Abstract
Objective
To clarify the spatio-temporal profile of cortical activity related to reaching movement in the posterior parietal cortex (PPC) in humans.
Methods
Four patients with intractable partial epilepsy who underwent subdural electrode implantation were studied as a part of pre-surgical evaluation. We investigated the Bereitschaftspotential (BP) associated with reaching and correlated the findings with the effect of electrical stimulation of the same cortical area.
Results
BPs specific for reaching, as compared with BPs for simple movements by the hand or arm contralateral to the implanted hemisphere, were recognized in all patients, mainly around the intraparietal sulcus (IPS), the superior parietal lobule (SPL) and the precuneus. BPs near the IPS had the earlier onset than BPs in the SPL. Electrical stimulation of a part of the PPC, where the reach-specific BPs were recorded, selectively impaired reaching.
Conclusions
Intracranial BP recording and cortical electrical stimulation delineated human reach-related areas in the PPC.
Significance
The present study for the first time by direct cortical recording in humans demonstrates that parts of the cortices around the IPS and SPL play a crucial role in visually-guided reaching.
Keywords: Bereitschaftspotential, cortical electrical stimulation, optic ataxia, posterior parietal cortex, reaching
1. Introduction
Optic ataxia is defined as a selective impairment of visually-guided hand movements and has been of great interest because this specific symptom is not explained by disturbance of any elementary cortical function such as motor, somatosensory, visual, oculomotor, perceptual and attention component alone. However, not much attention seems to be paid to this symptom in the field of functional neurosurgery and neurology, in which cortical functional mapping is often employed to identify eloquent cortices. Balint (Balint, 1909) originally reported a patient with bilateral parieto-occipital lesions showing optic ataxia and other attention impairments. Later, it was demonstrated that optic ataxia could occur in isolation (Garcin et al. , 1967). In this condition, misreaching is found in the peripheral visual field (Rondot et al. , 1977, Auerbach et al. , 1981, Jeannerod, 1986, Perenin et al. , 1988, Jakobson et al. , 1991, Roy et al. , 2004, Karnath et al. , 2005, Khan et al. , 2005). Common lesion sites have been found in the posterior parietal cortex (PPC), encompassing the intraparietal sulcus (IPS), the adjacent part of the inferior parietal lobule (IPL), the superior parietal lobule (SPL) and the precuneus (Auerbach et al., 1981, Perenin et al., 1988, Pisella et al. , 2000, Roy et al., 2004, Karnath et al., 2005, Khan et al., 2005). In the studies of patients with focal brain lesions, however, the location and the number or size of lesions vary among patients, and it is often associated with other parietal symptoms. Furthermore, the effect of functional compensation by other cortical areas is expected to modify the clinical picture. Virtual lesioning by transcranial magnetic stimulation (TMS) of the PPC may also produce misreaching in normal subjects (Desmurget et al. , 1999, MacDonald et al. , 2003, Della-Maggiore et al. , 2004, Glover et al. , 2005, van Donkelaar et al. , 2005, Vesia et al. , 2006), but its spatial resolution is limited to a sublobar level especially in the association areas like parietal lobe.
Electrophysiological studies in monkeys demonstrated involvement of various areas of the PPC, including the medial intraparietal area (MIP), V6A, areas 5, 7a and 7m in reaching movements (Kalaska et al. , 1983, Colby et al. , 1991, MacKay, 1992, Johnson et al. , 1996, Ferraina et al. , 1997, Battaglia-Mayer et al. , 2000, Fattori et al. , 2001, Ferraina et al. , 2001, Buneo et al. , 2002, Galletti et al. , 2003, Vesia et al., 2006). Recently, functional neuroimaging studies in humans, using functional MRI (fMRI) or PET, have also implicated the PPC in reaching or pointing movements (Kawashima et al. , 1995, Kertzman et al. , 1997, Inoue et al. , 1998, Desmurget et al. , 2001, Simon et al. , 2002, Astafiev et al. , 2003, Connolly et al. , 2003, Grefkes et al. , 2004, Prado et al. , 2005, Beurze et al. , 2007, Filimon et al. , 2007). However, neuroimaging techniques have insufficient temporal resolution to delineate the temporal profile of cortical activation during reaching. Within the PPC, little is known about the temporal dynamics of cortical activity involved in visually-guided behavior in humans. Some researchers hypothesized that information involved in visuo-manual transformation is transferred from parieto-occipital junction (POJ: including precuneus) to medial intraparietal area, especially in reaching to the target in the peripheral vision (Blangero et al. , 2008). However, recent human study using magnetoencephalography suggested that cortical activity was found first in the caudal PPC during preparation for reach, followed by the anterior IPS (Broadmann area 5) and medial wall of the PPC (Broadmann area 7m) and that POJ was active during execution of reach (Hinkley et al. , 2011).
