Abstract
Fulminant meningococcal sepsis has been termed the prototypical lipopolysaccharide (LPS)-mediated gram-negative septic shock. Systemic inflammation by activated complement and cytokines is important in the pathogenesis of this disease. We investigated the involvement of meningococcal LPS in complement activation, complement-dependent inflammatory effects, and cytokine or chemokine production. Whole blood anticoagulated with lepirudin was stimulated with wild-type Neisseria meningitidis H44/76 (LPS+), LPS-deficient N. meningitidis H44/76lpxA (LPS−), or purified meningococcal LPS (NmLPS) at concentrations that were relevant to meningococcal sepsis. Complement activation products, chemokines, and cytokines were measured by enzyme-linked immunosorbent assays, and granulocyte CR3 (CD11b/CD18) upregulation and oxidative burst were measured by flow cytometry. The LPS+ and LPS− N. meningitidis strains both activated complement effectively and to comparable extents. Purified NmLPS, used at a concentration matched to the amount present in whole bacteria, did not induce any complement activation. Both CR3 upregulation and oxidative burst were also induced, independent of LPS. Interleukin-1β (IL-1β), tumor necrosis factor alpha, and macrophage inflammatory protein 1α production was predominantly dependent on LPS, in contrast to IL-8 production, which was also markedly induced by the LPS− meningococci. In this whole blood model of meningococcal sepsis, complement activation and the immediate complement-dependent inflammatory effects of CR3 upregulation and oxidative burst occurred independent of LPS.
Invasive disease caused by the gram-negative bacterium Neisseria meningitidis is a life-threatening infection in children and young adults. Meningococcal septic shock (or fulminant meningococcal sepsis [FMS]), the prototype of overwhelming gram-negative sepsis, is feared especially because it is able to cause devastating disease with a high case fatality rate. The pathogenic mechanisms leading to FMS are thought to be the uncontrolled growth of meningococci in the circulation, resulting in a massive activation of diverse inflammatory systems, such as the complement system, the cytokine network, and the coagulation cascade (6, 35).
In FMS, high lipopolysaccharide (LPS) levels are correlated with an increased bacterial load, high levels of pro- and anti-inflammatory cytokines, increased complement activation, the induction of intravascular coagulation, and a poor outcome. Therefore, meningococcal LPS is regarded as the principal bacterial pathogenic element during FMS (3).
The complement cascade plays a dual role in the pathogenesis of meningococcal infections. A complement deficiency predisposes some individuals to meningococcal infection (10, 17, 28), but on the other hand, with FMS, extensive complement activation is correlated with severe disease and a poor outcome (4, 16). Thus, the complement system plays an important role in the first line of defense against meningococcal infection, whereas massive activation of the complement system contributes to the development of shock.
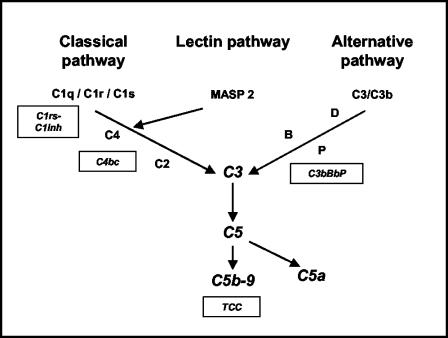
Complement is activated on the meningococcal surface by one or more of the three initial complement-activating pathways, i.e., the classical, the lectin, and the alternative pathways (Fig. 1). After the activation of complement factor 3 (C3) by any of these pathways, a C5 convertase is formed, which cleaves the pivotal C5 molecule into C5a and C5b. C5b is the initial molecule in the formation of the terminal C5b-9 complement complex (TCC). Membrane-associated TCC, also designated the C5b-9 membrane attack complex, leads to lysis of the bacterium, whereas C5a is a potent anaphylatoxin (24). Experimentally, the role of the complement system in inflammation by N. meningitidis and Escherichia coli was recently given more emphasis by our observation that granulocyte CR3 (CD11b/CD18) upregulation and the formation of reactive oxygen species in human whole blood, key elements in the induction of vascular damage during sepsis, are highly dependent on C5a (22, 29).
FIG. 1.
Schematic overview of the complement system. Boxes indicate specific complement activation products measured in the present study and their relation to specific complement activation pathways. C1rs-C1inh complexes reflect classical pathway activation, C4bc reflects both classical and lectin pathway activation, and C3bBbP reflects alternative pathway activation. TCC is an activation product of the final common and terminal pathways.
