Abstract
Recent studies have demonstrated that marine sponges and their active constituents exhibited several potential medical applications. This study aimed to evaluate the possible hepatoprotective role as well as the antioxidant effect of the Red Sea Suberea mollis sponge extract (SMSE) on carbon tetrachloride- (CCl4-) induced acute liver injury in rats. In vitro antioxidant activity of SMSE was evaluated by 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay. Rats were orally administered three different concentrations (100, 200, and 400 mg/kg) of SMSE and silymarin (100 mg/kg) along with CCl4 (1 mL/kg, i.p., every 72 hr) for 14 days. Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin were measured. Hepatic malondialdehyde (MDA), reduced glutathione (GSH), nitric oxide (NO), superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) were also measured. Liver specimens were histopathologically examined. SMSE showed strong scavenging activity against free radicals in DPPH assay. SMSE significantly reduced liver enzyme activities. Moreover, SMSE significantly reduced hepatic MDA formation. In addition, SMSE restored GSH, NO, SOD, GPx, and CAT. The histopathological results confirmed these findings. The results of this study suggested a potent protective effect of the SMSE against CCl4-induced hepatic injury. This may be due to its antioxidant and radical scavenging activity.
1. Introduction
Hepatotoxicity is a prevalent problem worldwide and represents 38% of all hepatic problems [1]. It is known that carbon tetrachloride (CCl4) causes acute liver toxicity in humans and experimental animals [2, 3]. Hepatotoxicity using CCl4 is a common model used to measure the efficiency of several antihepatotoxic drugs [4]. There are many previous in vivo and in vitro studies that documented the mechanism of CCl4-induced hepatocyte damage [5]. CCl4 is converted by cytochrome P450 (CYP) 2E1 to trichloromethyl (CCl3 •) free radical and trichloromethylperoxy radical (CCl3OO•). Both radicals covalently bond to cellular macromolecules, producing lipid peroxidation, protein degeneration, DNA damage, and apoptosis [4–6].
Marine sponges are considered to be a gold mine because of their diversity of secondary vital biological compounds, which are not present in terrestrial organisms [7, 8]. Many worldwide diseases could be treated by drugs extracted from the sponges [9]. Marine sponges of the order Verongida, including genus Suberea, attracted the attention of chemists specializing in marine-derived natural products. They possess an unusual chemical structure due to large amounts of sterols and a lack of terpenes and typical brominated compounds associated with tyrosine [10]. Members of the genus Suberea display diverse bioactivities, including antibacterial [11], antiviral [12], enzyme inhibition [13], and cytotoxic activity [14]. Prenylated toluquinone, hydroquinones, and naphthoquinones are examples of marine-derived natural products with reported antioxidant activities [15–18].
The present study aims to evaluate the possible hepatoprotective effect of the Red Sea Suberea mollis sponge extract (SMSE) against CCl4-induced hepatotoxicity as compared to silymarin, the most commonly known hepatoprotective agent. In addition, the mechanism of the suggested effect was studied regarding potential antioxidant properties of the organic extract of the sponge.
2. Materials and Methods
2.1. Sponge Collection
The sponge was collected from the Red Sea in 2011, between depths of 15 and 25 meters. The sponge was cylindrical in shape and had a low and a sharp conulose surface. The sharpness of the conules was due to the projection of strong fibers about 8–10 mm apart (Figure 1). The diameter of the oscules was about 1.0 cm, and they were located at the summit of the fragment. The interior of the sponge was cavernous. The fresh sponge had a green color with a yellowish interior, while the preserved sponge had a black color. The fresh sponge was frozen immediately after collection.
Figure 1.

Underwater (a) and in situ (b) photographs of the Suberea mollis sponge.
2.2. Identification of the Sponge
The sponge was kindly identified by Professor Rob van Soest at Naturalis Biodiversity Center, Department of Marine Zoology, RA Leiden, The Netherlands. The voucher specimen, measuring 3.5 cm, is incorporated in the collections of the Zoological Museum of the University of Amsterdam under registration number 16621. Another voucher specimen was deposited in the Red Sea Invertebrates Collection of the Department of Pharmacognosy, Suez Canal University, under the code number DY-8.
2.3. Extraction of the Sponge
The extraction was done using methanol (3 × 3000 mL) at room temperature after crushing the frozen sponge. The combined crude extracts evaporated under the reduced pressure.
2.4. Determination of In Vitro Antioxidant Activity (DPPH Test)
Different concentrations of the SMSE extract were dissolved in ethanol. 2 mL of each concentration was pipetted into a series of 5 mL volumetric flasks. 3 mL of DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) solution was added to each flask and mixed with extract solution. Methanol was used as a blank. The absorbance was measured at 517 nm after 10-minute incubation [19] using a Thermo Scientific GENESYS 10S UV-visible double beam spectrophotometer (USA). α-Tocopherol (vitamin E) was used as the positive control [20]. Higher free radical scavenging activity was indicated by lower absorbance of the reaction mixture. The following equation [20] shows the radical scavenging activity of the sample, which is expressed as the inhibition percentage: % inhibition = [(Ac −A sample)/Ac] × 100, where Ac is the absorbance of the control (DPPH in absence of extract) and A sample is the absorbance in the presence of the extract. All of the tests were performed in triplicate.
2.5. Animals
Male albino Sprague Dawley rats (200–220 g) were obtained from the Animal Resources Division of King Fahd Medical Research Center. The rats were housed at 22 ± 3°C and relative humidity of 44%–55% with a 12 h dark/light cycle and were provided with standard laboratory feed and water ad libitum. The use of experimental animals in this study was conducted under the guidance of the basic standards in the care and use of laboratory animals, which has been prepared and published by the National Institutes of Health. The study protocol has been approved by the Research Ethics Committee at King Abdulaziz University (Approval number 29-14).
