Abstract
A recent study demonstrated that polyclonal antibodies to Rickettsia conorii and monoclonal antibodies to outer membrane proteins A (OmpA) and B (OmpB) provided effective, Fc-dependent, passive immunity, even in severe combined immunodeficient mice with an established infection. In order to determine the mechanism of protection, mouse endothelial and macrophage-like cell lines were infected with R. conorii that had been exposed to polyclonal antibodies, monoclonal antibodies to OmpA or OmpB, Fab fragments of the polyclonal antibodies, or normal serum or that were left untreated. At 0 h, Fc-dependent antibody enhancement of R. conorii adherence to endothelial and macrophage-like cell lines was inhibited by the presence of normal serum, suggesting Fc receptor-mediated adherence of opsonized rickettsiae. At 3 h, the opsonized rickettsiae had been internalized. After 72 h, inhibited survival of rickettsiae exposed to polyclonal antibodies or monoclonal antibodies to OmpA or OmpB was evident compared with growth of untreated and normal serum-treated and polyclonal Fab antibody-treated R. conorii. Polyclonal antibodies and an anti-OmpB monoclonal antibody inhibited the escape of R. conorii from the phagosome, resulting in intraphagolysosomal rickettsial death. At 48 h of infection, rickettsicidal activity of macrophages by opsonized rickettsiae was inhibited by NG-monomethyl-l-arginine, superoxide dismutase, mannitol, or supplemental l-tryptophan, and endothelial rickettsicidal activity against opsonized rickettsiae was inhibited by NG-monomethyl-l-arginine, superoxide dismutase, catalase, or supplemental l-tryptophan. Thus, Fc-dependent antibodies protected against R. conorii infection of endothelium and macrophages by opsonization that inhibited phagosomal escape and resulted in phagolysosomal killing mediated by nitric oxide, reactive oxygen intermediates, and l-tryptophan starvation.
Knowledge of the existence and geographic distribution of spotted fever group (SFG) rickettsioses is expanding, and the virulence of Rickettsia rickettsii and R. conorii is unabated (17, 25). The continuing occurrence of fatalities when infections are misdiagnosed and treated with antibiotics that are ineffective against rickettsiae suggests that development of a vaccine that is protective against all SFG rickettsiae should be considered (8, 16). Agents of life-threatening rickettsioses are also the most virulent category B biologic agents of potential use for bioterrorism (26). Rational vaccine development requires knowledge of the mechanisms of immunity against a particular organism and of its components that stimulate protective immunity. For SFG rickettsiae, the emphasis during the last two decades has been on mechanisms of cellular immunity. In fact, the key rickettsicidal mechanisms are the activation of infected target cells, principally endothelium, by cytokines (gamma interferon, tumor necrosis factor alpha, interleukin-1β, and RANTES) to kill intracellular rickettsiae and the elimination of remaining infected target cells by CD8 cytotoxic T-lymphocyte-mediated apoptosis (5, 6, 7, 9, 27, 29). Cytokine-activated infected target cells kill intracellular rickettsiae by a combination of one or more mechanisms (nitric oxide, reactive oxygen intermediates, and degradation of tryptophan stores) (7).
Although knowledge of a modest beneficial effect of antiserum treatment of experimental animals and human Rocky Mountain spotted fever dates from the early research of ricketts until the dawn of the antibiotic era, humoral immunity to rickettsiae was only recently established in contemporary immunologic terms (18, 20, 21). Passive transfer of polyclonal antibodies or particular monoclonal antibodies to outer membrane protein A (OmpA) or OmpB, but not Fab fragments of polyclonal antibodies or monoclonal antibody to lipopolysaccharide, protected C3H severe combined immune-deficient mice against 10 50% lethal doses of R. conorii (9). The present study was undertaken to determine the effects of antibody treatment of R. conorii on the subsequent interactions with murine endothelial and macrophage-like cell lines. Experiments were designed to analyze the effect of antibodies on rickettsial attachment to the host cells and entry into them, escape from the phagosome into the cytosol, and survival and growth 72 h later as well as identification of the rickettsicidal mechanisms in each target cell type.
MATERIALS AND METHODS
Cell lines.
Vero cells (an African green monkey kidney cell line) obtained from the American Type Culture Collection (ATCC CCL 81, Manassas, Va.) were cultivated in minimum essential medium (MEM) (Gibco, Grand Island, N.Y.) containing 5% bovine calf serum (HyClone Inc., Logan, Utah), 2 mM l-glutamine, and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) (5-MEM). J774A.1 cells (a mouse monocyte-macrophage cell line) obtained from the American Type Culture Collection (ATCC T1B 67) were grown in Dulbecco's modified MEM (DMEM) containing 10% bovine calf serum, 2 mM l-glutamine, and 10 mM HEPES (10-DMEM). SVEC 4-10 cells (SVEC) (a simian virus 40-transformed mouse endothelial cell line), generously provided by Michael Edidin (Johns Hopkins University, Baltimore, Md.), were maintained in 10-DMEM.
Rickettsia.
