Abstract
Mucosal pathogens trigger a local innate host response by activating epithelial cells. Bacterial adherence and Toll-like receptor 4 (TLR4) signaling have been implicated as key events in this process. This study addressed the molecular basis of the epithelial response to gram-negative infection in the human urinary tract. Mucosal biopsies were obtained from kidneys, ureters, and bladders of patients undergoing urinary tract surgery, and epithelial TLR4 and CD14 expression was examined by immunohistochemistry. TLR4 was detected in epithelial cells lining the entire urinary tract and in the renal tubular epithelium. CD14, in contrast, was completely absent from the epithelial tissue. The response of the epithelial cells to infection was studied by in vitro challenge of the biopsies with uropathogenic Escherichia coli bacteria. A rapid cytokine response was observed, with production of interleukin-1β (IL-1β), IL-6, and IL-8 but not of IL-4 or gamma interferon. Adhering, P- or type 1-fimbriated E. coli activated IL-6 and IL-8 production more efficiently than the nonfimbriated control, as shown by cellular staining and analysis of secreted cytokines. The results demonstrate that human uroepithelial cells possess the molecular machinery needed to respond to uropathogenic E. coli. This includes recognition receptors for fimbriae and TLR4 for transmembrane signaling. We speculate that the lack of membrane-bound CD14 allows the epithelium to regulate its sensitivity to lipopolysaccharide and to discriminate between more-virulent and less-virulent strains.
Mucosal pathogens use diverse and highly sophisticated mechanisms to gain access to the tissues at their preferred site of infection (6, 8, 11, 35). Adherence is a crucial first step to establish tissue contact and to break the inertia of the mucosal barrier, but in addition, the molecular interactions between bacteria and host alert the host to the danger, and a host response is activated (1, 14). In urinary tract cell lines, epithelial cell activation by fimbriated Escherichia coli requires primary recognition receptors for fimbrial adhesins and Toll-like receptor 4 (TLR4) for transmembrane signaling (12). Human urinary tract epithelial cells express both glycosphingolipid and mannosylated surface glycoprotein receptors, which recognize the P fimbrial adhesins (29) and the type1 fimbriae, respectively (30). TLR4 is also expressed in murine urinary tract epithelium, and the tlr4 genotype was found to regulate the in vivo response to experimental urinary tract infection (UTI) caused by P- or type 1-fimbriated E. coli (12, 17, 36, 38).
The extent to which TLR4 is expressed by the human urinary tract epithelium remains controversial, however. In addition, there are contradictory reports concerning the lipopolysaccharide (LPS) responsiveness of uroepithelial cells and their expression of CD14. It is well established that cells of myeloid origin express CD14 and MD2 and recruit TLR4 for transmembrane signaling when exposed to LPS (5), but uroepithelial cell lines respond poorly to soluble LPS even though they express TLR4 (23). This has been attributed to the lack of CD14 expression by those cells (2, 26). More recent studies of TLR4 and CD14 expression in urinary epithelial cell lines either have failed to demonstrate TLR4 or CD14 on the epithelium (3, 7) or have detected CD14 and proposed that LPS responsiveness is important in the urinary bladder (37). There are reports that urine contains soluble CD14 (sCD14) (7), but whether the concentration in uninfected urine is high enough to support CD14-mediated recognition of LPS needs further study.
Cytokines are early markers of the epithelial response to infection and play a key role in the innate defense (39). Interleukin-6 (IL-6) may cause fever and trigger the acute-phase response, while chemokines such as CXCL8 (IL-8) recruit inflammatory cells to the site of infection. The urine cytokine levels are elevated in patients with UTI (22, 31-33), and epithelial cells have been identified as early producers of cytokines in the murine UTI model (9, 20, 36), but the epithelial cytokine response of the human mucosa in situ has not been investigated.
This study used human urinary tract biopsies to characterize TLR4 and CD14 expression in the intact human urinary tract epithelium and to study the epithelial cytokine response to infection.
MATERIALS AND METHODS
Reagents.
Monoclonal mouse anti-human IL-1β was from Genzyme, Cambridge, Mass.; anti-IL-4 and anti-gamma interferon (anti-IFN-γ) were from Mabtech AB, Stockholm, Sweden; anti-IL-6 (M16) was from Central Laboratory of The Netherlands' Red Cross Blood Transfusion Service, Amsterdam, The Netherlands; and anti-IL-8 (NAP-1) was from Sandoz, Vienna, Austria. Mouse immunoglobulin G1 (IgG1) normal rabbit immunoglobulin, rabbit anti-mouse immunoglobulins, mouse alkaline phosphatase-anti-alkaline phosphatase complexes, and alkaline phosphatase-conjugated goat anti-rabbit antibody were from Dakopatts AB, Älvsjö, Sweden. Fast-Red substrate was from Dako Corporation, Carpenteria, Calif. OCT compound was from Tissue Tek, Miles, Elkhart, Ind. Alkaline phosphatase-conjugated rabbit anti-mouse IgG and saponin were from Sigma, St. Louis, Mo. Poly-l-lysine-coated glass slides and Mayer hematoxylin were from Histolab, Västra Frölunda, Sweden. The peroxidase substrate diaminobenzidine kit was from Vector Laboratories Inc. Burlingame, CA, USA. Mount-quick “Aqueous” was from Daido Sangyo Co., Ltd., Tokyo, Japan. RPMI medium was from Flow, Göteborg, Sweden; monoclonal mouse anti-human TLR4 (IMG-320) was from Imgenex, San Diego, Calif.; polyclonal rabbit anti-human TLR4 was from Ebioscience, San Diego, Calif.; and monoclonal mouse anti-human CD14 clone (TUK4), monoclonal mouse anti-human cytokeratin, monoclonal mouse IgG1 control, monoclonal mouse IgG2a control, swine anti-rabbit-fluorescein isothiocyanate (FITC), and rabbit anti-mouse-FITC were from Dako. Monoclonal mouse anti-human CD14 (clone MY4) was from Beckman Coulter, Miami, Fla. Lymphoprep was from Axis-shield PoC AS, Oslo, Norway.
