Abstract
T-cell-mediated pathogenesis has been documented in various idiopathic and microbially induced intestinal disorders. Diffuse microvillous shortening seen in giardiasis is responsible for disaccharidase insufficiencies and malabsorption of electrolytes, nutrients, and water. Other mucosal changes include crypt hyperplasia and increased numbers of intraepithelial lymphocytes (IEL). A recent report using an athymic mouse model of infection showed that these epithelial injuries were dependent on T cells. The aim of the present study was to identify which subset of superior mesenteric lymph node (SMLN) T cells were responsible for mucosal alterations in giardiasis. CD4+ and CD8+ T cells, as well as whole lymphocyte populations, were isolated from SMLN of Giardia muris-infected mice for adoptive transfer. Jejunal segments of recipient mice were assessed for brush border ultrastructure, sucrase activity, crypt/villus ratio, and IEL numbers. Mice that received enriched CD8+ and whole SMLN lymphocytes, but not CD4+ T cells, from infected donors showed diffuse shortening of microvilli, loss of brush border surface area, impaired sucrase activity, and increased crypt/villus ratios compared to respective controls. Transfer of whole SMLN lymphocytes, as well as enriched CD4+ or CD8+ T cells, from infected donors led to increased IEL numbers in the recipient jejunum. The findings indicate that loss of intestinal brush border surface area, reduced disaccharidase activities, and increased crypt/villus ratios in giardiasis are mediated by CD8+ T cells, whereas both CD8+ and CD4+ SMLN T cells regulate the influx of IEL.
Intestinal infection with the parasite Giardia lamblia (synonyms, G. intestinalis and G. duodenalis) is the most common waterborne diarrheal disease in North America. Pathophysiological changes in giardiasis include digestive enzyme deficiencies, Na+ and glucose malabsorption, and microvillous brush border injury, which may or may not be associated with villous atrophy (3, 4, 6, 29, 41, 42, 44). These abnormalities result in maldigestion, malabsorption, and diarrhea. Similar pathophysiological processes also occur in food anaphylaxis (11, 18), celiac disease (40), Crohn's disease (2, 12, 14, 36), and bacterial enteritis (7, 19). The fact that similar mucosal structural and functional changes are seen in diseases with or without microbial involvement has led to the hypothesis that host factors are implicated in the pathogenesis of these disorders. These similarities make experimental giardiasis a useful model for studying the mechanism of intestinal epithelial injury and malfunction.
Nude athymic (nu−/nu−) mice, unlike their euthymic counterparts, fail to clear infections with Giardia (27, 39). Reconstitution of infected athymic mice with T cells leads to decreased parasite load (39). Moreover, specific depletion of CD4+ T cells allows the development of chronic giardiasis in mice (9, 45). In parallel with their role in parasite clearance, T cells have also been implicated in the pathogenesis of mucosal epithelial injury (16, 51). Previous studies using athymic nude mice have shown that villous atrophy and crypt cell hyperplasia in giardiasis are mediated by activated T cells, and reconstitution of T cells in athymic infected mice causes crypt-villus abnormalities (17, 39). Similarly, villous atrophy and crypt cell hyperplasia may be induced with T cells stimulated with either pokeweed mitogen or anti-CD3 antibody (32). Together, these observations suggest that intestinal villous atrophy and crypt cell hyperplasia can be mediated by activated T cells, regardless of the presence or absence of microbial pathogens, via mechanisms that remain obscure. Moreover, previous reports suggest that in giardiasis, the degree of epithelial microvillous abnormalities correlates with the severity of intestinal malfunction and clinical symptoms (6, 16). Recent findings have shown that microvillous injury and disaccharidase impairment develop in euthymic, but not athymic (nu−/nu−) mice, infected with G. muris, suggesting that brush border injury may be mediated at least in part by thymus-derived T cells (44). In contrast, increased numbers of intraepithelial lymphocytes (IEL) was seen in intestinal tissues from either group of infected animals (44). While this observation is consistent with previous studies that described increased numbers of IEL in the Giardia-infected intestine (19, 37, 48), the findings also imply that influx may in part depend on T cells of extrathymic origin.
In an attempt to better understand the mechanism of small intestinal abnormalities caused by giardiasis, the aim of this study was to assess the role of CD4+ and CD8+ T lymphocytes in intestinal injury and increase of IEL numbers.
MATERIALS AND METHODS
Animal model.
Four-week-old (17- to 20-g), female, specific-pathogen-free, immunocompetent, euthymic CD-1 mice (Charles River Laboratories, Wilmington, Mass.) were used in all experiments. Animals were housed in pairs and given water and commercial rodent chow ad libitum, and cages were covered with microisolator lids to prevent cross-contamination. The animal room environment was maintained at 22°C and 40% relative humidity with 12-h photoperiods. Maintenance of animals and all experimental procedures were carried out in accordance with guidelines of the Canadian Council on Animal Care and the University of Calgary Animal Life and Environmental Sciences Care Committee.
Isolation of T lymphocytes.