R. conorii (Malish 7 strain) was obtained from the American Type Culture Collection (ATCC VR 613) and has been cloned by plaque purification in our laboratory. The rickettsial stock was prepared as an infected 10% yolk sac suspension and stored at −80°C. In brief, the monolayer of Vero cells was infected with R. conorii from the 10% yolk sac stock (105 PFU per 150-cm2 flask). After 4 to 5 days of incubation at 37°C, the infected cells were harvested, and the host cells were broken by ultrasonication and treated with proteinase inhibitors (Complete Mini protease inhibitor cocktail [inhibiting a broad spectrum of serine, cysteine, and metalloproteases as well as calpain]; Roche Applied Science, Indianapolis, Ind.) and DNase (0.5 mg/ml). The cell debris was separated from the rickettsial bands by discontinuous density gradient centrifugation in renografin (10). The product used in these experiments was a mixture of the light and heavy bands of rickettsiae suspended in sucrose-phosphate-glutamic acid medium (0.218 M sucrose, 0.0038 M KH2PO4, 0.0072 M K2HPO4, 0.0049 M monosodium glutamic acid, pH 7.0; 1 ml per 10 original 150-cm2 flasks). The concentration of rickettsiae was 4 × 109 PFU/ml.
Antibodies.
Polyclonal antirickettsial antibody was prepared from mice immunized with a sublethal dose of R. conorii and boosted subsequently with a lethal dose of R. conorii. Ten days after booster immunization, the blood was collected and serum was separated. The globulin fraction was precipitated from the sera with 50% saturated ammonium sulfate and dialysed against phosphate-buffered saline. The immunofluorescent antibody titer of the polyclonal globulin solution was 1:2,560. The monoclonal antibodies U16 (anti-OmpA) and U14 (anti-OmpB) were obtained from the fusion of rickettsial immune splenic lymphocytes with the partner myeloma cells SP2/0-Ag 14 (ATCC CRL 8006). The immunofluorescent antibody titer and isotypes of the monoclonal antibodies were 1:2,560 immunoglobulin G2a (IgG2a) (U16) and 1:5,120 IgG2a (U14). All the sera or ascites fluids were inactivated for complement activity by incubation at 56°C for 30 min before use. The Fab fragment of polyclonal antibody was prepared by using a Fab fragment kit (Pierce, Rockford, Ill.) according to the instructions of the manufacturer.
Plaque assay.
The quantity of rickettsiae was measured by the plaque assay as described previously (28). In brief, the infected monolayers were scraped off the plates, disrupted by ultrasonication for 40 s, and centrifuged at low speed (400 × g for 10 min) to separate the cell debris. Serial 10-fold dilutions of the supernatant were prepared, and each dilution (0.2 ml/well) was placed onto a monolayer of Vero cells in 24-well plates in duplicate. The plates were centrifuged at 700 × g for 10 min to achieve synchronized contact of rickettsiae with the host cells and incubated at 37°C in 5% CO2 atmosphere for 2 h. Then, the plate was overlaid with 0.5% agarose in MEM containing 1% bovine calf serum (1-MEM) and 1 μg of cycloheximide/ml. Three days later, the plates were stained with 0.01% neutral red (Gibco) for 6 h, and the number of the plaques was counted under an inverted microscope.
Experimental design to evaluate the effect of antibody on rickettsial adherence, internalization, and growth in endothelial cells and macrophages.
Thirty microliters of a 1:5 suspension of density gradient-purified R. conorii was incubated with 30 μl of a monoclonal antibody against OmpA or OmpB, polyclonal anti-R. conorii antibodies, Fab of the polyclonal anti-R. conorii antibodies, normal serum, or phosphate-buffered saline for 1 h at room temperature. Subsequently, 140 μl of 1-MEM was added to each mixture of antibody-treated samples of rickettsiae. The excess antibodies were not washed away. The serum-treated rickettsiae were then inoculated onto monolayers of SVEC or J774A.1 cells in 24-well plates in duplicate. In order to determine the effect of antibody on rickettsial adherence to the cells, one plate was incubated at 6°C for 3 h, conditions under which attachment was observed but induced phagocytosis was markedly reduced. The monolayers were then washed three times with warm medium to remove unattached rickettsiae. Adherent cells were harvested by scraping the monolayers and sonicated to release individual rickettsiae, host cell debris was removed by low-speed centrifugation, and viable rickettsiae were quantified by plaque assay.
The other 24-well plates were incubated at 37°C for 3 h and then washed three times with warm medium to remove extracellular, nonadherent rickettsiae. Two plates containing R. conorii-infected SVEC and J774A.1 cells, respectively, were harvested and treated by sonication and low-speed centrifugation to release rickettsiae and remove host cell debris. Plaque assays were performed to determine the content of infectious rickettsiae that had been internalized during the 3-h incubation. Two plates containing R. conorii-infected endothelial cells and macrophages, respectively, were washed to remove unattached rickettsiae at 3 h, 1 ml of 1-MEM per well was added, and the infected monolayers were incubated at 37°C for 3 days to evaluate the role of antibody on intracellular growth of rickettsiae by plaque assay. All of these experiments were performed in duplicate, and the experiments were performed three times to assure reproducibility.
Determination of the intracellular killing mechanism(s) of antibody-opsonized rickettsiae in endothelial cells and macrophages.
