Abstract
Pertussis toxin (PT), a virulence factor secreted by Bordetella pertussis, contributes to respiratory tract infection and disease caused by this pathogen. By comparing a wild-type (WT) B. pertussis strain to a mutant strain with an in-frame deletion of the ptx genes encoding PT (ΔPT), we recently found that the lack of PT confers a significant defect in respiratory tract colonization in mice after intranasal inoculation. In this study, we analyzed serum antibody responses in mice infected with the WT or ΔPT strain and found that infection with the ΔPT strain elicited greater responses to several B. pertussis antigens than did infection with the WT, despite the lower colonization level achieved by the ΔPT strain. The same enhanced antibody response was observed after infection with a strain expressing an enzymatically inactive PT; but this response was not observed after infection with B. pertussis mutant strains lacking filamentous hemagglutinin or adenylate cyclase toxin, nor when purified PT was administered with the ΔPT inoculum, indicating a specific role for PT activity in this immunosuppressive effect. In particular, there were consistent strong serum antibody responses to one or more low-molecular-weight antigens after infection with the ΔPT strain. These antigens were Bvg independent, membrane localized, and also expressed by the closely related pathogens Bordetella parapertussis and Bordetella bronchiseptica. Two-dimensional gel electrophoresis and mass spectrometry were used to identify one of the immunodominant low-molecular-weight antigens as a protein with significant sequence homology to peptidoglycan-associated lipoprotein in several other gram-negative bacterial species. However, a serum antibody response to this protein alone did not protect mice against respiratory tract infection by B. pertussis.
Pertussis toxin (PT) is an important virulence factor produced exclusively by Bordetella pertussis, a gram-negative bacterial pathogen that colonizes the human respiratory tract and causes a disease known as whooping cough or pertussis. PT is a member of the AB5 structural class of bacterial toxins (41, 43), consisting of an enzymatically active A subunit (S1) that ADP-ribosylates the alpha subunit of the Gi family of heterotrimeric G proteins in mammalian cells (17, 30) and a pentameric B oligomer that binds unidentified glycoconjugate receptors on cells (5, 48). PT activity has a wide range of effects on signaling pathways and normal function in mammalian cells (35, 49). The administration of purified PT to experimental animals can reproduce almost all of the systemic symptoms associated with human pertussis disease, such as histamine sensitivity, lymphocytosis, and insulinemia (29, 31, 32), but its role in respiratory tract colonization and disease is uncertain. Several studies of mice intranasally infected with bacteria have shown that PT plays a role in the overall respiratory tract infection by B. pertussis (2, 10, 45, 46). Recently, it was found that PT is a significant colonization factor for respiratory tract infection of mice by B. pertussis and plays an early role in this host-pathogen interaction (6). We hypothesized that one potential target for PT activity in its ability to enhance infection is innate immunity, the mechanisms of which are elicited early in response to infection. Indeed, purified PT has been found to have several adverse effects on cells involved in innate immunity, such as macrophages (15), neutrophils (39), mast cells (26), and dendritic cells (4). However, clearance of and protection against B. pertussis infection is mediated, at least in part, by humoral and adaptive mechanisms of immunity, including antibodies (11, 21, 23, 24, 28), and purified PT has also been found to have various effects on lymphocytes, which are responsible for adaptive immunity and the production of antibodies (7, 15, 39). We therefore decided to analyze the serum antibody responses to respiratory tract infection of mice with either a wild-type (WT) or PT-deficient (ΔPT) strain of B. pertussis. A previous study indicated that intranasal administration of a ΔPT strain of B. pertussis resulted in a greater serum antibody response to filamentous hemagglutinin (FHA), a surface adherence factor, than that elicited by infection with the WT strain (27). Another study indicated that overall anti-Bordetella serum antibody responses elicited in response to the intranasal infection of mice with B. pertussis Tohama I (a WT strain) were barely detectable (11). Therefore, we hypothesized that PT produced by WT B. pertussis strains in the course of respiratory tract infection might suppress serum antibody responses to B. pertussis antigens, identifying another possible role for PT in this host-pathogen interaction. Indeed, we found that infection with our ΔPT strain elicited greater responses to several B. pertussis antigens than infection with the WT, in particular to low-molecular-weight (LMW) antigens, one of which we identified as a protein with significant sequence homology to peptidoglycan-associated lipoprotein (Pal) in several other gram-negative bacterial species.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bordetella strains used in this study were as follows: streptomycin- and nalidixic acid-resistant derivatives of B. pertussis Tohama I (16) and 18323 (ATCC 9797), Bordetella parapertussis strain BPP.P14 (3), and Bordetella bronchiseptica strain 7865 (3). The B. pertussis Tohama I ΔPT, FHA-deficient (ΔFHA), and cyclase-deficient (ΔCYA) derivatives were constructed as described below. The ΔBVG strain contains a deletion of the bvgA and bvgS genes, with replacement of this region by a kanamycin-resistance gene, and was constructed as previously described (25). Bordetella strains were grown on Bordet-Gengou (BG) agar (Difco) plates containing 15% defibrinated sheep blood and the following antibiotics where necessary: streptomycin (400 μg/ml), nalidixic acid (20 μg/ml), or gentamicin (10 μg/ml). Liquid cultures were grown in Stainer-Scholte medium (40) containing heptakis-dimethylcyclodextrin (Sigma). The Escherichia coli strains used were DH10B (34) for standard cloning experiments and S17.1 (38) for conjugation with B. pertussis, and these were grown on Luria-Bertani agar plates containing, where necessary, either gentamicin (10 μg/ml) or ampicillin (100 μg/ml).
Plasmid and strain construction.