Recent in vitro studies suggested that components of N. meningitidis other than LPS may also contribute to the inflammatory response of the host. Using a meningococcal mutant that was deficient for LPS in the outer membrane, we and others have shown that proinflammatory cytokines can be induced independent of LPS in cultured human cells (27, 30, 34). In addition, we showed that purified meningococcal LPS (NmLPS) is a poor activator of complement, whereas experiments with outer membrane vesicles that were depleted of or completely lacking LPS suggested that non-LPS components in the bacterial outer membrane are the principal complement activators in a whole blood model (1).
For the present study, LPS-containing N. meningitidis, LPS-deficient N. meningitidis, and purified meningococcal LPS were used in a human whole blood model of meningococcal sepsis to determine the role of LPS in complement activation, the complement-dependent inflammatory processes of CR3 upregulation and oxidative burst, and cytokine production.
(Part of this study was presented at the Joint Meeting of the Belgian and Dutch Societies for Immunology, Veldhoven, The Netherlands, 18 to 20 December 2002.)
MATERIALS AND METHODS
Equipment and reagents.
All materials used in the stimulation experiments were endotoxin-free. The polypropylene tubes used were either Nunc cryotubes (Nalgene Nunc, Roskilde, Denmark) or Falcon tubes (Becton Dickinson, Franklin Lanes, N.Y.). Phosphate-buffered saline (PBS) was produced in the laboratory, Dulbecco's medium was obtained from Invitrogen Corporation (Paisley, Scotland), and lepirudin (Refludan) was purchased from Hoechst (Frankfurt am Main, Germany). Flow cytometry was performed with a FACScalibur instrument (Becton Dickinson San Jose, Calif.), and optical densities were determined with an MRX microplate reader (Dynex Technologies, Denkendorf, Germany).
Bacterial strains.
N. meningitidis H44/76 (LPS+) is an isolate from a patient with invasive meningococcal disease (18). This strain is the production strain for the Norwegian group B OMV vaccine and is an international reference strain (11), serologically classified as B:15:P1.7,16, immunotype L3,7,9, as determined by the use of anti-LPS monoclonal antibodies (MAbs) in a dot blotting assay for immunotyping (40). The LPS-deficient strain N. meningitidis H44/76lpxA (LPS−) is a viable isogenic mutant of strain H44/76 that lacks LPS in the outer membrane and was a kind gift from Peter van der Ley and Liana Steeghs (Dutch Vaccine Institute, Bilthoven, The Netherlands). It was constructed by insertional inactivation of the lpxA gene, which is essential for the first committed step of biosynthesis of LPS, as described by Steeghs et al. (32). For the present study, batch suspensions of H44/76lpxA were tested and showed no reactivity in a Limulus amebocyte lysate (LAL) assay, a highly specific and sensitive test for LPS. As shown by Steeghs et al., the expression level of the integral outer membrane proteins by the LPS-deficient mutant is similar to that of the wild-type strain; however, the outer membrane phospholipid composition is altered, with a switch to mostly short-chain, saturated fatty acids (31). LPS-deficient mutant meningococci were grown overnight on Kellog's medium and resuspended in Hanks' balanced salts solution (Invitrogen). Bacteria were heat inactivated at 60°C for 40 min. The concentration of the bacteria was determined by measuring the optical density at 630 nm. NmLPS was isolated from strain H44/76 by phenol-water extraction (41) followed by DNase, RNase, and proteinase K treatment. The batch of LPS used for this study was previously shown to be highly reactive in the LAL assay and a potent inducer of cytokine production (2). Quantification of the amount of LPS in LPS+ N. meningitidis H44/76 bacteria was performed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining of LPS. The LPS level was determined by digital scanning, with purified H44/76 LPS used as the standard (30, 33).
Whole blood model.
A recently developed whole blood model for the study of N. meningitidis-induced inflammation was used (29). The model is based on anticoagulation with lepirudin, a recombinant hirudin which is a highly specific thrombin inhibitor that minimally influences complement activation or cytokine production. Whole blood from healthy, adult volunteers was collected in polypropylene tubes containing lepirudin (50-μg/ml final concentration). Informed consent was obtained from all donors prior to the experiments, and the human experimentation guidelines of the local ethics committee were followed in conducting the research. Immediately after being drawn, the whole blood (1 ml) was incubated with the stimulants (0.1 ml).
For the determination of granulocyte CR3 (CD11b/CD18) expression and oxidative burst, samples were incubated for 10 min at 37°C in a water bath, followed by immediate processing for flow cytometry.
For the detection of complement activation, samples were incubated for 1 h at 37°C in a Coulter mixer (Coulter, Luton, England). Complement activation was stopped by the addition of EDTA to a final concentration of 20 mM, after which samples were centrifuged at 1,400 × g for 10 min. The plasma was collected and stored at −70°C until samples were assayed in one batch.
Samples for the measurement of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-8, and macrophage inflammatory protein 1α (MIP-1α) were processed as described for complement activation, but the incubation time was increased to 2 h.