To evaluate the roles of nitric oxide, reactive oxygen species, and tryptophan starvation as antirickettsial mechanisms, medium containing NG-monomethyl-l-arginine (NGMMLA) (1 mM), superoxide dismutase (30 U/ml), catalase (100 μg/ml), d-mannitol (100 μg/ml), or l-tryptophan (80 μg/ml) was added to different wells after washing the monolayers three times to remove unattached rickettsiae as described above. The monolayers were incubated for 48 h and then harvested for plaque assay of the content of viable rickettsiae.
Ultrastructural analysis of phagosomal escape.
The experimental groups examined were R. conorii (Malish 7 strain) incubated with monoclonal antibody against OmpB (U14), polyclonal antibody against R. conorii, nonimmune serum, or buffer alone for 1 h prior to inoculation of cells. SVEC cells were inoculated with antibody-treated or untreated R. conorii at 4°C and harvested immediately or harvested after incubation at 37°C for 12, 24, or 48 h. Cells were immersed in Ito's fixative, a mixture of 1.25% formaldehyde, 2.5% glutaraldehyde, 0.03% trinitrophenol, 0.03% CaCl2, and 0.05 M cacodylate buffer at a pH of 7.3 (12). Cells were postfixed in 1% osmium tetroxide for 1 h and stained en bloc in 1% uranyl acetate in 0.1 M maleate buffer (pH 5.2) for 30 min at 60°C. Samples were then dehydrated by using a series of dilutions of ethanol and embedded in Poly/Bed 812 epoxy resin. Ultrathin sections (70 nm) were cut with a Reichert Ultracut S ultramicrotome, placed on copper grids, stained with uranyl acetate and lead citrate, and examined in a Phillips CM 100 electron microscope or a Phillips 201 electron microscope at 60 kV. The locations of rickettsiae were identified ultrastructurally as either intravacuolar (completely surrounded by the phagosomal membrane) or intracytosolic (free in the cytosol). Rickettsiae in the process of escaping from the vacuole were counted as present in the cytosol. Only intracellular rickettsiae were evaluated. At least 50 different rickettsiae were examined for each condition, and the experiment was repeated to assure reproducibility. The proportion of vacuole-bound rickettsiae in the untreated control (rickettsiae exposed to buffer alone) was compared statistically to the proportion of vacuole-bound rickettsiae for each experimental group at the same time point by the chi-square test (χ2) with the program epiInfo 2002. Values were considered significantly different when P was <0.05.
RESULTS
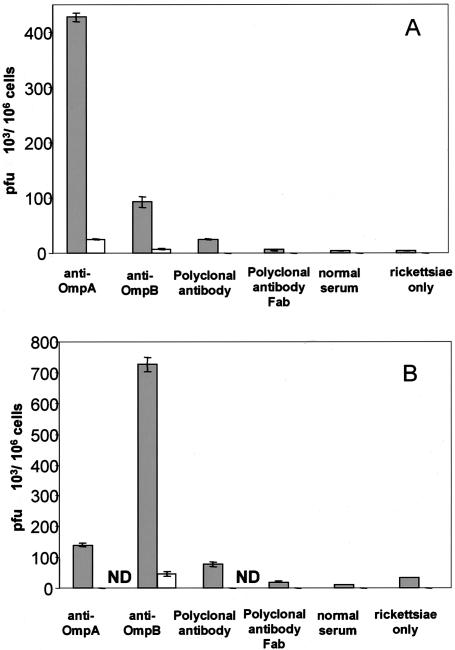
Polyclonal antibody to R. conorii and monoclonal antibodies to OmpA and OmpB increased the number of adherent and internalized R. conorii in mouse endothelial (SVEC) and macrophage (J774A.1) cell lines. The R. conorii treated with antibodies at room temperature for 1 h and then incubated with SVEC or J774A.1 cells at 6°C for 3 h, conditions at which the internalization of rickettsiae was slow, facilitated the study of adhesion of rickettsiae to the host cells. The adhesion of rickettsiae onto the SVEC endothelial cells was increased by 12.5-fold [(50 ± 4.3) × 103 PFU/106 cells; P < 0.001], 19-fold [(115 ± 8) × 103 PFU/106 cells; P < 0.001], and 14-fold [(58 ± 4.3) × 103 PFU/106 cells; P < 0.001] after treatment with polyclonal antibody and anti-OmpA and anti-OmpB monoclonal antibodies, respectively, compared with no antibody treatment [(4 ± 0.4) × 103 PFU/106 cells] (Fig. 1). This effect of antibody was Fc dependent. The Fab fragment of polyclonal antibody showed no effect on rickettsial adherence, and the antibody-enhanced rickettsial attachment was blocked if the host cells were pretreated with a 1:2 dilution (0.2 ml/well) of normal mouse serum in medium for 1 h before the antibody-treated rickettsiae were added. The normal serum-treated rickettsiae did not differ from untreated rickettsiae in their adherence to SVEC. In the mouse macrophage cell line (J774A.1), the results were similar to those observed in endothelial cells (Fig. 1). The attachment of rickettsiae to the J774A.1 cells was increased 8.9-fold [(170 ± 6.7) × 103 PFU/106 cells, P < 0.001], 4.3-fold [(82 ± 10.5) × 103 PFU/106 cells; P < 0.001], and 30-fold [(567 ± 12.5) × 103 PFU/106 cells; P < 0.001] with polyclonal, anti-OmpA, or anti-OmpB antibody treatment, respectively, compared with no antibody treatment [(19 ± 6) × 103 PFU/106 cells]. This effect was also Fc dependent and was blocked by pretreatment of the host cells with normal mouse serum.