Patients.
Biopsy specimens were obtained from 28 patients undergoing urinary tract surgery for a variety of diseases. The patients were divided into two groups, one with sterile urine at the time when the biopsy was obtained and one with asymptomatic bacteriuria (ABU). In the sterile urine group, nine patients underwent transurethral resection of the prostate due to benign prostate hyperplasia. Only bladder specimens were obtained from this group. Seven patients underwent nephroureterectomy, nephrectomy, or cystectomy due to cancer of the kidney, renal pelvis, or bladder. Bladder biopsies were obtained from two patients, renal pelvis biopsies were obtained from five patients, renal cortex biopsies were obtained from two patients, and urethral biopsies were obtained from two patients. Renal pelvis biopsies were also obtained from two patients undergoing pyeloplasty due to hydronephrosis and heminephrectomy due to a duplication of the renal pelvis.
In the bacteriuria group, all patients were asymptomatic, and only one had received antibiotic treatment during the last 30 days. Bladder biopsies were obtained from four patients undergoing transurethral resection due to benign prostate hyperplasia. These patients had indwelling catheters. One bladder biopsy was obtained from a patient undergoing cystectomy due to bladder cancer. One bladder biopsy was obtained from a patient with a neurogenic bladder disorder. Renal pelvis biopsies were obtained from three patients undergoing nephrectomy due to chronic pyelonephritis and from one patient undergoing nephrectomy due to renal cancer.
All biopsies from cancer patients were obtained from areas well outside tumor growth and were confirmed as tumor free by the pathologist.
This study was approved by the Ethics Committee of the Medical Faculty at Lund University.
Urine samples.
Morning urine was collected prior to surgery and before antibiotics were given. Samples were kept at 4°C until semiquantitatively cultured. The urine isolates were examined by Gram staining and were further identified by standard laboratory techniques.
Collection of biopsies.
The biopsies were obtained 10 to 30 min after cutting off the blood supply and were cut to pieces of 5 by 5 by 5 mm. The pieces were embedded in OCT compound, rapidly frozen in liquid nitrogen, and kept at −80°C or were stimulated with bacteria in vitro prior to freezing (see below).
Bacteria.
Uropathogenic E. coli isolates or recombinant strains differing in fimbrial expression were used for in vitro infection of the biopsies. E. coli Hu734 is a lac mutant of the wild-type pyelonephritis strain GR12 (serotype O75:K5:HO; hly ColV+ pap+ fim+) (16). E. coli HB101 (pap) is a K-12 derivative lacking most virulence genes. The strain was used as a host for the recombinant plasmid pPIL110-35 encoding P fimbriae (pap+). The plasmid carried a 16-kb EcoRI fragment from the AD110 pap gene cluster inserted in the EcoRI site of pACYC184 (41). The K-12 derivative E. coli AAEC 191A (fim) was used as a recipient for the recombinant plasmid pPKL4, encoding type 1 fimbriae. E. coli PKL4 (fim+) carried the entire fim gene cluster from E. coli PC31 inserted into the tetracycline site of pBR322 (27).
The strains were maintained on tryptic soy agar and subcultured overnight in Luria broth with appropriate selection at 37°C for in vitro infection. After centrifugation, the bacterial pellet was washed three times by repeated cycles of centrifugation at 2,800 × g for 10 min and resuspension in phosphate-buffered saline (PBS). The bacteria were finally diluted in RPMI to about 106 CFU per ml. Type 1 and P fimbrial expression was tested by receptor-specific hemagglutination (29).
Infection of biopsies.
All of the biopsies from bladder and pelvis that were large enough to be divided were included in the in vitro infection study. Fresh biopsy pieces were washed once in RPMI and transferred to 24-well plates in triplicate. Tissue culture medium or medium containing E coli bacteria (106 CFU/ml) was added, and the plates were incubated at 37°C in a 5% CO2 atmosphere with shaking. Tissue supernatants were harvested after 0, 2, and 6 h and centrifuged at 2,800 × g for 10 min, and supernatants were stored at −80°C for cytokine analysis. One piece each was collected at 0, 2, and 6 h and frozen as described above. For IL-8, additional biopsies were harvested after 30 min.
Cytokine analysis by immunohistochemistry.
The embedded tissues were sectioned in a cryostat (Carl Zeiss AB, Stockholm, Sweden) into 6-μm sections and mounted on poly-l-lysine-coated glass slides. For staining of intracellular cytokines, sections were fixed in freshly prepared 4% paraformaldehyde (34) for 15 min, rinsed in PBS (0.06 M, pH 7.2), air dried, and kept at −20°C until used. The sections were permeated with 0.1% saponin (34) in PBS containing 5% heat-inactivated normal human AB serum for 60 min at room temperature to reduce nonspecific binding. After being washed in PBS-0.1% saponin three times, the sections were incubated overnight at 4°C with a panel of cytokine-specific monoclonal antibodies at a concentration of 5 to 10 μg/ml. After being washed with 0.1% saponin in Tris-buffered saline (TBS-Sap), the sections were incubated with rabbit anti-mouse immunoglobulins for 60 min at room temperature in a moist chamber. The sections were washed again in TBS-Sap and incubated with mouse alkaline phosphatase-anti-alkaline phosphatase complexes in TBS-Sap for 60 min as described above. After three washes in TBS-Sap, the Fast-Red substrate with levamisole was added (according to the manufacturer's instructions) and left to incubate for 20 min. The sections were thereafter washed in TBS (pH 7.6), counterstained with hematoxylin for 5 to 10 s, and mounted with Mount-Quick “Aqueous.” Mouse IgG1 at a final concentration of 5 to 10 μg/ml and a normal rabbit immunoglobulin fraction were used as negative controls.
Three to five sections from each biopsy were inspected single blind by light microscopy. The intensity of cytokine staining was given as the number of positive epithelial cells after scoring of at least 200 cells per section.
TLR4 and CD14 analysis by immunohistochemistry.