Oxidative burst.
Oxidative burst was measured by using a commercially available Burst-test kit (Orpegen Pharma, Heidelberg, Germany). After incubation as described above, samples were treated according to the manufacturer's instructions and assayed by flow cytometry. Granulocytes were identified in a forward scatter-side scatter plot by their typical patterns.
CR3 (CD11b/CD18) expression.
After being incubated as described above, cells were washed once in sterile PBS and fixed with 0.5% (vol/vol) paraformaldehyde in an equal volume of PBS for 4 min at 37°C. Cells were stained with an anti-CD11b-PE or isotype control (γ2a) (Becton Dickinson) antibody for 15 min at room temperature. An anti-CD14-FITC (Becton Dickinson) antibody and the nuclear dye LDS-751 (Molecular Probes Inc., Eugene, Oreg.) were used to discriminate granulocytes in washed whole blood.
Enzyme immunoassays.
Complement activation was measured in plasma by enzyme immunoassays based on MAbs that were highly specific for the activation products; the native, nonactivated components did not interfere in the assays. Activation of the three initial pathways was selectively detected.
(i) C1rs-C1inh complexes.
Activation of the classical complement pathway was determined as described previously (12). C1rs-C1 inhibitor (C1rs-C1inh) complexes were measured by using MAb Kok-12, specific for a neoepitope exposed only when C1inh is in complex with its substrates. In brief, microtiter plates were coated with the Kok-12 antibody, the antibody was reacted with plasma or controls, and the complex was detected with a cocktail of anti-C1r and anti-C1s antibodies. Human serum activated with heat-aggregated immunoglobulin G was used as a standard and was defined to contain 1,000 arbitrary units (AU)/ml.
(ii) C4bc.
Activation of the classical and lectin pathways was determined by an assay using a MAb specific for a neoepitope that is exposed in activated C4 (42). The C4bc assay detects the sum of the activation products C4b, iC4b, and C4c. The same standard was used as for the C1rs-C1inh assay, defined to contain 1,000 AU/ml. Both the MAb for this assay and Kok-12 were a kind gift from C. E. Hack, Department of Immunopathology, Sanquin Research, Amsterdam, The Netherlands.
(iii) C3bBbP complexes.
Activation of the alternative pathway was detected by measuring the alternative convertase C3b-Bb-properdin (C3bBbP), as recently described (22). The capture antibody used was an antiproperdin antibody, and the detection antibody was a polyclonal anti-C3 antibody. The standard was normal human serum activated with zymosan and defined to contain 1,000 AU/ml.
(iv) TCC.
Activation of the terminal pathway was quantified by using MAb aE11, specific for C9 incorporated in the fluid-phase SC5b-9 complex, as described previously (23). The assay was modified, with a biotinylated anti-C6 antibody used as the detection antibody. Human serum activated with zymosan was used as the standard.
An overview of the complement activation markers measured is illustrated in Fig. 1.
Cytokines and chemokines.
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used according to the manufacturers' instructions to quantify TNF-α, IL-1β, IL-8 (Duo Set ELISA development system [R&D Systems, Minneapolis, Minn.] for all three), and MIP-1α (MIP-1α Cytoscreen [Biosource International, Cammarillo, Calif.]). The lower limits of detection were 8 pg/ml for TNF-α, IL-1β, and IL-8 and 2 pg/ml for MIP-1α.
LAL assay.
Quantification of the biological activity of LPS in the bacterial strains was performed as described previously (5). E. coli O55:B5 LPS was used as a reference. The lower limit of detection was 0.25 endotoxin units/ml.
Statistics.
A two-way analysis of variance (ANOVA) for repeated measures was used to test the data for statistically significant differences. P values of <0.05 were considered statistically significant.
RESULTS
Complement activation by LPS+ and LPS− N. meningitidis or purified NmLPS.
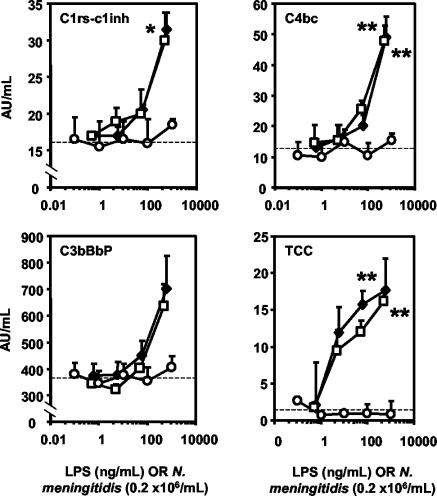
Assays for C1rs-C1inh (classical pathway), C4bc (classical and lectin pathways) and C3bBbP (alternative pathway) complexes were used to determine the initial complement activation pathways, whereas soluble TCC, the final product of the terminal pathway, was used as an indicator of total complement activation (Fig. 1).
