Abstract
Methanol extracts of Callistemon viminalis (Sol. Ex Gaertner) G.Don Ex Loudon fruits, bark and leaves were tested for molluscicidal activity. Snails were collected and kept in dechlorinated water under standard condition. Ten adults Biomphalaria Alexandrina, of the same size, were introduced in plastic acquaria for each experiment. The fruits, barks and leaves were extracted with methanol and the methanol extracts were kept for testing as molluscicides. Different extracts proved to have molluscicidal activity against the vector of schistosomiasis, B. alexandrina snails. LC50 values for C. viminalis fruits, bark and leaves were 6.2, 32 and 40 ppm respectively. The C. viminalis fruits extract showed the highest effect against the tested snails. Histopathological studies proved that the site of action of all tested extracts was localized in the digestive system and hermaphrodite gland.
Key Words: Callistemon viminalis, Molluscicides, Biomphalaria alexandrina, Histopathology, Snails
Introduction
Currently, there is an increased attention for the use of new molluscicides which are highly effective, rapidly biodegradable, less expensive, readily available and probably easily applicable with simple techniques than synthetic molluscicides. One of the new trends in the biological control of vectors of diseases is testing the toxicity of plant extracts, as alternatives to chemical molluscicides, which proved to be environmentally safe and have less residual activity. There are many restrictions of using toxic compounds (pesticides and molluscicides) with fresh water. Therefore, the safety of plant extracts to human being is an advantage for studying their effect against the snail vectors of schistosomiasis.
The botanical molluscicides are of economic importance, especially in developing countries (1). Also, there is a continuous need to search for new plant species with ideal molluscicidal properities (2-3). Different plants have been reported as molluscicides (4-7). In Egypt, screening of local plants for molluscicidal activity has received increasing attention (8-13).
The treatment of B. alexandrina snails with sublethal concentration of C. lanceolatus was effective in altering the amino acid profile of this snail species which could be contributed to the impairment of snails egg laying capacity, snail-schistosome miracidiae finding mechanisms and immune response of the molluscan hosts but has no effect on the mammalian skin penetration rate by schistosome cercariae (14). The genus Callistemon was reported to contain diverse chemical profile; steroids and triterpenes (15 - 18), flavonoids (15, 19 and 20), tannins and phenolic compounds (15, 19 and 21), tetradecahydroxanthenediones (22), in addition to the essential oils (23, 24). The diversity of chemical constitution of different Callistemon species reflects diversity in its biological activities; antibacterial and antifungal activities (25, 26), molluscicidal activity (14), Bio-repellents for land leeches, insecticidal and anthelmintic (27- 29), in addition to antioxidant and hepatoprotective activity (21), antithrombin (30), antidiabetic (31), anti-inflammatory (32), anti- alzheimers disease (20).
Reviewing the current literatures, C.viminalis was not previously investigated for mulloscicidal activity. So, the aim of the present study is to evaluate the efficacy of the methanolic extracts of C. viminalis (Sol. Ex Gaertner) G.Don Ex Loudon fruits, bark and leaves as molluscicides against Biomphalria alexandrina snails. Some histological parameters in snails tissues were determined.
Experimental
Materials and methods
Collection of snails
Fresh water snails were collected by using ordinary snail traps with long hand (33) from the irrigation and drainage canals in Mansoura, Dakahlia Governorate. The snails were individually isolated and placed in plastic bags with a suitable amount of water from the same source and immediately transported to the laboratory (34). The snails were identified according to Ibrahim et al. (35).
Maintenance of snails
Laboratory bred B. alexandrina snails were used in the work. The snails were washed several times and maintained in dechlorinated tap water to acclimatize to the laboratory conditions for four weeks before experimentation (36). The average size of the used adult snails was 10-14 mm in diameter. The snails were kept separately in plastic aquaria, 8-10 liters capacity, in dechlorinated tap water allowing 10 snails/liter to avoid crowding. The aquaria contained no mud, sand, gravel or any other substratum. Dechlorinated water was obtained by storing tap water in large containers for at least 48 hours up to one week. Water in the aquaria was changed as frequent as required to keep the snails in a good condition for experiments. Dead snails were removed every day, and no artificial aeration was used. The snails were fed on fresh boiled lettuce leaves, and they were kept under natural illumination. The temperature was the ordinary room temperature with its natural diurnal fluctuations (24-26 ± 2). Maintenance of snails was established in Parasitology Department, Faculty of Medicine, Mansoura University.