In all experiments, infection was established with an axenic inoculum of G. muris devoid of bacterial and/or viral contaminants as previously described (44). T-cell donor animals were separated into two groups: sham-treated control donor (CD) animals received 0.2 ml of sterile phosphate-buffered saline (PBS) by orogastric gavage; infected donor (ID) mice received 2 × 105 live, axenic G. muris trophozoites suspended in 0.2 ml of sterile PBS (106 trophozoites/ml). Lymphocytes were collected from the superior mesenteric lymph nodes (SMLNs) of donor mice 6 days after either inoculation with the axenic trophozoites suspension or sham vehicle. Day 6 was selected in light of the microvillous brush border and disaccharidase abnormalities seen at this time of a primary experimental infection (44). SMLNs were aseptically excised from the mice, pooled, and placed in chilled, sterile Hanks' balanced salt solution. A single-cell suspension was achieved by the gentle grinding of the lymph nodes between the frosted ends of two microscope slides. This cell suspension was centrifuged at 800 to 1,000 × g for 5 min at 4°C. The pelleted cells were then resuspended in 5 ml of sterile PBS, and viable cells were counted on a hemacytometer by trypan blue exclusion and adjusted to a concentration of 5 × 107 cells/ml.
Purification of CD4+ and CD8+ T lymphocyte population.
T-cell purification was performed using the MidiMACS magnetic cell separation system according to manufacturer's protocols (Miltenyi Biotec, Auburn, Calif.). Cells were suspended in MACS buffer (154 mM NaCl, 2.2 mM Na2HPO4, 8 mM NaH2PO4, 1.99 mM EDTA, 5% bovine serum albumin) to a concentration of 107 cells per 95 μl. Magnetic microbeads (5 μl/107 cells) specific for murine CD4 (L3T4) or murine CD8 (Ly-2), (both from Miltenyi Biotec) were used to capture the desired cells in the LS separation column (Miltenyi Biotec) and allowed the flowthrough of undesired cell types. The purity of each enriched population was assessed by fluorescence-activated cell sorting analysis and checked for contaminating aerobic and anaerobic bacteria using serial dilutions spread into plates with Columbia blood agar (5% defibrinated sheep blood, 37°C, 5% CO2 [for 24 and 48 h]), MacConkey agar (37°C and 5% CO2 [for 24 and 48 h]), or brucella blood agar (5% defibrinated sheep blood, 37°C [under anaerobic conditions for 48 and 72 h]) (all from Bacto, Difco Laboratories). The resulting purity of CD4+ and CD8+ T cells was over 92% as determined by flow cytometry (23, 38), and the cell preparations were devoid of bacterial contamination (data not shown).
T-cell adoptive transfer studies.
Naive recipient mice (CD-1 immunocompetent, nu+/nu+, female, 4 weeks old, 17 to 20 g) were assigned to the following experimental groups. Mice in the first of three paired groups received whole SMLN lymphocytes from either control or IDs. Mice in the other paired groups received either enriched CD4+ or enriched CD8+ SMLN T cells of CDs or IDs. All mice received 107 lymphocytes in 0.2 ml of sterile saline via tail vein injection and were killed 3 days later for analysis. Samples were collected for histology, transmission electron microscopy, and brush border surface area calculation, and sucrase activity as described previously (4, 6, 44). Trophozoite colonization of the jejunum was assessed in all recipient mice to verify their Giardia-free status. All transfer experiments were carried out twice to ensure the results were reproducible.
Transmission electron microscopy and brush border surface area determination.
Jejunal enterocyte microvillous brush border ultrastructure was measured with transmission electron microscopy as previously described (44). Briefly, intestinal segments (1 cm) taken 13 cm distal to the ligament of Treitz were removed and fixed in 5% glutaraldehyde in cacodylate buffer overnight at 4°C. Samples were cut into 1-mm2 squares and postfixed in 1% OsO4 for 2 h, dehydrated in ethanol, cleared with propylene oxide, and embedded in Spurr low-viscosity medium (Sigma, St. Louis, Mo.). Semithin sections were stained with basic toluidine blue for light microscopy to select midvillous areas for thin sectioning for electron microscopy. Sections (thickness, 80 nm) were double stained with saturated uranyl acetate in 50% ethanol and 0.4% lead citrate (aqueous). Sections were examined at 75 kV on a Hitachi H7000 transmission electron microscope.
Micrographs of midvillous enterocyte apical membranes from mice of each experimental group were obtained at the same magnification (×12,000), as previous studies have demonstrated that Giardia-induced microvillous injury in the midvillous area was representative of the changes along the entire villous axis (6, 44). Height, width, and density of the microvilli were calculated, and the surface area of the epithelial brush border was determined as previously described (5). To avoid observer bias, micrographs were coded and observations were recorded in a blind fashion. For each group, a total of 14 to 21 micrographs were obtained from three animals per group, and microvillous brush border surface area was calculated. In short, microvillous height and width were measured over 1 μm of epithelial surface, and the average values were used to determine the surface area of a cylinder (representing the shaft) and a circle (representing the tip of the microvillus), with the sum of these two calculated values representing the surface area of one average microvillus. The number of microvilli along one micrometer of epithelium (n) was determined and squared to obtain microvillar density per 1 μm2 of epithelial surface. Microvillar density was multiplied by the calculated microvillar surface area to determine the overall microvillar surface area per square micrometer of epithelium.
Disaccharidase activity.
As a number of previous studies have used disaccharidase impairment as a reliable marker of Giardia-induced mucosal injury, sucrase activity was measured in the present study to assess whether brush border function may be altered by the activated T cells. Jejunal segments (length, 12 cm) were collected from recipient mice, blotted dry, weighed, homogenized in 2.5 mM EDTA (4 ml/1 g of tissue), snap-frozen in liquid nitrogen, and stored at −70°C until needed. Sucrase activity was determined as previously described (6, 44) and expressed as units per gram of protein. Total protein content from mucosal samples was determined by the Bradford protein assay (Bio-Rad, Mississauga, Ontario, Canada).