The amount of LPS per 106 N. meningitidis cells was estimated by SDS-PAGE analysis; this showed that 106 bacteria contained approximately 5 ng of LPS. In Fig. 2 and 3, the x axes are calibrated for the numbers of LPS+ N. meningitidis cells as well as the amounts of LPS that are present in the bacteria. In this way, the activities of the bacteria and purified LPS can be compared quantitatively.
FIG. 2.
Complement activation by LPS+ and LPS− N. meningitidis and by purified NmLPS. The results are for the formation of C1rs-C1inh (classical pathway), C4bc (classical and lectin pathways), C3bBbP (alternative pathway), and TCC (final common pathway) after the stimulation of lepirudin-treated human whole blood for 1 h with LPS+ (diamonds) or LPS− (squares) N. meningitidis or purified NmLPS (circles). The x axes are calibrated for the numbers of LPS+ N. meningitidis cells as well as the amounts of NmLPS that are present in the LPS+ bacteria, according to SDS-PAGE analysis. Medians and upper quartiles are presented from four separate experiments. *, P < 0.05; **, P < 0.01, by two-way ANOVA. Dashed lines indicate baseline complement activation.
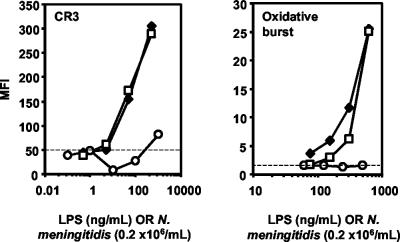
FIG. 3.
Granulocyte CR3 upregulation and oxidative burst by LPS+ and LPS− N. meningitidis and by purified NmLPS. The results are for CR3 (CD11b/CD18) upregulation and oxidative burst in human granulocytes after the stimulation of lepirudin-treated human whole blood for 10 min with LPS+ (diamonds) or LPS− (squares) N. meningitidis or purified NmLPS (circles). The x axes are calibrated for the numbers of LPS+ N. meningitidis cells as well as the amounts of NmLPS that are present in the bacteria, according to SDS-PAGE analysis. One representative of three separately performed experiments is shown. Dashed lines indicate baseline CR3 expression or oxidative burst.
The levels of the markers of initial pathway activation, C1rs-C1inh, C4bc, and C3bBbP, as well as that of TCC from the terminal pathway, were all increased from the baseline after stimulation with LPS+ or LPS− N. meningitidis (Fig. 2). The activation of initial complement activation products was seen at concentrations of 106 meningococci/ml or higher, which correspond to approximately 5 ng of NmLPS or more/ml. In contrast, purified NmLPS did not induce complement activation at concentrations up to 1,000 ng/ml. The combination of purified NmLPS and LPS− N. meningitidis induced complement activation to a similar extent as LPS+ or LPS− N. meningitidis (not shown). These data indicate that complement activation by N. meningitidis in human whole blood occurs independently of the LPS outer membrane component.
Granulocyte CR3 upregulation and oxidative burst by LPS+ and LPS− N. meningitidis or purified NmLPS.
Recently, the CR3 upregulation and oxidative burst induced by N. meningitidis in granulocytes were shown to be complement dependent (29). Therefore, we investigated the role of LPS in these processes.
Both LPS+ and LPS− N. meningitidis at concentrations of 106/ml induced marked granulocyte CR3 upregulation and oxidative burst. The addition of purified NmLPS to LPS-deficient N. meningitidis did not influence the CR3 upregulation or oxidative burst (not shown). Notably, purified NmLPS did not induce significant CR3 upregulation or oxidative burst at concentrations up to 1,000 ng/ml (Fig. 3). These results indicate that the upregulation of CR3 expression and the oxidative burst in whole blood granulocytes after stimulation with N. meningitidis are not dependent on NmLPS.
Cytokine and chemokine induction by LPS+ and LPS− N. meningitidis.