Plant materials
C. viminalis (Sol. Ex Gaertner) G.Don Ex Loudon fruits, bark and leaves. The plant identity was confirmed by Dr. Ibrahim Mashaly; Professor of systematic botany, Faculty of Science, Mansoura University, Egypt.
Preparation of plant extracts
The shade-dried fruits, bark and leaves (1 Kg, each) were powdered and extracted by percolation in two liters percolator with distilled methanol. The combined methanol extracts of each part were concentrated to a syrupy consistency under reduced pressure and then allowed to dry in a desiccator over anhydrous CaCl2. The dried extracts were kept for testing as molluscicides.
Bioassay
Ten adults B. alexandrina of the same size were introduced in plastic acquaria (25×13×7 cm) with the selected concentration of the tested extract. Three grams of each extract were transferred into 100 mL volumetric flasks, and then diluted with dechlorinated tap water to complete the volume. These solutions were used to prepare the required concentrations. Different concentrations of the tested extracts (10, 20 and 40 ppm) were used at three replicates and three containers were left as control. The results were recorded every 24 hours up to 5 days for the number of dead snails. Death of the snails was determined by lack of movement, lack of response to gentle prodding with a blunt object, discoloration of the shell, absence of bleeding when they are crushed, and finally by the standard method of immersing the snails in a small amount of 5% sodium hydroxide in a Petri-dish. If bubbles and blood come out of the shell, it is recorded as alive, and Vice-Versa (34, 37 - 39). After 24 hours, LC50 values for each extract were calculated according to Litchfield and Wilcoxon (40). The percentage of dead snails was calculated according to Abbot's formula, 1952:
Histological preparations
Treated and control snails were removed from their shells, washed thoroughly with distilled water and fixed in 10% formalin. The snail tissues were processed for paraffin sectioning after embedded in paraplast at 50 oC. The 7 µm sections were stained with iron haematoxylin and eosin, and examined for tissue changes with light microscopy.
Results and Discussion
Effect of the tested extracts on the snails
In Tables 1, 2, 3 and 4, the results of the effect of different C. viminalis extracts are listed. LC25 and LC50 values for each extract were listed in Table 5. Death of snails began after 24 hours from application at low concentrations. In the control experiment, one snail was died after 24 hours, while with the tested extracts at least 8 snails were died. This indicates the molluscicidal activity of the titled plant. Complete death of snails was observed after the third day with the extracts of bark and leaves at concentrations 20 and 40 ppm, while a concentration 10 ppm afforded 66.7% and 63.3% mortality for the extracts of bark and leaves respectively. On the other hand, complete death of snails was observed after the first day with the fruits extract even at a concentration 10 ppm. LC50 values for C. viminalis fruits bark and leaves were 6.2, 32 and 40 ppm respectively. Thus, C. viminalis fruits extract has the highest potential to kill the snails at low concentration and in short periods. According to the World Health Organization of plant molluscicide, screening must kill snails after 24 hours in a concentration 100 ppm or less at constant water temperature (39).
Table 1.
Death of snails in the control experiment
| Time (day) | Number of snails |
Control
|
|
|---|---|---|---|
| Death | % | ||
| 1 | 30 | 1 | 3.3 |
| 2 | 30 | 2 | 6.7 |
| 3 | 30 | 2 | 6.7 |
| 4 | 30 | 3 | 10 |
| 5 | 30 | 3 | 10 |
Table 2.
Effect of C. viminalis fruits extract on B. alexandrina snails (number 30).
| Time (day) | Number of snails |
5 ppm
|
10 ppm
|
20 ppm
|
40 ppm
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Death | % | Death | % | Death | % | Death | % | ||
| 1 | 30 | 10 | 33.3 | 30 | 100 | 30 | 100 | 30 | 100 |
| 2 | 30 | 17 | 56.7 | 30 | 100 | 30 | 100 | 30 | 100 |
| 3 | 30 | 21 | 70 | 30 | 100 | 30 | 100 | 30 | 100 |
| 4 | 30 | 26 | 86.7 | 30 | 100 | 30 | 100 | 30 | 100 |
| 5 | 30 | 29 | 96.7 | 30 | 100 | 30 | 100 | 30 | 100 |
Table 3.