Intestinal histology.
Jejunal segments (length, 2 cm, taken 11 cm distal to the ligament of Treitz) were removed and processed for light microscopy. Samples were fixed in 10% neutral buffered formalin (pH 7.3) and embedded in paraffin wax, and sections were stained with hematoxylin and eosin. Crypt and villus length were measured from well-oriented, complete, crypt-villus units, using a scale built in the optical lens of the light microscope. The average of the calculated ratio of crypt to villus was determined for each mouse in the different groups. The number of IEL was also determined along villus units and expressed as the number of IEL per 100 epithelial cells (% IEL).
Statistical analysis.
Results were expressed as mean ± standard error of the mean (SEM). Means were compared by paired t test or one-way analysis of variance and Tukey's compromise test for multiple comparison where applicable. Significance was established at P < 0.05.
RESULTS
Brush border ultrastructure.
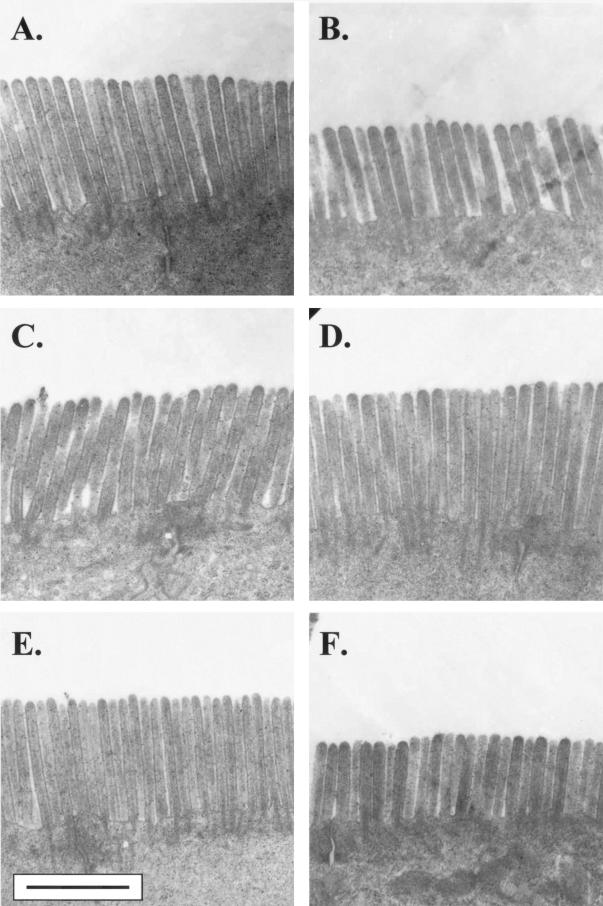
Six days postinfection, the total number of lymphocytes isolated from the SMLNs of infected mice (32.1 × 106 ± 6.2 × 106) was significantly higher (approximately 2.2-fold) than that of controls (14.4 × 106 ± 1.7 × 106). To determine the role of CD8+ and CD4+ lymphocytes in the brush border injury and disaccharidase deficiencies caused by giardiasis, T-cell-adoptive transfer studies were performed. Representative electron photomicrographs of the intestinal epithelial cells of naive recipient mice 3 days posttransfer are shown in Fig. 1. Transfer of whole SMLN lymphocyte populations and CD8+-enriched population of T cells from infected mice induced a diffuse shortening of brush border microvilli in recipient animals. Transfer of CD4+ T cells from infected animals did not alter microvillous ultrastructure (Fig. 1). Specific measurements of the brush border architecture confirmed these observations (Table 1). In naive recipients given whole lymphocyte populations or enriched CD8+ T cells, the overall epithelial surface area was reduced by approximately 30% compared to controls. In both cases, the loss of brush border surface area was a direct result of microvillous shortening (Table 1). In mice that received purified CD4+ T cells from infected animals, jejunal microvillous architecture was not different from that of controls (Table 1).
FIG. 1.
Representative transmission electron micrographs of the jejunal microvillous brush border from naive mice which received lymphocytes from donors infected with Giardia (B, D, and F) or from sham-inoculated CDs (A, C, and E). In separate experiments, mice received either whole SMLN lymphocytes (A and B), enriched SMLN CD4+ T cells (C and D), or enriched SMLN CD8+ T cells (E and F). Brush border shortening, when present, was diffuse and was seen at sites of trophozoite colonization as well as in other areas. Bar equals 1 μm for all micrographs.
TABLE 1.