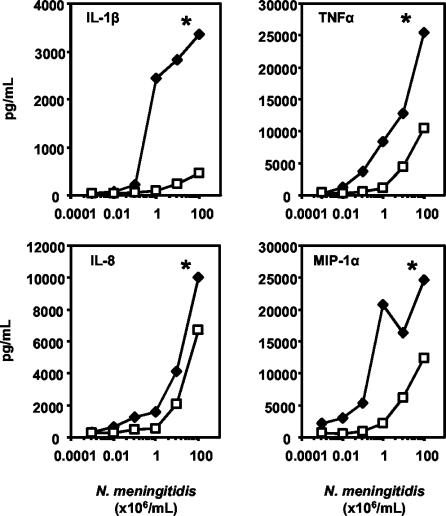
Cytokine and chemokine induction in a human whole blood model of meningococcal sepsis was assessed after 2 h of incubation with LPS+ or LPS− N. meningitidis (Fig. 4). No cytokine or chemokine production was seen in this whole blood model in the absence of a stimulus. LPS+ N. meningitidis was a potent inducer of IL-1β, TNF-α, IL-8, and MIP-1α production, as it was able to induce cytokine and chemokine production at concentrations higher than 104 meningococci/ml. IL-1β, TNF-α, IL-8, and MIP-1α production by LPS− meningococci was significantly reduced compared to that by LPS+ N. meningitidis (P < 0.05 for all cytokines and chemokines measured). However, marked IL-8, and to a lesser extent, TNF-α and MIP-1α production was also induced by LPS− N. meningitidis, suggesting that these mediators can also be induced in the absence of LPS (Fig. 4).
FIG. 4.
Cytokines and chemokines induced by LPS+ and LPS− N. meningitidis. The results are for the production of IL-1β, TNF-α, IL-8, and MIP-1α after the stimulation of lepirudin-treated human whole blood for 2 h with LPS+ (diamonds) or LPS− (squares) N. meningitidis. Mean cytokine or chemokine production levels from three separate experiments are presented. *, P < 0.05, by two-way ANOVA.
DISCUSSION
The principal finding of the present study was that complement activation and the complement-dependent inflammatory events of CR3 upregulation and oxidative burst are independent of LPS in a whole blood model of meningococcal sepsis.
The construction of the LPS-deficient meningococcal mutant H44/76lpxA has made it possible to study the relative importance of the LPS moiety of a gram-negative bacterium in diverse inflammatory processes (32). We used LPS-deficient N. meningitidis to assess the involvement of meningococcal LPS in complement activation, complement-dependent inflammatory effects, and cytokine or chemokine production in a human whole blood model of meningococcal sepsis. The lack of reactivity of the LPS− strain in a LAL assay confirmed the absence of LPS in the batch used for the experiments. The estimate that 106 LPS+ meningococci contained approximately 5 ng of meningococcal LPS was in line with previous estimations (2, 30).
N. meningitidis is exclusively a human commensal or pathogen, and none of the animal models available accurately simulate FMS. Therefore, we used an in vitro experimental system approaching, as closely as possible, the human in vivo situation (22, 29). The principle of this model is to keep all ambient inflammatory systems intact so that they can be activated and mutually interact but still to avoid coagulation. Because most anticoagulants, such as EDTA, citrate, and heparin, interact with critical steps in the inflammatory network, this model uses the highly specific thrombin inhibitor lepirudin, a recombinant hirudin analogue, as an anticoagulant. CR3 upregulation and oxidative burst were found to be induced to a similar extent by live or heat-inactivated bacteria in this model. Since these processes are highly complement dependent, this indicates that complement activation is also not affected by inactivation of the bacteria. Therefore, heat-inactivated bacteria were used because of practical considerations, as live meningococci constitute a significant health hazard (29).
LPS is present in solution in the form of micellar structures, which is quite distinct from its organization when it is present in the outer membrane. Hypothetically, the physicochemical presentation of LPS could affect the complement-activating ability, favoring membrane-bound LPS as the stronger complement activator. However, the observation that LPS-containing meningococci activate complement to a similar extent as LPS-deficient meningococci suggested that this is not the case, as did the absence of an increase in the complement-activating ability when isolated LPS was added back to the LPS-deficient strain. This is supported by a previous study in which neisserial outer membrane vesicles (OMVs) containing LPS were equipotent to OMVs deficient for LPS (1).
Complement activation by LPS+ or LPS− N. meningitidis was seen at concentrations of 106/ml or higher. This is a concentration of meningococci that is frequently found during FMS (3, 13); 106 LPS+ N. meningitidis cells contain approximately 5 ng of LPS. In contrast, complement was not activated by purified LPS at concentrations up to 1,000 ng/ml. This suggests that during meningococcal sepsis, complement activation is not induced by LPS, but by other constituents of N. meningitidis.
Members of our laboratory previously reported that the upregulation of CR3 and the induction of oxidative burst in granulocytes in this whole blood model are dependent on complement activation (22, 29). When complement is activated, C5a is formed, and this anaphylatoxin reacts with the C5a receptor on granulocytes and monocytes to induce the upregulation of CR3. CR3 is the principal receptor involved in the phagocytosis of C3b-coated bacteria, and when upregulated, it will enhance phagocytosis of the bacteria and in this way stimulate the oxidative burst process. Our results indicate that CR3 expression and oxidative burst are mediated by non-LPS components of N. meningitidis.