Effect of Callistemon viminalis bark extract on Biomphalaria alexandrina snails (number 30).
| Time (day) | Number of snails |
10 ppm
|
20 ppm
|
40 ppm
|
|||
|---|---|---|---|---|---|---|---|
| Death | % | Death | % | Death | % | ||
| 1 | 30 | 9 | 30 | 12 | 40 | 17 | 56.66 |
| 2 | 30 | 16 | 53.3 | 23 | 76.7 | 25 | 83.3 |
| 3 | 30 | 20 | 66.7 | 30 | 100 | 30 | 100 |
| 4 | 30 | 25 | 83.3 | 30 | 100 | 30 | 100 |
| 5 | 30 | 28 | 93.3 | 30 | 100 | 30 | 100 |
Table 4.
Effect of C. viminalis leaves extract on B. alexandrina snails (number 30).
| Time (day) | Number of snails |
10 ppm
|
20 ppm
|
40 ppm
|
|||
|---|---|---|---|---|---|---|---|
| Death | % | Death | % | Death | % | ||
| 1 | 30 | 8 | 26.7 | 13 | 43.3 | 15 | 50 |
| 2 | 30 | 15 | 50 | 21 | 70 | 24 | 80 |
| 3 | 30 | 19 | 63.3 | 30 | 100 | 30 | 100 |
| 4 | 30 | 23 | 76.7 | 30 | 100 | 30 | 100 |
| 5 | 30 | 27 | 90 | 30 | 100 | 30 | 100 |
Table 5.
LC50 values for C. viminalis extracts against B. alexandrina snails
| C. viminalis extract | LC 50 |
|---|---|
| Fruits | 6.2 |
| Bark | 32 |
| Leaves | 40 |
In the control test, 3 snails out of 30 were dead during the period of the experiment (10%). These results are in agreement with the results of Youssif et al. (41) who reported that the daily mortality of B. alexandrina was 2.2%.
Histopathological changes
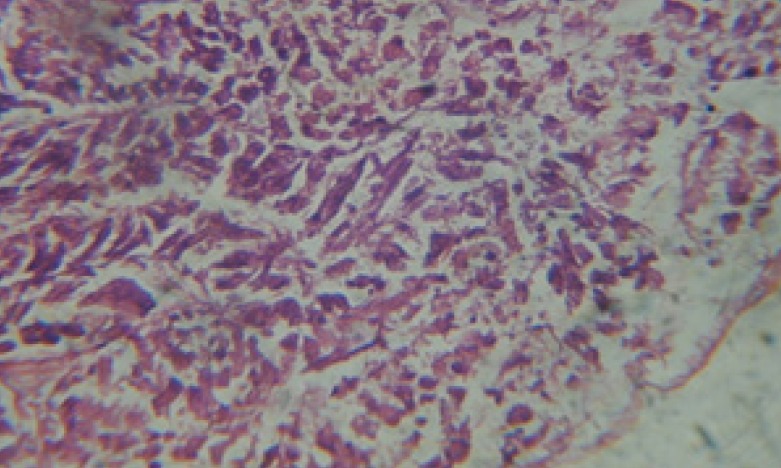
Treatment of snails with C. viminalis fruits, leaves and bark extracts showed great histopathological signs to the hermaphrodite gland and the digestive tract of the snails. The harmful histopathological changes were a function of each extract concentrations. Treated B. alexandrina with a concentration 40 ppm of C. viminalis fruits extract showed large vacuoles and degeneration in the hermaphrodite gland, destruction in the follicular membrane and the mature ovum showed losing of the nucleolus (Figure 2).
Figure 2.
T.S. in treated B. alexandrina with 40 ppm fruits extract (Hermaphrodite region). D: degeneration V: vacuoles X= 200
Figure 1.