2.6. Estimation of SMSE Dose
The extract (300 mg/kg) was orally administered to three rats, which were fasted overnight. The animals were observed daily for 14 days for mortality [21]. The procedure was repeated for higher doses up to 2000 mg/kg bw. 1/20, 1/10, and 1/5 of highly tolerated doses (2000 mg/kg) were selected (100, 200, and 400 mg/kg, resp.) for assessment of hepatoprotective activity [22].
2.7. Experimental Design
Three different doses of the SMSE (100, 200, and 400 mg/kg) were tested for their hepatoprotective effect against CCl4-induced hepatotoxicity. A total of 30 rats were divided into six groups (n = 5). (1) Control: rats in this group were orally administered 1 mL/kg of dimethyl sulfoxide (DMSO) daily for 14 days. (2) CCl4: this group served as the model of hepatotoxicity. The rats in this group were intraperitoneally (i.p.) administered CCl4 in olive oil (1 mL/kg, 1 : 1 v/v) every 72 hours for 14 days [23]. (3) CCl4 + silymarin (100 mg/kg): this group served as a positive control. Rats in this group were administered silymarin (Sigma Chemicals Company, USA) (100 mg/kg, orally through a feeding tube) in DMSO daily for 14 days [24]. CCl4 in olive oil was also given to this group every 72 hours for 14 days. (4) CCl4 + SMSE (100 mg/kg): SMSE (100 mg/kg) dissolved in DMSO was given orally daily for 14 days, and CCl4 in olive oil (1 mL/kg, i.p. 1 : 1 v/v) was given every 72 hours for 14 days. (5) CCl4 + SMSE (200 mg/kg): SMSE (200 mg/kg) dissolved in DMSO was given orally daily for 14 days, and CCl4 in olive oil (1 mL/kg, i.p. 1 : 1 v/v) was given every 72 hours for 14 days. (6) CCl4 + SMSE (400 mg/kg): SMSE (400 mg/kg) dissolved in DMSO was given orally daily for 14 days, and CCl4 in olive oil (1 mL/kg, i.p. 1 : 1 v/v) was given every 72 hours for 14 days. Two days after the last dose, blood from all of the rats was collected via retroorbital sinus plexus under mild ether anesthesia [24]. Rats were sacrificed by cervical dislocation. Blood was allowed to clot at room temperature and the serum was separated by centrifuging at 4000 rpm for 15 min and was kept at −20°C for further biochemical analysis. The liver was dissected and used for histopathological (formalin fixed) and biochemical (frozen −80°C) studies.
2.8. Determination of Alanine Amino Transaminase (ALT), Aspartate Amino Transaminase (AST), Alkaline Phosphatase (ALP), and Total Bilirubin
Various liver marker enzymes, such as ALT, AST, ALP, and total bilirubin, were measured in plasma using an automated analyzer (Flexor EL200, France).
2.9. Sample Preparation for Biochemical Analysis
The liver samples were homogenized in 2% Triton X-100 containing 0.32 M sucrose solution for SOD determination. Other liver portions were homogenized in 50 Mm potassium phosphate pH 7.5 and 1 Mm EDTA for MDA, GSH, NO, GPx, and CAT measurements. Homogenized tissues were subjected to a sonication procedure twice with 30 s intervals at 4°C. After the sonication process, homogenized tissues were centrifuged at 4000 rpm/min for 10 min at 4°C [25].
2.10. Determination of Lipid Peroxide (Measured as MDA)
MDA was determined in the liver homogenates using kits provided by Biodiagnostic, Egypt. MDA was determined according to the method of Uchiyama and Mihara [26]. The adducts were formed following the reaction of thiobarbituric acid with tissue homogenate in a boiling water bath and were extracted with n-butanol. Tissue MDA content was measured by the difference in optical density developed at two distinct wavelengths, 535 nm and 525 nm. Tissue MDA content was expressed as nmol/g tissue.
2.11. Determination of Reduced Glutathione (GSH)
GSH was determined in the liver homogenates using kits provided by Biodiagnostic, Egypt. GSH was determined according to the method described earlier by Ellman [27]. The principle of this procedure is based on the formation of 2-nitro-5-mercaptobenzoic acid from reduction of bis(3-carboxy-4-nitrophenyl) disulfide reagent by the SH group, which has a deep yellow color that was measured spectrophotometrically at 412 nm [28]. Tissue GSH content was expressed as nmol/g tissue.
2.12. Determination of Nitric Oxide (NO)
NO was determined in the liver homogenates using kits provided by Biodiagnostic, Egypt. Initially, nitrate was converted into nitrite by the enzyme nitrate reductase, followed by quantitation of nitrite using Griess reagent at the absorbance of 550 nm, as previously described [29]. NO was assayed by measuring total nitrate plus nitrite (NO3 − + NO2 −), the stable end products of NO metabolism. Results were expressed as μmol/g tissue.
2.13. Determination of Glutathione Peroxidase (GPx)
GPx activity was determined in the liver homogenates using kits provided by Biodiagnostic, Egypt. GPx activity was determined in a coupled assay with glutathione reductase by measuring the rate of NADPH oxidation at 340 nm using H2O2 as the substrate [30]. GPx activity was expressed in mU/g tissue.
2.14. Determination of Superoxide Dismutase (SOD)
The activity of SOD was determined in the liver homogenates using kits provided by Biodiagnostic, Egypt. SOD was determined according to the method described earlier by Nishikimi et al. [31]. This assay relies on the ability of the enzyme to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye. SOD activity was expressed in U/g tissue.