Sections were fixed on polylysine-coated slides in acetone for 3 min, air dried, and kept at −80°C until used. Sections were incubated with primary antibody (diluted in PBS) for 60 min at room temperature. After incubation, they were washed twice in PBS. The sections were incubated with the secondary antibody (alkaline phosphatase-conjugated rabbit anti-mouse antibody diluted in 5% AB serum) for 60 min and washed twice in PBS. The sections were then incubated with Fast-Red substrate for 20 min and were further treated as were the cytokine stainings described above.
Analysis of TLR4 and CD14 on excreted urinary cells by flow cytometry.
Urine samples were received from four healthy females. The urine was centrifuged at 260 × g for 30 min to pellet the shed epithelial cells. The cells were washed in PBS-0.1% bovine serum albumin (BSA), and 2 × 105 cells were incubated with the primary antibody (rabbit anti-human TLR4, mouse anti-human CD14, or control antibody) diluted in 100 μl of PBS-0.1% BSA for 1 h at 4°C. The cells were washed twice in PBS-0.1% BSA and incubated with the secondary antibody (swine anti-rabbit-FITC or rabbit anti-mouse-FITC). The cells were washed twice and analyzed in a flow cytometer (FACScalibur; Becton Dickinson).
Antibody specificity.
The specificity of the anti-TLR4 monoclonal antibody was confirmed by using native HEK-293 cells lacking tlr4 (CRL-1573; American Type Culture Collection, Manassas, Va.) and the tlr4-transfected HEK-293 cell line (43). The specificity of the anti-CD14 antibodies was confirmed by using human peripheral blood monocytes. The mononuclear cells were isolated with Lymphoprep according to the manufacturer's instruction. Adherent cells were enriched by adsorption to a plastic slide, followed by washing three times to remove nonadherent cells. The monocytes were detached with PBS-EDTA and stained with anti-CD14 antibodies or an isotype-matched negative control antibody.
Quantitation of IL-6 and IL-8.
Secreted IL-8 was quantified by enzyme-linked immunosorbent assay with monoclonal mouse anti-human IL-8 IgG (1 to 5 μg/ml) as the primary antibody and polyclonal goat anti-mouse IgG conjugated to alkaline phosphatase as the secondary antibody. The lower detection limit of the assay was 7.5 pg/ml. IL-6 was quantified by enzyme-linked immunosorbent assay with monoclonal mouse anti-human IL-6 IgG (M16) (2 mg/ml) as the primary antibody and polyclonal goat anti-mouse IgG conjugated to biotin as the secondary antibody. The lower detection limit of this assay was 7.5 pg/ml.
Statistical analysis.
Fisher's exact test, the Spearman rank correlation test, and the unpaired t test were used. Differences were considered significant for P values of <0.05.
RESULTS
TLR4 expression in the human urinary tract mucosa.
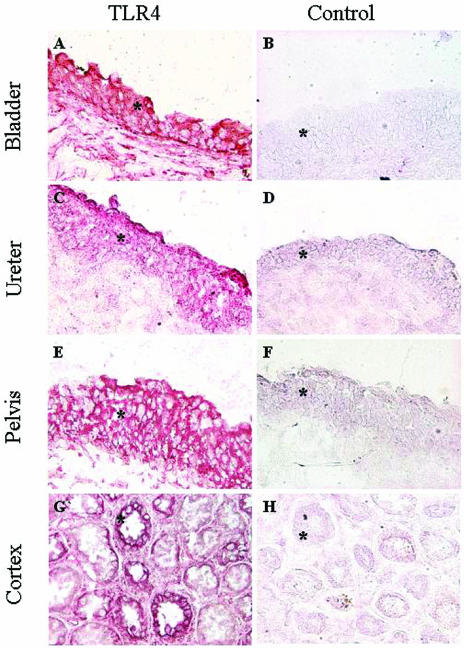
TLR4 expression in biopsies from five patients with sterile urine was examined. Sections were stained with a monoclonal anti-TLR4 antibody, and the results are shown in Fig. 1. TLR4 staining was detected throughout the epithelium, with the strongest staining in the cells closest to the lumen. Epithelial staining was observed in the urinary bladder, the ureter, and the renal pelvis. TLR4 staining was also detected in the tubular epithelium of the renal cortex and in the medulla of the kidney, but the tubular staining was heterogeneous, with stronger staining in some areas. In addition, some TLR4 staining was observed in subepithelial tissues and in the connective tissue surrounding the tubuli. Similar results were obtained with a polyclonal anti-TLR4 antibody (not shown).
FIG. 1.
TLR4 expression in the human urinary tract epithelium. (Left panels) Biopsies from the human urinary tract were stained with monoclonal anti-human TLR4 antibody (IMG-320) and visualized with Fast-Red substrate. TLR4 (red) was detected in the epithelium (asterisks) lining the bladder, ureter, pelvis, and cortex. (Right panels) The isotype control antibody did not stain the sections. Magnification, ×200.
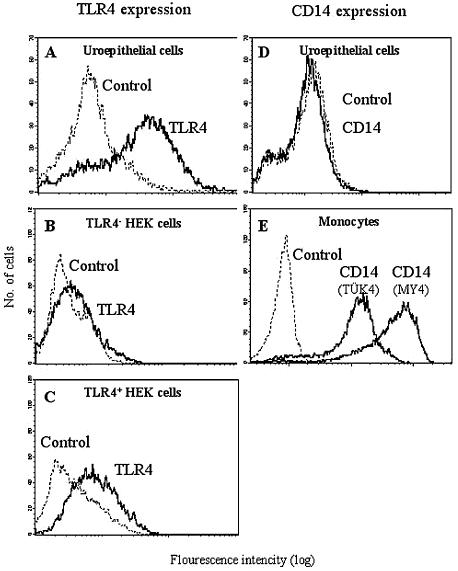
Epithelial TLR4 expression was confirmed by flow cytometry analysis of exfoliated cells in urine specimens from healthy individuals (Fig. 2A). The specificity of the anti-TLR4 antibodies was confirmed by comparing TLR4-negative HEK-293 cells with a tlr4-transfected cell line (Fig. 2B and C).