An in-frame deletion of the ptx genes (encoding the five PT subunits) was derived as previously described (6). The resulting strain (ΔPT) and a parental strain (WT) emerging from the same conjugation were used for further experiments (the ΔPT strain differs from the WT putatively only by the lack of PT production). An in-frame deletion of the cyaA gene (encoding adenylate CYA toxin) was constructed by PCR amplification of two fragments from the cya region, an upstream fragment (by oligonucleotide primers 958 [5′-GATATCTAGACGGACGGAAGCATGACAT-3′] and 959 [5′-GATAGAATTCGTAACCAGCCTGATGCGAT-3′] and containing the sequence encoding the first 9 amino acids of CyaA) and a downstream fragment (by oligonucleotide primers 960 [5′-GATAGAATTCGATCACCGGGTGGAAAT-3′] and 961 [5′-GATAGGTACCGGACGCTGGATGAGTA-3′] and containing the sequence encoding the last 57 amino acids of CyaA). The fragments were doubly digested with XbaI/EcoRI and EcoRI/KpnI, respectively, and ligated with XbaI/KpnI-digested pJHC1 (20) to derive the plasmid pJ-ΔCYA. Similarly, an in-frame deletion of the fhaB gene (encoding FHA) was constructed by PCR amplification of two fragments from the fha region, an upstream fragment (by oligonucleotide primers 1008 [5′-GATATCTAGACCTCGTGCAGGTTCTC-3′] and 1006 [5′-GATTGGATCCTGTACAGGTTCGTGTTCA-3′] and containing the sequence encoding the first seven amino acids of FhaB) and a downstream fragment (by oligonucleotide primers 1007 [5′-GATAGGATCCTCTTCTATGAAACCAACAAATAG-3′] and 1009 [5′-GATAGGTACCGCAGGCGTAAACCATCCCT-3′] and containing the sequence encoding the last six amino acids of FhaB). The fragments were doubly digested with XbaI/BamHI and BamHI/KpnI, respectively, and ligated with XbaI/KpnI-digested pJHC1 to derive the plasmid pJ-ΔFHA. The correct deletion and flanking sequences on these plasmids were confirmed by restriction enzyme digestion and DNA sequencing. The plasmids were transformed into E. coli S17.1 and then introduced into the B. pertussis chromosome by conjugation and allelic exchange as described previously (42). Exconjugants were screened by PCR amplification to identify strains with the appropriate deletion. A Pal construct with 15 additional residues (Pal+15) at the C terminus was obtained by overlap extension PCR (14) using oligonucleotides 1126 (5′-TGTCTGCCCTCCGATTTTCCGCAAAGTCGGCGCGCTGATAAACGATATCGG-3′) and 1127 (5′-AAATCGGAGGGCAGACATCGTCTACCAGCGGTAAGTCCTCGCGACGCCAA-3′) to insert the additional 15 residues at the C terminus (a repeat of the C-terminal 15 residues). The fragment was inserted into pJHC1, and the correct insertion was confirmed by DNA sequencing and introduced into the B. pertussis chromosome by conjugation and allelic exchange. Exconjugants were screened by PCR amplification to identify strains with the appropriate insertion.
GST fusion construction and purification.
The Pal open reading frame from codon 22 (putatively the second codon of the mature protein) to the C terminus was amplified by PCR using oligonucleotides 1091 (5′-GATAAGATCTAGTTCCGTCCCTCTCGAC-3′) and 1090 (5′-GATAGAATTCCGTGGTGGGGCTGCTT-3′). This fragment was doubly digested with BglII and EcoRI, ligated with the BamHI and EcoRI doubly digested vector pGEX-2T (Pharmacia), and transformed into E. coli DH10B. The correct fusion was confirmed by DNA sequencing. For induction of the fusion protein, the strain was grown in Luria-Bertani broth to an A600 of 1.0, and then IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a concentration of 0.5 mM. Samples were taken just before the addition of IPTG and then 1 and 2 h after adding IPTG. Cells were centrifuged, resuspended in sample buffer, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. The glutathione transferase (GST)-Pal fusion protein was purified from a 250-ml culture induced as above. Cells were lysed in bacterial protein extraction reagent (Pierce), cleared by centrifugation, and passed through a GSTrap column (Pharmacia). The fusion protein was eluted in reduced glutathione buffer, dialyzed against phosphate-buffered saline (PBS), and analyzed by SDS-PAGE and bicinchoninic acid assay (Pierce) to determine protein concentration.
Western blotting.
Bacterial whole-cell lysates or cellular fractions were run on SDS-12% PAGE or SDS-15% PAGE gels and transferred to nitrocellulose membranes. Proteins were visualized on the membranes with Ponceau stain and, when necessary, cut into strips of the individual sample lanes. Membranes were blocked in 5% milk in Tris-buffered saline (TBS)-Tween buffer and then incubated in primary antibody (either mouse serum or a monoclonal antibody), followed by secondary horseradish peroxidase-conjugated anti-mouse immunoglobulin (Ig) antibody. Proteins bound by the antibodies were visualized by enhanced chemiluminescence.
ELISA.
For the enzyme-linked immunosorbent assay (ELISA), 96-well plates were coated for 3 h with either heat-killed B. pertussis Tohama I WT (3 × 109 CFU/ml in TBS, pH 9.6) or GST-Pal fusion protein (5 μg/ml in TBS, pH 9.6), and plates were blocked overnight with 5% bovine serum albumin (BSA). Twofold dilutions of sera (starting at 1:25) were assayed, and endpoint titers were those with optical density at 450 nm (OD450) values at least 0.05 units above background.
PT preparation.
PT was prepared from B. pertussis culture supernatants by the fetuin affinity method of Kimura et al. (19), resuspended in PBS, and stored at −80°C until use. The protein concentration was determined by bicinchoninic acid assay (Pierce), and the presence or absence of toxin activity was assessed by a CHO cell clustering assay (13).
Mouse infection.
Six-week-old female BALB/c or C57BL6 mice (Charles River) were used for infection and immunization experiments. Preparation of inocula, intranasal inoculation, and assessment of respiratory tract colonization were performed as previously described (6). At the indicated time points, mice were sacrificed by carbon dioxide inhalation, and blood was obtained by cardiac puncture. Serum was obtained by allowing the blood to clot for 3 h at room temperature; samples were then subjected to centrifugation, and sera were stored at −20°C.
Two-dimensional PAGE.
The B. pertussis ΔBVG strain was resuspended from BG agar plates in PBS to an OD600 of 0.3. A 3-ml aliquot was washed twice with cold PBS and once with 50 mM Tris, pH 8.0. Cells were boiled for 2 min in 80 μl of SB1 (40 mM Tris [pH 8.0], 200 mM dithiothreitol, 0.3% [wt/vol] SDS). Next, 8 μl of SB2 (500 mM Tris [pH 8.0], 50 mM MgCl2, 1 mg of DNase I ml−1, 0.25 mg of RNase A ml−1) was added, and cells were incubated on ice for 30 min. Acetone was added to 80% (vol/vol), and proteins were precipitated by centrifugation. The pellet was resuspended in 80 μl of SB1 and precipitated again with acetone. After air drying, the pellet was resuspended in 60 μl of SB1 and 240 μl of SB3 (9.9 M urea, 100 mM dithiothreitol, 4% [vol/vol] Triton X-100, 2.2% [vol/vol] polyampholytes [pH 3to 10]) (Genomic Solutions). Two-dimensional PAGE was performed with a Genomic Solutions Investigator 2-D electrophoresis system, following the manufacturer's protocol. Proteins (50 μl of lysate) were separated in the first dimension by using an isoelectric focusing tube gel (pH 3 to 10) and in the second dimension in a 15% polyacrylamide gel. Gels were stained with SilverQuest silver staining solutions (Invitrogen), proteins of interest were cut out of the gels, and mass spectrometry analysis of trypsin-digested proteins was performed at the W. M. Keck Biomedical Mass Spectrometry Laboratory at the University of Virginia Biomolecular Research Facility.
Cell fractionation.