2.15. Determination of Catalase (CAT)
The activity of CAT was determined in the liver homogenates using kits provided by Biodiagnostic, Egypt. This enzyme was measured according to Aebi [32]. H2O2 reacts with a known quantity of CAT. After adding catalase inhibitor, the reaction was stopped after exactly one minute. The remaining H2O2 reacts with 3,5-dichloro-2-hydroxybenzene sulfonic acid (DHBS) and 4-aminophenazone (AAP) to form a chromophore with color intensity inversely proportional to the amount of CAT in the original sample. The absorbance of samples was read at 510 nm against a standard blank. CAT activity was expressed in U/g tissue.
2.16. Histopathological Examination
For a histopathological study of the liver, the animals were dissected via abdominal incision, and the livers of all groups were extracted. Slices of liver 2 × 2 cm were excised and fixed in 10% neutral buffered formalin for further processing using the paraffin technique. Five-micron paraffin sections were stained with hematoxylin and eosin (H&E) and examined by a light microscope connected to a digital camera. Photographs of the liver at different magnifications were screened for features of hepatotoxicity and the hypothesized protective efficacy.
2.17. Statistical Analysis
All data were presented as mean ± SD. The results were statistically analyzed by an ANOVA test utilizing SPSS10.0 statistical software. Statistical differences of P ≤ 0.05 were considered significant.
3. Results
3.1. Effect of SMSE on In Vitro Antioxidant Activity (DPPH Test)
The scavenging activity of SMSE (125, 250, 500, 1000, and 2000 μg/mL) against DPPH free radicals was demonstrated in Figure 2. SMSE had a strong DPPH radical inhibition and their percentage of inhibition (89%) nearly reached the control percentage inhibition (90%) at 500 and 1000 μg/mL.
Figure 2.
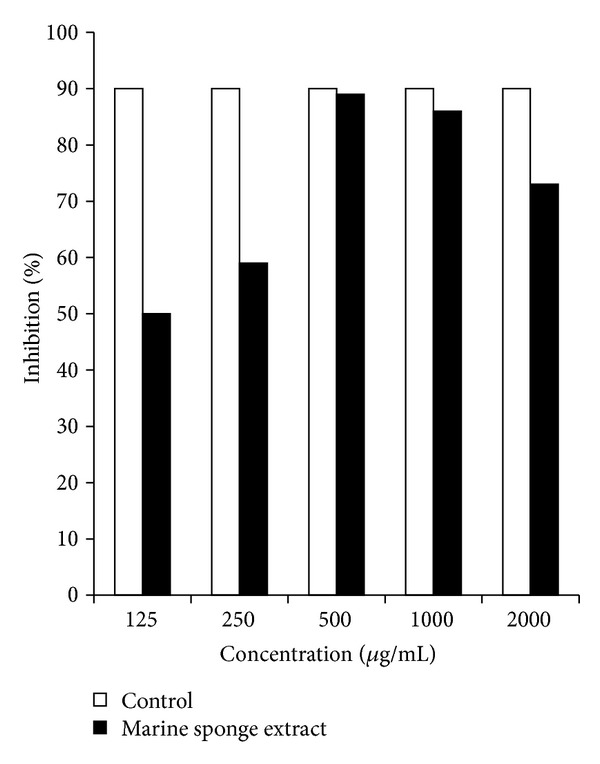
Total antioxidant capacity of different concentrations of Suberea mollis sponge extract (SMSE). Expressed as percent inhibition toward DPPH-induced oxidative stress in vitro.
3.2. Effect of SMSE and Silymarin on Liver Functions Measured as ALT, AST, ALP, and Bilirubin
The results of the liver function tests are shown in Table 1. Treatment of rats with CCl4 caused a significant increase in ALT by 266%, AST by 57.47%, ALP by 152.91%, and total bilirubin by 126.6% compared to the control group (P = 0.001). Treatment of CCl4-injected rats with silymarin significantly decreased all measured serum biochemical activities (ALT 60.75%, AST 29.87%, ALP 52.41%, and total bilirubin 51.47%) compared to the CCl4 group (P = 0.001). Treatment of CCl4-injected rats with the SMSE (100 mg/kg) significantly decreased the percentage of liver marker enzymes and total bilirubin: ALT 47.32% (P = 0.001), AST 12.67%, ALP 29.78% (P = 0.001), and total bilirubin 42.27% (P = 0.001) compared to the CCl4 group. On the other hand, the percentage protection was increased at the dose of 200 and 400 mg/kg: ALT 56.11% (P = 0.001), 71.56% (P = 0.001); AST 26% (P = 0.01), 31.18% (P = 0.001); ALP 41.77% (P = 0.001), 45.13% (P = 0.001); and total bilirubin 53.3% (P = 0.001), 54% (P = 0.001), respectively.
Table 1.
Effects of the Suberea mollis sponge extract (SMSE) and silymarin on ALT, AST, ALK, and total bilirubin levels measured in CCl4-induced hepatotoxicity in rats.
| Treatment regimen | ALT | AST | ALP | Total bilirubin |
|---|---|---|---|---|
| Control | 44.2 ± 5.89 | 145.8 ± 13.08 | 123.6 ± 24.35 | 1.2 ± 0.45 |
| CCl4 | 161.8 ± 13.71a | 229.6 ± 19.48a | 312.6 ± 49.06a | 2.72 ± 0.19a |
| CCl4 + silymarin (100 mg/kg) | 63.5 ± 18.59b | 161 ± 29.8b | 148.75 ± 37.6b | 1.32 ± 0.12b |
| CCl4 + SMSE (100 mg/kg) | 85.25 ± 15.2b | 200.5 ± 34.8 | 219.5 ± 41.34b | 1.57 ± 0.3b |
| CCl4 + SMSE (200 mg/kg) | 71 ± 13.31b | 169.7 ± 37.52b | 182 ± 16.58b | 1.27 ± 0.2b |
| CCl4 + SMSE (400 mg/kg) | 46 ± 16b | 158 ± 13.56b | 171.5 ± 14.7b | 1.25 ± 0.5b |
Data are mean ± SD of five animals.
aSignificantly different from control group (P ≤ 0.05).
bSignificantly different from the CCl4 group (P ≤ 0.05).
