) | 75 | 65 | 69 | 60 | 0.24 |
| HOMA score | 1.66 (1.25–1.65) | 1.78 (1.35–2.04) | 3.34 (2.84.4.40) | 4.43 (3.04–6.39) | 0.001 |
| Hs-CRP (mg/L) | 1.7 (1.07–2.3) | 6.2 (4–11.2) | 1.63 (1–2.3) | 6.2 (4.3–12.7) | 0.001 |
|
| |||||
| ATP III MS CRITERIA | |||||
| - INCREASED WAIST | 77% | 86% | 73% | 82% | 0.21 |
| - HIGH | 73% | 43% | 67% | 58% | 0.01 |
| TRIGLYCERIDES | |||||
| - LOW HDL-C | 54% | 63% | 60% | 48% | 0.24 |
| - HYPERTENSION | 100% | 100% | 97% | 98% | 0.36 |
| - HYPERGLYCEMIA | 31% | 39% | 61% | 71% | 0.001 |
|
| |||||
| WAIST (cm) | 96±9° | 99±9° | 98±10° | 101±8 | 0.01 |
| TRIGLYCERIDES (mg/dL) | 169 (139–198) | 139 (103–176) | 170 (138–213) | 161 (119–216) | 0.001 |
| HDL-C (mg/dL) | 48±10 | 47±12 | 47±11 | 46±12 | 0.79 |
| SBP (mmHg) | 154±18 | 155±17 | 156±21 | 156±18 | 0.79 |
| DBP (mmHg) | 83±7 | 86±9 | 86±8 | 85±9 | 0.12 |
| FASTING | 93±18*° | 97±23*° | 112±33 | 121±41 | 0.001 |
| GLUCOSE (mg/dL) | |||||
| LDL-C (mg/dL) | 143±41 | 138±34 | 138±38 | 136±33 | 0.71 |
| URIC ACID (mg/dL) | 5.4±1.1 | 5.4±1.5 | 5.5±1.4 | 5.7±1.7 | 0.58 |
| BMI (Kg/m2) | 28.6±4.0° | 29.6±4.2 | 29.3±3.7° | 30.9±4.3 | 0.005 |
| CREATININE (mg/dL) | 0.91±0.3 | 0.96±0.5 | 0.91±0.2 | 0.96±0.2 | 0.42 |
|
| |||||
| DIABETES (%) | 10.4 | 12.0 | 26.3 | 28.3 | 0.01 |
| CHD (%) | 6.3 | 10.0 | 7.6 | 13.6 | 0.39 |
| STROKE (%) | 2.1 | 2.0 | 6.5 | 11.0 | 0.08 |
| PAD (%) | 10% | 23% | 12% | 21% | 0.10 |
| SMOKING (%) | |||||
| Never | 67 | 61 | 71 | 64 | |
| Former | 29 | 32 | 23 | 27 | |
| Current | 4 | 8 | 7 | 9 | 0.29 |
All p < 0.01 # vs group 2 ;
vs group 3;
vs group 4
Participants without MS
The principal characteristics of 700 older subjects without MS, according to IR/LGSI classification are reported in TABLE 3. Among them, those with no IR nor LGSI were the most frequent (34.2%), followed by those with either IR or LGSI (24.6% and 23.6%, respectively). Diabetes and hyperglycemia were more frequent in subjects with IR (group 3 and 4), while low HDL-C and previous stroke were more frequent in subjects presenting LGSI (group 2 and 4). Subjects with IR and LGSI were more often affected by PAD, and had higher BMI.