FIG. 1.
Comparison of the effects of antibody on the adherence of R. conorii to mouse endothelial cells (A) and macrophages (B). Host cells which were preincubated with normal mouse serum (open bars) had many fewer adherent viable rickettsiae than cells which were not preincubated with normal serum (filled bars). Greater quantities of viable rickettsiae treated with polyclonal antibody or monoclonal anti-OmpA or anti-OmpB adhered to both endothelial cells and macrophages than untreated or normal serum-treated or Fab antibody-treated rickettsiae (P < 0.001). ND, no rickettsiae detected. These results represent the average of three separate experiments.
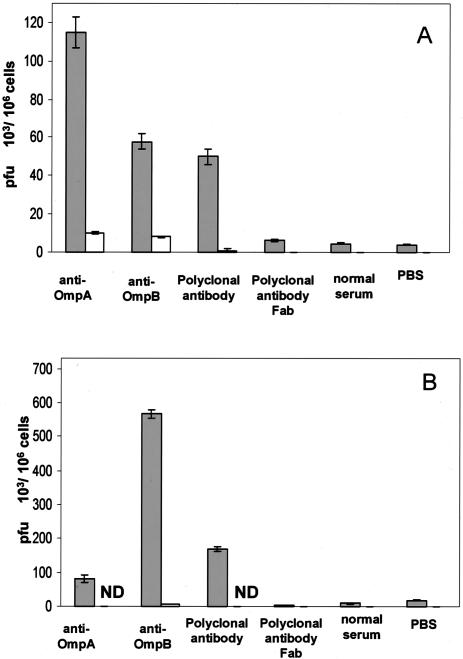
The study of the effect of antibody on the internalization of rickettsiae into the host cells revealed that the antibody-treated rickettsiae incubated at 37°C with the host cells for 3 h showed increased internalization into endothelial cells and macrophages to a similar degree to the enhancement of rickettsial attachment. The rickettsial entry of organisms reacted with polyclonal anti-R. conorii, monoclonal anti-OmpA, or anti-OmpB was increased 5.6-fold [(25 ± 1.0) × 103 PFU/106 cells; P < 0.001], 95-fold [(428 ± 7.5) × 103 PFU/106 cells; P < 0.001], and 20-fold [(93 ± 9.8) × 103 PFU/106 cells; P < 0.001] compared with no antibody treatment [(4.5 ± 0.25) × 103 PFU/106 cells] in SVEC cells and was increased 2.3-fold [(78 ± 7.5) × 103 PFU/106 cells; P < 0.001], 4-fold [(141 ± 6.8) × 103 PFU/106 cells; P < 0.001], and 21-fold [(727 ± 23.3) × 103 PFU/106 cells; P < 0.001] compared with no antibody treatment [(34 ± 0.8) × 103 PFU/106 cells] in J774A.1 cells (Fig. 2). The increased internalization was also Fc dependent and was blocked by the pretreatment of the host cells with normal serum.
FIG. 2.
Comparison of the effects of antibody on the internalization of R. conorii into mouse endothelial cells (A) and macrophages (B). Host cells which were preincubated with normal mouse serum (open bars) had many fewer viable internalized rickettsiae than cells which were not preincubated with normal serum (filled bars). Greater quantities of viable internalized rickettsiae that had been treated with polyclonal antibody or monoclonal anti-OmpA or anti-OmpB were present in both endothelial cells and macrophages at 3 h after infection than those of untreated or normal serum-treated or Fab antibody-treated rickettsiae (P < 0.001). ND, no rickettsiae detected. These results represent the average of three separate experiments.
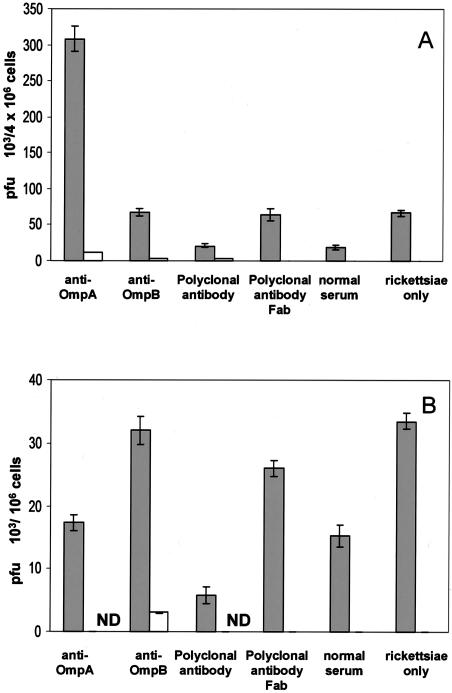
Intracellular rickettsial growth and survival were diminished by antibody treatment in both endothelial cells and macrophages.