A slow surface-negative electroencephalographic (EEG) potential preceding self-initiated movements was discovered and named the Bereitschaftspotential (BP) by Kornhuber and Deecke (Kornhuber et al. , 1965). The BP is composed of an early slope (early BP) and a late steeper slope (late BP) (Shibasaki et al. , 2006, Shibasaki, 2011). The BP is generated from the frontal cortices such as the primary sensorimotor area (Neshige et al. , 1988), the supplementary motor area (SMA) (Ikeda et al. , 1992), the pre-SMA (Yazawa et al. , 2000) and the lateral non-primary motor areas (Kunieda et al. , 2004). In the present study of direct epicortical recording in patients with medically intractable partial epilepsy originating from parietal lobes, we recorded the BP associated with reach-to-grasp movements and investigated the effect of a train of high frequency repetitive electrical stimulation of the same cortical areas. The combined use of BP recording and electrical stimulation provides high temporal as well as spatial resolution to delineate reach-related areas in the PPC and a temporal pattern of cortical activity in these areas.
This study, for the first time by direct cortical recording in humans, demonstrated the precise spatio-temporal profile of cortical areas involved in reaching movements.
2. Methods
2.1. Subjects
We studied four patients (15-27 years of age, three men and one woman) with medically intractable parietal lobe epilepsy who underwent chronic implantation of subdural electrodes for pre-surgical evaluation (Table 1). Subdural electrodes were placed over the cortical surface including the PPC (on the left in Patient 1 and on the right in other three patients). Electrodes made of platinum (Ad-Tech Co., Racine, WI, USA) were arranged on a grid or strip with the center-to-center inter-electrode distance of 1 cm. Each electrode was 2.3 mm in diameter. Two patients were right-handed and the other two were ambidextrous as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Patient 3 had frequent myoclonus in the left hand. The other patients had no neurological deficits. Patient 3 underwent surgical resection of the right anterior temporal lobe 19 years previously, and Patient 4 had resection of a part of the right inferior parietal lobule 5 years previously, both for intractable partial epilepsy. A localized lesion was revealed by MRI in the medial aspect of the left parietal lobe in Patient 1 and in the lateral aspect of the right parietal lobe in Patient 3. In all patients, focal cortical dysplasia was pathologically proven after surgery. One of the patients (Patient 4) was reported elsewhere for entirely different purposes (Matsumoto et al. , 2003, Matsuhashi et al. , 2004).
Table 1.
Patient profile.
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age/Gender | 15/F | 27/M | 17/M | 22/M |
| Neurological symptoms/signs | SPS (motor) | SPS (motor), SGTCS |
Myoclonus, SPS (motor), SGTCS |
SPS (visual), CPS, SGTCS |
| Handedness | R | Ambidextrous | R | Ambidextrous |
| Pathological diagnosis | FCD | FCD | FCD | FCD |
| Implanted hemisphere | L | R | R | R |
| Number of implanted electrodes | ||||
| Total | 50 | 46 | 56 | 34 |
| PPC | 28 | 23 | 33 | 22 |
| SPL | 9 | 7 | 7 | 1 |
| Peri-IPS | 7 | 5 | 8 | 6 |
| IPL | 12 | 11 | 11 | 15 |
| Precuneus | none | none | 7 | none |
F: female, M: male, SPS: simple partial seizure, CPS: complex partial seizure, SGTCS: secondarily generalized tonic-clonic seizure, R: right, L: left, FCD: focal cortical dysplasia, PPC: posterior parietal cortex, SPL: superior parietal lobule, IPS: intraparietal sulcus, IPL: inferior parietal lobule.