TABLE 3.
characteristics of 700 older subjects without metabolic syndrome, according to presence or absence of insulin resistance and low grade systemic inflammation (IR / LGSI group) [one way ANOVA, Bonferroni post-hoc test].
| GROUP 1 CONTROLS |
GROUP 2 LGSI ONLY |
GROUP 3 IR ONLY |
GROUP 4 IR AND LGSI |
||
|---|---|---|---|---|---|
| N° (%) | 240 (34.2%) | 165 (23.6%) | 172 (24.6%) | 123 (17.6%) | |
|
| |||||
| AGE | 75±7.5 | 77±8.7* | 74±6.6 | 76±6.9 | 0.002 |
| GENDER (F%) | 56 | 46 | 51 | 51 | 0.22 |
| HOMA score | 1.48 (1.11–1.84) | 1.56 (1.09–1.89) | 3.04 (2.65–3.51) | 3.15 (2.67–3.70) | 0.001 |
| Hs-CRP (mg/L) | 1.3 (0.9–2.0) | 6.03 (4.3–11) | 1.25 (0.8–2.06) | 6.0 (4.3–9.3) | 0.001 |
|
| |||||
| ATP III MS CRITERIA | |||||
| - INCREASED WAIST | 21% | 31% | 27% | 30% | 0.10 |
| - HIGH | 10% | 9% | 6% | 13% | 0.26 |
| TRIGLYCERIDES | |||||
| - LOW HDL-C | 6% | 15% | 4% | 14% | 0.001 |
| - HYPERTENSION | 87% | 93% | 91% | 89% | 0.22 |
| - HYPERGLYCEMIA | 7.5% | 5.5% | 21% | 18% | 0.001 |
|
| |||||
| WAIST (cm) | 88±9#° | 91±8 | 89±10° | 92±9 | 0.001 |
| TRIGLYCERIDES (mg/dL) | 97 (76–128) | 97 (76–128) | 98 (76–123) | 108 (89–127) | 0.007 |
| HDL-C (mg/dL) | 63±16#° | 56±14* | 61±13° | 56±13 | 0.001 |
| SBP (mmHg) | 147±19.7 | 150±19 | 149±20 | 149±19 | 0.44 |
| DBP (mmHg) | 83±8.6 | 84±8.6 | 84±8.6 | 84±7.7 | 0.47 |
| FASTING | 85±12*° | 85±12*° | 95±24 | 94±20 | 0.001 |
| GLUCOSE (mg/dL) | |||||
| LDL-C (mg/dL) | 132±34 | 133±36 | 134±32 | 141±34 | 0.11 |
| URIC ACID (mg/dL) | 4.8±1.2# | 5.3±1.7* | 4.7±1.2° | 5.2±1.3 | 0.001 |
| BMI (Kg/m2) | 25.9±3.2° | 26.4±3.4 | 25.8±3.5° | 27.4±3.4 | 0.001 |
| CREATININE (mg/dL) | 0.89±0.18# | 0.96±0.26 | 0.92±0.17 | 0.94±0.21 | 0.03 |
|
| |||||
| DIABETES | 5.1 | 4.3 | 14.1 | 7.0 | 0.002 |
| CHD (%) | 6.4 | 6.2 | 6.5 | 7.8 | 0.95 |
| STROKE (%) | 2.5 | 11 | 4.7 | 8.7 | 0.003 |
| PAD (%) | 6.7% | 9.1% | 8.1% | 17.9% | 0.006 |
| SMOKING (%) | |||||
| Never | 62 | 53 | 55 | 58 | |
| Former | 27 | 37 | 35 | 25 | |
| Current | 11 | 10 | 10 | 17 | 0.08 |
All p <0.01 # vs group 2;
vs group 3;
vs group 4
Whole sample
The prevalence of MS was 17% in subjects without IR and LGSI (group 1), 24% in subjects with isolated LGSI, 35% in those with isolated IR, and 49% in those with IR and LGSI (p for trend<0.001). Compared to participants without MS, those with MS had higher prevalence of IR (68% versus 42%) and LGSI (55% versus 41%). Nevertheless, despite being recognized as two “core features” of MS, we found that the overlap between IR and LGSI and MS phenotype was only partial. Indeed, 31.8% of subjects with MS phenotype had no IR, while 45.3% of them had no LGSI; on the other hand, it has to be underlined that 51% of subjects with both IR and LGSI didn’t display an MS phenotype.