It is commonly accepted that LPS extracted from diverse gram-negative bacteria activates complement readily via classical and alternative pathway-dependent mechanisms (25, 38). In addition, LPS has been shown to be able to upregulate CR3 expression and to prime neutrophils for oxidative burst (8, 9, 19, 21). However, for the experiments presented in these studies, very high concentrations of LPS were applied (>5 to 10 μg/ml), incubation times of longer than 30 min were employed for granulocyte activation, or isolated cell cultures or plasma were used. In addition, it was recently shown that a contaminating substance of commercially available LPS is, at least in part, responsible for the upregulation of neutrophil responses (20). We claim that the conditions used in our study more closely resemble the in vivo situation than those used in previous studies. In our model, purified NmLPS was tested in whole blood at concentrations that are relevant for the situation encountered during FMS. The relatively short incubation times mimic the function of the complement cascade system, the components of which are present in plasma and able to be immediately activated by an invading pathogen, in this way avoiding the contribution of activation processes secondary to other inflammatory events.
We propose that the observed weak complement-activating property of NmLPS is not specific for this type of LPS, as it has a relatively short saccharide side chain, but that other types of LPS are also weak complement activators at relevant concentrations. Preliminary data from our laboratory suggest that this is the case for E. coli LPS in the same in vitro model. In addition, in primate models, complement is not activated after E. coli LPS infusion, but after whole E. coli cell infusion, marked complement activation occurs within 15 min (15, 37). Also, in a human endotoxemia model, no complement activation is observed, whereas significant cytokine production, endothelial cell activation, and activation of the coagulatory and fibrinolytic systems are found (36a).
The bacterial components that are involved in the activation of complement, the upregulation of CR3 expression, and oxidative burst remain elusive and will be subjected to further investigation. It was previously reported that OMVs isolated from LPS-deficient N. meningitidis induce substantial complement activation (1). This suggests that the non-LPS components that induce complement activation reside in the outer membrane. The lectin pathway seems to be an important player in complement activation by meningococci when they are grown on Kellog's medium and studied in the whole blood model (1, 29). Since mannose-binding lectin binding to the bacteria occurs via the outer membrane proteins porin and opacity protein (OpA) independently of LPS (9a), it is reasonable to suggest that the porins and OpA are responsible, at least in part, for the observed complement activation and subsequent CR3 upregulation and oxidative burst.
The proinflammatory cytokines TNF-α and IL-1β are primary cytokine mediators during meningococcal sepsis, whereas IL-8 and MIP-1α are important chemotactic proteins that are important for pathogenesis (14, 36, 39). We found that LPS− N. meningitidis induced marked IL-8, and to a lesser extent, TNF-α and MIP-1α production in the whole blood model, indicating that bacterial components other than LPS are also proficient at inducing cytokine or chemokine production. However, LPS+ N. meningitidis was more potent in the induction of these proinflammatory mediators, in particular TNF-α and MIP-1α, indicating that LPS is an important factor involved in the regulation of these processes, which is in agreement with previous reports (27, 30, 34). It is tempting to speculate that the observed LPS-independent induction of IL-8, and possibly also TNF-α and MIP-1α, is mediated or potentiated by the activation of complement factor C5a, as has been suggested previously (7, 22, 26).
We conclude that complement activation occurs independently of the LPS moiety of N. meningitidis. Therefore, CR3 upregulation and oxidative burst, previously shown to be highly complement dependent, are also independent of LPS. Cytokine and chemokine production can be induced via LPS-dependent as well as LPS-independent mechanisms.
Acknowledgments
We thank Anne Pharo, Gunni Ulvund, Hilde Fure, Karin Bolstad, and Berit Nyland for their excellent technical assistance. Peter van der Ley and Liana Steeghs are thanked for supplying the LPS-deficient meningococcal strain.
Financial support was provided by The Research Council of Norway (International Scholarship Section), Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) grant number 920-03-176, The Norwegian Foundation for Health and Rehabilitation, The Norwegian Council on Cardiovascular Disease, Medinnova SF, and the Research Council of the Rikshospitalet University Hospital.
