Abstract
Numerous inflammatory cells are recruited in response to Cryptosporidium parvum infection. These cells include interferon gamma-producing T lymphocytes, which are of major importance for the resolution of infection. Here, we show that β7 integrin is not essential for the control of infection in mice but that β7-deficient neonatal mice are more susceptible during the early stages of infection.
Cryptosporidium parvum is a protozoan parasite that infects intestinal epithelial cells. In mammals, including humans and domestic animals, C. parvum infection causes acute watery diarrhea and weight loss. The severity of the infection depends on the immune status of the host. Neonatal mice, which have an immature immune system, are infected by the parasite but recover naturally within about 3 weeks. Immunocompetent adult mice are, on the other hand, resistant to severe C. parvum infection unless they are immunodeficient, like SCID mice or knockout mice (which lack CD40, CD154, gamma interferon [IFN-γ]) (6). This highlights the importance of immune system development in young animals and of the recruitment of key cells, such as IFN-γ-producing T cells, involved in protection processes.
The homing of lymphocytes to normal tissues and sites of inflammation is partly regulated by the differential expression of cell surface homing receptors and their selective interactions with tissue-selective vascular adhesion molecules at sites of lymphocyte recruitment from the blood (1). In mice, lymphocyte homing to the intestinal lamina propria involves a single-chain 60-kDa glycoprotein, the mucosal addressin cell adhesion molecule 1 (MAdCAM-1). The heterodimeric α4β7 integrin acts on leukocytes as a ligand for MAdCAM-1 (16). In the case of intestinal inflammation, MAdCAM-1 expression increases and is thought to allow inflammatory cells to reach the site of inflammation (5). Another member of the β7 integrin family, αEβ7, is produced in large quantities by more than 90% of intraepithelial lymphocytes (IEL) (15). αEβ7 mediates their adherence to and retention in the intestinal epithelium by interacting with E-cadherin. In addition to the high expression of the α4β7 integrin, effector T cells homing to the small intestine also express high levels of the chemokine receptor CCR9 (2), whose ligand CCL25 is selectively secreted by small intestine epithelial cells (10). β7 integrins and CCL25 are important for T-cell localization to intestinal effector sites, as β7-deficient T cells are severely impaired in their ability to localize to the intestinal mucosa and as neutralizing antibodies to CCL25 partially block T-cell localization to the small intestine epithelium (13, 17). A previous study examined the expression of chemokines in the mucosa of C. parvum-infected neonates and underlined the importance of the broad spectrum of chemokines released upon the recruitment and activation of T lymphocytes (11). Here, we addressed the importance of the β7 integrin in the selective recruitment of lymphocytes to the intestinal mucosa and the subsequent control of infection.
C. parvum, which was initially isolated from an infected child, was maintained by regular passage in newborn calves and was purified by filtration and diethyl ether sedimentation as described previously (12). C57BL/6 β7-deficient mice (β7−/−) and wild-type C57BL/6 mice were kindly provided by Norbert Wagner (19). The dams and their pups were housed in individual cages under pathogen-free conditions. Food and water were available ad libitum. Three-day-old pups were orally infected with 106 oocysts and were killed 4, 9, or 16 days postinfection (p.i.) for immunofluorescence staining or intestinal C. parvum oocyst quantification. Immunofluorescence staining was performed as described previously (11). Briefly, 7-μm-thick acetone-fixed frozen ileum sections were incubated with a rat anti-mouse β7 integrin monoclonal antibody (Pharmingen, San Diego, Calif.) and then with a monoclonal anti-rat biotin conjugate (Sigma, Saint Quentin Fallavier, France), and finally they were incubated with an ExtrAvidin fluorescein isothiocyanate conjugate (Sigma).