Participants with MS had significantly higher values of HOMA compared to those without MS, overall and among those with isolated IR or LGSI or both conditions (p<0.01). Moreover, among participants with IR only, those with MS had higher CRP levels than those without the MS (p<0.01). After adjustment for age, gender, and diabetes, MS was not an independent predictor of CVD mortality (HR: 1.29; 95%C.I.:0.92–1.81), nor of total mortality (HR: 1.07; 95%C.I.:0.86–1.34). Instead, IR (HR: 1.68; 95%C.I.:1.21–2.32) but not LGSI (HR: 1.29; 95%C.I.:0.98–1.69) significantly predicted CVD mortality, while IR and LGSI were both significant risk factors for total mortality (age and gender adjusted HR: 1.24; 95%C.I.:1.009–1.53, and 1.27; 95%C.I.:1.07–1.52, respectively).
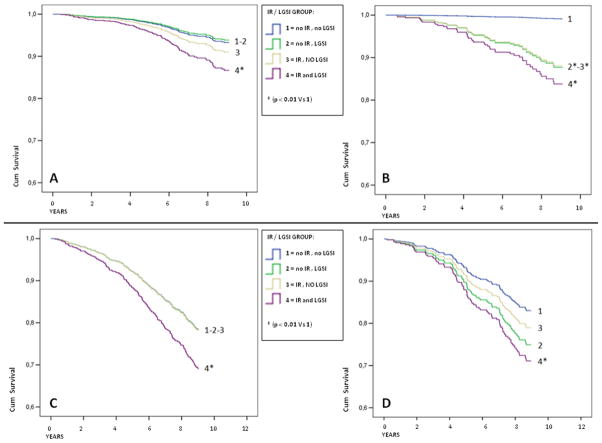
TABLE 4-A shows event rates and adjusted HR for CVD mortality, according to IR/LGSI classification, in individuals without and with MS. In people without MS (FIGURE 1-A), the HR was similar between group 1 (no IR, no LGSI) and group 2 (isolated LGSI), with a non-significant increase in group 3 (isolated IR). Only in group 4 (IR and LGSI) the risk was higher compared to group 1 (HR 2.07; 95%C.I.:1.12–3.72). Among subjects with MS, the event rate was unexpectedly low in individuals without IR and LGSI (FIGURE 1-B). Compared with these individuals, a significant increase in the HR for CVD mortality was observed not only in subjects with both LGSI and IR (HR 9.88; 95%C.I.:2.18–44), but also in those with IR (HR 6.90; 95%C.I.:1.45–32) or LGSI (HR 7.56; 95%C.I.:1.63–35).
TABLE 4-A.
Event rates and Hazard Ratios for cardiovascular mortality, according to presence or absence of insulin resistance and low grade systemic inflammation (IR / LGSI group), among 1011 community-dwelling older individuals with or without metabolic syndrome.
| Without Metabolic Syndrome
|
With Metabolic Syndrome
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95 % C.I.)
|
Hazard Ratio (95 % C.I.)
|
|||||||
| N. | Events per 1000 persons- year | Age-Sex Adjusted | Multivariable* Adjustment | N. | Events per 1000 persons- year | Age-Sex Adjusted | Multivariable† Adjustment | |
|
|
|
|||||||
| HOMA/CRP group | ||||||||
| 1. NO IR, NO LGSI | 239 | 15.3 | 1 | 1 | 48 | 4.6 | 1 | 1 |
| 2. LGSI, NO IR | 165 | 18.8 | 1.15 (0.69–1.92) | 0.90 (0.47–1.73) | 51 | 26.0 | 7.44 (1.65–33.60) | 7.56 (1.63–35.01) |
| 3. IR, NO LGSI | 172 | 14.8 | 1.28 (0.75–2.21) | 1.36 (0.77–2.41) | 93 | 14.5 | 6.67 (1.46–30.52) | 6.90 (1.45–32.22) |
| 4. IR + LGSI | 124 | 21.6 | 1.95 (1.14–3.33) | 2.07 (1.12–3.72) | 119 | 24.2 | 9.60 (2.23–41.33) | 9.88 (2.18–44.58) |
Adjusted for: age and gender
Adjusted for: age, gender, diabetes, total cholesterol, BMI, smoking habit, coronary heart disease, and previous stroke
Figure 1.