Description of brush border of midvillus enterocytes in the jejunum of micea
| Cells and mouse group | Ht (μm) | Width (μm) | Density (no./μm2 of epithelium) | Surface area (μm2/μm2 of epithelial surface) |
|---|---|---|---|---|
| Whole cells | ||||
| CD (n = 20) | 1.362 ± 0.047 | 0.132 ± 0.004 | 60.43 ± 3.78 | 33.00 ± 1.74 |
| ID (n = 18) | 0.963 ± 0.072b | 0.129 ± 0.003 | 58.74 ± 2.06 | 23.98 ± 1.76b |
| T cells | ||||
| CD CD4+ (n = 15) | 1.132 ± 0.054 | 0.129 ± 0.003 | 60.68 ± 2.52 | 28.42 ± 1.45 |
| ID CD4+ (n = 14) | 1.195 ± 0.083 | 0.131 ± 0.003 | 59.85 ± 2.94 | 29.92 ± 2.25 |
| CD CD8+ (n = 13) | 1.088 ± 0.044 | 0.129 ± 0.003 | 60.76 ± 3.42 | 27.34 ± 1.38 |
| ID CD8+ (n = 18) | 0.798 ± 0.058§§ | 0.127 ± 0.002 | 62.62 ± 2.56 | 20.77 ± 1.72c |
Animals received whole superior mesenteric lymph node (SMLN) lymphocytes, enriched SMLN CD4+ T cells, or enriched SMLN CD8+ T cells from control (CD) or previously infected (ID) donor mice. Values are mean ± SEM.
P < 0.05 versus respective control.
P < 0.01 versus paired control.
Sucrase activities.
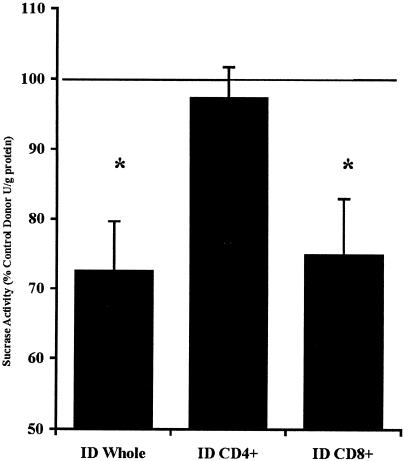
Sucrase activity was assessed in order to determine whether loss of jejunal brush border surface area was associated with impaired function. Sucrase activity was significantly decreased (P < 0.05) in naive mice that received either whole SMLN cell preparations (143 ± 14 U/g of protein) or enriched CD8+ T cells (186 ± 11 U/g of protein) from IDs compared to mice receiving whole SMLN preparations (197 ± 13 U/g of protein) or CD8+ cells (249 ± 21 U/g of protein) from uninfected CDs. Sucrase activity was not different in recipients of purified CD4+ T cells from IDs (194 ± 19 U/g of protein) versus their respective controls (189 ± 16 U/g of protein). Results in Fig. 2 illustrate sucrase activities in recipient animals as percentages versus their respective controls.
FIG. 2.
Jejunal sucrase activity in naive mice that received T-lymphocytes from donors. Donor mice were infected with G. muris (ID) or inoculated with medium alone (CD; solid line). Values (units per gram of protein) are expressed as a percentage of sucrase activity (mean ± SEM [error bars]) versus paired control (set at 100%, solid line). *, P < 0.05 versus paired CD (n = 8 to 12 mice per group).
Crypt/villus ratio.
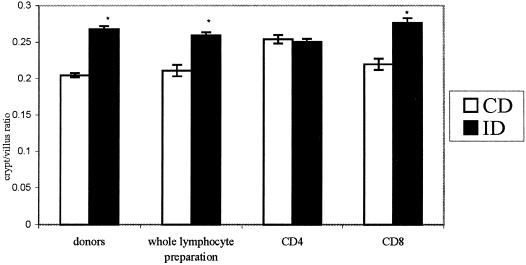
The effect of specific subpopulations of T cells in the regulation of crypt/villus ratios was determined. Increased crypt/villus ratio was found in the jejunum of Giardia-infected donor mice compared to uninfected donor controls (Fig. 3). Naive mice that received whole SMLN lymphocytes, or CD8+ SMLN T cells, but not CD4+ T cells, from IDs showed increased crypt/villus ratios compared to their paired controls (Fig. 3).
FIG. 3.
Crypt/villus ratios in donor mice (donors) or in naive mice that received T-lymphocytes from donors 3 days posttransfer (whole lymphocyte preparation, CD4, CD8). Empty bars represent results from CDs or from naive recipients that received lymphocytes from CDs; solid bars represent results from IDs or from naive recipients that received lymphocytes from ID. Values are expressed as crypt/villus ratio (mean ± SEM [error bars]) per group. *, P < 0.05 versus paired CD group (n = 5 to 10 mice per group).
Jejunal IEL numbers.
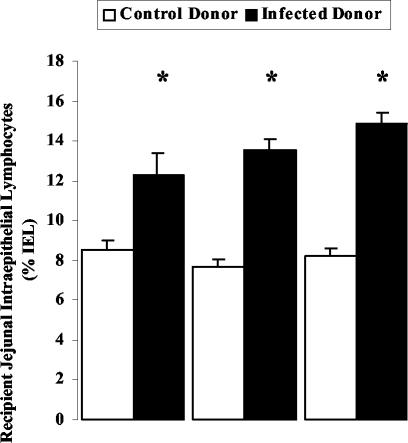
Previous experiments using the same model have demonstrated that 6 days postinfection, when Giardia-induced brush border microvillous shortening and disaccharidase impairment are evident, there is a concurrent increase in IEL numbers (44). Therefore, the present study also assessed the effects of lymphocyte transfer with whole SMLN population, CD4+- or CD8+-T-cell-enriched populations, on IEL numbers. Figure 4 illustrates that all mice that received lymphocytes from IDs, regardless of cell population phenotype, exhibited significant increases in jejunal IEL numbers, compared to animals given lymphocytes from uninfected CDs or compared to naive unmanipulated animals (8.7% ± 0.5%; data not shown). Moreover, enhanced IEL numbers were of similar magnitude in all groups (Fig. 4).