FIG. 2.
TLR4 and CD14 surface expression on excreted urinary tract epithelial cells. Urinary epithelial cells were harvested from healthy individuals, and the expression of TLR4 and CD14 was analyzed by flow cytometry. The shed cells stained strongly for TLR4 (A), but CD14 was not detected (D). Epithelial cells were identified by using the cytokeratin antibody MNF116 (not shown). The specificity of the anti-TLR4 antibody was confirmed by using HEK 293 cells that did not express tlr4 (B) and HEK 293 cells transfected with tlr4 (C). CD14-expressing blood monocytes were used as positive controls for anti-CD14 antibodies (E).
CD14 is not expressed in the urinary tract epithelium.
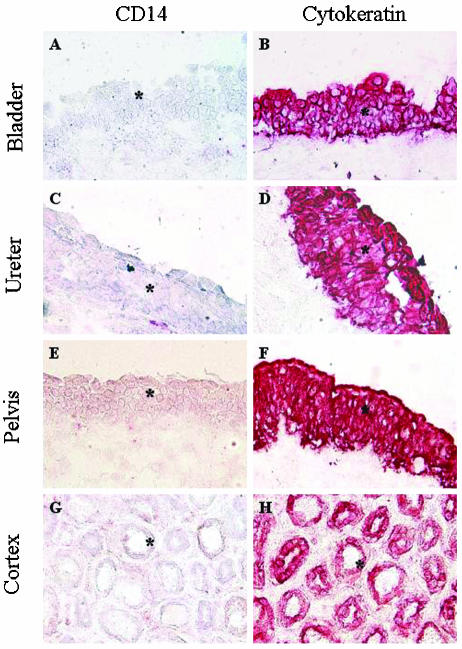
The tissue sections were subsequently stained for CD14, using two different monoclonal antibodies (clones TÜK4 [Fig. 3] and MY4 [data not shown]). CD14 was not detected in epithelial cells from any part of the urinary tract, but occasional cells in the subepithelial compartment were CD14 positive. The specificity of the anti-CD14 antibodies was confirmed by staining of peripheral blood monocytes (Fig. 2E).
FIG. 3.
CD14 is not expressed by the human urinary tract epithelium. (Left panels) Biopsies were stained with the anti-CD14 antibody TÜK4 (epithelium is marked by asterisks). CD14 was not detected in the epithelium of the bladder, ureter, renal pelvis, or cortex. Weak CD14 staining was observed between the tubuli (G), probably reflecting the presence of serum. Occasional CD14-positive myeloid cells were detected under the epithelium. Similar results were obtained by staining with the CD14-specific antibody MY4 (not shown). (Right panels) Control sections were stained with the epithelial cell cytokeratin antibody MNF116. Magnification, ×200.
To examine the effect of infection on epithelial CD14 expression, biopsies were challenged with a virulent E. coli strain for 6 h in vitro, sectioned, and examined for CD14 expression. There was no epithelial CD14 response in the infected biopsies (data not shown).
Mucosal cytokine profile in native biopsies.
Epithelial cytokine expression in the urinary bladder and renal pelvis was examined in serial sections of biopsies from patients with sterile urine or bacteriuria. The epithelial cytokine content was characterized by immunohistochemistry and quantified by counting of 200 epithelial cells in each sample (Table 1). Examples of epithelial staining are given in Fig. 4A to C. Preformed IL-8 was detected in the epithelial cells of all of the sterile biopsies, with a cellular frequency ranging from about 50 to 100%. Some individuals showed IL-1β staining, and one showed IL-6 staining in the renal pelvis, but there was no expression of IL-4 or IFN-γ.
TABLE 1.
Epithelial cytokine expression in urinary tract biopsies
| Urine | Biopsy location (n) | No. of positive samples/total (% cytokine-expressing epithelial cells in positive samples)
|
||||
|---|---|---|---|---|---|---|
| IL-1β | IL-6 | IL-8 | IL-4 | IFN-γ | ||
| Sterile | Bladder (8) | 2/8 (15-17.5) | 0/8 | 8/8 (49-100) | 0/8 | 0/8 |
| Pelvis (5) | 3/5 (15-100) | 1/5 (9) | 5/5 (44-100) | 0/5 | 0/5 | |
| ABU | Bladder (6)a | 6/6 (23.5-73) | 1/6 (17.5) | 6/6 (40-100) | 0/6 | 0/6c |
| Pelvis (4)b | 3/4 (34-100) | 1/4 (16) | 3/4 (44-100) | 0/4 | 0/4 | |
| P valued | <0.05 | NSe | NS | NS | NS | |
Two patients had gram-positive bacteriuria, and four had gram-negative bacteriuria.
One patient had gram-positive bacteriuria, and three had gram-negative bacteriuria.
One sample contained nonepithelial IFN-γ positive cells.
Sterile urine versus ABU.
NS, not significant.
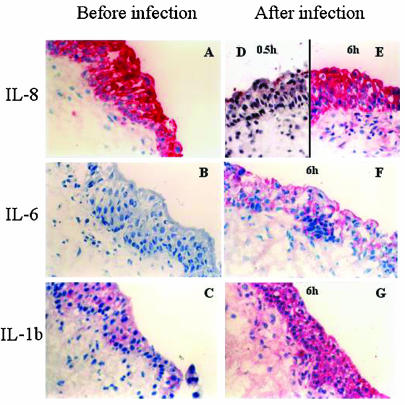
FIG. 4.
Epithelial cytokine response to bacterial challenge. Human biopsies were challenged with a uropathogenic E. coli strain. Sections were stained with monoclonal anti-IL-8 (A, D, and E), IL-6 (B and F), and IL-1β (C and G) antibodies. Tissue sections are shown before (left panels) or after (right panels) infection with E. coli Hu734. Magnification, ×200.