In SVEC cells, rickettsial proliferation was inhibited or intracellular rickettsicidal activity was more effective against antibody-exposed R. conorii. After 3 days of incubation, the titer of untreated rickettsiae had increased 15-fold [(67.3 ± 4.5) × 103 PFU/106 cells versus (4.5 ± 0.25) × 103 PFU/106 cells]. In contrast, the content of viable rickettsiae in the cells incubated with antibody-treated rickettsiae was less than that present 3 h after the rickettsiae were internalized into the host cells. The quantity of viable R. conorii was 80% [(21.5 ± 2.5) × 103 PFU/106 cells versus (25 ± 1.0) × 103 PFU/106 cells], 72% [(308 ± 17.5) × 103 PFU/106 cells versus (428 ± 7.5) × 103 PFU/106 cells], and 73% [(68 ± 5) × 103 PFU/106 cells versus (93 ± 9.8) × 103 PFU/106 cells] of the titer of intracellular rickettsiae present 69 h earlier in the polyclonal, monoclonal anti-OmpA, or monoclonal anti-OmpB antibody-treated rickettsiae, respectively. (Fig. 2 and 3). Instead of substantial rickettsial growth, actual rickettsial killing had occurred in the endothelial cells containing antibody-treated rickettsiae.
FIG. 3.
Comparison of the effects of antibody on the survival and growth of R. conorii in mouse endothelial cells (A) and macrophages (B). Host cells which were pretreated with normal mouse serum (open bars) contained many fewer viable rickettsiae than cells that were not pretreated with normal serum (filled bars). The quantities of viable rickettsiae that had been treated with polyclonal antibody or monoclonal anti-OmpB were reduced at 72 h after infection compared with the quantities of viable intracellular rickettsiae under the corresponding treatment conditions at 3 h after infection. In contrast, the untreated and normal serum-treated or Fab antibody-treated rickettsiae proliferated in the interval between 3 and 72 h of infection. ND, no rickettsiae detected. These results represent the average of three separate experiments.
In J774A.1 cells, the rickettsial quantity in the cells inoculated with untreated rickettsiae after 3 days of incubation was similar to the content of infectious rickettsiae after 3 h of internalization. But in the case of polyclonal, monoclonal anti-OmpA, or monoclonal anti-OmpB antibody-treated rickettsiae, the quantity of viable rickettsiae had decreased 13-fold [(5.8 ± 1.4) × 103 PFU/106 cells versus (78 ± 7.5) × 103 PFU/106 cells], 8.3-fold [(17 ± 1.3) × 103 PFU/106 cells versus (141 ± 6.8) × 103 PFU/106 cells], and 22.7-fold [(32 ± 2.2) × 103 PFU/106 cells versus (727 ± 23.3) × 103 PFU/106 cells], respectively, compared with that after the first 3 h of internalization (Fig. 2 and 3). These results suggested that the intracellular killing mechanisms were more effective in the macrophage, especially when the internalized rickettsiae were treated with antibodies.
Mechanisms of intracellular killing of antibody-treated rickettsiae in endothelial cells and macrophages.
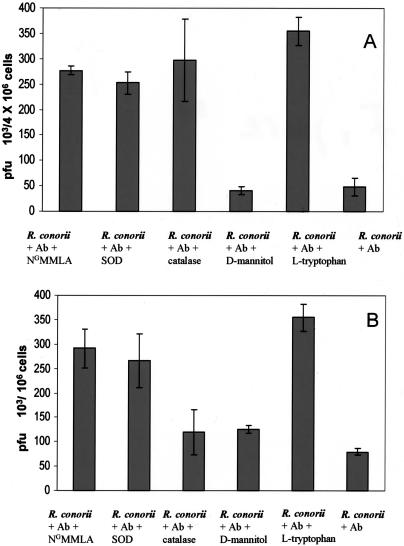
NGMMLA (a competitive inhibiting analog of l-arginine that blocks the synthesis of nitric oxide), superoxide dismutase, catalase and mannitol, scavengers of superoxide anion, hydrogen peroxide, and hydroxyl radicals were incubated with the SVEC cells and J774A.1 cells infected with antibody-treated rickettsiae to investigate the roles of reactive nitrogen and oxygen species in rickettsial killing. Antibody-treated rickettsiae in endothelial cells exposed to NGMMLA [(277.5 ± 8.5) × 103 PFU/106 cells], superoxide dismutase [(252.5 ± 22) × 103 PFU/106 cells], and catalase [(297.5 ± 81.8) × 103 PFU/106 cells] compared with controls [(48.8 ± 17) × 103 PFU/106 cells] (P < 0.001 for all) showed increased intracellular survival and growth (Fig. 4). Similarly, J774A.1 macrophage-like cells exposed to NGMMLA [(291 ± 40) × 103 PFU/106 cells], superoxide dismutase [(266 ± 55) × 103 PFU/106 cells], and mannitol [(125 ± 8) × 103 PFU/106 cells] (P < 0.001 for all), but not catalase [(120 ± 47) × 103 PFU/106 cells], showed decreased killing of antibody-treated R. conorii [(80 ± 6.3) × 103 PFU/106 cells], indicating that nitric oxide, superoxide, and hydroxyl radicals are effectors of antirickettsial activity against opsonized rickettsiae by murine macrophages (Fig. 4).
FIG. 4.