T.S. in control B. alexandrina (Hermaphrodite region). Anl= ancel's layer Sp= sperms S= spermatocytes O= oocyte X= 200
Large vacuoles and great destruction was observed in the digestive acini and the columnar epithelial cells (Figure 13). In the digestive epithelia, there observed large evacuated epithelial cells (Figure 24). While treatment with concentrations 20 and 10 ppm of C. viminalis fruits extract showed small vacuoles and little degeneration in the hermaphrodite gland (Figure 3 and 4). The size of vacuoles was not affected by the decreasing in concentrations of the extract and the destruction in the digestive acini and the columnar epithelial cells was less affected (Figure 14 and 15). The evacuation of the epithelial cells in the digestive epithelia was moderate (Figure 25 and 26). On the other hand, treatment with concentration 5 ppm of C. viminalis fruits extract showed small vacuoles in the hermaphrodite gland (Figure 5), normal digestive acini and normal columnar epithelial cells (Figure 16) and normal digestive epithelia (Figure 27). This indicates that mild toxicity afforded with the low concentration of the fruit extract. Treated B. alexandrina with a concentration 40 ppm of both C. viminalis bark and leaves extracts showed small vacuoles and moderate degeneration in the hermaphrodite gland (Figures 6 and 9), small vacuoles in the digestive acini and the columnar epithelial cells (Figures 17 and 20) and small evacuated epithelial cells in the digestive epithelia (Figures 28 and 31) while treatment with concentrations 20 and 10 ppm of both C. viminalis bark and leaves extracts was comparable with that of 5 ppm of the fruits extract; small vacuoles in the hermaphrodite gland (Figures 7, 8, 10 and 11), normal digestive acini and normal columnar epithelial cells (Figures 18, 19, 21 and 22) and normal digestive epithelia (Figures 29, 30, 32 and 33).
Figure 13.
T.S. in treated B. alexandrina with 40 ppm fruits extract (digestive acini). X= 200
Figure 24.
T.S. in treated B. alexandrina with 40 ppm fruits extract showing digestive epithelia. A.E.c: evacuated epithelial cells X = 200
Figure 3.
T.S. in treated B. alexandrina with 20 ppm fruits extract (Hermaphrodite region). D: degeneration V: vacuoles X= 200
Figure 4.
T.S. in treated B. alexandrina with 10 ppm fruits extract (Hermaphrodite region). D: degeneration V: vacuoles X= 200
Figure 14.
T.S. in treated B. alexandrina with 20 ppm fruits extract (digestive acini). X= 200
Figure 15.
T.S. in treated B. alexandrina with 10 ppm fruits extract (digestive acini). X= 200.
Figure 25.
T.S. in treated B. alexandrina with 20 ppm fruits extract showing digestive epithelia. A.E.c: evacuated epithelial cells X = 200
Figure 26.
T.S. in treated B. alexandrina with 10 ppm fruits extract showing digestive epithelia. A.E.c: evacuated epithelial cells X = 200
Figure 5.
T.S. in treated B. alexandrina with 5 ppm fruits extract (Hermaphrodite region). X=200.
Figure 16.
T.S. in treated B. alexandrina with 5 ppm fruits extract (digestive acini). X=200.
Figure 27.
T.S. in treated B. alexandrina with 5 ppm fruits extract showing digestive epithelia. A.E.c: evacuated epithelial cells X = 200
Figure 6.
T.S. in treated B. alexandrina with 40 ppm bark extract (Hermaphrodite region). D: degeneration V: vacuoles X= 200.
Figure 9.
T.S. in treated B. alexandrina with 40 ppm leaves extract (Hermaphrodite region). D: degeneration V: vacuoles X= 200.
Figure 17.
T.S. in treated B. alexandrina with 40 ppm bark extract (digestive acini). X= 200
Figure 20.
T.S. in treated B. alexandrina with 40 ppm leaves extract (digestive acini). X= 200
Figure 28.
T.S. in treated B. alexandrina with 40 ppm bark extract showing digestive epithelia. A.E.c: evacuated epithelial cells X = 200
Figure 31.
T.S. in treated B. alexandrina with 40 ppm leaves extract showing digestive epithelia. A.E.c: evacuated epithelial cells X = 200
Figure 7.
T.S. in treated B. alexandrina with 20 ppm bark extract (Hermaphrodite region). V: vacules X= 200.
Figure 8.
T.S. in treated B. alexandrina with 10 ppm bark extract (Hermaphrodite region). X= 200.
Figure 10.
T.S. in treated B. alexandrina with 20 ppm leaf extract (Hermaphrodite region). V: vacuoles X= 200.
Figure 11.
T.S. in treated B. alexandrina with 10 ppm leaf extract (Hermaphrodite region). X= 200.
Figure 18.