Biopsies were also collected from 10 patients who had significant bacteriuria at the time of surgery but no symptoms of infection. Five patients carried E. coli, and five carried gram-positive bacteria. The epithelial cytokine content of their biopsies is summarized in Table 1. All but one of the bacteriuric patients showed epithelial IL-8 staining, with a frequency of positive cells ranging from 40 to 100%. IL-6 was detected in only two biopsies, which also contained some inflammatory cells. IL-1β staining was detected in all but one of the biopsies from the bacteriuric patients (9 of 10, compared to 5 of 13 biopsies from patients with sterile urine [P < 0.05]). IFN-γ was detected in one infected patient, but these cells did not show epithelial morphology. IL-4 was not detected at all. Thus, except for IL-1β, there was no significant difference in epithelial cytokine content between biopsies from patients with sterile urine and those from patients with ABU.
Infection stimulates epithelial cytokine production.
The mucosal cytokine response to infection was examined by bacterial challenge of individual biopsies from the urinary bladder and renal pelvis, and the epithelial cytokine content was characterized by immunohistochemistry. The response to infection was defined as the change in epithelial cytokine content after bacterial challenge, by counting of 200 epithelial cells in each section (Fig. 4D to G and 5).
FIG. 5.
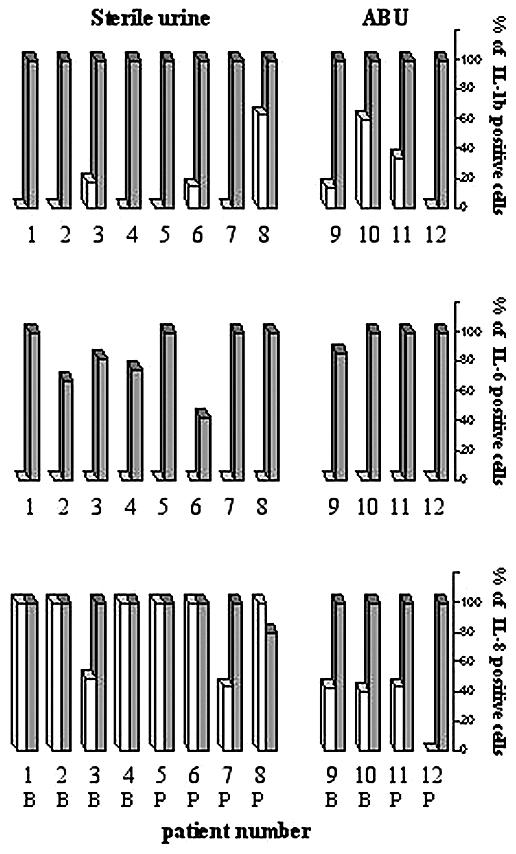
Cytokine response to in vitro infection of the biopsies. IL-1β-, IL-6-, and IL-8-positive epithelial cells were quantified before (white bars) and after (stippled bars) infection with E. coli Hu734. (The percentage of stained cells out of 200 epithelial cells scored is shown.) The biopsies were obtained from bladder (B) and pelvis (P).
The biopsies were first exposed to the uropathogenic E. coli strain Hu734. Prior to infection, IL-8 was present in the epithelial lining in all of the biopsies, with the most intense staining in the apical compartment of the epithelial cells. Shortly after infection (30 min), there was a decrease in the epithelial IL-8 content, suggesting that infection triggers IL-8 secretion (see example in Fig. 4D). After 6 h, IL-8 had accumulated in the epithelium, indicating de novo synthesis. The release of IL-8 was confirmed by analysis of cytokines in tissue supernatants obtained before infection and at 2 and 6 h after infection (P < 0.001 compared to the uninfected controls) (Table 2).
TABLE 2.
Epithelial cytokine response to in vitro challenge with E. coli
| Strain | Cytokine | h | Cytokine secretion (pg/ml)a
|
||
|---|---|---|---|---|---|
| Challenge | Controlb | P value | |||
| Hu734 | IL-6 | 0 | <50 | <50 | NSc |
| 2 | 612 ± 73 | 185 ± 13 | <0.001 | ||
| 6 | 2,692 ± 395 | 337 ± 24 | <0.001 | ||
| IL-8 | 0 | <50 | <50 | NS | |
| 2 | 934 ± 240 | 132 ± 7 | <0.001 | ||
| 6 | 11,460 ± 1,238 | 223 ± 10 | <0.001 | ||
| pap | IL-6 | 0 | <50 | <50 | NS |
| 2 | 1,060 ± 233 | 524 ± 194 | NS | ||
| 6 | 4,342 ± 152 | 998 ± 136 | <0.001 | ||
| IL-8 | 0 | 119 ± 36 | 53 ± 7 | NS | |
| 2 | 433 ± 132 | 235 ± 18 | 0.057 | ||
| 6 | 14,352 ± 647 | 2,059 ± 284 | <0.001 | ||
| fim | IL-6 | 0 | <50 | <50 | NS |
| 2 | 1,451 ± 307 | 494 ± 81 | 0.029 | ||
| 6 | 4,284 ± 190 | 994 ± 107 | <0.001 | ||
| IL-8 | 0 | 100 ± 4 | 51 ± 13 | 0.03 | |
| 2 | 1,538 ± 967 | 346 ± 37 | NS | ||
| 6 | 16,896 ± 744 | 2,673 ± 743 | <0.001 | ||
Cytokines in supernatants obtained at various times after in vitro challenge of the biopsies were quantified. Values are means ± standard errors of the means.
Control biopsies were incubated in RPMI. Controls for the pap+ and fim+ strains were pap- and fim-negative strains, respectively.
NS, not significant.
The epithelial IL-6 response to in vitro infection with virulent E. coli is shown in Fig. 4F and 5. IL-6 was detected within 2 h, and after 6 h, the epithelial IL-6 content had increased further. There was no preformed IL-6, but secretion increased with time after E. coli infection (Table 2) (P < 0.001 compared to the uninfected controls). Low-level IL-1β expression was detected in uninfected epithelium, but after in vitro infection, a strong increase in epithelial IL-1β was observed (Fig. 4C and G and 5). Release of IL-1β was not measured, but other studies have shown that this cytokine is not secreted from epithelial cells of the urinary tract (13, 21, 28). IL-4 and IFN-γ were not detected in the epithelium after in vitro infection (not shown).