3.3. Effect of SMSE and Silymarin on CCl4-Induced Changes in Liver MDA, GSH, and NO
The treatment of rats with CCl4 caused a significant increase (63%) in liver MDA contents compared to the control group (P = 0.002) (Figure 3). On the other hand, the treatment of rats with CCl4 caused a significant decrease (28% and 51%) in liver GSH and NO contents, respectively, compared to the control group (P = 0.012 and 0.019) (Figures 4 and 5). Treatment of CCl4-injected rats with silymarin significantly decreased (43%) liver MDA contents and increased (28% and 170%) liver GSH and NO contents, respectively, compared to the CCl4 group (P = 0.001, 0.016, and 0.004) (Figures 3, 4, and 5). Treatment of CCl4-injected rats with the SMSE (100, 200, and 400 mg/kg) significantly decreased (22%, 42%, and 43%) liver MDA contents, respectively, compared to the CCl4 group (P = 0.024, 0.001, and 0.002) (Figure 3). In addition, treatment of CCl4-injected rats with the SMSE (100, 200, and 400 mg/kg) significantly increased liver contents of both GSH (35%, 44%, and 51%) (P = 0.029, 0.002, and 0.000) and NO (124%, 171%, and 187%) (P = 0.005, 0.001, and 0.001), respectively, compared to the CCl4 group (Figures 4 and 5).
Figure 3.
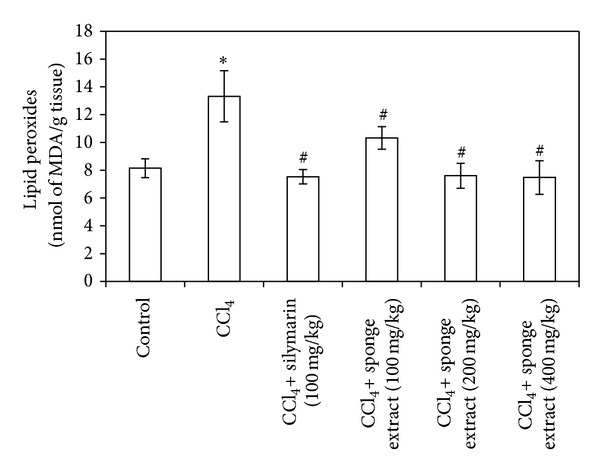
Effect of Suberea mollis sponge extract (SMSE) and silymarin on liver lipid peroxides in CCl4-induced hepatotoxicity in rats. The effect of SMSE (100, 200, and 400 mg/kg) and silymarin (100 mg/kg) on liver lipid peroxides (measured as MDA) concentration measured in CCl4-induced hepatotoxicity in rats. Each point represents the mean ± SD of five rats. ∗Significant difference compared with the control group (P ≤ 0.05). #Significant difference compared with the CCl4 group (P ≤ 0.05).
Figure 4.
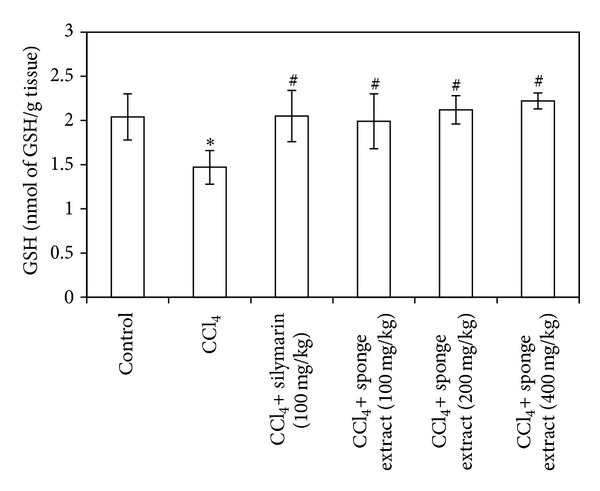
Effect of Suberea mollis sponge extract (SMSE) and silymarin on liver GSH in CCl4-induced hepatotoxicity in rats. The effect of SMSE (100, 200, and 400 mg/kg) and silymarin (100 mg/kg) on liver GSH contents measured in CCl4-induced hepatotoxicity in rats. Each point represents the mean ± SD of five rats. ∗Significant difference compared with the control group (P ≤ 0.05). #Significant difference compared with the CCl4 group (P ≤ 0.05).
Figure 5.
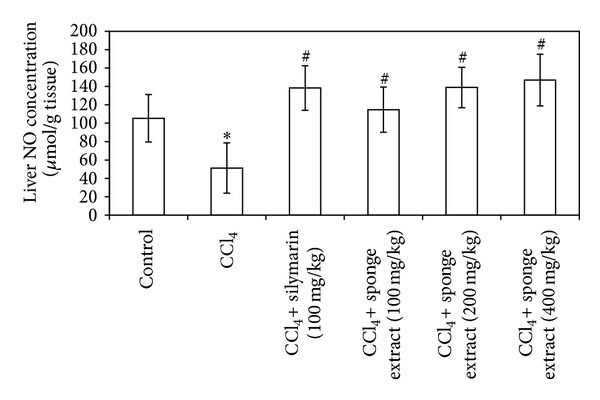
Effect of Suberea mollis sponge extract (SMSE) and silymarin on liver NO in CCl4-induced hepatotoxicity in rats. The effect of SMSE (100, 200, and 400 mg/kg) and silymarin (100 mg/kg) on liver NO concentration measured in CCl4-induced hepatotoxicity in rats. Each point represents the mean ± SD of five rats. ∗Significant difference compared with the control group (P ≤ 0.05). #Significant difference compared with the CCl4 group (P ≤ 0.05).