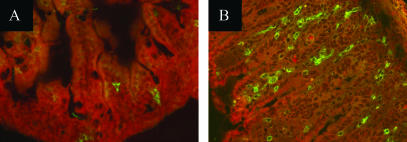
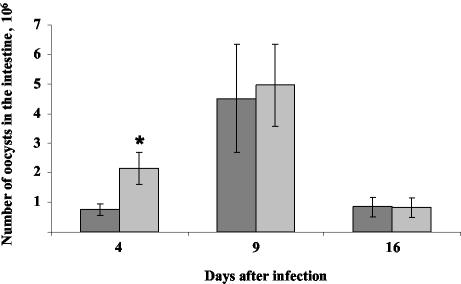
Following C. parvum infection, β7-integrin-positive cells were observed in the villi and at intraepithelial locations in the mucosa of infected wild-type neonates (Fig. 1). This reflects the recruitment of activated T (11) and B lymphocytes and possibly NK cells bearing the β7 integrin. We next investigated the effect of the absence of the β7 integrin on the level of infection at different times following infection. Whole intestines of neonates were removed and individually homogenized in 1 ml of water with an Ultra-turrax. Oocysts were quantified in Sheather's solution by using a Thoma cell. On day 4 p.i., β7−/− mice contained about threefold more oocysts than wild-type mice, whereas no difference was observed at later time points (Fig. 2). This experiment was repeated four times, with similar results each time (Table 1). These data suggest that although β7 integrin plays a role, its absence is rapidly compensated for by alternative mechanisms, allowing the normal resolution of infection by neonates. Fujimori et al. recently showed, by using an intravital microscope, that the accumulation of lamina propria lymphocytes in villus tips is almost completely inhibited by anti-β7 integrin antibodies (3). The importance of the β7 integrin in other enteric diseases affecting neonates, such as rotavirus disease, has also been investigated. Adoptive transfer experiments into Rag-2-deficient mice showed that the B (9) and CD8+ T cells that protect against rotavirus are members of the α4β7 high population (8). However, β7−/− mice can clear primary rotavirus infection as quickly as wild-type animals. Depletion and transfer experiments select cell populations, whereas experiments using β7-deficient individuals help to show the importance of the integrin. However, the cells that usually bear the integrin are still present and functional in β7−/− mice. Other groups have reported similar findings, showing the discrepancy obtained in experiments using monoclonal antibodies blocking β7 integrin and β7−/− mice (7, 18). It is possible that the β7 antibodies used cross-reacted with molecules other than the β7 integrin. This apparent contradiction may also be explained by the existence of a β7-independent mechanism responsible for lymphocyte migration to and/or retention in the intestine. In neonates, in which the immune system is immature (4), the absence of the β7 integrin is more crucial for the first step of the immune response and leads to significantly increased parasite development. Accordingly, wild-type adult β7−/− mice do not develop significant infection (data not shown). Therefore, β7 integrin is not essential for the control of C. parvum infection.
FIG. 1.
C. parvum induces the recruitment of β7-integrin-positive cells in the ileum. Immunofluorescence in 7-μm frozen ileum sections showing β7 integrin expression in wild-type neonates on day 9 p.i. (B) and their age-matched controls (A). Three-day-old mice were infected with 106 oocysts. Sections were counterstained with Evans blue. Magnification, 200×.
FIG. 2.
Course of infection in wild-type and β7−/− neonate mice infected with C. parvum. Three-day-old wild-type (gray) and β7−/− (light gray) mice were inoculated with 106 oocysts and were sacrificed 4, 9, or 16 days later. The parasite load in the intestine was then determined. Each point represents the mean ± standard deviation of the number of oocysts (n = 6 to 9). A significant difference was observed at day 4 p.i. (*, P < 0.01 [Mann-Whitney test]).
TABLE 1.