The B. pertussis WT cells were resuspended from BG agar plates in 50 ml of PBS to an OD600 of 2.0, centrifuged, resuspended in 10 ml of 10 mM Tris (pH 8), 20% sucrose, 1 mM EDTA, and 0.1 mg of lysozyme/ml and incubated on ice for 10 min. The cell suspension was then frozen in a dry ice-ethanol bath and thawed immediately, and cells were lysed in a French press. The lysate was cleared by centrifugation at 6,000 × g for 10 min, and then the membrane fraction was pelleted by centrifugation at 125,000 × g for 30 min and resuspended in 1 ml of PBS. Soluble and membrane fractions were stored at −20°C until use.
Indirect immunofluorescence analysis.
WT cells were spread onto coverslips previously coated with poly-l-lysine (0.1% in PBS), and the coverslips were rinsed in PBS. Bacteria were fixed in 3% paraformaldehyde in PBS for 10 min and washed in PBS before staining with antiserum from a mouse immunized subcutaneously with the GST-Pal fusion protein (1:100 dilution in PBS containing 1% BSA) for 20 min at room temperature. A control sample remained in PBS without primary antibody staining. After washing in PBS, bacteria were stained with fluorescein isothiocyanate-conjugated goat anti-mouse Ig (1:100 dilution in PBS containing 1% BSA; Jackson ImmunoResearch Laboratories) for 15 min at room temperature in the dark. After washing in PBS, coverslips were mounted onto slides on mounting solution (0.1 M N-propyl gallate in glycerol) and observed with a fluorescence microscope (Zeiss) equipped with the ×100 objective. Images were captured with a CCD camera (Sensys) equipped with IPlab software (Signal Analytics Corp.).
RESULTS
Serum antibody responses to respiratory tract infection with the WT and ΔPT strains.
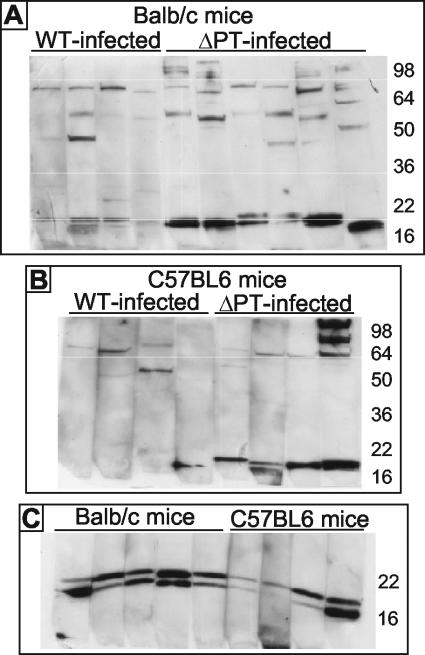
Six-week-old female BALB/c mice were intranasally inoculated with the Tohama I-derived WT or ΔPT (approximately 5 × 105 CFU) strain, and serum was obtained from groups of four mice on days 4, 7, 10, 14, and 21 postinoculation. Colonization profiles of these two strains were as previously reported (6), with the ΔPT strain showing a significant colonization defect at almost all time points. Sera from mice within the groups were pooled and used in Western blotting of whole-cell lysates of B. pertussis WT. There were few antigens to which an antibody response could be detected at any time point, but with day 21 postinoculation sera there appeared to be a relatively strong response to one or more LMW antigens in mice infected with the ΔPT strain but not in mice infected with the WT (data not shown). To determine whether this difference in antibody response was somehow related to the different colonization levels achieved by the WT and ΔPT strains, we inoculated groups of mice with higher (approximately 5 × 106 CFU) and lower (approximately 5 × 104 CFU) doses of the WT and with a high (approximately 107 CFU) dose of the ΔPT strain. From this group of mice, sera (pooled within groups) was obtained on days 21 and 28 postinoculation and analyzed by Western blotting as before. None of the groups of mice inoculated with the WT demonstrated a strong response to the LMW antigens (although a detectable response occurred with the higher dose), whereas the group inoculated with the higher dose of the ΔPT strain showed the same strong response as mice inoculated with the standard dose (data not shown), demonstrating that this effect was strain dependent and not dose dependent. To determine whether the response to the LMW antigens detected in the pooled sera was due to an aberrant response in a single mouse in the groups infected with the ΔPT strain or a representative response of individual mice, we infected mice with approximately 5 × 105 CFU of the WT or ΔPT strain as before and collected sera from these mice 28 days postinoculation. The individual sera were then tested by Western blot analysis as before, and the result showed that the strong response to the LMW antigens was seen in almost all of the BALB/c mice infected with the ΔPT strain but in none of the WT-infected mice (Fig. 1A). This was also true in C57BL6 mice (Fig. 1B), demonstrating that this phenomenon was not specific to a particular mouse genetic background. Western blot analysis of lysates run on an SDS-15% PAGE gel (instead of the previous 12% gel) probed with sera from the mice infected with the ΔPT strain revealed the presence of two strongly reacting LMW (approximately 15 to 20 kDa) antigens, the relative responses against which were different for different mice (Fig. 1C). There were no other consistently strong antibody responses detected in this assay, although in general responses were greater and more numerous in mice infected with the ΔPT strain than in WT-infected mice (Fig. 1). ELISA experiments with the individual sera from the BALB/c and C57BL6 mice infected with the WT or ΔPT strain revealed that there was an approximately twofold greater mean anti-B. pertussis antibody response in the ΔPT strain-infected mice than in the WT-infected mice (data not shown).
FIG. 1.
Serum antibody response of individual BALB/c or C57BL6 mice to respiratory tract infection with the Tohama I WT or ΔPT strain. (A and B) Western blots of whole-cell lysates of WT run on SDS-12% PAGE gels probed with individual sera (1:50 dilution) from BALB/c or C57BL6 mice obtained 28 days after infection with either the WT or ΔPT strain. (C) Western blots of whole-cell lysates of the WT run on SDS-15% PAGE gels probed with individual sera (1:50 dilution) from BALB/c or C57BL6 mice obtained 28 days after infection with the ΔPT strain. The molecular mass (kDa) of protein markers is indicated at right.
Serum antibody responses to respiratory tract infection with other mutants.
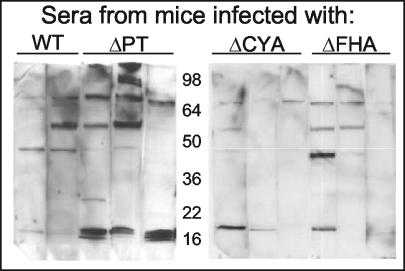
To determine whether the strong response to the LMW antigens was specific to infection with the ΔPT strain or might be elicited by infection with other B. pertussis mutants with various degrees of attenuation in this infection model, we inoculated groups of BALB/c mice with approximately 5 × 105 CFU of either the WT, ΔPT, ΔCYA, or ΔFHA strain. The ΔCYA strain has an approximately 10-fold reduction in peak respiratory tract colonization compared to the WT, while the reduction for the ΔFHA strain is only about twofold, and both strains secrete normal levels of PT (our unpublished data). We collected sera from individual mice 28 days postinoculation and analyzed the sera by Western blotting as before. As seen in Fig. 2, only infection with the ΔPT strain elicited a consistently strong response to the LMW antigens, demonstrating that this effect was specific to infection with the ΔPT strain and not due to attenuated infection with any B. pertussis mutant.