Cox survival curves according to insulin resistance/low grade systemic inflammation (IR/LGSI) group. Group 1 = no IR, no LGSI ; Group 2 = no IR , LGSI ; Group 3 = IR , no LGSI ; Group 4 = IR and LGSI. Panel A–B: Survival curves for cardiovascular mortality in individuals without (A) or with (B) MS. Adjusted for age, gender, diabetes, total cholesterol, BMI, smoking habit, coronary heart disease, and previous stroke. Panel C–D: Survival curves for total mortality in individuals without (C) or with (D) MS. Adjusted for age, gender, diabetes, total cholesterol, BMI, and smoking habit.
HR for CVD mortality according to IR/LGSI, were no significantly difference between subjects with or without MS. Only in subjects of group 1 (without IR neither LGSI) MS seemed associated with lower CVD mortality compared with those without MS (4.6 vs 15.3/1000 persons-year; p: 0.25).
TABLE 4-B reports event rates and adjusted HR for total mortality according to IR/LGSI classification, separately for participants with and without MS. Interesting, while mortality rates in IR/LGSI groups were quite different, within the same group the diagnosis of MS made only small differences. Compared to participants without IR and LGSI, participants with both IR and LGSI, but not those with IR or LGSI alone, had increased HR for mortality, and this was true both in those with and without MS. (FIGURE 1-C and 1-D).
TABLE 4-B.
Event rates and Hazard Ratios for total mortality, according to presence or absence of insulin resistance and low grade systemic inflammation (IR / LGSI group), among 1011 community dwelling older individuals with or without metabolic syndrome.
| Without Metabolic Syndrome
|
With Metabolic Syndrome
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95 % C.I.)
|
Hazard Ratio (95 % C.I.)
|
|||||||
| N. | Events per 1000 persons- year | Age- Sex Adjusted | Multivariable* Adjustment | N. | Events per 1000 persons- year | Age-Sex Adjusted | Multivariable† Adjustment | |
|
|
|
|||||||
| HOMA/CRP group | ||||||||
| 1. NO IR, NO LGSI | 239 | 38.1 | 1 | 1 | 48 | 39.2 | 1 | 1 |
| 2. LGSI, NO IR | 165 | 52.5 | 1.19 (0.87–1.64) | 1.00 (0.69–1.45) | 51 | 50.2 | 1.56 (0.83–2.96) | 1.54 (0.79–3.01) |
| 3. IR, NO LGSI | 172 | 32.9 | 0.99 (0.69–1.41) | 1.01 (0.70–1.48) | 93 | 28.7 | 1.29 (0.68–2.48) | 1.17 (0.66–2.33) |
| 4. IR + LGSI | 124 | 49.3 | 1.63 (1.16–2.30) | 1.51 (1.02–2.28) | 119 | 48.6 | 1.81 (1.02–3.22) | 1.72 (1.001–3.17) |
Adjusted for: age and gender
Adjusted for: age, gender, diabetes, total cholesterol, BMI, and smoking habit
DISCUSSION
In this study we hypothesized that in the elderly population MS phenotype might lose its values in predicting CVD/total mortality risk, while conversely IR and/or LGSI would not.
Metabolic syndrome and mortality
We found no association between MS and 9-years CVD/total mortality risk. Unlike what has been reported in young/middle-age population, the association between MS and mortality has not been consistently demonstrated in older people. Our findings are different from those reported in other populations; however, most of studies reporting a significant association (5–7) used the 2002 NCEP-ATP III MS criteria (33) with a higher cut-point for elevated fasting glucose (EFG: ≥110 mg/dL) compared to 2005 updated criteria (EFG: ≥100 mg/dL) used in this study (32). In the Cardiovascular Health Study (5) mortality risk was evaluated by using both NCEP-ATP III cut points for EFG (5); when the lower EFG cut-off was used, the association between MS and mortality was weaker (RR: 1.12 vs RR: 1.22); moreover, MS didn’t predict mortality in the absence of EFG. It is possible that by using a higher cut-off these authors indentified a “MS phenotype” closer to the “diabetic phenotype”; this might justify the higher mortality, since diabetes preserves its association with mortality in the elderly (34). Additionally, our population was older compared to those populations, and this indirectly suggests the possible weakness of MS as predictor of mortality in late life (5;6).