No significant differences in the cytokine response to in vitro challenge could be seen between biopsies from the urinary bladder and renal pelvis or between biopsies from patients with sterile urine and ABU.
P and type 1 fimbriae amplify the mucosal cytokine response.
The biopsies were subjected to in vitro infection with recombinant E. coli strains differing in P or type 1 fimbrial expression. The fimbriated pap+ (Fig. 6) and fim+ (data not shown) strains caused strong and rapid IL-6 responses. Increases in epithelial staining were detected after 2 h, with further increases after 6 h. The nonfimbriated control strains caused a lower and delayed response.
FIG. 6.
Epithelial IL-6 response following in vitro infection with recombinant E. coli strains differing in fimbrial expression. Biopsies were infected with the P-fimbriated recombinant strain E. coli HB101(pPIL110-35) (pap+) (right panels). E. coli HB101 (pap) (left panels) was the nonfimbriated control. Magnification, ×200.
The results were confirmed by analysis of cytokine secretion in the supernatants from the biopsies infected with fimbriated bacteria. The kinetics are shown in Table 2. After 6 h there was a significant difference in the concentrations of IL-6 and IL-8 compared to control strains.
DISCUSSION
The urinary tract is frequently challenged by invading microorganisms, but in most individuals, sterility is rapidly regained. An efficient mucosal sensing machinery identifies the pathogens and recruits the innate antimicrobial defense. The epithelial cells, which form the critical barrier to microbial attack, are the first source of defense molecules, and the molecular interactions that determine epithelial cell activation are fundamental to pathogenesis. In the murine model, tlr4 has been shown to control epithelial responses to virulent E. coli bacteria (12, 17, 38). The present study examined the molecular basis for epithelial responses to infection in the urinary tract epithelium. TLR4 expression was detected in epithelial cells along the entire human urinary tract, thus providing the basis for TLR4 to control the innate mucosal response to infection also in humans.
As most uropathogens are gram negative, LPS would be the expected ligand/agonist triggering TLR4 signaling. Paradoxically, uroepithelial cells respond poorly to LPS challenge in vitro (26). The present study demonstrated that CD14 is absent from the human urinary tract epithelium, thus providing an explanation for the poor response both to soluble LPS and to avirulent strains of E. coli. This poor response to avirulent strains has been documented in large clinical studies. Most patients with ABU carry between 105 and 108 CFU/ml of urine, and still there is no evidence of mucosal inflammation. Fewer than 20% of the ABU strains express P fimbriae, and they adhere poorly to uroepithelial cells despite possessing the genetic machinery required to express type 1 fimbriae (11, 18, 19). Virulent strains, on the other hand, were able to activate a response, and fimbria-mediated adherence was one of the virulence factors needed to break the inertia of the mucosal barrier. Recent studies with the human urinary tract have confirmed this concept. P-fimbriated E. coli triggers a mucosal response, and host activation was shown to require the PapG tip adhesin. A PapG deletion mutant failed to stimulate cytokine responses and did not cause pyuria (4).
Epithelial cell responses to bacteria have been extensively investigated in cell lines and animal models, and partly conflicting results have been obtained (10, 23). The use of human urinary tract biopsies has numerous advantages over previous models. Most importantly, the biopsies provided information about the status of nontransformed cells in their normal tissue environment in the human host. While this model allows us to examine the very first response of the mucosa, it is not suitable for studies of later and more complex interactions involving recruited inflammatory cells and humoral factors. The biopsies were obtained during urinary tract surgery due to unrelated disorders, as biopsies may not be taken from healthy controls or patients with acute infection. All biopsies were carefully controlled to ensure that the sampled area was free of pathology. Furthermore, the functional integrity of the host response machinery was assessed by in vitro challenge of the biopsies with relevant bacterial strains. The biopsies permit the direct assessment of the epithelial response, in the absence of humoral factors, and thus isolate the mucosal compartment. The results show that human uroepithelial cells are armed with the molecular machinery needed to respond to invading pathogens, in the absence of circulating soluble or cellular factors. This initial response of the urinary tract mucosa sets the stage for the continued local and/or systemic inflammatory cascades (14, 39).
TLR4 expression by the human urinary tract epithelium has remained controversial. TLR4 is needed to activate mucosal inflammation in the murine UTI model (17), and TLR4 is expressed in certain human uroepithelial cell lines (12, 26), but some studies have failed to detect TLR4 in the kidney epithelium (3). The present study resolved this controversy by examining TLR4 expression in mucosal biopsies from the human urinary tract, showing that TLR4 is expressed in the epithelium and in renal tubular cells. A second unresolved question concerns epithelial CD14 expression. Early studies failed to detect CD14 in the kidney epithelial cell line A498, and the cells were shown to respond poorly to soluble LPS (23, 26). More recently, CD14 was detected in bladder cell lines, and LPS was proposed to trigger the mucosal response to urinary tract infection via the CD14-dependent TLR4 pathway (37). The present study showed that CD14 is absent from the human urinary tract epithelium and that infection fails to trigger CD14 expression in those cells.
The lack of membrane-bound CD14 in the epithelium is consistent with the nonmyeloid origin of those cells. Furthermore, the epithelium was shown to respond to E. coli challenge also in the absence of CD14. These results do not exclude the involvement of CD14 in mucosal responses, however. The soluble form of CD14 has been shown to enhance epithelial LPS responses in vitro (2, 40). sCD14 may be secreted into the urine during infection and may enhance the continued in vivo response to infection (2, 7). Bussolati et al. (7) showed that urinary LPS-CD14 complexes from proteinuric patients could activate a primary kidney epithelial cell line, and LPS was shown to activate epithelial cell lines in vitro in the presence of both sCD14 and LBP. It remains to be studied whether the concentration of sCD14 and LPS-binding protein in the urine of uninfected patients is sufficient for an LPS-LBP-CD14-dependent signal to occur. As the concentration of sCD14 increases after infection, the CD14-dependent pathways may take over during later stages of infection.