We studied BP and cortical electrical stimulation as for reaching for research purpose. All patients gave their written informed consent prior to the implantation of electrodes according to clinical research protocol No. 79 approved by the Ethics Committee of Kyoto University Graduate School of Medicine.
2.2. Recording of electrocorticographic potentials associated with reaching movement and simple motor task
2.2.1. Tasks
The patients were seated or laid in the supine position comfortably on a bed with the arm used for the task resting on a pillow while video and EEG were continuously monitored in the Epilepsy Monitoring Unit. A training session preceded an actual recording session in order to confirm proper execution of the task by the patients. In all patients, two sessions of the reaching task were employed for the left and right hand, and two sessions of the simple movement were employed for the hand contralateral to implanted hemisphere. In the reaching task, the patients were instructed to reach and grasp the handle of a cup placed at a comfortable reaching distance as quickly as possible at a self-paced interval of about 10 s. Once they grasped it, then they quickly released and returned their hands to the resting position. The patients were requested to keep fixating on the cup throughout each recording session. For simple movements as control tasks, wrist extension or shoulder abduction of the side contralateral to the implanted hemisphere was employed in otherwise the same condition. The number of trials used for BP analysis in reaching with the contralateral hand was 166, 147, 180 and 121 in Patient 1, 2, 3, and 4, respectively, while the number of trials in wrist extension was 186, 129, 179 and 142 and the number of trials in shoulder abduction was 227, 151, 146 and 185.
The task performance was monitored with electromyograms (EMG) of the effector muscles. Verbal instruction was given to the patients whenever their performance was found inadequate. The patients were told to avoid eye movements and blinks in association with each task execution. In Patient 1, all recordings were made on the same day. Recordings were completed in three days in Patient 2 and 3 and in two days in Patient 4.
2.2.2. Data acquisition
Electrocorticograms (ECoGs) were recorded from 34 to 56 intracranial subdural electrodes with digital sampling rate of 500 Hz (Patient 4), 1000 Hz (Patient 1 and 2) and 2000 Hz (Patient 3) using a digital electroencephalograph (EEG2100 and 1100, Nihon Kohden, Tokyo, Japan). All subdural electrodes were referenced to a scalp electrode placed on the skin over the mastoid process contralateral to the implanted hemisphere. EMGs were bipolarly recorded with a pair of shallow Ag-AgCl cup electrodes placed 3 cm apart on the skin over the corresponding deltoid and the extensor carpi radialis (ECR) muscles. In a reaching task of Patient 2, EMGs were acquired only from the deltoid muscle. The bandpass filter for data acquisition of both ECoG and EMG was set to 0.016 – 120 Hz (Patient 4), 300 Hz (Patient 1 and 2) and 600 Hz (Patient 3).
2.2.3. Data analysis
Data analysis was accomplished in an off-line manner on a PC workstation using Matlab version 7.5 (Mathworks, Inc., Natick, MA, USA). After applying rectification and high-pass filter of 5 Hz to the EMG data, the EMG onset of each task was visually identified and used as fiducial points. ECoGs were averaged from 3 s before to 2 s after the fiducial points. We excluded trials containing obvious artifacts or an EMG onset that was not brisk enough to be clearly identified. The baseline was corrected for the first 0.5 s of the analysis window. For reaching tasks, the EMG onset of the deltoid muscle was used for the analysis because the results estimated from ECR and deltoid muscles were almost the same and also because EMG waveforms were relatively more uniform in the deltoid muscle than the ECR. Two sessions of each task were separately averaged and plotted together on the figure to confirm the reproducibility. In the shoulder abduction task of Patient 4, since movement-related artifacts always occurred on the reference electrode, one of the intracranial electrodes, on which apparently no slow potentials were observed in either reaching or wrist extension tasks, was adopted as the reference before the final analysis.