Editor: J. N. Weiser
REFERENCES
- 1.Bjerre, A., B. Brusletto, T. E. Mollnes, E. Fritzsonn, E. Rosenqvist, E. Wedege, E. Namork, P. Kierulf, and P. Brandtzaeg. 2002. Complement activation induced by purified Neisseria meningitidis lipopolysaccharide (LPS), outer membrane vesicles, whole bacteria and an LPS-free mutant. J. Infect. Dis. 15:220-228. [DOI] [PubMed] [Google Scholar]
- 2.Bjerre, A., B. Brusletto, E. Rosenquist, E. Namork, P. Kierulf, R. Ovstebo, G. B. Joo, and P. Brandtzaeg. 2000. Cellular activating properties and morphology of membrane-bound and purified meningococcal lipopolysaccharide. J. Endotoxin Res. 6:437-445. [PubMed] [Google Scholar]
- 3.Brandtzaeg, P., A. Bjerre, R. Ovstebo, B. Brusletto, G. B. Joo, and P. Kierulf. 2001. Neisseria meningitidis lipopolysaccharides in human pathology. J. Endotoxin Res. 7:401-420. [PubMed] [Google Scholar]
- 4.Brandtzaeg, P., T. E. Mollnes, and P. Kierulf. 1989. Complement activation and endotoxin levels in systemic meningococcal disease. J. Infect. Dis. 160:58-65. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., R. Ovstebo, and P. Kierulf. 2000. Quantitative detection of bacterial lipopolysaccharides in clinical specimens, p. 427-439. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal disease. Humana Press, Totowa, N.Y. [DOI] [PubMed]
- 6.Brandtzaeg, P., and M. van Deuren. 2002. Current concepts in the role of the host response in Neisseria meningitidis septic shock. Curr. Opin. Infect. Dis. 15:247-252. [DOI] [PubMed] [Google Scholar]
- 7.Cavaillon, J. M., C. Fitting, and N. Haeffner-Cavaillon. 1990. Recombinant C5a enhances interleukin 1 and tumor necrosis factor release by lipopolysaccharide-stimulated monocytes and macrophages. Eur. J. Immunol. 20:253-257. [DOI] [PubMed] [Google Scholar]
- 8.Chateau, M. T., and R. Caravano. 1997. The oxidative burst triggered by Salmonella typhimurium in differentiated U937 cells requires complement and a complete bacterial lipopolysaccharide. FEMS Immunol. Med. Microbiol. 17:57-66. [DOI] [PubMed] [Google Scholar]
- 9.Dahinden, C., C. Galanos, and J. Fehr. 1983. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J. Immunol. 130:857-862. [PubMed] [Google Scholar]
- 9a.Estabrook, M. M., D. L. Jack, N. J. Klein, and G. A. Jarvis. 2004. Mannose-binding lectin binds to two major outer membrane proteins, opacity protein and porin, of Neisseria meningitidis. J. Immunol. 172:3784-3792. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa, J. E., and P. Densen. 1991. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, and U. Rye. 1991. Production, characterization, and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed] [Google Scholar]
- 12.Fure, H., E. W. Nielsen, C. E. Hack, and T. E. Mollnes. 1997. A neoepitope-based enzyme immunoassay for quantification of C1-inhibitor in complex with C1r and C1s. Scand. J. Immunol. 46:553-557. [DOI] [PubMed] [Google Scholar]
- 13.Hackett, S. J., M. Guiver, J. Marsh, J. A. Sills, A. P. J. Thomson, E. B. Kaczmarski, and C. A. Hart. 2002. Meningococcal bacterial DNA load at presentation correlates with disease severity. Arch. Dis. Child. 86:44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halstensen, A., M. Ceska, P. Brandtzaeg, H. Redl, A. Naess, and A. Waage. 1993. Interleukin-8 in serum and cerebrospinal fluid from patients with meningococcal disease. J. Infect. Dis. 167:471-475. [DOI] [PubMed] [Google Scholar]
- 15.Hangen, D. H., J. H. Stevens, P. S. Satoh, E. W. Hall, P. T. O'Hanley, and T. A. Raffin. 1989. Complement levels in septic primates treated with anti-C5a antibodies. J. Surg. Res. 46:195-199. [DOI] [PubMed] [Google Scholar]
- 16.Hazelzet, J. A., R. de Groot, G. van Mierlo, K. F. M. Joosten, E. van der Voort, A. Eerenberg, M. H. Suur, W. C. J. Hop, and C. E. Hack. 1998. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect. Immun. 66:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibberd, M. L., M. Sumiya, J. A. Summerfield, R. Booy, M. Levin, and the Meningococcal Research Group. 1999. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet 353:1049-1053. [DOI] [PubMed] [Google Scholar]
- 18.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes, J. E., J. Stewart, G. R. Barclay, and J. R. W. Govan. 1997. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia cepacia. Infect. Immun. 65:4281-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurt-Jones, E. A., L. Mandell, C. Whitney, A. Padgett, K. Gosselin, P. E. Newburger, and R. W. Finberg. 2002. Role of Toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood 100:1860-1868. [PubMed] [Google Scholar]