Effect of β7 integrin on parasite load in intestine of wild-type and β7−/− neonates at day 4 p.i.
| Expt no. | No. of oocysts in the intestine on day 4 p.i. (105)a
|
Fold increase | |
|---|---|---|---|
| Wild-type neonate | β7−/− neonate | ||
| 1 | 7.65 ± 1.97 (n = 5) | 21.54 ± 5.29** (n = 6) | 2.81 |
| 2 | 7.97 ± 8.02 (n = 12) | 14.61 ± 5.82** (n = 16) | 1.83 |
| 3 | 6.19 ± 5.08 (n = 21) | 10.28 ± 4.95** (n = 34) | 1.66 |
| 4 | 4.89 ± 3.82 (n = 13) | 10.63 ± 8.17* (n = 27) | 2.17 |
P values were calculated by using the Mann-Whitney test (**, P < 0.01; *, P < 0.05).
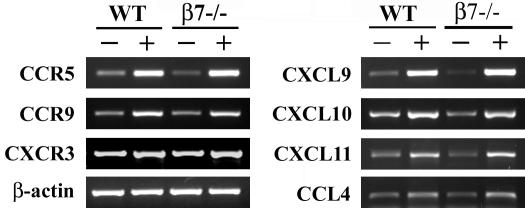
β7 deficiency results in a defect in the recruitment and/or retention of T cells in the absence of infection (19). We therefore studied the influence of β7 deficiency on T-lymphocyte recruitment during C. parvum infection, as T cells are important for the control of cryptosporidiosis (14). Immunohistochemistry is not an appropriate method for following the recruitment of T cells in the mucosa, because the infection is not homogeneously spread in the ileum in the early stage of infection and because very few CD3+ cells can be observed on 7-day-old neonates infected 4 days earlier (data not shown). Thus, we decided to use quantitative real-time PCR (RT-PCR) to measure the amount of CD3-γ chain mRNA present in the ileum. Ilea from neonates were individually crushed in 1 ml of Trizol (Gibco-BRL, Life Technology, Cergy Pontoise, France) with an Ultra-turrax. RNA was then purified as recommended by the supplier. CD3-γ chain mRNA was quantified on a Light-Cycler (Roche Biochemicals, Mannheim, Germany) with the SYBR Green dye and the Titanium Taq DNA polymerase (Roche). The sequences of the primers are indicated in Table 2. The experiment was performed three times on pools of RNA from neonates from different litters (for each pool, n = 6). Values from one representative experiment are given after normalization to β-actin. Ilea of 7-day-old uninfected β7−/− neonates contained 2.18 times less CD3-γ mRNA than did those of wild-type neonates. This is consistent with the defect in the recruitment and/or retention of T cells observed by Wagner et al. for β7−/− mice (19). In contrast, 4 days p.i. the ilea of 7-day-old infected β7−/− neonates contained 2.20 times more CD3-γ mRNA than did those of wild-type neonates, suggesting that the number of CD3-γ-positive cells in the ileum reflects the severity of C. parvum infection. This hypothesis is strengthened by the fact that at 9 days p.i., wild-type and β7−/− neonates infected with similar amounts of parasites (Fig. 2) contained equal amounts of CD3-γ mRNA. During inflammation, CD3+ cells are therefore recruited and/or retained effectively in the mucosa by a β7-integrin-independent mechanism. The nature of the alternative mechanism for the recruitment and/or retention of lymphocytes in the absence of β7 integrin is unknown but may involve chemokine receptors present on Th1-type lymphocytes generated during C. parvum infection. The increase of chemokine mRNA expression as soon as 4 days after C. parvum infection was previously shown by quantitative RT-PCR (11). These chemokines include CXCL9, CXCL10, and CXCL11, all of which bind to CXCR3, and CCL4, which binds to CCR5 (11). The CCR9 chemokine receptor is strongly expressed and is associated with α4β7-positive intestine-homing T cells (20), and CCL25, its ligand, is selectively and constitutively expressed in the small intestine (10). CCR9, CXCR3, and CCR5 are therefore prime candidates for controlling tissue-specific T-lymphocyte recruitment in the C. parvum-infected mucosa. To evaluate the effect of infection on the presence of cells bearing chemokine receptors, we carried out RT-PCR on the same samples used to quantify CD3-γ chain expression. Our results suggest that CCR5-, CCR9-, and, to a lesser extent, CXCR3-positive cells are recruited during C. parvum infection (Fig. 3). This recruitment is most probably due to chemokines released in the infected mucosa. In both wild-type and β7−/− neonates, we observed a strong increase of mRNA expression for CCL4 and the IFN-γ-induced chemokines CXCL9, CXCL10, and CXCL11 (Fig. 3), whereas CCL25 expression is constitutive (data not shown).