T.S. in treated B. alexandrina with 20 ppm bark extract (digestive acini). X= 200.
Figure 19.
T.S. in treated B. alexandrina with 10 ppm bark extract (digestive acini). X= 200.
Figure 21.
T.S. in treated B. alexandrina with 20 ppm leaves extract (digestive acini). X= 200
Figure 22.
T.S. in treated B. alexandrina with 10 ppm leaves extract (digestive acini). X= 200
Figure 29.
T.S. in treated B. alexandrina with 20 ppm bark extract showing digestive epithelia. X = 200.
Figure 30.
T.S. in treated B. alexandrina with 10 ppm bark extract showing digestive epithelia. X = 200
Figure 32.
T.S. in treated B. alexandrina with 20 ppm leaves extract showing digestive epithelia. X = 200
Figure 33.
T.S. in treated B. alexandrina with 10 ppm leaves extract showing digestive epithelia. X = 200
Figure 12.
T.S. in control B. alexandrina (digestive acini) showing normal columnar epithelial cells. X= 200.
Figure 23.
T.S. in control B. alexandrina showing digestive epithelia. X = 200
The tested plant extracts proved positive molluscicidal activity against B. alexandrina snails. It seems that the target tissues, for the tested extracts, were the hermaphrodite gland and the digestive tract. Destruction of the epithelial layer, vaculation and degeneration of secretory cells are the histopathological signs detected after treatment with the tested extracts.
References
- 1.McCullough FS, Gayral P, Duncan J, Christie J. Molluscicides in schistosomiasis control. Bulletin of the World Health Organization . 1980;58:681–689. [PMC free article] [PubMed] [Google Scholar]
- 2.Tantawy A, Mostafa BB, Sharaf El-Din AT. Molluscicidal activity of Synadenium grandii (Euphorbiaceae) against Biomphalaria alexandrina and Bulinus truncates the intermediate host snails of schistosomiasis in Egypt and their infectivity with the parasite. Egyptian J. Sci. 2004;14:183–196. [Google Scholar]
- 3.Bakry FA, Hamdi SA. Molluscicidal activity of latex aqueous solution of Euphorbia acetonitril and Euphorbia granulate against the intermediate host of schistosomiasis and fascioliosis. J. uni. Arab Biol. 2007;27:101–126. [Google Scholar]
- 4.Marston A, Hostettmann K. Review article No 6: Plant Mulloscicides. Phytochem . 1986;24:639–652. [Google Scholar]
- 5.Li J, Xu H. Bioactive compounds from the bark of Eucalyptus exserta F Muell. Industrial crops and Products . 2012;40:302–306. [Google Scholar]
- 6.Marston A, Maillard M, Hostettmann K. Search for antifungal, molluscicidal and larvicidal compounds from African medicinal plants. J. Ethnopharmacol. 1993;38:209–214. doi: 10.1016/0378-8741(93)90018-z. [DOI] [PubMed] [Google Scholar]
- 7.Parkhurst RM, Mthupha BM, Liang Y-S, Bruce JI, Lambert JDH, Collier TL, ApSimon JW, Wolde-Yohannes L, Heath GE, Jones WO, Stobaeus JK, Machubu LP. Molluscicidal activity of Phytolacca dodecandra I Location of the activating esterase. Biochem. Biophysic. Res. Cummunicat. 1989;158:436–439. doi: 10.1016/s0006-291x(89)80066-x. [DOI] [PubMed] [Google Scholar]
- 8.Rawi SM, El-Gindy H, Haggag AM, Abou El Hassan A, Abdel Kader A. New possible molluscicides from Calendula micrantha officinalis and Ammi majus plants I. Physiological effect on B. alexandrina and B. truncates. J. Egyptian German Socie. Zoolog. 1995;6:69–75. doi: 10.1006/eesa.1996.0109. [DOI] [PubMed] [Google Scholar]
- 9.Rawi SM, El-Gindy H, Abdel Kader A. New possible molluscicides from Calendula micrantha officinalis and Ammi majus II. Molluscicidal, physiological and egg laying effects against Biomphalaria alexandrina and Bulinus truncates. J. Ecol. Toxical. Environ. Safety . 1996;35:261–270. doi: 10.1006/eesa.1996.0109. [DOI] [PubMed] [Google Scholar]