For identifying BP, once it was found reproducible in the two sequential sessions, slow potential shifts starting before the EMG onset and exceeding 50% of the maximal amplitude in each polarity among the PPC electrodes for each task were accepted regardless of polarity. Waveform of BP at each electrode was compared between the reaching and the simple motor tasks, and a BP was defined ‘reach-specific’ when the amplitude of BP in the reaching task was larger (> 200% in amplitude) than the amplitude of larger BPs between two simple movement tasks at the same electrode by the same hand or when the polarity of BP was opposite (i.e., positive vs negative). The onset of the slow potentials at each electrode was determined with the aid of a linear regression line on the averaged data of the two sessions. The regression line was calculated from the point where the averaged signal exceeded ±2 S.D. of the baseline period to the peak of slow potential. When the linear regression line did not fit well with the actual waveform, the final determination of the onset was made visually (Nagamine et al. , 1996).
In this paper, we emphasized the posterior parietal areas more than the prefrontal and peri-central areas for analysis, because the precentral area showed BP in all the tasks employed and also because the PPC has been reported to play a crucial role for reaching in functional imaging and lesion studies (Perenin et al., 1988, Connolly et al., 2003, Karnath et al., 2005).
2.3. Functional mapping by direct cortical electrical stimulation
In three patients (Patient 1, 3 and 4), functional cortical mapping was done by electrical stimulation of subdural electrodes for the purpose of pre-surgical evaluation. The method has been reported in detail elsewhere (Luders et al. , 1987, Ikeda et al., 1992). In brief, constant electrical current of square wave pulse with alternating polarity (pulse duration 0.3 ms) was delivered to a pair of adjacent electrodes at a frequency of 50 Hz. Current intensity and stimulus duration were gradually increased up to 15 mA and 5 s, respectively. When any positive symptoms were elicited or after-discharges persisted at the submaximum intensity, the stimulation was terminated at that intensity. In two patients (Patient 1 and 3), the effect of stimulation of the PPC at and around the areas, where reach-specific BP was identified, was investigated for research purpose in the following tasks: 1) repetitive finger tapping with the contralateral hand while arms were held extended forward, 2) contralateral shoulder abduction, 3) visual field test by confrontation, 4) reaching task to the central visual field (toward the foveally fixated target), and 5) reaching to the peripheral visual field (25 degrees apart horizontally from the fixation point) contralateral to the stimulated hemisphere. The patients’ performance was evaluated by a board-certified neurophysiologist (MI) during cortical electrical stimulation and later confirmed on video by another board-certified neurophysiologist (RM). If the patient could not successfully (=no hesitation, smooth trajectory) reach the object of the cup upon cortical electrical stimulation, and it was reproducible by the similar stimulation, and reaching was normally performed upon sham stimulation (with no electric current while the test was performed in otherwise the same way), then we defined it as misreaching elicited by cortical electrical stimulation.
2.4. Anatomical localization of subdurally placed electrodes and nomenclature of PPC subregions
In order to localize subdural electrodes precisely, anatomical MR images were obtained while electrodes were in place. Anatomical location of each electrode was identified using 2D MRIs based upon its round hypointensity spot or signal void due to the property of platinum alloy (Matsumoto et al. , 2004). Structural image of each patient (FSPGR or MPRAGE) was acquired while electrodes were in place and was co-registered to the pre-surgical structural image using both linear and non-linear transformation algorithm (FLIRT, FNIRT) from Functional Magnetic Resonance Imaging of the Brain’s Software Library (www.fmrib.ox.ac.uk/fsl). The transformation matrix or coefficient was then applied to the electrode coordinates. Additionally, the pre-surgical images were registered into Montreal Neurological Institute (MNI) standard space (Collins et al. , 1994) and electrode coordinates were transformed. When the electrode coordinate was situated above the surface of the brain, the electrode location was shifted perpendicularly toward the cortical surface by <5 mm. The methods have been reported elsewhere (Matsumoto et al. , 2011).