TABLE 2.
Primer sequences used for PCR analysisa
| Target mRNA | Accession no. | Sense primer | Antisense primer | Size of PCR products (bp) |
|---|---|---|---|---|
| CD3-γ | BC027528 | 5′-TCTGGGCAACAATGCCAAAGAC-3′ | 5′-CCTCTCTGCTTGCAGTCTACC-3′ | 449 |
| CCR5 | AF022990 | 5′-ACCTTCCAGGAATTCTTTGGACT-3′ | 5′-TATACCCGATCCACAGGAGAAC-3′ | 261 |
| CCR9 | AJ131357 | 5′-GTCAGCTGTCTTGATCCTGAAG-3′ | 5′-CATAGAGAACTGGGTTCAGACAA-3′ | 320 |
| CXCR3 | AF045146 | 5′-CTGCTGGTCTCCAGAGGCCA-3′ | 5′-GTCAGTGCATCCTGGCAGCAAA-3′ | 501 |
The sequences of the primers used to amplify β-actin, CCL4, CXCL9, CXCL10, and CXCL11 have been previously described (11).
FIG. 3.
Increased chemokine and chemokine receptor mRNA expression in the C. parvum-infected ileum. Three-day-old wild-type (WT) and β7−/− mice were infected with 106 oocysts. RNA was extracted from the ileum of neonates 4 days p.i. (+) and from their age-matched controls (−), and RT-PCRs were performed on pools of RNA samples from neonates of different litters (n = 6). Data are from one representative experiment. Number of PCR cycles for each chemokine: CCR5, 30; CCR9 and CXCR3, 32; β-actin, 25; CXCL9, CXCL10, CXCL11, and CCL4, 35.
In conclusion, β7 integrin is not essential for the control of infection in mice, but β7−/− neonates are more susceptible to the early stages of C. parvum infection than their wild-type counterparts. In the absence of β7 integrin, the recruitment of lymphocytes in the infected mucosa may be sufficiently driven by the interaction between the chemokines released by intestinal epithelial cells and inflammatory cells and the chemokine receptors present on the surface of lymphocytes.
Acknowledgments
We thank Norbert Wagner (Institute for Genetics, University of Cologne, Germany) for kindly providing β7−/− mice and Jean Lorieux for mouse breeding.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Brandtzaeg, P., I. N. Farstad, and G. Haraldsen. 1999. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol. Today 20:267-277. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, D. J., and E. C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimori, H., S. Miura, S. Koseki, R. Hokari, S. Komoto, Y. Hara, S. Hachimura, S. Kaminogawa, and H. Ishii. 2002. Intravital observation of adhesion of lamina propria lymphocytes to microvessels of small intestine in mice. Gastroenterology 122:734-744. [DOI] [PubMed] [Google Scholar]
- 4.Garcia, A. M., S. A. Fadel, S. Cao, and M. Sarzotti. 2000. T cell immunity in neonates. Immunol. Res. 22:177-190. [DOI] [PubMed] [Google Scholar]
- 5.Hatanaka, K., R. Hokari, K. Matsuzaki, S. Kato, A. Kawaguchi, S. Nagao, H. Suzuki, K. Miyazaki, E. Sekizuka, H. Nagata, H. Ishii, and S. Miura. 2002. Increased expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and lymphocyte recruitment in murine gastritis induced by Helicobacter pylori. Clin. Exp. Immunol. 130:183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayward, A. R., M. Cosyns, M. Jones, and E. M. Ponnuraj. 2001. Marrow-derived CD40-positive cells are required for mice to clear Cryptosporidium parvum infection. Infect. Immun. 69:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellersmann, R., A. Lazarovits, D. Grant, B. Garcia, B. Chan, A. Kellersmann, H. Wang, A. Jevnikar, N. Wagner, W. Muller, K. Ulrichs, A. Thiede, and R. Zhong. 2002. Monoclonal antibody against β7 integrins, but not β7 deficiency, attenuates intestinal allograft rejection in mice. Transplantation 74:1327-1334. [DOI] [PubMed] [Google Scholar]