Investigation of the mechanisms of intracellular killing of antibody-exposed R. conorii in endothelial cells and macrophages in vitro. The reduction in rickettsial survival by polyclonal antibody (Ab) against R. conorii in mouse endothelial cells (A) was inhibited by the presence of NGMMLA, superoxide dismutase (SOD), catalase, or supplemental l-tryptophan (P ≤ 0.001). Antibody-associated rickettsicidal activity of macrophage-like cells (B) was inhibited by NGMMLA, SOD, mannitol, or supplemental l-tryptophan (P < 0.001).
It has been documented that one of the intracellular antirickettsial mechanisms is mediated by the induction of a catabolic enzyme, indoleamine-2,3-dioxygenase, which catalyzes the degradation of tryptophan to kynurenine and formyl kynurenine, resulting in the limitation of the availability of intracellular tryptophan (7). The addition of supplemental l-tryptophan in the medium of cells containing antibody-exposed rickettsiae inhibited, at least in part, the rickettsicidal activity. In the presence of supplemental tryptophan, survival and growth of antibody-treated rickettsiae were increased [(355 ± 28.5) × 103 PFU/106 cells versus (48.8 ± 17.3) × 103 PFU/106 cells; P < 0.001] in SVEC cells and [(355.8 ± 57) × 103 PFU/106 cells versus (80 ± 6.3) × 103 PFU/106 cells; P < 0.001] in J774A-1 cells. These results imply that tryptophan starvation also played a role in intracellular rickettsial killing in both endothelial cells and macrophages (Fig. 4).
Polyclonal antibodies and monoclonal anti-OmpB inhibit rickettsial escape from the phagosome.
Rickettsiae were observed in ultrathin sections intracellularly in the infected SVEC cells at all time points and conditions except at 0 h, as expected. At 12 to 48 h postinfection, the proportion of vacuole-bound rickettsiae was significantly higher when rickettsiae had been incubated with the anti-OmpB monoclonal antibody or with polyclonal antibodies to R. conorii compared with normal serum-treated or untreated rickettsiae (P < 0.05) (Table 1). There was no significant difference (P > 0.10) in the proportion of vacuole-bound rickettsiae between the untreated controls and the rickettsiae incubated with normal serum at any time points tested.
TABLE 1.
Effect of antibody exposure on ultrastructural localization of Rickettsia conorii in endothelial cells in vitro
| Experimental treatment of rickettsiae | % Intravacuolar (mean ± SD) at time after infection (h)
|
||
|---|---|---|---|
| 12 | 24 | 48 | |
| Untreated | 18 ± 4.24 | 14.8 ± 1.1 | 6.3 ± 1.1 |
| Polyclonal anti-R. conorii | 58.5 ± 8.1a | 43.6 ± 5.1a | 54.5 ± 12.0a |
| Monoclonal anti-OmpB | 46.7 ± 3.7a | 42.5 ± 0.7a | 32.5 ± 5.0a |
| Normal serum | 19.4 ± 6.5 | 18.2 ± 2.6 | 14.7 ± 1.8 |
P < 0.05 compared with untreated rickettsiae.
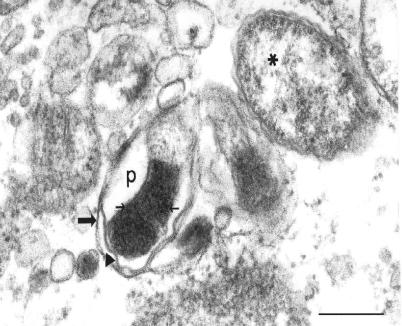
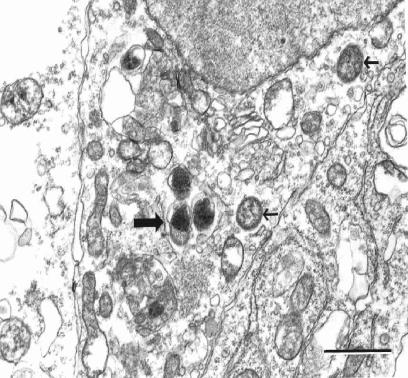
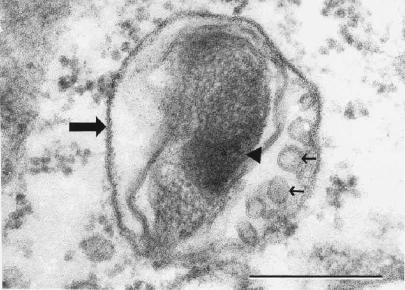
Rickettsiae free in the cytosol possessed typical rickettsial morphology and dimensions (Fig. 5). Nearly all of the intravacuolar rickettsiae demonstrated morphological characteristics consistent with different stages of rickettsial death and phagolysosomal digestion, including increased nucleoplasmic densities (Fig. 5, 6, and 7), loss of bacillary morphology (Fig. 5), increased cell wall waviness (Fig. 5 and 6), dilation of the periplasmic space (Fig. 5), cell wall blebs (Fig. 7), and overall shrunken appearance (Fig. 5). Occasionally, several rickettsiae were observed undergoing apparent digestion within a single vacuole (Fig. 6).
FIG. 5.