Anatomical landmarks such as the central, postcentral, intraparietal and parieto-occipital sulci were identified on the pre-surgical MRI in reference to the anatomical atlas (Ono et al. , 1990). The PPC was situated between the occipital lobe and the postcentral gyrus and were divided into two subregions, superior parietal lobule (SPL) and inferior parietal lobule (IPL), by intraparietal sulcus (IPS). On the lateral surface, we included the areas around the parieto-occipital border into the PPC because the boundary could not always be determined with precision due to its variability. On the mesial surface, the precuneus was defined as the area posterior to the paracentral lobule, anterior to the parieto-occipital sulcus, and superior to the subparietal sulcus. In this study we referred to an electrode whose center was located on the cortical surface within 5 mm from the IPS as ‘peri-IPS’ electrode.
3. Results
The number of electrodes placed over the PPC ranged from 22 to 33 among patients. Across the patients, a total of 24 electrodes were located in the SPL, 26 in the peri-IPS area, 49 in the IPL and 7 in the precuneus (Table 1).
3.1. Cortical potentials associated with the reaching task
3.1.1. Location of active cortical areas for reaching and simple movements
Both reaching and simple movements of the hand contralateral to the implanted hemisphere were preceded by a slow potential (BP) in the pre- and postcentral areas. BP with the reaching task was also observed on the PPC in all patients. The BP fields with the reaching task recorded from the central area and the PPC were spatially isolated from each other (Fig. 1). Among the patients, the maximal BPs in the PPC were observed 4-6 cm apart from the maximal frontal BPs. The number of electrodes where the BP was recorded in the reaching with the contralateral hand was nine, eight, four and two in Patient 1, 2, 3 and 4, respectively (Table 2). BP with simple movements was also identified in the PPC in three patients (Table 2). The PPC is considered to be involved also in other behaviors such as tool-use or somatosensory-guided actions and also in simple actions. Therefore, we similarly consider that BP in the PPC could be generated not only by visually guided reaching but also by other, even in simple actions. Indeed, in monkey, it was reported that self-paced movements (lifting a lever, finger extension, toe and trunk movements) elicited pre-movement slow potentials (Gemba et al. , 2004). In the present study, we defined reach-specific BP, using BP for simple movements as control and did not further discuss BP with simple movement tasks.
Figure 1.
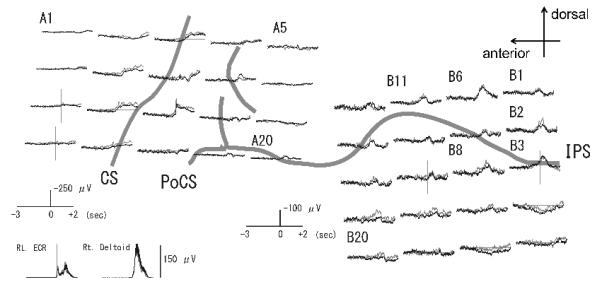
Waveforms of Bereitschaftspotential (BP) in the reaching task recorded from the left frontal (left panel) and parietal (right panel) cortex in Patient 1.Two subaverages of electrocorticograms (ECoGs) are shown in superimposition as the dark and light grey lines. Waveforms are shown from 3 s before to 2 s after the EMG onset of the deltoid muscle. Note different amplitude scale between the anterior and posterior grids. BP is formed by the initial slow and the following steeper pre-movement potential. The frontal and parietal BPs are spatially separate from each other. Electrode locations are shown on 3D-MRI in Figure 2.
CS: central sulcus, PoCS: postcentral sulcus, IPS: intraparietal sulcus, ECR: extensor carpi radialis muscle.
Table 2.
Cortical areas active for BP with reaching and simple tasks in four patients
| Patient | 1 | 2 | 3 | 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subregions | SPL | Peri- IPS |
IPL | SPL | Peri-IPS | IPL | SPL | Peri- IPS |
PCu | SPL | Peri- IPS |
IPL |
| Motor task | ||||||||||||
| Reach-c | A14, A15, B2, B6 |
A20, B3, B11 |
B4, B8 |
A5, A10, A15 |
A2, A8, A14, A20 |
A7 | - | B3, B8 | C6, C13 | A5 | A4 | - |
| Reach-i | B2, B6 | A19, A20, B11 |
B4, B5 |
A5, A10 | A2 | A6, A7 | - | B8 | C13 | - | - | - |
| Simple: shoulder | A4, A5, A14, A15, B6 |
A19 | B4 | A15 | A14, A20 | A6, A7, A17, A19 |
- | - | - | - | - | - |
| Simple: wrist | A4, A9, A15 | B17 | - | A5, A15 | A14, A20 | - | - | - | - | - | - | A16, B1, B10 |
Labels of electrodes are the same as those in Figs.2-5. Electrodes with ‘reach-specific BPs’ are underlined. Reach-c: reaching with the contralateral hand, Reach-i: reaching with the ipsilateral hand, Simple: simple movement (contralateral shoulder abduction and wrist extension), SPL: superior parietal lobule, IPS: intraparietal sulcus, IPL: inferior parietal lobule, PCu: precuneus.