- 8.Kuklin, N. A., L. Rott, J. Darling, J. J. Campbell, M. Franco, N. Feng, W. Muller, N. Wagner, J. Altman, E. C. Butcher, and H. B. Greenberg. 2000. α(4)β(7) independent pathway for CD8(+) T cell-mediated intestinal immunity to rotavirus. J. Clin. Investig. 106:1541-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuklin, N. A., L. Rott, N. Feng, M. E. Conner, N. Wagner, W. Muller, and H. B. Greenberg. 2001. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J. Immunol. 166:1894-1902. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel, E. J., J. J. Campbell, G. Haraldsen, J. Pan, J. Boisvert, A. I. Roberts, E. C. Ebert, M. A. Vierra, S. B. Goodman, M. C. Genovese, A. J. Wardlaw, H. B. Greenberg, C. M. Parker, E. C. Butcher, D. P. Andrew, and W. W. Agace. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacroix-Lamande, S., R. Mancassola, M. Naciri, and F. Laurent. 2002. Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect. Immun. 70:2090-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent, F., L. Eckmann, T. C. Savidge, G. Morgan, C. Theodos, M. Naciri, and M. F. Kagnoff. 1997. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect. Immun. 65:5067-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefrancois, L., C. M. Parker, S. Olson, W. Muller, N. Wagner, M. P. Schon, and L. Puddington. 1999. The role of β7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald, V. 2000. Host cell-mediated responses to infection with Cryptosporidium. Parasite Immunol. 22:597-604. [DOI] [PubMed] [Google Scholar]
- 15.Schon, M. P., A. Arya, E. A. Murphy, C. M. Adams, U. G. Strauch, W. W. Agace, J. Marsal, J. P. Donohue, H. Her, D. R. Beier, S. Olson, L. Lefrancois, M. B. Brenner, M. J. Grusby, and C. M. Parker. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J. Immunol. 162:6641-6649. [PubMed] [Google Scholar]
- 16.Shaw, S. K., and M. B. Brenner. 1995. The beta 7 integrins in mucosal homing and retention. Semin. Immunol. 7:335-342. [DOI] [PubMed] [Google Scholar]
- 17.Svensson, M., J. Marsal, A. Ericsson, L. Carramolino, T. Broden, G. Marquez, and W. W. Agace. 2002. CCL25 mediates the localization of recently activated CD8αβ(+) lymphocytes to the small-intestinal mucosa. J. Clin. Investig. 110:1113-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sydora, B. C., N. Wagner, J. Lohler, G. Yakoub, M. Kronenberg, W. Muller, and R. Aranda. 2002. β7 integrin expression is not required for the localization of T cells to the intestine and colitis pathogenesis. Clin. Exp. Immunol. 129:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner, N., J. Lohler, E. J. Kunkel, K. Ley, E. Leung, G. Krissansen, K. Rajewsky, and W. Muller. 1996. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature 382:366-370. [DOI] [PubMed] [Google Scholar]
- 20.Zabel, B. A., W. W. Agace, J. J. Campbell, H. M. Heath, D. Parent, A. I. Roberts, E. C. Ebert, N. Kassam, S. Qin, M. Zovko, G. J. LaRosa, L. L. Yang, D. Soler, E. C. Butcher, P. D. Ponath, C. M. Parker, and D. P. Andrew. 1999. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]