Two studies specifically compared mortality risk in middle age versus elderly MS individuals. Sundstrom et al. evaluated the same sample of men at ages 50 and 70, reporting a lower prognostic impact for MS at age 70 (14), while Hildrum et al. found that MS was not associated with the risk of total/CVD mortality after 60 years of age (12). Besides methodological arguments, our results agree with other longitudinal studies (9–11). Different reasons might explain our negative findings. In particular, a different significance of the single risk factors composing MS phenotype (e.g. abdominal fat accumulation, hypertension, increased fasting glycaemia) might be present in advanced age, in relation to “usual” modifications related to the aging process; this would lead to misclassification bias, i.e. to the inclusion into MS phenotype of subjects that actually have not MS. Moreover, a selection due to early mortality among MS adult individuals surviving to advance age might be also present; finally, the late onset of MS in some/most of individuals might reduce significantly the exposure to the risk factors over time, and as result their clinical significance.
Insulin resistance, low grade systemic inflammation, and 9-years mortality
We also tested the association between IR/LGSI and mortality risk; indeed, these conditions represent the “pathophysiological core” of MS, and have been correlated themselves with CVD/overall mortality (15–18;23–28). We found that isolated IR or LGSI were not associated with total mortality risk, whereas they were associated with higher CVD mortality, but only in subjects with MS phenotype. On the other hand, subjects with both IR and LGSI had higher CVD/total mortality, independently of the presence of MS phenotype. Thus, while MS phenotype did not, IR together with LGSI identified a sub-group of individuals with the highest risk of CVD/overall mortality.
Previous studies reported that IR or LGSI were, considered apart, independent risk factors for mortality (15;16;26;35;36). Perseghin et al. found a significant association of HOMA-IR with CVD (HR:1.05, 95%CI:1.03–1.08) and overall mortality (HR:1.05, 95%CI:1.03–1.07) after 15 years follow up (16). Harris et al. (26) reported that higher CRP and/or IL-6 levels were independently associated with increased CVD/non-CVD death in older individuals (26). Laaksonen et al. found an increased risk for CVD/total mortality associated with higher CRP levels, after multivariate adjustment including fasting glucose and insulin; nevertheless, these authors did not evaluate the simultaneous presence of IR and LGSI (36). The reason why in our sample IR and LGSI would separately affect CVD mortality only in individuals with MS phenotype is not clear, and two explanations are possible in our view: 1. We observed that among individuals with either IR or LGSI, higher HOMA (and hs-CRP) were associated with higher probability of having not only MS phenotype, but also dyslipidemia, diabetes, central obesity, and finally of having increased CVD mortality; 2. this phenomenon might partially depend also on the very low mortality rate found in MS individuals without IR/LGSI (reference group). Thus, it seems that MS phenotype might be helpful in identifying, among subjects with either IR or LGSI, a sub-group with increased CVD mortality risk. Referring to the “overflow-ectopic fat” model (37;38), our data support the hypothesis that, among older subjects with IR and/or LGSI, MS phenotype might identify those individuals in which larger amounts of adipose tissue had been deposited at undesirable sites (i.e. liver, skeletal muscle, and visceral fat) determining higher CVD mortality.
Finally, some important limitations of the study must be acknowledged. First, we used an indirect measure of insulin resistance (HOMA), while a direct measure such as the hyperinsulinemic euglycemic clamp would be much more precise. However, HOMA has been validated with the hyperinsulinemic euglycemic clamp (39). Second, the sample of subjects with MS was not very large; of consequence, the size of the IR/LGSI groups was small and this caused large confidence intervals in Cox analysis. Third, we did not consider the rate of CVD events, and this might be an additional important outcome in older people affected by MS. Conversely, we would like to underline the strength of our research; indeed to our knowledge this is the first study that methodically analysed the combined effect of IR and LGSI, finding a relevant association with mortality in older population.