FIG. 2.
Serum antibody response of mice to respiratory tract infection with Tohama I WT or different mutant strains. Western blots of whole-cell lysates of the WT strain run on SDS-12% PAGE gels probed with individual sera (1:50 dilution) from BALB/c mice obtained 28 days after infection with the indicated strain.
Effect of PT on serum antibody response to the LMW antigens.
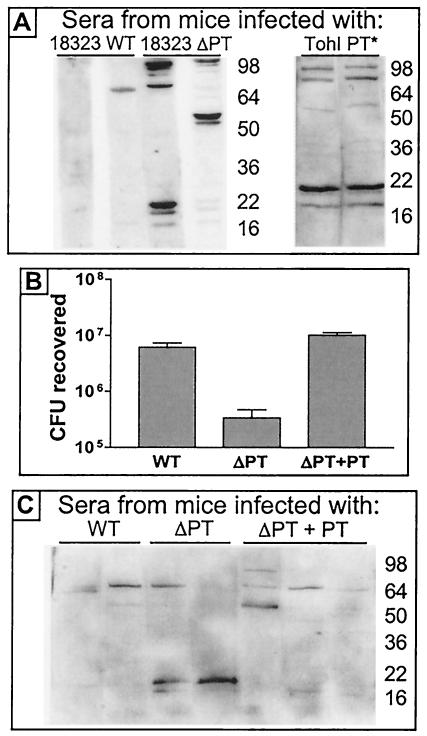
We hypothesized that the increased serum antibody response elicited in mice by infection with the ΔPT strain versus infection with the WT was due to the lack of PT production by the ΔPT strain and that PT produced by the WT somehow suppressed this antibody response. However, another possibility was that our ΔPT strain was antigenically different from the WT, and this difference caused the different antibody response independently of the presence or absence of PT. We took several approaches to distinguish between these possibilities. First we compared lysates of the WT and ΔPT strains by Western blotting with the mouse serum containing high levels of antibodies to the LMW antigens and observed no difference in the bands detected by this serum (data not shown), demonstrating that there was no major difference in the antigenic makeup of the two strains (at least with respect to the LMW antigens). We next analyzed serum antibody responses to infection with WT or ΔPT derivatives of a different B. pertussis background strain, 18323. Sera from BALB/c mice obtained 21 days after infection with approximately 5 × 105 CFU of strain 18323 WT or ΔPT derivatives showed a similar pattern as in previous experiments, with a clearly observable response to the LMW antigens in sera from mice infected with the ΔPT strain (though the response in one mouse was relatively weak) but little or no such response in sera from WΤ-infected mice (Fig. 3A). In addition, we analyzed serum antibody responses to infection with a B. pertussis strain (Tohama I background) expressing an inactive mutant form of PT, PT-9K/129G (33), and once again found a strong response to the LMW antigens in serum obtained 21 days postinfection (Fig. 3A). Together these data strongly suggested that the lack of active PT during B. pertussis infection of the mouse respiratory tract, rather than an antigenic change in the ΔPT strain, was responsible for the increased serum antibody responses observed. To confirm this idea, we infected BALB/c mice with approximately 5 × 105 CFU of the original ΔPT strain mixed with 10 ng of purified PT. We previously found that intranasal coadministration of this amount of purified PT restored the colonization of the ΔPT strain to WT levels (6), and we hypothesized that if the serum antibody responses to the LMW antigens were due to the lack of PT during infection, then coadministration of PT with the ΔPT strain would suppress this response. We first confirmed that colonization of the ΔPT strain 7 days postinfection was increased to WT levels by PT coadministration in these mice (Fig. 3B) and then analyzed antibody responses in sera collected 21 days postinoculation from these groups of mice by Western blotting as before. As seen in Fig. 3C, the strong responses to the LMW antigens were indeed suppressed by coadministration of PT with the bacteria, whereas infection with the ΔPT strain without added PT once again elicited the typical strong response. This result confirms that the serum antibody response to the LMW antigens is due to the lack of PT production by the ΔPT strain and that PT produced by WT bacteria (or administered as purified protein) suppresses this response, at least up to 28 days postinoculation.
FIG. 3.
Effect of PT on the serum antibody response of mice to respiratory tract infection with B. pertussis. (A) Western blots of whole-cell lysates of the Tohama I WT strain run on SDS-12% PAGE gels probed with individual sera (1:50 dilution) from BALB/c mice obtained 21 days after infection with either the 18323 WT, 18323 ΔPT, or Tohama I strain expressing inactive mutant PT9K/129G (TohI PT*). (B) Respiratory tract colonization levels of BALB/c mice 7 days after infection with 5 × 105 CFU of the Tohama I WT or ΔPT strain or the ΔPT strain mixed with 10 ng of PT (ΔPT+PT). (C) Western blots of whole-cell lysates of Tohama I WT run on SDS-12% PAGE gels probed with individual sera (1:50 dilution) from BALB/c mice obtained 21 days after infection with the Tohama I WT, ΔPT, or ΔPT+PT strain.
Characterization of the immunodominant LMW antigens.
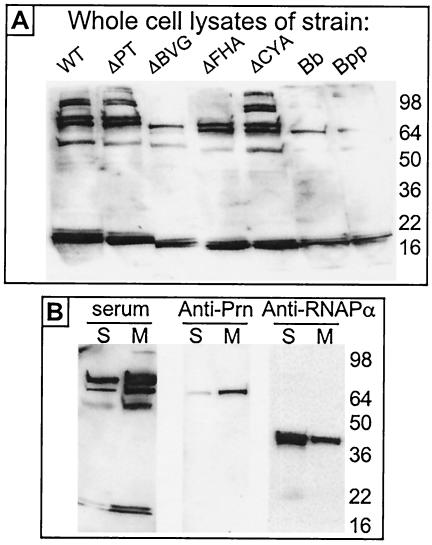
In a preliminary analysis of the nature of these immunodominant LMW antigens, we addressed the possibility that they were different forms of lipopolysaccharide (LPS), which can exist as at least two antigenically different LMW forms in B. pertussis (1). However, in addition to migrating at a different position on SDS-PAGE gels (LPS migrated faster), the LMW antigens were proteinase K susceptible, whereas LPS was proteinase K resistant (data not shown), thus identifying the antigens as proteins and not forms of LPS. To determine whether the expression of the LMW antigens was dependent upon the Bvg proteins (which control expression of all known virulence factors in B. pertussis), we used Western blotting to probe a lysate of the ΔBVG strain (lacking Bvg and Bvg-dependent proteins) with the mouse serum from mice infected with the ΔPT strain. As seen in Fig. 4A, the LMW antigens were present in the ΔBVG strain lysate, identifying them as Bvg-independent proteins, while some higher-molecular-weight antigens (presumably Bvg-dependent proteins, including FHA-derived proteins by comparison to the pattern of bands in the ΔFHA strain lysate) detected by the serum in a WT lysate were missing from the ΔBVG strain lysate. Furthermore, the LMW antigens were detected in lysates of B. parapertussis and B. bronchiseptica strains (Fig. 4A), indicating that they are proteins common to the major Bordetella species and not unique to B. pertussis. In addition, we performed cell lysis and fractionation into soluble and membrane components of the WT strain, followed by Western blotting with the mouse serum from the mice infected with the ΔPT strain or other control antibodies. As seen in Fig. 4B, the LMW antigens were enriched in the membrane fraction, similar to the known membrane protein pertactin and in contrast to the cytoplasmically located RNA polymerase alpha subunit, indicating that they are most likely membrane proteins.