Electron photomicrograph demonstrating an intravacuolar rickettsia with morphology consistent with phagolysosomal digestion (vacuolar membrane, large arrow). Note the increased waviness of the rickettsial outer membrane (arrowhead), increased electron density of the rickettsial nucleoplasm (small arrows), dilation of the periplasmic space (p), and overall loss of bacillary morphology. An adjacent, intracytosolic rickettsia appears healthy by comparison (asterisk). Anti-OmpB antibody-treated rickettsiae at 24 h after infection of SVEC endothelial cells are shown. Bar, 0.25 μm.
FIG. 6.
Electron photomicrograph demonstrating three rickettsiae enclosed within a vacuolar membrane (large arrow) with large rickettsial nucleoplasmic electron densities and increased rickettsial outer membrane waviness as ultrastructural characteristics of rickettsial death. Note the two healthy-appearing rickettsiae free in the cytosol (small arrows). Anti-OmpB antibody-treated rickettsiae in an SVEC endothelial cell at 12 h after infection are shown. Bar, 1.0 μm.
FIG. 7.
Electron photomicrograph demonstrating a damaged intravacuolar rickettsia with the morphological characteristics of early destruction (large arrow, vacuolar membrane). Note the large rickettsial nucleoplasmic electron density (arrowhead) and rickettsial cell wall membrane blebs (small arrows). Anti-OmpB antibody-treated rickettsiae after 24 h in an SVEC endothelial cell are shown. Bar, 0.25 μm.
DISCUSSION
These in vitro studies confirmed the in vivo observations that antibody protection against R. conorii is Fc dependent, that antibodies to epitopes of OmpA and OmpB are protective, and that rickettsial death is intraphagolysosomal (9). These experiments extended our knowledge to the cellular level for endothelial cells and macrophages. The observations in this study revealed several particularly relevant and novel findings: escape from the phagosome and growth of opsonized rickettsiae are inhibited, and the neutralization of OmpB is a component of the mechanism of inhibition of phagosomal escape. The mechanism of rickettsial escape from the phagosome remains to be elucidated. Unlike Listeria monocytogenes, for which listeriolysin O is established as the mechanism of bacterial escape into the host cell cytosol, the rickettsial gene product responsible for phagosomal escape is known for neither R. conorii nor R. prowazekii, for which complete genome sequences are available (1, 4, 15). It is anticipated that an investigation of the anti-OmpB inhibition of phagosomal escape may lead to the elucidation of this key pathogenetic step.
Although it was not unexpected that opsonized rickettsiae would be killed by macrophages utilizing mechanisms involving superoxide, hydroxyl radicals, and nitric oxide, it is remarkable that endothelial cells were also able to restrict the survival of intracellular opsonized R. conorii by a mechanism(s) involving nitric oxide, superoxide, and hydrogen peroxide in the absence of exogenous cytokines. That the limitation of tryptophan availability to rickettsiae would be a mechanism of control of infection by opsonized R. conorii in both endothelial cells and macrophages was also a noteworthy and unpredicted antirickettsial effect that had been observed previously only after cytokine activation of the host target cells (7).
A surprising result in this study was the marked increase in adhesion of opsonized R. conorii to the endothelial cells. The observations that normal serum inhibited the attachment of opsonized rickettsiae and that the Fab fragment of attachment-enhancing antibodies did not increase the attachment of R. conorii to endothelial cells suggested that the great augmentation of rickettsial attachment was mediated by an Fc receptor on the endothelial cells. Some vascular endothelial cells express Fc receptors such as FcγRIIA, a low-affinity receptor that could bind opsonized rickettsiae with strong avidity, owing to the interactions of numerous rickettsial surface molecules with antibodies that could interact with multiple Fc receptors. Although the expression of Fc receptors on human endothelial cells has been described for infections involving endothelium, it seems likely that this phenomenon would not be important in vivo, as the presence of normal serum mitigated the effect, as would presumably occur in the presence of abundant circulating IgG (2, 3, 24).
Rickettsial growth is strictly intracellular. During the course of even the most severe rickettsiosis, Rocky Mountain spotted fever, nearly all rickettsiae are found in the cytosol of vascular endothelium; few bacteria (median, 0.6 bacteria/ml) are present free in the circulating blood (13). Even highly sensitive PCR often fails to detect rickettsiae in the blood (19, 22) where some of the organisms are present within detached circulating endothelial cells rather than extracellularly (14). Moreover, SFG rickettsiae possess the ability to stimulate actin-based mobility that effects cell-to-cell spread unless they are restricted to phagosomes (11). Nonetheless, polyclonal antibodies and monoclonal antibodies to OmpA and OmpB exert a strong antirickettsial effect even in SCID mice which have an established infection and no means of developing acquired immunity (9). Presumably, antibodies bind to organisms during their brief extracellular transit from one cell to another. Thus, the identification of rickettsial B-cell epitopes that contribute to protective immunity is an important aim toward the goal of developing a protective subunit vaccine against SFG rickettsiae.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Andersson, S. G. E., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. M. Alsmark, R. M. Podowski, A. K. Naslund, A.-S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 2.Bengualid, V., V. B. Hatcher, B. Diamond, E. A. Blumberg, and F. D. Lowy. 1990. Staphylococcus aureus infection of human endothelial cells potentiates Fc receptor expression. J. Immunol. 145:4279-4283. [PubMed] [Google Scholar]
- 3.Cines, D. B., A. P. Lyss, and M. Bina. 1982. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J. Clin. Investig. 69:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelson, B. T., and E. R. Unanue. 2001. Intracellular antibody neutralizes Listeria growth. Immunity 14:503-512. [DOI] [PubMed] [Google Scholar]
- 5.Feng, H.-M., V. L. Popov, and D. H. Walker. 1994. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 62:1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng, H.-M., V. L. Popov, G. Yuoh, and D. H. Walker. 1997. Role of T-lymphocyte subsets in immunity to spotted fever group rickettsiae. J. Immunol. 158:5314-5320. [PubMed] [Google Scholar]
- 7.Feng, H.-M., and D. H. Walker. 2000. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 68:6729-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng, H.-M., and D. H. Walker. 2003. Cross-protection between distantly related spotted fever group rickettsiae. Vaccine 21:3901-3905. [DOI] [PubMed] [Google Scholar]
- 9.Feng, H.-M., T. Whitworth, J. P. Olano, V. L. Popov, and D. H. Walker. 2003. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 72:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson, B. A., C. L. Wisseman, Jr., A. Waddell, and D. J. Silverman. 1981. Some characteristics of heavy and light bands of Rickettsia prowazekii on renografin gradients. Infect. Immun. 34:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito, S., and Y. Rikihisa. 1981. Techniques for electron microscopy of rickettsiae, p. 213-227. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, N.Y.