3.4. Effect of SMSE and Silymarin on CCl4-Induced Changes in Liver GPx, SOD, and CAT Activities
The results of enzymatic antioxidant analyses are shown in Table 2. Briefly, the activities of GPx, SOD, and CAT were significantly decreased (78%, 39%, and 41%) in the CCl4 treated group compared to the control group (P = 0.002, 0.05, and 0.002). On the other hand, treatment of CCl4-injected rats with either silymarin or SMSE (100, 200, and 400 mg/kg) significantly increased GPx (3-, 6-, 6-, and 8-fold) (P = 0.011, 0.004, 0.008, and 0.000), respectively, compared to the CCl4 group. Treatment of CCl4-injected rats with either silymarin or SMSE (400 mg/kg) significantly increased SOD (59% and 190%) (P = 0.02 and 0.001), respectively, compared to the CCl4 group. In addition, treatment of CCl4-injected rats with either silymarin or SMSE (200 and 400 mg/kg) significantly increased CAT (73%, 59%, and 65%) (P = 0.002, 0.007, and 0.006), respectively, compared to the CCl4 group.
Table 2.
Effect of Suberea mollis sponge extract (SMSE) and silymarin on liver GPx, SOD, and CAT measured in CCl4-induced hepatotoxicity in rats.
| Treatment regimen | GPx (mU/g tissue) |
SOD (U/g tissue) |
CAT (U/g tissue) |
|---|---|---|---|
| Control | 24.8 ± 6.9 | 18.3 ± 4.8 | 8.2 ± 0.2 |
| CCl4 | 5.3 ± 9.2a | 11.13 ± 3.6a | 4.8 ± 1.3a |
| CCl4 + silymarin (100 mg/kg) | 15.5 ± 4.7b | 17.8 ± 2.3b | 8.3 ± 0.3b |
| CCl4 + SMSE (100 mg/kg) | 29.1 ± 10.1b | 12.7 ± 2.8 | 4.5 ± 1.3 |
| CCl4 + SMSE (200 mg/kg) | 32.5 ± 13.6b | 16.8 ± 3.6 | 7.65 ± 0.5b |
| CCl4 + SMSE (400 mg/kg) | 42.3 ± 9.9b | 32.7 ± 6.5b | 7.9 ± 0.7b |
Data are mean ± SD of five animals.
aSignificantly different from control group (P ≤ 0.05).
bSignificantly different from the CCl4 group (P ≤ 0.05).
3.5. Histopathological Microscopic Study
The histological architecture of the control rat livers was similar to normal rat livers (Figure 6(a)). CCl4 administration caused central perivenular cell necrosis and fibrous bridging. Bile duct proliferation, portal vein congestion, and inflammatory cell infiltrate were also observed. Karyomegaly and fatty infiltration of hepatocytes were observed in central regions of the hepatic lobules. Some samples showed increased degeneration of apoptotic cells (Figures 6(b), 6(c), and 6(d)). Silymarin provided protection against fatty infiltration, nuclear changes, and bile duct proliferation (Figure 6(e)). Administration of 100 mg/kg SMSE resulted in potential protection against fatty infiltration. Lymphocytes were frequent within hepatic sinusoids (Figure 6(f)). On the other hand, histopathological findings showed that protection of rat liver against CCl4 hepatotoxicity was more profound in rats that received 200 mg/kg of SMSE when compared to the previous low dose group. There was an absence of fatty infiltration, and portal changes and apoptotic hepatocytes were less frequent (Figure 6(g)). Liver sections of rats treated with SMSE at a dose of 400 mg/kg showed healthy hepatocytes with active vesicular nuclei. Signs of fatty changes, fibrosis, or portal tract changes were not observed (Figure 6(h)).
Figure 6.