CONCLUSIONS
In conclusion, we found that in a population of community dwelling older individuals the simultaneous presence of IR and LGSI, but not MS phenotype, was associated with a significant increase of 9-years overall and CVD mortality risk. Our findings do not question the usefulness of MS phenotype in identifying a cluster of vascular risk factors, but strongly underline the concept that its clinical predictive value is essentially related, at least in the elderly, to the presence of IR and LGSI. Compared to adulthood, there might be a reduced “overlap” between MS phenotype and its physiopathological core (IR and LGSI) in older age, perhaps because of the confounding effect of some “usual” modification of aging itself, such as visceral fat accumulation, increase fasting glycaemia, and hypertension. Our study suggest that the definition of MS should be more holistic in advanced age, and probably comprise also the measurement of IR and LGSI.
Highlights.
We studied 1011 elderly community-dwelling subjects with 9-years follow up
MS was not associated with CVD or total mortality
Instead, insulin resistance plus Inflammation were associated with increased mortality
Increased CRP and HOMA might be good clinical predictors of mortality in elderly
In advanced age MS definition might comprise the measurement of IR and LGSI.
Acknowledgments
Sources of support: The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health, and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336). The Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the Follow-up 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002). Supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose
G.Z., M.L.M., S.V., M.M., A.C., D.F., S.B., G.P., J.M.G. and L.F. takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004 Sep 7;110(10):1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 3.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010 Sep 28;56(14):1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002 Jan 16;287(3):356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Kamineni A, Prineas RJ, Siscovick DS. Metabolic syndrome and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2008 May 12;168(9):969–78. doi: 10.1001/archinte.168.9.969. [DOI] [PubMed] [Google Scholar]
- 6.Zambon S, Zanoni S, Romanato G, Corti MC, Noale M, Sartori L, et al. Metabolic syndrome and allcause and cardiovascular mortality in an Italian elderly population: the Progetto Veneto Anziani (Pro.V.A.) Study. Diabetes Care. 2009 Jan;32(1):153–9. doi: 10.2337/dc08-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbaraly TN, Kivimaki M, Ancelin ML, Barberger-Gateau P, Mura T, Tzourio C, et al. Metabolic syndrome, its components, and mortality in the elderly. J Clin Endocrinol Metab. 2010 Nov;95(11):E327–E332. doi: 10.1210/jc.2010-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rachas A, Raffaitin C, Barberger-Gateau P, Helmer C, Ritchie K, Tzourio C, et al. Clinical usefulness of the metabolic syndrome for the risk of coronary heart disease does not exceed the sum of its individual components in older men and women. The Three-City (3C) Study. Heart. 2012 Apr;98(8):650–5. doi: 10.1136/heartjnl-2011-301185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Ruotsalainen S, Moilanen L, Lepisto P, Laakso M, Kuusisto J. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007 Apr;28(7):857–64. doi: 10.1093/eurheartj/ehl524. [DOI] [PubMed] [Google Scholar]
- 10.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008 Jun 7;371(9628):1927–35. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 11.Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006 Apr 18;47(8):1595–602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 12.Hildrum B, Mykletun A, Dahl AA, Midthjell K. Metabolic syndrome and risk of mortality in middleaged versus elderly individuals: the Nord-Trondelag Health Study (HUNT) Diabetologia. 2009 Apr;52(4):583–90. doi: 10.1007/s00125-009-1271-5. [DOI] [PubMed] [Google Scholar]
- 13.Thomas F, Pannier B, Benetos A, Vischer UM. The impact of the metabolic syndrome--but not of hypertension--on all-cause mortality disappears in the elderly. J Hypertens. 2011 Apr;29(4):663–8. doi: 10.1097/HJH.0b013e32834320dc. [DOI] [PubMed] [Google Scholar]