FIG. 4.
Jejunal IEL counts in mice that received lymphocytes from previously infected donors or from sham-inoculated CDs. Lymphocytes used were whole SMLN lymphocyte preparation, enriched CD4+ T cells, or enriched CD8+ T cells, and counts were performed 3 days after transfer. Values are expressed as number of IEL per 100 enterocytes (% IEL) (mean ± SEM [error bars]). (* P < 0.05 versus paired CD group; n = 5 to 10 mice per group).
DISCUSSION
Diffuse brush border abnormalities responsible for maldigestion and malabsorption in giardiasis have been reported in humans as well as animal models (4-6, 16). Increased IEL numbers have also been associated with Giardia infection in numerous reports (19, 37). The mechanisms responsible for these alterations remain unclear. Results from previous studies indicate that the diffuse loss of epithelial brush border surface area and the decrease of sucrase and maltase activities in giardiasis are mediated by thymus-dependent T cells, whereas increased number of IEL may be due at least in part to T cells of extrathymic origin (44). Findings from the present study demonstrate that transfer of whole SMLN lymphocytes or purified CD8+ T cells, but not CD4+ T cells, from previously infected mice elicits brush border injury, sucrase insufficiency, and increased crypt/villus ratios in the jejunum of naive recipient animals. Moreover, transfer of either CD8+ or CD4+ SMLN T cells from infected mice is capable of inducing IEL influx in naive recipients. The findings indicate that SMLN CD8+, but not CD4+, T-lymphocytes are responsible for the pathogenesis of epithelial microvillous injury and increased crypt/villus ratios in giardiasis. In contrast, both CD4+ and CD8+ T cells are implicated in the increase of IEL numbers during infection.
Results from the present study demonstrate that infection with G. muris induces a significant increase in the total number of SMLN lymphocytes. A previous study of G. lamblia-infected mice showed that T cells from Peyer's patches proliferate in response to Giardia antigens, whereas no response was seen in spleen and inguinal lymph node lymphocytes (20). Previous research has suggested that during the course of Giardia infection, there is a gradual transition from suppressor or cytotoxic to helper or inducer T cell in the intraepithelial and lamina propria compartments (48). Together with the present findings and other reports on cellular and humoral immunity to giardiasis (21, 47), these observations suggest that Giardia elicits a local mucosal immune reaction, which implicates CD8+ as well as CD4+ T lymphocytes.
Direct evidence for the role of CD4+ T cells in parasite expulsion has been demonstrated in murine giardiasis. Depletion of CD4+ helper or inducer T lymphocytes in G. muris-infected mice results in chronic infection (26, 45). In contrast, infected mice depleted of CD8+ suppressor or cytotoxic T-cell subtypes show normal eradication (26). The expulsion of G. muris coincides with the appearance and level of secreted immunoglobulin A antibody in infected mice (46). Moreover, immunoglobulin A-mediated defense against Giardia is impaired by treatment of mice with anti-CD4 antibodies, further suggesting that CD4+ T cells contribute to the clearance of Giardia parasites (25). The role of CD8+ T cells in giardiasis has remained more elusive.
Findings from the present study show that adoptive transfer of whole activated SMLN lymphocytes or CD8+ T cells, but not CD4+ T cells, from ID mice induces brush border injury in naive recipient animals. In these animals, shortening of microvillous length and loss of reduced brush border surface area coincided an impairment of sucrase activity. In contrast, sucrase activity in mice that received enriched CD4+ T cells was not different from controls. Moreover, increased crypt/villus ratio was reported in the jejunum of naive mice that received whole SMLN lymphocytes or CD8+ T cells from IDs compared to their paired CD groups. No changes were seen in mice that received SMLN CD4+ T cells from infected and CDs. Hence, the alterations in crypt/villus ratio appear to be regulated by CD8+ T cells, similarly to the changes seen in the epithelial brush border. These findings indicate that activated CD8+ T cells are responsible for the ultrastructural, architectural, and functional changes of the jejunal mucosa observed in the acute phase of giardiasis.
Diffuse pathogenic brush border abnormalities have also been documented in Crohn's disease (2, 12-14, 36, 42), celiac disease (40), food anaphylaxis (11, 18), and bacterial enteritis (7, 19). CD4+ T cells are known to be involved in the pathogenesis of Crohn's disease, and adoptive transfer of CD4+ CD45 RBhigh cells into SCID mice induces inflammation in the intestine and lesions similar to Crohn's disease (31). In addition, significant infiltration of CD8+ T cells has also been documented in the small intestinal mucosa of Crohn's disease patients, and it has been suggested that increased cytolytic activity of CD8+ T cells in the intraepithelial compartment of the patients may be involved in the induction of epithelial tissue damage (28, 34, 35). Consistent with this hypothesis, findings from the present study demonstrated that CD8+ lymphocytes are responsible for the brush border injury and malfunction seen in giardiasis. Immunostaining studies have shown that, in Giardia-infected human intestinal tissues, the IEL, which are mostly CD8+ T cells, are positive for T-cell restricted intracellular antigen-1, but negative for granzyme B, implying that these CD8+ T cells are resting cytotoxic cells (37). Furthermore, CD8+ IEL isolated from TCR transgenic mice exhibit antigen-specific perforin- and FasL-mediated cytotoxic activity toward intestinal epithelial cells and T cells (10, 33). Potent FasL-dependent cytolytic activity of CD8+ IEL toward enterocytes was also reported in graft-versus-host disease (30). The signaling events implicating CD8+ T cells in the brush border injury during giardiasis and other disorders of the small intestinal warrant further investigations.