In Patient 1 (right-handed, implanted to left hemisphere), out of the above nine electrodes, six were reach-specific; two in the SPL (B2 and B6), three in the peri-IPS (B3, B11 and A20) and one in the IPL (B8) (Fig. 2, Table 2). In Patient 2 (ambidextrous, implanted to right hemisphere), four out of the above eight electrodes were found reach-specific, two each in the SPL (A5 and A10) and in the peri-IPS (A2 and A8) (Fig. 3, Table 2). In Patient 3 (right-handed, implanted to right hemisphere), two electrodes in the peri-IPS (B3 and B8) and two in the precuneus (C6 and C13) were found reach-specific (Fig. 4, Table 2). In Patient 4 (ambidextrous, implanted to right hemisphere), one each in the peri-IPS (A4) and the SPL (A5) was found reach-specific (Fig. 5, Table 2).
Figure 2.
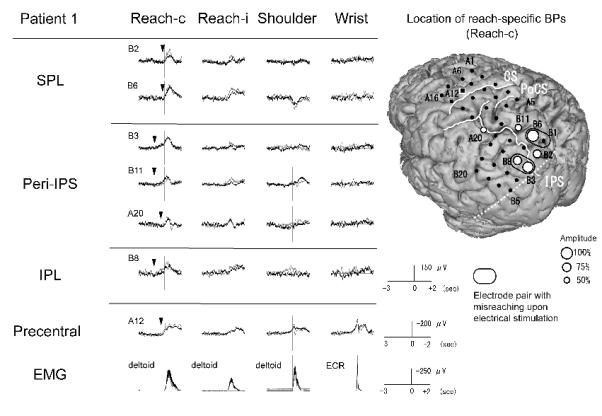
BPs recorded from the left parietal cortex in association with four different tasks and the location of reach-specific electrode sites in Patient 1. Only electrodes that showed reach-specific BP in the PPC (B2, B6, B3, B11, A20 and B8) and a representative electrode in the precentral gyrus (A12) are shown. Note that the reach-specific BP with the contralateral hand (Reach-c) in the peri-IPS area and the IPL starts earlier than in the SPL. Arrowheads indicate the onset of reach-specific BPs. Among reach-specific sites, BP for reaching with the left hand (Reach-i) is also found at four electrodes (B2, B6, B11 and A20). In 3D-MRI, reach-specific BPs (white dots) are located mainly in the middle to posterior part of the PPC along the IPS, involving the SPL. A square indicates representative electrode in the precentral gyrus. Size of the white dots is proportional to the maximal amplitude of reach-specific BP at each electrode along the entire time window. Small black dots show the location of the subdural electrodes. Electrode pairs with stimulation effect for reaching (i.e., misreaching) are highlighted by a black ellipsoid. The dashed line indicates the anterior border of the occipital lobe on the lateral surface. (Reach-c: reaching with the contralateral hand, Reach-i: reaching with the ipsilateral hand, Shoulder: contralateral shoulder abduction, Wrist: contralateral wrist extension, CS: central sulcus, PoCS: postcentral sulcus, IPS: intraparietal sulcus).
Figure 3.