P or type 1 fimbriae were shown to enhance the epithelial cytokine response of the human biopsies. This is consistent with in vitro studies in human cell lines and with results from the murine UTI model and confirms the effect of adherence on epithelial cell activation (12, 15, 23, 26). In contrast, the mucosa was quite inert to the nonfimbriated control strains. Two mechanisms have been offered to explain how fimbria-mediated adherence can break mucosal inertia. One involves TLR4 activation through modification of the recognition receptor for the fimbriae (25). The second involves fimbriae as presenters of LPS, which compensate for the lack of membrane-bound CD14 by delivering LPS in the proximity of TLR4. This mechanism was previously examined by mutational detoxification of lipid A in P- or type 1-fimbriated E. coli strains (24, 26). msbB mutations did not reduce the response to P-fimbriated bacteria, suggesting that TLR4 activation is through a partly LPS-independent mechanism. By using the same experimental approach, type 1 fimbriae were shown to differ from P fimbriae in that they can constitute LPS delivery vehicles (24). These experiments illustrate the diversity of molecular cross talk between pathogens and epithelial cells.
The present study further emphasizes the difference in the handling of gram-negative bacteria between the mucosal and the systemic compartments. Macrophages and other TLR4/CD14-positive cells respond promptly to LPS (42) but are less able than the epithelial cells to discriminate between more or less pathogenic gram-negative bacteria. Such indiscriminate recognition may be desirable in the systemic compartment, where the main defense function is to maintain tissue sterility at any cost and where the presence of even the least virulent organism poses a potential threat. Epithelial cells, on the other hand, encounter a diverse gram-negative flora and need to separate LPS-bearing, nonpathogenic strains from the pathogens. The presence of TLR4 and the lack of membrane CD14 may explain why ABU strains often fail to trigger a mucosal host response in the human urinary tract, despite their LPS content.
Acknowledgments
We thank I. Lindley and M. Ceska (Sandoz, Vienna, Austria) for kindly supplying recombinant IL-8, monoclonal mouse anti-human IL-8, and polyclonal rabbit anti-human IL-8 antibody and Christina Andersson, Department of Pathology, Lund University, for the diagnosis of tissues.
This work was supported by the Medical Faculty, University of Lund; the Swedish Medical Research Council (grant 7934); the program Glycoconjugates in Biological Systems, sponsored by the Swedish Foundation for Strategic Research; the Österlund, Crawford, and Lundberg Foundations; the Royal Physiographic Society; the Finnish Cultural Foundation; and the Finnish Academy. C.S. is a recipient of a Bristol Myer's Squibb unrestricted grant.
Editor: A. D. O'Brien
REFERENCES
- 1.Agace, W. W., S. R. Hedges, M. Ceska, and C. Svanborg. 1993. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J. Clin. Investig. 92:780-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed, F., L. Meijer, S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14. Cell Microbiol. 4:493-501. [DOI] [PubMed] [Google Scholar]
- 3.Backhed, F., M. Soderhall, P. Ekman, S. Normark, and A. Richter-Dahlfors. 2001. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell Microbiol. 3:153-158. [DOI] [PubMed] [Google Scholar]
- 4.Bergsten, G., M. Samuelsson, B. Wullt, I. Leijonhufvud, H. Fischer, and C. Svanborg. PapG dependent adhesion breaks mucosal inertia and triggers the innate host response. J. Infect. Dis., in press. [DOI] [PubMed]
- 5.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 6.Bleves, S., and G. R. Cornelis. 2000. How to survive in the host: the Yersinia lesson. Microbes Infect. 2:1451-1460. [DOI] [PubMed] [Google Scholar]
- 7.Bussolati, B., S. David, V. Cambi, P. S. Tobias, and G. Camussi. 2002. Urinary soluble CD14 mediates human proximal tubular epithelial cell injury induced by LPS. Int. J. Mol. Med. 10:441-449. [PubMed] [Google Scholar]
- 8.Cossart, P., and H. Bierne. 2001. The use of host cell machinery in the pathogenesis of Listeria monocytogenes. Curr. Opin. Immunol. 13:96-103. [DOI] [PubMed] [Google Scholar]
- 9.de Man, P., C. van Kooten, L. Aarden, I. Engberg, H. Linder, and C. Svanborg Eden. 1989. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect. Immun. 57:3383-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckmann, L., H. C. Jung, C. Schurer-Maly, A. Panja, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1993. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 105:1689-1697. [DOI] [PubMed] [Google Scholar]
- 11.Eden, C. S., L. A. Hanson, U. Jodal, U. Lindberg, and A. S. Akerlund. 1976. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet i:490-492. [PubMed] [Google Scholar]
- 12.Frendeus, B., C. Wachtler, M. Hedlund, H. Fischer, P. Samuelsson, M. Svensson, and C. Svanborg. 2001. Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol. Microbiol. 40:37-51. [DOI] [PubMed] [Google Scholar]
- 13.Funfstuck, R., S. Franke, M. Hellberg, U. Ott, B. Knofel, E. Straube, M. Sommer, and J. Hacker. 2001. Secretion of cytokines by uroepithelial cells stimulated by Escherichia coli and Citrobacter spp. Int. J. Antimicrob. Agents 17:253-258. [DOI] [PubMed] [Google Scholar]
- 14.Godaly, G., G. Bergsten, L. Hang, H. Fischer, B. Frendeus, A. C. Lundstedt, M. Samuelsson, P. Samuelsson, and C. Svanborg. 2001. Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection. J. Leukoc. Biol. 69:899-906. [PubMed] [Google Scholar]
- 15.Godaly, G., B. Frendeus, A. Proudfoot, M. Svensson, P. Klemm, and C. Svanborg. 1998. Role of fimbriae-mediated adherence for neutrophil migration across Escherichia coli-infected epithelial cell layers. Mol. Microbiol. 30:725-735. [DOI] [PubMed] [Google Scholar]