- 10.Bakry FA, Ragab FM, Sakran AM. Effect of some plant extracts with molluscicidal properities on some biological and physiological parameters of Biomphalaria alexandrina snails. J. Egyptian German Soci. Zool. 2002;38:101–111. [Google Scholar]
- 11.Bakry FA, Sakran AM, Ismail NM. Molluscicidal effect of fungicide, herbicide and plant extract on some biological and physiological parameters of Biomphalaria alexandrina. J. Egyptian Socie. Parasitol. 2002;32:821–835. [PubMed] [Google Scholar]
- 12.Sakran AM. Biological and physiological studies on Biomphalaria alexandrina snails exposed to two herbicides. Egyptian J. Zool. 2004;42:205–215. [Google Scholar]
- 13.Hussein KT. Suppressive effects of Calendula micrantha essential oil and gibberelic acid (PGR) on reproductive potential of the Mediterranean fruit fly Ceratitis capitata Wied (Diptera: Tephritidae) J. Egyptian Soci. Parasitol. 2005;35:365–377. [PubMed] [Google Scholar]
- 14.Soliman MS, El-Ansary A. Induced changes in the amino acid profile of Biomphalaria alexandrina molluscan host to Schistosoma mansoni using sublethal concentrations of selected plant molluscicides. J. Applied Sci. 2007;7:2881–2885. [Google Scholar]
- 15.Gohar AA, Maatooq GT, Gadara SR, Aboelmaaty WA. One new pyrroline compound from Callistemon viminalis (Sol. Ex Gaertner) G.Don Ex Loudon. Natural Product Research. 2012:1–7. doi: 10.1080/14786419.2012.718771. [DOI] [PubMed] [Google Scholar]
- 16.Lee NH, Kim JH. Wrinkle-preventing cosmetics containing Callistemon lanceolatus extracts. Republic Korean: Kongkae Taeho Kongbo; 2011. [Google Scholar]
- 17.Jeong W, Hong SS, Kim N, Yang YT, Shin YS, Lee C, Hwang BY, Lee D. "Bioactive triterpenoids from Callistemon lanceolatus". Arch. Pharmacal. Res. 2009;32:845–849. doi: 10.1007/s12272-009-1605-3. [DOI] [PubMed] [Google Scholar]
- 18.Djoukeng JD. Study phytochemical and biological activities of four Cameroon species of the family Myrtaceae: Eucalyptus saligna Sm. 2005. Callistemon viminalis W., Syzygium guineense W. and Syzygium aromaticum Mr. and P. [Google Scholar]
- 19.Khatoon S, Singh H, Goel AK. Use of HPTLC to establish the chemotype of a parasitic plant, Dendrophthoe falcata (Linn. f.) etting. (Loranthaceae), growing on different substrates. J. Planar Chromatography-Modern TLC. 2011;24:60–65. [Google Scholar]
- 20.Park SY, Lim JY, Jeong W, Hong SS, Yang YT, Hwang BY, Lee D. C-methylflavonoids isolated from Callistemon lanceolatus protect PC12 cells against Ab-induced toxicity. Planta Medica . 2010;76:863–868. doi: 10.1055/s-0029-1240801. [DOI] [PubMed] [Google Scholar]
- 21.El Dib RA, El-Shenawy SM. Phenolic constituents and biological activities of the aerial parts of Callistemon viminalis (Sol. Ex Gaertner) G.Don ex Loudon. Bulletin of the Faculty of Pharmacy (Cairo University) 2008;46:223–235. [Google Scholar]
- 22.Khambay BP, Beddie DG, Hooper AM, Simmonds MS, Green PW. New insecticidal tetradecahydroxanthenediones from Callistemon viminalis. Natural Products . 1999;62:1666–1667. [Google Scholar]
- 23.Silva CJ, Barbosa LC, Demuner AJ, Montanari RM, Pinheiro AL, Dias I, Andrade NJ. Chemical composition and antibacterial activities from the essential oils of Myrtaceae species planted in Brazil. Quimica Nova . 2010;33:104–108. [Google Scholar]
- 24.Liu B, Dong X, Lin X, Mo J, Zhang Y, Su X. Chemical components of the essential oil from Callistemon rigidus R. Br.Qinghua Daxue Xuebao, Ziran Kexueban . 2010;50:1437–1439. [Google Scholar]