FIG. 4.
(A) Western blots of whole-cell lysates of the indicated strains (WT and mutants of Tohama I, B. bronchiseptica [Bb], and B. parapertussis [Bpp]) run on SDS-12% PAGE gels probed with serum (1:30 dilution) from a BALB/c mouse obtained 28 days after infection with the Tohama I ΔPT strain. (B) Western blots of soluble (S) or membrane (M) fractions of Tohama I WT probed with serum (1:30 dilution) from a BALB/c mouse obtained 28 days after infection with the Tohama I ΔPT strain (serum) or the indicated monoclonal antibodies.
Identification of one of the LMW antigens.
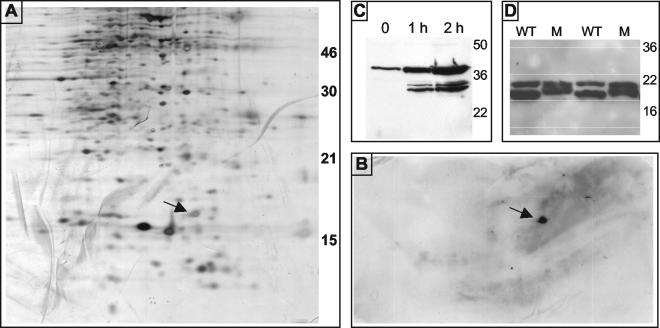
To determine the identity of the immunodominant LMW proteins identified by serum from mice infected with the ΔPT strain, we performed two-dimensional gel electrophoresis on lysates of the B. pertussis ΔBVG strain. One gel was silver stained to visualize protein spots (Fig. 5A), and another equivalent gel was transferred to nitrocellulose and probed with serum from the mice infected with the ΔPT strain, which detected a single spot of approximately 15 kDa (Fig. 5B). The corresponding protein spot (Fig. 5A, arrow) was excised from the silver-stained gel and sent to the University of Virginia Biomedical Research Facility for mass spectrometry analysis and identification. The sequences of peptides identified after trypsin digestion of the protein are shown in Table 1 and correspond to the predicted protein product of an open reading frame identified from the B. pertussis Tohama I genome sequence (http://www.sanger.ac.uk/Projects/B_pertussis/). A BLAST search of homologies to this predicted protein identified highly significant matches to Pal from several gram-negative bacterial species, from which we concluded that our protein was the B. pertussis equivalent of Pal. This open reading frame corresponds to the gene identified as BP3342 in the Tohama I genome annotation, and the predicted protein contains a consensus lipoprotein sequence at the putative signal peptide cleavage site (between residues 20 and 21). To confirm that this protein was indeed one of the immunodominant LMW antigens detected by serum from the mice infected with the ΔPT strain, we first attempted to make an in-frame deletion of the corresponding gene on the Tohama I chromosome. However, repeated attempts at making such a deletion were unsuccessful, from which we concluded that the expression of this protein is likely essential for B. pertussis viability. We next attempted to insert an inducible promoter (the ptx promoter) upstream of the pal gene, but once again we were unsuccessful after multiple attempts. As an alternative to knocking out expression of Pal in B. pertussis, we constructed a fusion of the Pal open reading frame (without the predicted signal peptide) with GST in the plasmid vector pGEX-2T. E. coli DH10B strains containing this fusion plasmid (pGEX-Pal) or vector alone were grown to mid-log phase, and then 0.5 mM IPTG was added to induce expression of the GST-Pal fusion protein. Aliquots of the cultures were removed just before the addition of IPTG and then at 1 and 2 h after adding IPTG, and whole-cell lysates of these aliquots were analyzed by SDS-PAGE and Western blotting (Fig. 5C). Probing with serum from mice infected with the ΔPT strain revealed a strongly reacting band of approximately 38 kDa (the predicted size of a GST-Pal fusion protein), visible at low levels before IPTG induction and greatly increasing in intensity after IPTG induction, as well as lower-molecular-weight bands presumably corresponding to degradation products derived from the fusion protein. ELISA experiments using plates coated with the GST-Pal fusion protein revealed that that there was an approximately fivefold greater mean anti-Pal antibody response in the sera of mice infected with the ΔPT strain than in sera of WT-infected mice (corresponding to the sera tested as described in Fig. 1; data not shown).
FIG. 5.
(A) Silver-stained two-dimensional SDS-15% PAGE gel of the ΔBVG strain whole-cell lysate. Arrow indicates protein spot that was excised and identified. (B) Western blot of the lower half of a gel probed with serum (1:30 dilution) from a BALB/c mouse obtained 28 days after infection with the Tohama I ΔPT strain. The arrow indicates a protein spot that reacted with the serum. (C) Western blot of whole-cell lysates of E. coli DH10B pGEX-Pal after the IPTG induction of fusion protein expression for the indicated times run on SDS-12% PAGE gels and probed with serum (1:100 dilution) from a BALB/c mouse obtained 28 days after infection with the Tohama I ΔPT strain. (D) Western blot of whole-cell lysates of Tohama I WT or two different isolates of the Pal+15 strain (M) run on SDS-15% PAGE gels probed with serum (1:50 dilution) from a BALB/c mouse obtained 28 days after infection with the Tohama I ΔPT strain.
TABLE 1.
Sequences and masses of peptides derived from a trypsin-digested protein spot isolated from a two-dimensional gel
| Sequence | Mass (Da) |
|---|---|
| AGQAGGSGQGSASGQILDPFNPQSILAQQR | 2,940.5 |
| MMTLLGVSDNQIETISFGK | 2,084.0 |
| SVYFDFDSYTVSEQYR | 2,005.9 |
| ATGSSEADFAENR | 1,354.6 |
| GGAEYNLALGQR | 1,248.6 |
| IKIEGNTDER | 1,174.6 |
| RADIVYQR | 1,020.6 |
| YLASNNQQR | 1,093.5 |
| IEGNTDER | 933.43 |
| ADIVYQR | 864.46 |
| GLVETHAR | 882.48 |
| RADAVR | 687.39 |
To determine which of the two LMW antigens detected by most sera from mice infected with the ΔPT strain (Fig. 1B) was Pal, we constructed a larger derivative of the Pal protein by duplicating the C-terminal 15 amino acids at the C terminus of the protein and replacing the WT copy of Pal with this construct (Pal+15) by allelic exchange. Lysates of two independent isolates of this strain were analyzed by Western blotting with serum from the mice infected with the ΔPT strain. As seen in (Fig. 5D), the lower-molecular-weight band was increased in apparent molecular weight compared to the same band from the WT strain, while the higher-molecular-weight band was unchanged, indicating that Pal is the lower-molecular-weight protein of this pair. The identity of the other protein, which was not detected by Western blotting of the two-dimensional gel, remains undetermined.