- 16.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg Eden. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagberg, L., R. Hull, S. Hull, J. R. McGhee, S. M. Michalek, and C. Svanborg Eden. 1984. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46:839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagberg, L., U. Jodal, T. K. Korhonen, G. Lidin-Janson, U. Lindberg, and C. Svanborg Eden. 1981. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect. Immun. 31:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson, S., D. Caugant, U. Jodal, and C. Svanborg-Eden. 1989. Untreated asymptomatic bacteriuria in girls. I. Stability of urinary isolates. Br. Med. J. 298:853-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haraoka, M., L. Hang, B. Frendeus, G. Godaly, M. Burdick, R. Strieter, and C. Svanborg. 1999. Neutrophil recruitment and resistance to urinary tract infection. J Infect. Dis. 180:1220-1229. [DOI] [PubMed] [Google Scholar]
- 21.Hedges, S., W. Agace, M. Svensson, A. C. Sjogren, M. Ceska, and C. Svanborg. 1994. Uroepithelial cells are part of a mucosal cytokine network. Infect. Immun. 62:2315-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges, S., K. Stenqvist, G. Lidin-Janson, J. Martinell, T. Sandberg, and C. Svanborg. 1992. Comparison of urine and serum concentrations of interleukin-6 in women with acute pyelonephritis or asymptomatic bacteriuria. J Infect. Dis. 166:653-656. [DOI] [PubMed] [Google Scholar]
- 23.Hedges, S., M. Svensson, and C. Svanborg. 1992. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect. Immun. 60:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedlund, M., B. Frendeus, C. Wachtler, L. Hang, H. Fischer, and C. Svanborg. 2001. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39:542-552. [DOI] [PubMed] [Google Scholar]
- 25.Hedlund, M., M. Svensson, A. Nilsson, R. D. Duan, and C. Svanborg. 1996. Role of the ceramide-signaling pathway in cytokine responses to P-fimbriated Escherichia coli. J. Exp. Med. 183:1037-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedlund, M., C. Wachtler, E. Johansson, L. Hang, J. E. Somerville, R. P. Darveau, and C. Svanborg. 1999. P fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine responses. Mol. Microbiol. 33:693-703. [DOI] [PubMed] [Google Scholar]
- 27.Klemm, P., B. J. Jorgensen, I. van Die, H. de Ree, and H. Bergmans. 1985. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli, cloning and genetic organization. Mol. Gen. Genet. 199:410-414. [DOI] [PubMed] [Google Scholar]
- 28.Ko, Y. C., N. Mukaida, S. Ishiyama, A. Tokue, T. Kawai, K. Matsushima, and T. Kasahara. 1993. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect. Immun. 61:1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffler, H., and C. Svanborg-Edén. 1980. Chemical identification of a glycosphingolipid receptor for Escherichia coli attaching to human urinary tract epithelial cells and agglutinating human erythrocytes. FEMS Microbiol. Lett. 8:127-134. [Google Scholar]
- 30.Ofek, I., D. Mirelman, and N. Sharon. 1977. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature 265:623-625. [DOI] [PubMed] [Google Scholar]
- 31.Olszyna, D. P., S. M. Opal, J. M. Prins, D. L. Horn, P. Speelman, S. J. van Deventer, and T. van der Poll. 2000. Chemotactic activity of CXC chemokines interleukin-8, growth-related oncogene-alpha, and epithelial cell-derived neutrophil-activating protein-78 in urine of patients with urosepsis. J. Infect. Dis. 182:1731-1737. [DOI] [PubMed] [Google Scholar]
- 32.Olszyna, D. P., J. M. Prins, P. E. Dekkers, E. De Jonge, P. Speelman, S. J. Van Deventer, and T. Van Der Poll. 1999. Sequential measurements of chemokines in urosepsis and experimental endotoxemia. J. Clin. Immunol. 19:399-405. [DOI] [PubMed] [Google Scholar]
- 33.Otto, G., J. Braconier, A. Andreasson, and C. Svanborg. 1999. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J. Infect. Dis. 179:172-179. [DOI] [PubMed] [Google Scholar]
- 34.Sander, B., J. Andersson, and U. Andersson. 1991. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol. Rev. 119:65-93. [DOI] [PubMed] [Google Scholar]
- 35.Sansonetti, P. 2001. Phagocytosis of bacterial pathogens: implications in the host response. Semin. Immunol. 13:381-390. [DOI] [PubMed] [Google Scholar]
- 36.Schilling, J. D., S. M. Martin, C. S. Hung, R. G. Lorenz, and S. J. Hultgren. 2003. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 100:4203-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schilling, J. D., S. M. Martin, D. A. Hunstad, K. P. Patel, M. A. Mulvey, S. S. Justice, R. G. Lorenz, and S. J. Hultgren. 2003. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect. Immun. 71:1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahin, R. D., I. Engberg, L. Hagberg, and C. Svanborg Eden. 1987. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J. Immunol. 138:3475-3480. [PubMed] [Google Scholar]
- 39.Svanborg, C., G. Godaly, and M. Hedlund. 1999. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 2:99-105. [DOI] [PubMed] [Google Scholar]
- 40.Thieblemont, N., and S. D. Wright. 1999. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J. Exp. Med. 190:523-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Die, I., H. I. van den Hondel, H. W. Hoekstrer, and H. Bergmans. 1983. Studies on fimbriae of an Escherichia coli O6:K2:H1:F7strain: molecular cloning of a DNA fragment encoding a fimbriae antigen responsible for mannose-resistant hemagglutination of human erythrocytes. FEMS Microbiol. Lett. 19:77-92. [Google Scholar]
- 42.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 43.Yang, H., D. W. Young, F. Gusovsky, and J. C. Chow. 2000. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J. Biol. Chem. 275:20861-20866. [DOI] [PubMed] [Google Scholar]