- 25.Seyyed MS, Masumeh N, Ismaieel D, Hossein M. Antibacterial Activity of Hydroalcoholic Extract of Callistemon citrinus and Albizia lebbeck. American J. Appli. Sci. 2010;7:13–16. [Google Scholar]
- 26.Dongmo BN, Dongmo PM, Ngoune LT, Kwazou NL, Zollo PH, Menut C. Antifungal activities of essential oils of some Cameroonian Myrtaceae on Aspergillus flavus Link ex. Fries. Asian J. Exper. Biological. Sci. 2010;1:907–914. [Google Scholar]
- 27.Nath DR, Das NG, Das SC. Bio-repellents for land leeches. Defense Sci. J. 2002;52:73–76. [Google Scholar]
- 28.Ndomo AF, Tapondjou LA, Ngamo LT, Hance T. Insecticidal activities of essential oil of Callistemon viminalis applied as fumigant and powder against two bruchids. J. Appl. Entomol. 2010;134:333–341. [Google Scholar]
- 29.Pal D, Pathak AK. Evaluation of anthelmintic activity of leaves of Callistemon citrinus Curtis. Asian J. Chem. 2007;19:2839–2842. [Google Scholar]
- 30.Chistokhodova N, Nguyen C, Calvino T, Kachirskaia I, Cunningham G, Howard MD. Antithrombin activity of medicinal plants from central Florida. J. Ethnopharmacol. 2002;81:277–280. doi: 10.1016/s0378-8741(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi K, Ishihara T, Khono E, Miyase T, Yoshizaki F. Constituents of stem bark of Callistemon rigidus showing inhibitory effects on mouse α-amylase activity. Biological &Pharmaceutical Bulletin . 2006;29:1275–1277. doi: 10.1248/bpb.29.1275. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Kumar V, Prakash OM. Pharmacognostic study and anti-inflammatory activity of Callistemon lanceolatus leaf. Asian Pacific J. Tropical Biomedicine . 2011;1:177–178. doi: 10.1016/S2221-1691(11)60022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashmawy K, Abuel-Wafa SA, El-Bahy MM, Diab MR. Incidence and ecology of fresh water snails in Behara Province. Assuit Vetrinary Med. J. 1993;30:101–113. [Google Scholar]
- 34.Allam AF, El-Sayed MH, Khalid SS. Laboratory assessment of the molluscicidal activity Commiphora molmol (Myrrh) on Biomphalaria alexandrina, Bulinus truncates and Lymnaea cailliaudi. J. Egyptian Soc. Parasitol. 2001;31:683–690. [PubMed] [Google Scholar]
- 35.Ibrahim NM, Bishai HM, Khalil MT. Freshwater Molluscs of Egypt: Egyptian Environmental Affairs Agency (EEAA) Publication of National Biodiversity Unit. 1999;(10) [Google Scholar]
- 36.Yousif F, El-Emam M, El-Sayed K. Effect of six non-target snails on Schistosoma mansoni miracidial host finding and infection of Biomphalaria alexandrina under laboratory conditions. J. Egyptian Soc. Parasitol. 1998;28:559–568. [PubMed] [Google Scholar]
- 37.El-Shazly AM, Motawea SM, El-Gilany A, Salama O, Massoud A, Gaballah M. Preliminary study of the molluscicidal action of Myrrh. J. Envir. Sci. 2001;21:153–162. [Google Scholar]
- 38.Al-Jalaud N, Al-Kasas N, Radi MH. Comparative study on the effect of some plant extracts on the biology, body tissues and protein contents of two madically important snails. Global J. Biotechnol. Biochem. 2007;2:63–73. [Google Scholar]
- 39.WHO Molluscicide screening and evaluation. Bull. WHO . 1965;33:567–581. [PMC free article] [PubMed] [Google Scholar]
- 40.Litchfield JT, Wilocoxon F. A simplified method of evaluating dose-effect experiment. J. Pharmacol. Exper. Therapeutics . 1949;96:99–113. [PubMed] [Google Scholar]
- 41.Yousif F, Kamel G, El-Emam M, Mohamed SA. Population dynamics and schistosomal infection of Biomphalaria alexandrina in four irrigation canals in Egypt. J. Egyptian Soc. Parasitol. 1993;23:621–628. [PubMed] [Google Scholar]