- 14.Sundstrom J, Riserus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006 Apr 15;332(7546):878–82. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care. 2010 Jun;33(6):1179–85. doi: 10.2337/dc09-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perseghin G, Calori G, Lattuada G, Ragogna F, Dugnani E, Garancini MP, et al. Insulin resistance/hyperinsulinemia and cancer mortality: the Cremona study at the 15th year of followup. Acta Diabetol. 2012 Jan 4; doi: 10.1007/s00592-011-0361-2. [DOI] [PubMed] [Google Scholar]
- 17.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007 Feb;30(2):318–24. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- 18.de Boer IH, Katz R, Chonchol MB, Fried LF, Ix JH, Kestenbaum B, et al. Insulin resistance, cystatin C, and mortality among older adults. Diabetes Care. 2012 Jun;35(6):1355–60. doi: 10.2337/dc11-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Festa A, D'Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000 Oct;58(4):1703–10. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 20.Hassinen M, Lakka TA, Komulainen P, Gylling H, Nissinen A, Rauramaa R. C-reactive protein and metabolic syndrome in elderly women: a 12-year follow-up study. Diabetes Care. 2006 Apr;29(4):931–2. doi: 10.2337/diacare.29.04.06.dc05-2508. [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002 Mar 5;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 22.Libby P. Inflammation in atherosclerosis. Nature. 2002 Dec 19;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 23.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009 Oct 6;151(7):483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 24.Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004 Jul 27;110(4):380–5. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 25.Elkind MS, Luna JM, Moon YP, Liu KM, Spitalnik SL, Paik MC, et al. High-sensitivity C-reactive protein predicts mortality but not stroke: the Northern Manhattan Study. Neurology. 2009 Oct 20;73(16):1300–7. doi: 10.1212/WNL.0b013e3181bd10bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999 May;106(5):506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 27.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005 Jul 5;112(1):25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 28.Giovannini S, Onder G, Liperoti R, Russo A, Carter C, Capoluongo E, et al. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc. 2011 Sep;59(9):1679–85. doi: 10.1111/j.1532-5415.2011.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrucci L, Bandinelli S, Benvenuti E, Di IA, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000 Dec;48(12):1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 30.Zuliani G, Galvani M, Maggio M, Volpato S, Bandinelli S, Corsi AM, et al. Plasma soluble gp130 levels are increased in older subjects with metabolic syndrome. The role of insulin resistance. Atherosclerosis. 2010 Nov;213(1):319–24. doi: 10.1016/j.atherosclerosis.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson TA, Bazzarre TL, Daniels SR, Fair JM, Fortmann SP, Franklin BA, et al. American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, healthcare providers, and health policy makers from the American Heart Association Expert Panel on Population and Prevention Science. Circulation. 2003 Feb 4;107(4):645–51. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 34.Barnett KN, McMurdo ME, Ogston SA, Morris AD, Evans JM. Mortality in people diagnosed with type 2 diabetes at an older age: a systematic review. Age Ageing. 2006 Sep;35(5):463–8. doi: 10.1093/ageing/afl019. [DOI] [PubMed] [Google Scholar]
- 35.Lee JK, Bettencourt R, Brenner D, Le TA, Barrett-Connor E, Loomba R. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One. 2012;7(4):e34218. doi: 10.1371/journal.pone.0034218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laaksonen DE, Niskanen L, Nyyssonen K, Punnonen K, Tuomainen TP, Salonen JT. C-reactive protein in the prediction of cardiovascular and overall mortality in middle-aged men: a population-based cohort study. Eur Heart J. 2005 Sep;26(17):1783–9. doi: 10.1093/eurheartj/ehi237. [DOI] [PubMed] [Google Scholar]
- 37.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec 14;444(7121):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 38.Boren J, Taskinen MR, Olofsson SO, Levin M. Ectopic lipid storage and insulin resistance: a harmful relationship. J Intern Med. 2013 Jul;274(1):25–40. doi: 10.1111/joim.12071. [DOI] [PubMed] [Google Scholar]
- 39.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000 Jan;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]