Increased numbers of IEL have been reported in a variety of intestinal diseases, including giardiasis, cryptosporidiosis, salmonellosis, and celiac disease (1, 15, 19, 24, 37, 45, 48, 50). Recent findings suggest that the origin of enhanced numbers of IEL in giardiasis may be partly extrathymic (44). Similarly, in cryptosporidiosis, the source of proliferative IEL is both thymic and extrathymic (1). In the present study, transfer of Giardia-activated SMLN CD4+ or CD8+ T cells, regardless of their subset, induced a modest, albeit significant, increase of IEL numbers in recipient mice. The results suggest that both subtypes of T cells may be regulating the proliferation and/or the increased homing of lymphocytes into the intraepithelial compartment during giardiasis. Celiac disease is characterized by a striking increase in γδ versus αβ T cells (43, 49). Similarly, increased numbers and activation of the CD8+ TCRγδ+ T cells have been reported in the IEL compartment during intestinal infections with Salmonella spp., Listeria spp., or Cryptosporidium spp. (8, 15, 22, 24). Studies of Giardia-infected tissues reveal a much more subtle increase in IEL numbers than that seen in celiac disease (27, 37). Whether this may suggest that unlike in celiac disease, the pathophysiology of giardiasis may be mediated by cytotoxic T cells other than γδ IEL has yet to be investigated. Recent studies have found that in athymic mice, which still harbor functional extrathymic γδ IEL, Giardia-induced brush border injury is abolished (44). Future experiments will determine whether thymus-derived CD8+ TCRαβ+ T cells, rather than γδ T cells as seen in celiac disease, may be responsible for the microvillous injury seen in giardiasis.
In summary, findings from this study demonstrate that brush border injury, disaccharidase deficiency, and increased crypt/villus ratio, which cause malabsorptive diarrhea in giardiasis and other intestinal diseases, are dependent on SMLN-derived CD8+ T cells, but not CD4+ T cells. Moreover, transfer of either CD4+or CD8+ T cells from Giardia-infected donors increased the numbers of IEL in recipient mice, suggesting that both subpopulations of T cells may regulate the influx of IEL in giardiasis. Future investigations will assess whether and how CD8+ T cells may signal for epithelial brush border injury and malfunction from within the IEL compartment, in giardiasis as well as in other malabsorptive disorders of the small intestine.
Acknowledgments
We are grateful for the financial support of the Natural Sciences and Engineering Research Council of Canada and the Crohn's Colitis Foundation of Canada. L. C. H. Yu is recipient of a fellowship cosponsored by the Canadian Association of Gastroenterology, the Canadian Institute of Health Research, and AstraZeneca.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Adjei, A. A., J. T. Jones, and F. J. Enriquez. 2000. Differential intra-epithelial lymphocyte phenotypes following Cryptosporidium parvum challenge in susceptible and resistant athymic strains of mice. Parasitol. Int. 49:119-129. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis, C. 1979. Abnormalities of jejunal mucosal enzymes in ulcerative colitis and Crohn's disease. Digestion 19:259-266. [DOI] [PubMed] [Google Scholar]
- 3.Belosevic, M., G. M. Faubert, and J. D. MacLean. 1989. Disaccharidase activity in the small intestine of gerbils (Meriones unguiculatus) during primary and challenge infections with Giardia lamblia. Gut 30:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buret, A., D. G. Gall, and M. E. Olson. 1990. Effects of murine giardiasis on growth, intestinal morphology, and disaccharidase activity. J. Parasitol. 76:403-409. [PubMed] [Google Scholar]
- 5.Buret, A., D. G. Gall, and M. E. Olson. 1991. Growth, activities of enzymes in the small intestine, and ultrastructure of microvillous border in gerbils infected with Giardia duodenalis. Parasitol. Res. 77:109-114. [DOI] [PubMed] [Google Scholar]
- 6.Buret, A., J. A. Hardin, M. E. Olson, and D. G. Gall. 1992. Pathophysiology of small intestinal malabsorption in gerbils infected with Giardia lamblia. Gastroenterology 103:506-513. [DOI] [PubMed] [Google Scholar]
- 7.Buret, A., M. E. Olson, D. G. Gall, and J. A. Hardin. 1998. Effects of orally administered epidermal growth factor on enteropathogenic Escherichia coli infection in rabbits. Infect. Immun. 66:4917-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai, J. Y., S. M. Guk, H. K. Han, and C. K. Yun. 1999. Role of intraepithelial lymphocytes in mucosal immune responses of mice experimentally infected with Cryptosporidium parvum. J. Parasitol. 85:234-239. [PubMed] [Google Scholar]
- 9.Chung, B. M., L. E. Wallace, J. A. Hardin, and D. G. Gall. 2002. The effect of epidermal growth factor on the distribution of SGLT-1 in rabbit jejunum. Can. J. Physiol. Pharmacol. 80:872-878. [DOI] [PubMed] [Google Scholar]