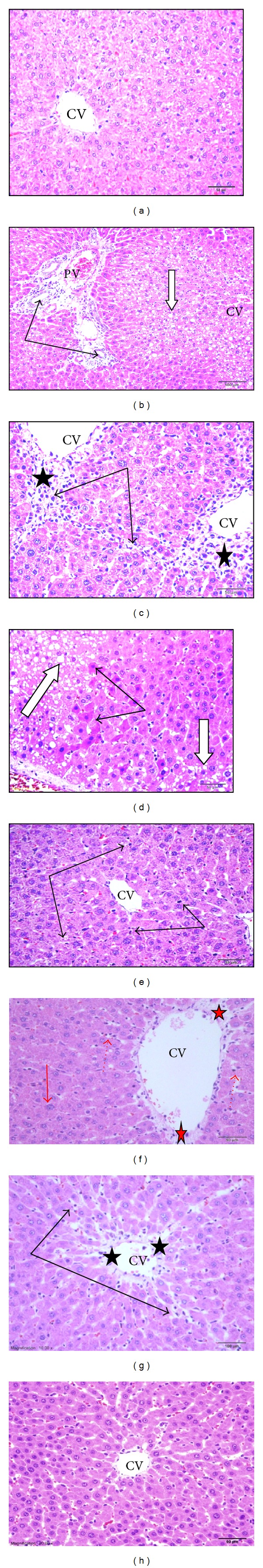
Histopathological study of liver tissue in control, CCl4, silymarin, and Suberea mollis sponge extract (SMSE) groups of rats. (a) Control group showed normal liver architecture. (b, c, and d) CCl4 group: (b) showed portal areas with dilated congested vein (PV) and proliferating bile ducts (arrows) and inflammatory cells. Notice fatty infiltration of hepatocytes (white arrow). (c) revealed cell necrosis (stars) near central veins (CV) with fibrous bridging (thin arrows). (d) showed increased dark degenerated apoptotic hepatocytes (black arrows) and fatty infiltration (white arrow). (e) CCl4 + silymarin (100 mg/kg) showed normal hepatocytes around the central vein with an absence of fatty infiltration. Few cells showed karyomegaly and dark degenerated nuclei (black arrow). (f) CCl4 + SMSE (100 mg/kg) showed normal hepatocytes around the central vein (CV) with few cells showing karyomegaly (red arrows). Blood sinusoids showed lymphocyte (dotted arrows) infiltration. Mild perivenular fibrosis (star) could be seen. (g) CCl4 + SMSE (200 mg/kg) showed normal hepatocytes (black arrows) with no signs of fatty changes around the central vein (CV) and mild perivenular fibrosis (star). (h) CCl4 + SMSE (400 mg/kg): region near central vein (CV) showed normal hepatocytes with an absence of any signs of fatty infiltration. Absence of perivenular fibrosis (H&E stain).
4. Discussion
The data presented in this study demonstrate that the crude SMSE protected against CCl4-induced liver injury. This was reflected by the significant decrease in serum levels of ALT, AST, ALP, and total bilirubin and histopathologically by the protection against CCl4-induced liver degeneration. Similarly, Abdel-Monem et al. [33] recently found that Trichurus spiralis extract isolated from Hippospongia communis sponges possesses a hepatoprotective activity against heavy-metal-mixture-induced liver damage. It is clear that the hepatotoxicity of CCl4 increased with both the depletion of GSH and the increase of free radicals and lipid oxidation [34, 35]. In the present study, the treatment of rats with CCl4 resulted in the depletion of hepatic GSH and NO and a decrease in the activity of GPx, SOD, and CAT. It also increased the liver lipid peroxidation product, MDA. This observation was in agreement with the recent findings of Simeonova et al. [36]. On the other hand, pretreatment with SMSE resulted in a significant decrease of MDA quantity and increase in GSH content, as well as a significant increase in the activity of GPx, SOD, and CAT. GSH serves as the main cytosolic antioxidants; it has a central role in sulfhydryl homeostasis [37, 38]. Recently, Lind et al. [39] found that barettin isolated from the marine sponge Geodia barretti possesses antioxidant properties in ferric reducing antioxidant power (FRAP) assays and lipid peroxidation cell assays. In addition, Dupont et al. [40] reported that the crude extract of the marine sponge Phorbastenacior exhibited effects against DPPH-induced oxidative stress. Suberea mollis in the order Verongida in the family Aplysinellidae afforded two new brominated arginine-derived alkaloids: subereamines A and B; a new brominated phenolic compound has exhibited strong antioxidant potential compared to vitamin E and ascorbic acid; moreover, Suberea mollis contain subereaphenol D and the known compounds dichloroverongiaquinol, aerothionin, and purealdin L [41, 42]. Subereaphenol was reported to be a free radical scavenger in the DPPH assay [41, 42]. Previous findings are consistent with the scavenging activity of SMSE against DPPH-induced oxidative stress in this study, which reaches 89%, near to the standard antioxidant control (90%). The result suggested that SMSE at a concentration of 500 μg/mL was found to have the highest inhibitory activity against DPPH. However, the decrease in radical scavenging activity was observed with increasing SMSE concentration (1000 and 2000 μg/mL) indicating a possible prooxidative effect of SMSE at high concentration. This result was in accordance with Kiokias and Gordon [43] and Chen and Yen [44], who found the autoxidation of carotenoids and guava leaf extracts, respectively, at a high concentration in vitro.
Nitric oxide (NO) is an important biological mediator and has been shown to be involved in diverse physiological and pathological processes [45]. It has various functional messengers in numerous vertebrates. The liver is an organ that is clearly influenced by NO [46]. Using different models of liver damage, the role of NO in the liver remains controversial and it may be associated with both beneficial and detrimental consequences [47]. NO was found to have a protective role in mild oxidative hepatotoxicity models, although this was not the case in severe hepatotoxicity models. In the hepatocytes, there is a balance between the generation rate of NO and its degradation. The disturbance in this balance led to harmful free radical formation, which may be responsible for liver toxicity [45]. NO is a highly reactive oxidant synthesized from L-arginine by inducible nitric oxide synthase (NOS II) and produced from parenchymal and nonparenchymal liver cells [48, 49]. In this study, the treatment of rats with CCl4 led to a significant unexpected decrease of liver total nitrates contents compared to the rats in the control group. In addition, the histopathological results showed enhanced severe liver damage, suggesting that the decreased NO release in the liver is attributable to CCl4-induced hepatotoxicity. Similarly, Laskin et al. [50] found that CCl4-induced hepatotoxicity was increased in mice lacking the gene for NO synthesis. On the other hand, pretreatment of rats with SMSE and silymarin resulted in a significant increase in liver NO content. Previous data suggested that the hepatoprotective effects of nitric oxide in this model may be due in part to the inhibition of TNF-α [51]. The attenuation of metal/peroxide oxidative chemistry, as well as lipid peroxidation, appears to be the major chemical mechanisms by which NO may limit oxidative injury [52].