- 13.Kaplowitz, L. G., J. V. Lange, J. J. Fischer, and D. H. Walker. 1983. Correlation of rickettsial titers, circulating endotoxin, and clinical features in Rocky Mountain spotted fever. Arch. Intern. Med. 143:1149-1151. [PubMed] [Google Scholar]
- 14.La Scola, B., and D. Raoult. 1996. Diagnosis of Mediterranean spotted fever by cultivation of Rickettsia conorii from blood and skin samples using the centrifugation-shell vial technique and by detection of R. conorii in circulating endothelial cells: a 6-year follow-up. J. Clin. Microbiol. 34:2722-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogata, H., S. Audic, P. Renesto-Audiffren, P.-E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J.-M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 16.Paddock, C. D., P. W. Greer, T. L. Ferebee, J. Singleton, Jr., D. B. McKechnie, T. A. Treadwell, J. W. Krebs, M. J. Clarke, R. C. Holman, J. G. Olson, J. E. Childs, and S. R. Zaki. 1999. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J. Infect. Dis. 179:1469-1476. [DOI] [PubMed] [Google Scholar]
- 17.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricketts, H. T. 1908. Studies on immunity in Rocky Mountain spotted fever. J. Infect. Dis. 5:221-224. [Google Scholar]
- 19.Sexton, D. J., S. S. Kanj, K. Wilson, G. R. Corey, B. C. Hegarty, M. G. Levy, and E. B. Breitschwerdt. 1994. The use of a polymerase chain reaction as a diagnostic test for Rocky Mountain spotted fever. Am. J. Trop. Med. Hyg. 50:59-63. [PubMed] [Google Scholar]
- 20.Topping, N. H. 1940. Rocky Mountain spotted fever. Treatment of infected laboratory animals with immune rabbit serum. Public Health Rep. 55:41-46. [Google Scholar]
- 21.Topping, N. H. 1943. Rocky Mountain spotted fever: further experience in the therapeutic use of immune rabbit serum. Public Health Rep. 58:757-775. [Google Scholar]
- 22.Tzianabos, T., B. E. Anderson, and J. E. McDade. 1989. Detection of Rickettsia rickettsii DNA in clinical specimens by using polymerase chain reaction technology. J. Clin. Microbiol. 27:2866-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valbuena, G., H.-M. Feng, and D. H. Walker. 2002. Mechanisms of immunity against rickettsiae. New perspectives and opportunities offered by unusual intracellular parasites. Microbes Infect. 4:625-633. [DOI] [PubMed] [Google Scholar]
- 24.Vielma, S., G. Virella, A. J. Gorod, and M. F. Lopes-Virella. 2002. Chlamydophila pneumoniae infection of human aortic endothelial cells induces the expression of FC γ receptor II (FcγRII). Clin. Immunol. 104:265-273. [DOI] [PubMed] [Google Scholar]
- 25.Walker, D. H. 2002. Rickettsia rickettsii: as virulent as ever. Am. J. Trop. Med. Hyg. 66:448-449. [DOI] [PubMed] [Google Scholar]
- 26.Walker, D. H. 2003. Principles of the malicious use of infectious agents to create terror: reasons for concern for organisms of the genus Rickettsia. Ann. N. Y. Acad. Sci. 990:739-742. [DOI] [PubMed] [Google Scholar]
- 27.Walker, D. H., J. P. Olano, and H.-M. Feng. 2001. Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect. Immun. 69:1841-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker, D. H., V. L. Popov, J. Wen, and H.-M. Feng. 1994. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab. Investig. 70:358-368. [PubMed] [Google Scholar]
- 29.Walker, D. H., V. L. Popov, P. A. Crocquet-Valdes, C. J. R. Welsh, and H.-M. Feng. 1997. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab. Investig. 76:129-138. [PubMed] [Google Scholar]