- 10.Corazza, N., S. Muller, T. Brunner, D. Kagi, and C. Mueller. 2000. Differential contribution of Fas- and perforin-mediated mechanisms to the cell-mediated cytotoxic activity of naive and in vivo-primed intestinal intraepithelial lymphocytes. J. Immunol. 164:398-403. [DOI] [PubMed] [Google Scholar]
- 11.Curtis, G. H., M. K. Patrick, A. G. Catto-Smith, and D. G. Gall. 1990. Intestinal anaphylaxis in the rat: effect of chronic antigen exposure. Gastroenterology 98:1558-1566. [DOI] [PubMed] [Google Scholar]
- 12.D'Inca, R., G. C. Sturniolo, D. Martines, L. Di, V., A. Cecchetto, C. Venturi, and R. Naccarato. 1995. Functional and morphological changes in small bowel of Crohn's disease patients. Influence of site of disease. Dig. Dis. Sci. 40:1388-1393. [DOI] [PubMed] [Google Scholar]
- 13.Dunne, W. T., W. T. Cooke, and R. N. Allan. 1977. Enzymatic and morphometric evidence for Crohn's disease as a diffuse lesion of the gastrointestinal tract. Gut 18:290-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak, A. M., and G. R. Dickersin. 1979. Crohn's disease: electron microscopic studies. Pathol. Annu. 14:259-306. [PubMed] [Google Scholar]
- 15.Emoto, M., M. Miyamoto, Y. Emoto, J. Zerrahn, and S. H. Kaufmann. 2001. A critical role of T-cell receptor gamma/delta cells in antibacterial protection in mice early in life. Hepatology 33:887-893. [DOI] [PubMed] [Google Scholar]
- 16.Farthing, M. J. 1993. Diarrhoeal disease: current concepts and future challenges. Pathogenesis of giardiasis. Trans. R. Soc. Trop. Med. Hyg. 87(Suppl. 3):17-21. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira, R. C., L. E. Forsyth, P. I. Richman, C. Wells, J. Spencer, and T. T. MacDonald. 1990. Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T-cell-mediated response in human small intestine. Gastroenterology 98:1255-1263. [DOI] [PubMed] [Google Scholar]
- 18.Freier, S., M. Eran, and R. Goldstein. 1985. The effect of immediate-type gastrointestinal allergic reactions on brush border enzymes and gut morphology in the rat. Pediatr. Res. 19:456-459. [DOI] [PubMed] [Google Scholar]
- 19.Gillon, J., D. Al Thamery, and A. Ferguson. 1982. Features of small intestinal pathology (epithelial cell kinetics, intraepithelial lymphocytes, disaccharidases) in a primary Giardia muris infection. Gut 23:498-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottstein, B., G. R. Harriman, J. T. Conrad, and T. E. Nash. 1990. Antigenic variation in Giardia lamblia: cellular and humoral immune response in a mouse model. Parasite Immunol. 12:659-673. [DOI] [PubMed] [Google Scholar]
- 21.Gottstein, B., N. I. Stocks, G. M. Shearer, and T. E. Nash. 1991. Human cellular immune response to Giardia lamblia. Infection 19:421-426. [DOI] [PubMed] [Google Scholar]
- 22.Guk, S. M., T. S. Yong, and J. Y. Chai. 2003. Role of murine intestinal intraepithelial lymphocytes and lamina propria lymphocytes against primary and challenge infections with Cryptosporidium parvum. J. Parasitol. 89:270-275. [DOI] [PubMed] [Google Scholar]
- 23.Hamelmann, E., A. Oshiba, J. Paluh, K. Bradley, J. Loader, T. A. Potter, G. L. Larsen, and E. W. Gelfand. 1996. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a marine model of airway sensitization. J. Exp. Med. 183:1719-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara, T., Y. Mizuno, K. Takaki, H. Takada, H. Akeda, T. Aoki, M. Nagata, K. Ueda, G. Matsuzaki, Y. Yoshikai, et al. 1992. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J. Clin. Investig. 90:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyworth, M. F. 1989. Intestinal IgA responses to Giardia muris in mice depleted of helper T lymphocytes and in immunocompetent mice. J. Parasitol. 75:246-251. [PubMed] [Google Scholar]
- 26.Heyworth, M. F., J. R. Carlson, and T. H. Ermak. 1987. Clearance of Giardia muris infection requires helper/inducer T lymphocytes. J. Exp. Med. 165:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyworth, M. F., R. L. Owen, and A. L. Jones. 1985. Comparison of leukocytes obtained from the intestinal lumen of Giardia-infected immunocompetent mice and nude mice. Gastroenterology 89:1360-1365. [DOI] [PubMed] [Google Scholar]
- 28.Honma, J., H. Mitomi, K. Murakami, M. Igarashi, K. Saigenji, and K. Toyama. 2001. Nodular duodenitis involving CD8+ cell infiltration in patients with ulcerative colitis. HepatoGastroenterology 48:1604-1610. [PubMed] [Google Scholar]
- 29.Koudela, B. 1994. The ultrastructure of the intestinal microvillous border in the common vole (Microtus arvalis) naturally infected with Giardia microti. Fol. Parasitol. (Prague) 41:241-245. [PubMed] [Google Scholar]