5. Conclusion
This study suggested a protective effect of the extract of SMSE against CCl4-induced hepatotoxicity. The mechanisms for the hepatoprotective effect center on the increased GSH synthesis, increased GPx, CAT, and SOD activities, and decreased MDA generation. In addition, a potential mechanism for the hepatoprotective role of the crude SMSE against CCl4-induced hepatotoxicity may center on its role in NO homeostasis.
Acknowledgment
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant no. 141-012-D1434. The authors therefore acknowledge, with thanks, DSR technical and financial support.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Khan RA, Khan MR, Sahreen S, Shah NA. Hepatoprotective activity of Sonchus asper against carbon tetrachloride-induced injuries in male rats: a randomized controlled trial. BMC Complementary and Alternative Medicine. 2012;12, article 90 doi: 10.1186/1472-6882-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin A, Mahmoud-Ghoneim D. Zizyphus spina-christi protects against carbon tetrachloride-induced liver fibrosis in rats. Food and Chemical Toxicology. 2009;47(8):2111–2119. doi: 10.1016/j.fct.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Kim J, Choi J, et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice. Journal of Pharmacological Sciences. 2010;112(1):105–112. doi: 10.1254/jphs.09234fp. [DOI] [PubMed] [Google Scholar]
- 4.Recknagel RO, Glende EA, Jr., Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacology and Therapeutics. 1989;43(1):139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 5.Weber LWD, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Critical Reviews in Toxicology. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 6.Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. Journal of Environmental Science and Health C: Environmental Carcinogenesis and Ecotoxicology Reviews. 2007;25(3):185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 7.Belarbi EH, Contreras Gómez A, Chisti Y, García Camacho F, Molina Grima E. Producing drugs from marine sponges. Biotechnology Advances. 2003;21(7):585–598. doi: 10.1016/s0734-9750(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 8.Koopmans M, Martens D, Wijffels RH. Towards commercial production of sponge medicines. Marine Drugs. 2009;7(4):787–802. doi: 10.3390/md7040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sipkema D, Franssen MCR, Osinga R, Tramper J, Wijffels RH. Marine sponges as pharmacy. Marine Biotechnology. 2005;7(3):142–162. doi: 10.1007/s10126-004-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu YZ. Recent natural products based drug development: a pharmaceutical industry perspective. Journal of Natural Products. 1998;61(8):1053–1071. doi: 10.1021/np9800102. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda M, Sakuma Y, Kobayashi J. Suberedamines A and B, new bromotyrosine alkaloids from a sponge Suberea species. Journal of Natural Products. 2001;64(7):980–982. doi: 10.1021/np010077g. [DOI] [PubMed] [Google Scholar]
- 12.Gunasekera SP, Cross SS. Fistularin 3 and 11-ketofistularin 3. Feline leukemia virus active bromotyrosine metabolites from the marine sponge Aplysina archeri. Journal of Natural Products. 1992;55(4):509–512. doi: 10.1021/np50082a020. [DOI] [PubMed] [Google Scholar]
- 13.Hirano K, Kubota T, Tsuda M, Watanabe K, Fromont J, Kobayashi J. Ma'edamines A and B, cytotoxic bromotyrosine alkaloids with a unique 2(1H)pyrazinone ring from sponge Suberea sp. Tetrahedron. 2000;56(41):8107–8110. [Google Scholar]
- 14.Bowden BF, McCool BJ, Willis RH. Lihouidine, a novel spiro polycyclic aromatic alkaloid from the marine sponge Suberea n. sp. (Aplysinellidae, Verongida) The Journal of Organic Chemistry. 2004;69(23):7791–7793. doi: 10.1021/jo0498819. [DOI] [PubMed] [Google Scholar]
- 15.Takamatsu S, Hodges TW, Rajbhandari I, Gerwick WH, Hamann MT, Nagle DG. Marine natural products as novel antioxidant prototypes. Journal of Natural Products. 2003;66(5):605–608. doi: 10.1021/np0204038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song L, Li T, Yu R, Yan C, Ren S, Zhao Y. Antioxidant activities of hydrolysates of Arca subcrenata prepared with three proteases. Marine Drugs. 2008;6(4):607–619. doi: 10.3390/md6040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunassee SN, Davies-Coleman MT. Cytotoxic and antioxidant marine prenylated quinones and hydroquinones. Natural Product Reports. 2012;29(5):513–535. doi: 10.1039/c2np00086e. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Wu W, Wang J, Lan M. Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Marine Drugs. 2012;10(1):119–130. doi: 10.3390/md10010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Science and Technology. 1995;28(1):25–30. [Google Scholar]
- 20.Marxen K, Vanselow KH, Lippemeier S, Hintze R, Ruser A, Hansen U-P. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors. 2007;7(10):2080–2095. doi: 10.3390/s7102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handa SS, Sharma A. Hepatoprotective activity of Andrographolide from andrographis paniculata against carbontetrachloride. The Indian Journal of Medical Research B: Biomedical Research Other Than Infectious Diseases. 1990;92:276–283. [PubMed] [Google Scholar]
- 22.Singhal KG, Gupta GD. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pacific Journal of Tropical Medicine. 2012;5(9):677–685. doi: 10.1016/S1995-7645(12)60106-0. [DOI] [PubMed] [Google Scholar]
- 23.Gupta RK, Hussain T, Panigrahi G, et al. Hepatoprotective effect of Solanum xanthocarpum fruit extract against CCl4 induced acute liver toxicity in experimental animals. Asian Pacific Journal of Tropical Medicine. 2011;4(12):964–968. doi: 10.1016/S1995-7645(11)60227-7. [DOI] [PubMed] [Google Scholar]