- 21.Mirlashari, M. R., E. A. Hoiby, J. Holst, and T. Lyberg. 2002. Outer membrane vesicles from Neisseria meningitidis. APMIS 110:193-204. [DOI] [PubMed] [Google Scholar]
- 22.Mollnes, T. E., O. L. Brekke, M. Fung, H. Fure, D. Christiansen, G. Bergseth, V. Videm, K. T. Lappegard, J. Kohl, and J. D. Lambris. 2002. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 100:1869-1877. [PubMed] [Google Scholar]
- 23.Mollnes, T. E., T. Lea, S. S. Froland, and M. Harboe. 1985. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand. J. Immunol. 22:197-202. [DOI] [PubMed] [Google Scholar]
- 24.Mollnes, T. E., W. C. Song, and J. D. Lambris. 2002. Complement in inflammatory tissue damage and disease. Trends Immunol. 23:61-64. [DOI] [PubMed] [Google Scholar]
- 25.Morrison, D. C., and L. F. Kline. 1977. Activation of the classical and properdin pathways of complement activation by bacterial lipopolysaccharides (LPS). J. Immunol. 118:362-368. [PubMed] [Google Scholar]
- 26.Okusawa, S., K. B. Yancey, J. W. van der Meer, S. Endres, G. Lonnemann, K. Hefter, M. M. Frank, J. F. Burke, C. A. Dinarello, and J. A. Gelfand. 1988. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J. Exp. Med. 168:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 2001. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signal via Toll-like receptor (TLR) 2, but not via TLR4/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 28.Ross, S. C., and P. Densen. 1984. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 63:243-273. [PubMed] [Google Scholar]
- 29.Sprong, T., P. Brandtzaeg, M. Fung, A. M. Pharo, E. A. Hoiby, T. E. Michaelsen, A. Aase, J. W. van der Meer, M. van Deuren, and T. E. Mollnes. 2003. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 102:3702-3710. [DOI] [PubMed] [Google Scholar]
- 30.Sprong, T., N. Stikkelbroeck, P. van der Ley, L. Steeghs, L. van Alphen, N. Klein, M. G. Netea, J. W. M. van der Meer, and M. van Deuren. 2001. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 70:283-288. [PubMed] [Google Scholar]
- 31.Steeghs, L., H. de Cock, E. Evers, B. Zomer, J. Tommassen, and P. van der Ley. 2001. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 20:6927-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 33.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 34.Uronen, H., A. J. Williams, G. Dixon, S. R. Andersen, P. Van der Ley, M. Van Deuren, R. E. Callard, and N. Klein. 2000. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide (LPS). Clin. Exp. Immunol. 22:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Deuren, M., P. Brandtzaeg, and J. W. M. Van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Deuren, M., J. van der Ven-Jongekrijg, E. Vannier, R. van Dalen, G. Pesman, A. K. M. Bartelink, C. A. Dinarello, and J. W. M. van der Meer. 1997. The pattern of interleukin-1β (IL-1β) and its modulating agents IL-1 receptor antagonist and IL-1 soluble receptor type II in acute meningococcal infections. Blood 90:1101-1108. [PubMed] [Google Scholar]
- 36a.Van Deventer, S. J., H. R. Buller, J. W. ten Cate, L. A. Aarden, C. E. Hack, and A. Sturk. 1990. Experimental endotixaemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 76:2520-2526. [PubMed] [Google Scholar]
- 37.van Leenen, D., T. van der Poll, M. Levi, H. Ten Cate, S. J. van Deventer, C. E. Hack, L. A. Aarden, and J. W. Ten Cate. 1993. Pentoxifylline attenuates neutrophil activation in experimental endotoxaemia in chimpanzees. J. Immunol. 151:2318-2325. [PubMed] [Google Scholar]
- 38.Vukajlovich, S. W., J. Hoffman, and D. C. Morrison. 1987. activation of human serum complement by bacterial lipopolysaccharides: structural requirements for antibody independent activation of the classical and alternative pathways. Mol. Immunol. 24:319-331. [DOI] [PubMed] [Google Scholar]
- 39.Waage, A., A. Halstensen, and T. Espevik. 1987. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet i:355-357. [DOI] [PubMed] [Google Scholar]
- 40.Wedege, E., R. Dalseg, D. A. Caugant, J. T. Poolman, and L. O. Froholm. 1993. Expression of an inaccessible P1.7 subtype epitope on meningococcus class 1 proteins. J. Med. Microbiol. 38:23-28. [DOI] [PubMed] [Google Scholar]
- 41.Westphal, O., and J. K. Jann. 1965. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 42.Wolbink, G. J., J. Bollen, J. W. Baars, R. J. M. Tenberge, A. J. G. Swaak, J. Paardekooper, and C. E. Hack. 1993. Application of a monoclonal antibody against a neoepitope on activated C4 in an ELISA for the quantification of complement activation via the classical pathway. J. Immunol. Methods 163:67-76. [DOI] [PubMed] [Google Scholar]