To determine whether Pal is surface exposed, we also performed indirect immunofluorescence microscopy on intact WT bacteria by using antiserum from a mouse immunized subcutaneously with the GST-Pal fusion protein (see below). Fluorescent staining of the intact bacteria indicated that Pal is indeed surface exposed (data not shown), consistent with the previous conclusion that the LMW antigens are membrane proteins (Fig. 4B). A control sample in which the primary antiserum was omitted and bacteria were stained only with the secondary antibody was negative in this assay, demonstrating that bacteria were not simply binding the fluorescent secondary antibody.
Immunization with Pal and protection against B. pertussis respiratory tract infection.
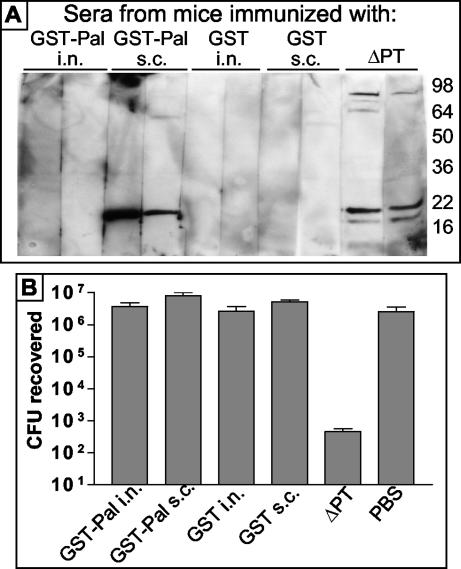
To determine whether a serum antibody response to Pal would be sufficient to confer protection against respiratory tract colonization by B. pertussis, we immunized groups of 6-week-old BALB/c mice with the GST-Pal fusion protein or with GST as a control. Mice were immunized with 20 μg of protein suspended in PBS (without adjuvant) either intranasally or subcutaneously on day 0 and then administered a booster dose on day 21. Additional control mice were infected intranasally either with 5 × 105 CFU of the ΔPT strain or with PBS on day 0. Sera were obtained from two mice per group on day 28 and analyzed by Western blotting of B. pertussis lysates; as seen in Fig. 6A, subcutaneous immunization with GST-Pal was successful in eliciting a serum antibody response to Pal, similar to infection with the ΔPT strain, while intranasal inoculation elicited no detectable response. Mice were challenged with 2 × 106 CFU of the WT strain intranasally inoculated on day 29, and respiratory tract colonization levels were assessed 4 days later. The results (Fig. 6B) showed that there was no protection afforded by immunization with the GST-Pal fusion protein, despite the anti-Pal serum antibody response in the subcutaneously immunized group, whereas infection with the ΔPT strain affords significant protection against colonization.
FIG. 6.
(A) Western blots of whole-cell lysates of Tohama I WT run on an SDS-12% PAGE gel probed with sera (1:50 dilution) from BALB/c mice immunized with the indicated protein by the indicated route or obtained 28 days after infection with the ΔPT strain. (B) Respiratory tract colonization levels of BALB/c mice previously immunized with the indicated protein or infected with the Tohama I ΔPT strain (or PBS as a control) 4 days after infection with 2 × 106 CFU of Tohama I WT. i.n., intranasal; s.c., subcutaneous.
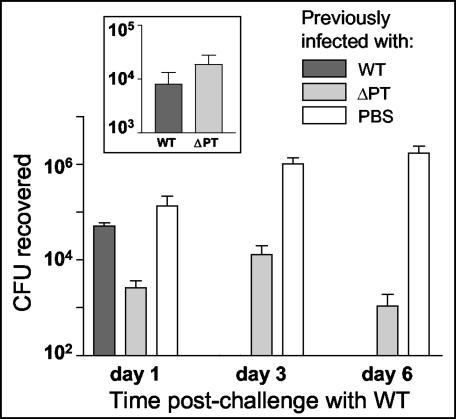
In addition, we compared the protective effects against respiratory tract colonization given by previous infection with equivalent levels of either the WT or ΔPT strain (or no infection) to test whether the increased serum antibody responses, particularly to Pal and the other LMW protein, elicited by infection with the ΔPT strain might provide superior protection. BALB/c mice were intranasally inoculated with either 104 CFU of the WT or 2 × 105 CFU of the ΔPT strain (or administered PBS as a control), and colonization levels assessed on three mice per group at day 7 showed that approximately equal colonization levels were achieved by these inoculations (Fig. 7, inset). Serum was obtained from two mice per group on day 28 postinoculation, and serum antibody responses assessed by Western blotting showed the usual pattern, with significantly stronger responses to the LMW proteins in mice infected with the ΔPT strain than in WT-infected mice (data not shown). Two mice per group were sacrificed, and lungs were homogenized and plated to confirm clearance of the initial infection on day 34; the remaining mice were challenged with 7 × 105 CFU of the WT strain. Respiratory tract colonization levels were assessed on days 1, 3, and 6 postinoculation, and the results are shown in Fig. 7. On day 1 postinoculation, there was significantly greater protection against colonization by previous infection with the ΔPT strain than by infection with the WT, but by day 3 postinoculation, mice previously infected with the WT had completely cleared the challenge infection, whereas mice previously infected with the ΔPT strain still had a low-level infection that persisted on day 6 postinoculation. Therefore, infection with the ΔPT strain does not appear to provide significantly greater overall protection against respiratory tract colonization by B. pertussis than infection with the WT strain, despite the differences in serum antibody responses elicited.
FIG. 7.
Protection of mice against B. pertussis respiratory tract infection by previous infection with either the Tohama I WT or ΔPT strain. BALB/c mice were infected with 104 CFU of the WT or 2 × 105 CFU of the ΔPT strain (or administered PBS as a control), and day 7 colonization levels were assessed (inset). On day 34 postinoculation, all mice were challenged with 7 × 105 CFU of Tohama I WT, and respiratory tract colonization levels were assessed on the indicated days postchallenge.