- 24.Tsai C, Hsu Y, Chen W, et al. Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food and Chemical Toxicology. 2009;47(8):2031–2036. doi: 10.1016/j.fct.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Uraz S, Tahan V, Aygun C, et al. Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Digestive Diseases and Sciences. 2008;53(4):1071–1077. doi: 10.1007/s10620-007-9949-3. [DOI] [PubMed] [Google Scholar]
- 26.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 27.Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Akerboom TPM, Sies H. [48] Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods in Enzymology. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- 29.Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2004;286(3):R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 30.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 31.Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 32.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Monem NM, Abdel-Azeem AM, El-Ashry EH, Ghareeb DA, Nabil-Adam A. Pretreatment hepatoprotective effect of the marine fungus derived from sponge on hepatic toxicity induced by heavy metals in rats. BioMed Research International. 2013;2013:15 pages. doi: 10.1155/2013/510879.510879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy KNC, Rajesha J, Swamy MM, Ravishankar GA. Comparative evaluation of hepatoprotective activity of carotenoids of microalgae. Journal of Medicinal Food. 2005;8(4):523–528. doi: 10.1089/jmf.2005.8.523. [DOI] [PubMed] [Google Scholar]
- 35.Kuriakose GC, Kurup GM. Antioxidant activity of Aulosira fertilisima on CCl4 induced hepatotoxicity in rats. Indian Journal of Experimental Biology. 2008;46(1):52–59. [PubMed] [Google Scholar]
- 36.Simeonova R, Kondeva-Burdina M, Vitcheva V, Krasteva I, Manov V, Mitcheva M. Protective effects of the apigenin-O/C-diglucoside saponarin from Gypsophila trichotoma on carbone tetrachloride-induced hepatotoxicity in vitro/in vivo in rats. Phytomedicine. 2014;21(2):148–154. doi: 10.1016/j.phymed.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Jones TW, Thor H, Orrenius S. Cellular defense mechanisms against toxic substances. Archives of Toxicology. 1986;59(9):259–271. doi: 10.1007/978-3-642-71248-7_41. [DOI] [PubMed] [Google Scholar]
- 38.Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochemical Pharmacology. 1992;44(10):1905–1915. doi: 10.1016/0006-2952(92)90091-v. [DOI] [PubMed] [Google Scholar]
- 39.Lind KF, Hansen E, Østerud B, et al. Antioxidant and anti-inflammatory activities of barettin. Marine Drugs. 2013;11(7):2655–2666. doi: 10.3390/md11072655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupont S, Carre-Mlouka A, Descarrega F, et al. Diversity and biological activities of the bacterial community associated with the marine sponge Phorbas tenacior (Porifera, Demospongiae) Letters in Applied Microbiology. 2014;58(1):42–52. doi: 10.1111/lam.12154. [DOI] [PubMed] [Google Scholar]
- 41.Abou-Shoer MI, Shaala LA, Youssef DTA, Badr JM, Habib AM. Bioactive brominated metabolites from the red sea sponge Suberea mollis. Journal of Natural Products. 2008;71(8):1464–1467. doi: 10.1021/np800142n. [DOI] [PubMed] [Google Scholar]
- 42.Shaala LA, Bamane FH, Badr JM, Youssef DTA. Brominated arginine-derived alkaloids from the red sea sponge suberea mollis. Journal of Natural Products. 2011;74(6):1517–1520. doi: 10.1021/np200120d. [DOI] [PubMed] [Google Scholar]
- 43.Kiokias S, Gordon M. Properties of carotenoids in vitro and in vivo. Food Reviews International. 2004;20:99–121. [Google Scholar]
- 44.Chen H, Yen G. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chemistry. 2007;101(2):686–694. [Google Scholar]
- 45.Chen T, Zamora R, Zuckerbraun B, Billiar TR. Role of nitric oxide in liver injury. Current Molecular Medicine. 2003;3(6):519–526. doi: 10.2174/1566524033479582. [DOI] [PubMed] [Google Scholar]
- 46.Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: friend, foe, or just passerby? Annals of the New York Academy of Sciences. 2002;962:275–295. doi: 10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 47.Koerber K, Sass G, Kiemer AK, Vollmar AM, Tiegs G. In vivo regulation of inducible NO synthase in immune-mediated liver injury in mice. Hepatology. 2002;36(5):1061–1069. doi: 10.1053/jhep.2002.36155. [DOI] [PubMed] [Google Scholar]
- 48.Farghali H, Hynie S, Vohnikova Z, Masek K. Possible dual role of nitric oxide in oxidative stress injury: a study in perfused hepatocytes. International Journal of Immunopharmacology. 1997;19(9-10):599–605. doi: 10.1016/s0192-0561(97)00048-9. [DOI] [PubMed] [Google Scholar]
- 49.Geller DA, Lowenstein CJ, Shapiro RA, et al. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laskin DL, Heck DE, Gardner CR, Feder LS, Laskin JD. Distinct patterns of nitric oxide production in hepatic macrophages and endothelial cells following acute exposure of rats to endotoxin. Journal of Leukocyte Biology. 1994;56(6):751–758. doi: 10.1002/jlb.56.6.751. [DOI] [PubMed] [Google Scholar]
- 51.Morio LA, Chiu H, Sprowles KA, et al. Distinct roles of tumor necrosis factor-α and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicology and Applied Pharmacology. 2001;172(1):44–51. doi: 10.1006/taap.2000.9133. [DOI] [PubMed] [Google Scholar]
- 52.Wink DA, Miranda KM, Espey MG, et al. Mechanisms of the antioxidant effects of nitric oxide. Antioxidants & Redox Signaling. 2001;3(2):203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]