- 30.Lin, T., T. Brunner, B. Tietz, J. Madsen, E. Bonfoco, M. Reaves, M. Huflejt, and D. R. Green. 1998. Fas ligand-mediated killing by intestinal intraepithelial lymphocytes. Participation in intestinal graft-versus-host disease. J. Clin. Investig. 101:570-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Z., K. Geboes, S. Colpaert, L. Overbergh, C. Mathieu, H. Heremans, M. de Boer, L. Boon, G. D'Haens, P. Rutgeerts, and J. L. Ceuppens. 2000. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40-CD154 interactions. J. Immunol. 164:6005-6014. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, T. T., and J. Spencer. 1988. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J. Exp. Med. 167:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melgar, S., A. Bas, S. Hammarstrom, and M. L. Hammarstrom. 2002. Human small intestinal mucosa harbours a small population of cytolytically active CD8+ alphabeta T lymphocytes. Immunology 106:476-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller, S., J. Lory, N. Corazza, G. M. Griffiths, K. Z'graggen, L. Mazzucchelli, A. Kappeler, and C. Mueller. 1998. Activated CD4+ and CD8+ cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am. J. Pathol. 152:261-268. [PMC free article] [PubMed] [Google Scholar]
- 35.Nussler, N. C., B. Stange, R. A. Hoffman, W. H. Schraut, A. J. Bauer, and P. Neuhaus. 2000. Enhanced cytolytic activity of intestinal intraepithelial lymphocytes in patients with Crohn's disease. Langenbecks Arch. Surg. 385:218-224. [DOI] [PubMed] [Google Scholar]
- 36.Nyhlin, H., and R. Stenling. 1984. The small-intestinal mucosa in patients with Crohn's disease assessed by scanning electron and light microscopy. Scand. J. Gastroenterol. 19:433-440. [PubMed] [Google Scholar]
- 37.Oberhuber, G., H. Vogelsang, M. Stolte, S. Muthenthaler, A. J. Kummer, and T. Radaszkiewicz. 1996. Evidence that intestinal intraepithelial lymphocytes are activated cytotoxic T cells in celiac disease but not in giardiasis. Am. J. Pathol. 148:1351-1357. [PMC free article] [PubMed] [Google Scholar]
- 38.Petrow, P. K., K. Thoss, D. Katenkamp, and R. Brauer. 1996. Adoptive transfer of susceptibility to antigen-induced arthritis into severe combined immunodeficient (SCID) mice: role of CD4+ and CD8+ T cells. Immunol. Investig. 25:341-353. [DOI] [PubMed] [Google Scholar]
- 39.Roberts-Thomson, I. C., and G. F. Mitchell. 1978. Giardiasis in mice. I. Prolonged infections in certain mouse strains and hypothymic (nude) mice. Gastroenterology 75:42-46. [PubMed] [Google Scholar]
- 40.Rubin, W., L. L. Ross, M. H. Sleisenger, and E. Weser. 1966. An electron microscopic study of adult celiac disease. Lab. Investig. 15:1720-1747. [PubMed] [Google Scholar]
- 41.Samra, H. K., N. K. Ganguly, U. C. Garg, J. Goyal, and R. C. Mahajan. 1988. Effect of excretory-secretory products of Giardia lamblia on glucose and phenylalanine transport in the small intestine of Swiss albino mice. Biochem. Int. 17:801-812. [PubMed] [Google Scholar]
- 42.Samra, H. K., U. C. Garg, N. K. Ganguly, and R. C. Mahajan. 1987. Effect of different Giardia lamblia inocula on glucose and amino acids transport in the intestinal brush border membrane vesicles of infected mice. Ann. Trop. Med. Parasitol. 81:367-372. [DOI] [PubMed] [Google Scholar]
- 43.Savilahti, E., A. Arato, and M. Verkasalo. 1990. Intestinal gamma/delta receptor-bearing T lymphocytes in celiac disease and inflammatory bowel diseases in children. Constant increase in celiac disease. Pediatr. Res. 28:579-581. [DOI] [PubMed] [Google Scholar]
- 44.Scott, K. G., M. R. Logan, G. M. Klammer, D. A. Teoh, and A. G. Buret. 2000. Jejunal brush border microvillous alterations in Giardia muris-infected mice: role of T lymphocytes and interleukin-6. Infect. Immun. 68:3412-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer, S. M., and T. E. Nash. 2000. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect. Immun. 68:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snider, D. P., and B. J. Underdown. 1986. Quantitative and temporal analyses of murine antibody response in serum and gut secretions to infection with Giardia muris. Infect. Immun. 52:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkatesan, P., R. G. Finch, and D. Wakelin. 1996. Comparison of antibody and cytokine responses to primary Giardia muris infection in H-2 congenic strains of mice. Infect. Immun. 64:4525-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinayak, V. K., R. Khanna, and K. Kum. 1991. Kinetics of intraepithelium and lamina propria lymphocyte responses during Giardia lamblia infection in mice. Microb. Pathog. 10:343-350. [DOI] [PubMed] [Google Scholar]
- 49.Westerholm-Ormio, M., J. Garioch, I. Ketola, and E. Savilahti. 2002. Inflammatory cytokines in small intestinal mucosa of patients with potential coeliac disease. Clin. Exp. Immunol. 128:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyatt, C. R., E. J. Brackett, and W. J. Barrett. 1999. Accumulation of mucosal T lymphocytes around epithelial cells after in vitro infection with Cryptosporidium parvum. J. Parasitol. 85:765-768. [PubMed] [Google Scholar]
- 51.Yu, L. C. H., and M. H. Perdue. 2001. Immunologically mediated transport of ions and macromolecules. Ann. N. Y. Acad. Sci. 915:247-259. [DOI] [PubMed] [Google Scholar]