DISCUSSION
In our attempts to elucidate the role of PT in infection and disease caused by B. pertussis, we hypothesized that PT may act to suppress immune responses by the host and promote optimal bacterial colonization of the respiratory tract. The findings in this study support the idea that PT suppresses antibody responses to B. pertussis antigens, at least as measured by serum antibody levels to whole-cell lysates. Initially, we found that infection of mice with our ΔPT strain elicited greater serum antibody responses to B. pertussis antigens, particularly a pair of LMW proteins, than infection with the WT strain, despite the fact that WT colonization is approximately 100-fold higher than that of the ΔPT strain at the doses used in these studies (6). Several lines of evidence supported the conclusion that it was the presence or absence of PT during infection that caused this effect, rather than an antigenic difference between the WT and ΔPT strains. First, there was no apparent difference in the expression levels of the LMW proteins between the two strains. Second, both a strain producing an inactive form of PT and a ΔPT mutant in another B. pertussis background strain elicited the same strong response to the LMW proteins after infection of the mouse respiratory tract, demonstrating that the effect was not peculiar to the original ΔPT strain but most likely was caused by the absence of active PT. Third and most definitively, the addition of purified PT to the ΔPT strain inoculum elicited a response similar to that elicited by the WT, with no apparent response to the LMW antigens.
The mechanism of suppression of antibody responses by PT is unclear. Several reports in the literature demonstrate that PT has adjuvant activities, including increasing antibody responses (36, 37, 47). However, this result occurred in the absence of the bacteria or other B. pertussis antigens and may have involved an artificially high concentration of PT. Other reports demonstrate that PT has immunosuppressive effects on lymphocytes, including the inhibition of B-cell responses to LPS (15), inhibition of lymphocyte chemotaxis (39), and inhibition of IgM production and proliferation by mouse splenocytes (44). Therefore, PT produced by B. pertussis during infection may inhibit any or all of several mechanisms promoting antibody responses, including phagocytosis by antigen-presenting cells, antigen processing and presentation, trafficking of antigen-presenting cells to lymph nodes, activation of T and B lymphocytes, or maturation of B lymphocytes to antibody-secreting plasma cells. It is also unclear whether PT-mediated suppression of antibody responses plays a role in the evolutionary biology of the B. pertussis-host interaction. The ΔPT strain begins to show a colonization defect relative to WT within 1 to 2 days postinoculation (6), and therefore antibody responses, which typically start to appear at least 6 to 7 days after exposure to novel antigens, cannot contribute to this early defect. Instead, the primary target for PT activity may be innate immune responses, evidence for which was obtained in a previous study (6). However, PT-mediated suppression of antibody responses may lengthen the infection period, and therefore promote transmission of the bacteria to susceptible hosts, or may reduce the level or longevity of the immunity afforded by B. pertussis infection, leading to greater susceptibility in the long term. In addition, if the suppression of antibody responses is not limited to responses to B. pertussis antigens, then a corollary of this effect may be to increase the susceptibility of pertussis patients to secondary infections, an apparent problem in such patients (9).
The most obvious difference in antibody responses to infection with the WT and ΔPT strains was a response to at least two LMW proteins. This was a consistent response in individual mice infected with ΔPT strains but not with any other strain. The proteins were found to be Bvg-independent, membrane-localized proteins (at least one of them surface exposed) expressed also by B. parapertussis and B. bronchiseptica. It is not clear why the absence of PT results in such a strong response to these particular proteins. Presumably, they are highly antigenic proteins whose immunodominance is only manifested in the absence of PT activity. One of the proteins was identified as Pal, a putative lipoprotein that has not previously been characterized in the bordetellae. Pal has been identified and characterized in several other gram-negative bacteria. In E. coli, Pal contributes to the maintenance of cell wall integrity (22) and causes inflammation after release into the bloodstream in gram-negative sepsis (12). The expression of Pal is required for virulence in experimental Haemophilus ducreyi infection (8), and Legionella pneumophila Pal has been proposed as a possible diagnostic antigen for Legionnaires' disease (18). The identity of the other LMW protein, which for unknown reasons was not identified by Western blotting of the two-dimensional gel, is yet to be determined.
An anti-Pal serum antibody response was elicited by subcutaneous, but not intranasal, immunization of mice with a GST-Pal fusion protein. The level of this response was comparable to that elicited by infection with the ΔPT strain. However, we found no evidence that this response alone protected mice against respiratory tract colonization by B. pertussis, and so from these preliminary data Pal does not appear to be a strongly protective antigen. Whether Pal might enhance protection afforded by subunit acellular vaccine compositions for B. pertussis remains to be determined. In addition, mice previously infected with the ΔPT strain were not protected to a greater overall extent against early respiratory tract colonization by B. pertussis than mice previously infected with an equivalent level of the WT, and so the greater antibody responses elicited by infection with the ΔPT strain may not contribute to superior immunity to subsequent infection. However, interpretation of this experiment is complicated by the potential anti-PT protective response generated by WT infection and not by infection with the ΔPT strain. Challenge with the ΔPT strain instead of the WT strain or a comparison of the protection given by the WT and the PT-9K/129G-producing strains may help to resolve this issue.
Acknowledgments
This work was supported by PHS grants AI50022 and AI45732. Mass spectrometry analysis was performed at the W. M. Keck Biomedical Mass Spectrometry Laboratory and the University of Virginia Biomedical Research Facility, which are funded by a grant from the University of Virginia Pratt Committee.
We thank Scott Stibitz, Andrew Preston, and Richard Burgess for antibodies; Jun Zhang and Crystal Kearney for help with strain construction; and Andrew Preston, Carl Brinkley, and Carolyn Machamer for technical advice. We also thank one of the anonymous reviewers for helpful suggestions in improving the manuscript and providing interesting discussion points.
Editor: A. D. O'Brien
REFERENCES
- 1.Allen, A., and D. Maskell. 1996. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol. Microbiol. 19:37-52. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, S., K. Pethe, N. Mielcarek, D. Raze, and C. Locht. 2001. Role of ADP-ribosyltransferase activity of pertussis toxin in toxin-adhesin redundancy with filamentous hemagglutinin during Bordetella pertussis infection. Infect. Immun. 69:6038-6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aricò, B., and R. Rappuoli. 1987. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J. Bacteriol. 169:2847-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J. Leukoc. Biol. 72:962-969. [PubMed] [Google Scholar]
- 5.Brennan, M. J., J. L. David, J. G. Kenimer, and C. R. Manclark. 1988. Lectin-like binding of pertussis toxin to a 165-kilodalton Chinese hamster ovary cell glycoprotein. J. Biol. Chem. 263:4895-4899. [PubMed] [Google Scholar]
- 6.Carbonetti, N. H., G. V. Artamonova, R. M. Mays, and Z. E. V. Worthington. 2003. Pertussis toxin plays an early role in respiratory tract colonization by Bordetella pertussis. Infect. Immun. 71:6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cyster, J. G., and C. C. Goodnow. 1995. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J. Exp. Med. 182:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan, V. N., and T. V. Murphy. 1990. Pertussis in hospitalized children. Am. J. Dis. Child. 144:1130-1134. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin, M. S., and A. A. Weiss. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58:3445-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellman, J., J. D. Roberts, Jr., M. M. Tehan, J. E. Allaire, and H. S. Warren. 2002. Bacterial peptidoglycan-associated lipoprotein in released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J. Biol. Chem. 277:14274-14280. [DOI] [PubMed] [Google Scholar]
- 13.Hewlett, E. L., K. T. Sauer, G. A. Myers, J. L. Cowell, and R. L. Guerrant. 1983. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 40:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 15.Jakway, J. P., and A. L. DeFranco. 1986. Pertussis toxin inhibition of B cell and macrophage responses to bacterial lipopolysaccharide. Science 234:743-746. [DOI] [PubMed] [Google Scholar]
- 16.Kasuga, T., Y. Nakase, K. Ukishima, and K. Takatsu. 1954. Studies on Haemophilus pertussis. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch. Exp. Med. 27:57-62. [PubMed] [Google Scholar]
- 17.Katada, T., M. Tamura, and M. Ui. 1983. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch. Biochem. Biophys. 224:290-298. [DOI] [PubMed] [Google Scholar]
- 18.Kim, M. J., J. W. Sohn, D. W. Park, S. C. Park, and B. C. Chun. 2003. Characterization of a lipoprotein common to Legionella species as a urinary broad-spectrum antigen for diagnosis of Legionnaire's disease. J. Clin. Microbiol. 41:2974-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura, A., K. T. Mountzouros, P. A. Schad, W. Cieplak, and J. L. Cowell. 1990. Pertussis toxin analog with reduced enzymatic and biological activities is a protective immunogen. Infect. Immun. 58:3337-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinnear, S. M., P. E. Boucher, S. Stibitz, and N. H. Carbonetti. 1999. Analysis of BvgA activation of the pertactin gene promoter in Bordetella pertussis. J. Bacteriol. 181:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 71:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazzaroni, J.-C., and R. Portalier. 1992. The excC gene of Escherichia coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (PAL). Mol. Microbiol. 6:735-742. [DOI] [PubMed] [Google Scholar]
- 23.Leef, M., K. L. Elkins, J. Barbic, and R. D. Shahin. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahon, B. P., B. J. Sheahan, F. Griffin, G. Murphy, and K. H. Mills. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J. Exp. Med. 186:1843-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marques, R. R., and N. H. Carbonetti. 1997. Genetic analysis of pertussis toxin promoter activation in Bordetella pertussis. Mol. Microbiol. 24:1215-1224. [DOI] [PubMed] [Google Scholar]
- 26.Mielcarek, N., E. H. Hornquist, B. R. Johansson, C. Locht, S. N. Abraham, and J. Holmgren. 2001. Interaction of Bordetella pertussis with mast cells, modulation of cytokine secretion by pertussis toxin. Cell. Microbiol. 3:181-188. [DOI] [PubMed] [Google Scholar]
- 27.Mielcarek, N., G. Riveau, F. Remoue, R. Antoine, A. Capron, and C. Locht. 1998. Homologous and heterologous protection after single intranasal administration of live attenuated recombinant Bordetella pertussis. Nat. Biotechnol. 16:454-457. [DOI] [PubMed] [Google Scholar]
- 28.Mills, K. H. 2001. Immunity to Bordetella pertussis. Microbes Infect. 3:655-677. [DOI] [PubMed] [Google Scholar]
- 29.Morse, S. I., and J. H. Morse. 1976. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J. Exp. Med. 143:1483-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss, J., S. J. Stanley, D. L. Burns, J. A. Hsia, D. A. Yost, G. A. Myers, and E. L. Hewlett. 1983. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J. Biol. Chem. 258:11879-11882. [PubMed] [Google Scholar]
- 31.Munoz, J. J., H. Arai, R. K. Bergman, and P. L. Sadowski. 1981. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect. Immun. 33:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittman, M. 1979. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough: a hypothesis. Rev. Infect. Dis. 1:401-412. [DOI] [PubMed] [Google Scholar]
- 33.Pizza, M., A. Covacci, A. Bartoloni, M. Perugini, L. Nencioni, M. T. DeMagistris, L. Villa, D. Nucci, R. Manetti, M. Bugnoli, F. Giovannoni, R. Olivieri, J. T. Barbieri, H. Sato, and R. Rappuoli. 1989. Mutants of pertussis toxin suitable for vaccine development. Science 246:497-500. [DOI] [PubMed] [Google Scholar]
- 34.Raleigh, E. A., N. E. Murray, H. Revel, R. M. Blumenthal, D. Westaway, A. D. Reith, P. W. Rigby, J. Elhai, and D. Hanahan. 1988. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 16:1563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reisine, T. 1990. Pertussis toxin in the analysis of receptor mechanisms. Biochem. Pharmacol. 39:1499-1504. [DOI] [PubMed] [Google Scholar]
- 36.Ryan, M., L. McCarthy, R. Rappuoli, B. P. Mahon, and K. H. G. Mills. 1998. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int. Immunol. 10:651-662. [DOI] [PubMed] [Google Scholar]
- 37.Samore, M. H., and G. R. Siber. 1996. Pertussis toxin enhanced IgG1 and IgE responses to primary tetanus immunization are mediated by interleukin-4 and persist during secondary responses to tetanus alone. Vaccine 14:290-297. [DOI] [PubMed] [Google Scholar]
- 38.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 39.Spangrude, G. J., F. Sacchi, H. R. Hill, D. E. Van Epps, and R. A. Daynes. 1985. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J. Immunol. 135:4135-4143. [PubMed] [Google Scholar]
- 40.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 41.Stein, P. E., A. Boodhoo, G. D. Armstrong, S. A. Cockle, M. H. Klein, and R. J. Read. 1994. The crystal structure of pertussis toxin. Structure 2:45-57. [DOI] [PubMed] [Google Scholar]
- 42.Stibitz, S., W. Black, and S. Falkow. 1986. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene 50:133-140. [DOI] [PubMed] [Google Scholar]
- 43.Tamura, M., L. Nogimori, S. Murai, M. Yajima, K. Itio, T. Katada, M. Ui, and S. Ishii. 1982. Subunit structure of the islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 21:5516-5522. [DOI] [PubMed] [Google Scholar]
- 44.Vogel, F. R., T. W. Klein, W. E. Stewart II, T. Igarishi, and H. Friedman. 1985. Immune suppression and induction of gamma interferon by pertussis toxin. Infect. Immun. 49:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss, A. A., and M. S. Goodwin. 1989. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect. Immun. 57:3757-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J. Infect. Dis. 150:219-222. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, A. D., A. Robinson, L. Irons, and C. R. Stokes. 1993. Adjuvant action of cholera toxin and pertussis toxin in the induction of IgA antibody response to orally administered antigen. Vaccine 11:113-118. [DOI] [PubMed] [Google Scholar]
- 48.Witvliet, M. H., D. L. Burns, M. J. Brennan, J. T. Poolman, and C. R. Manclark. 1989. Binding of pertussis toxin to eukaryotic cells and glycoproteins. Infect. Immun. 57:3324-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong, W. S. F., and P. M. Rosoff. 1996. Pharmacology of pertussis toxin B-oligomer. Can. J. Physiol. Pharmacol. 74:559-564. [DOI] [PubMed] [Google Scholar]