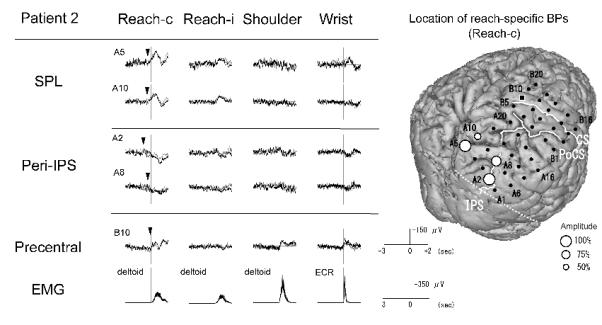
BPs recorded from the right parietal cortex in association with four different tasks and the location of reach-specific electrode sites in Patient 2. Only electrodes that showed reach-specific BP in the PPC (A5, A10, A2 and A8) and a representative electrode in the precentral gyrus (B10) are shown. The reach-specific BP (Reach-c) in the peri-IPS area has the earlier onset compared with the SPL. The reach-specific BP in the SPL is negative in polarity while that in the peri-IPS area is positive. Among reach-specific sites, BP for reaching with the right hand (Reach-i) is also found at three electrodes (A5, A10 and A2). In 3D-MRI, reach-specific BPs are located both in the SPL and near the IPS in the middle to posterior part of the PPC.
Conventions are the same as for Fig. 2.
Figure 4.
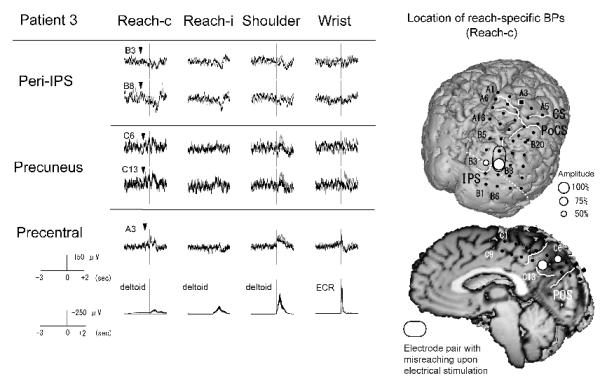
BPs recorded from the right parietal cortex in association with four different tasks and the location of reach-specific electrode sites in Patient 3. Only electrodes that showed reach-specific BP in the PPC (B3, B8, C6 and C13) and a representative electrode in the precentral gyrus (A3) are shown. The reach-specific BP (Reach-c) in the precuneus is negative in polarity while that in the peri-IPS area is positive. Among reach-specific sites, BP for reaching with the right hand (Reach-i) is also found at two electrodes (B3 and C13). In the pre-surgical 3D-MRI, reach-specific BPs are located both near the IPS in the posterior part of the PPC and in the precuneus. Electrode pair with stimulation effect for reaching (i.e., misreaching) are highlighted by a black ellipsoid.
Conventions are the same as for Fig. 2.
Figure 5.
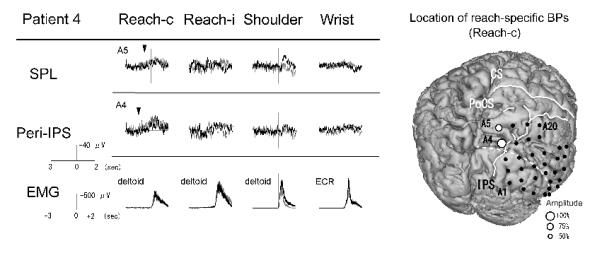
BPs recorded from the right parietal cortex in association with four different tasks and the location of reach-specific electrode sites in Patient 4. Only electrodes that showed reach-specific BP in the PPC (A4, A5) are shown. Reach-specific BP (Reach-c) in the peri-IPS area starts earlier than in the SPL area. In the reaching task with the right hand, BP (Reach-i) is not observed. In the pre-surgical 3D-MRI, reach-specific BPs are located both in the SPL and near the IPS. Conventions are the same as for Fig. 2.
Among electrode sites active for reaching, the reach-specific areas were located at the posterior part of the PPC. Reach-specific BP in the SPL had negative polarity while those in the peri-IPS area had either positive or negative polarity. Reaching with the hand ipsilateral to the implanted hemisphere was also preceded by BP at the PPC similar to that of the contralateral hand with regard to waveform and localization, but no pre-movement activities were found in the precentral areas (Figs. 2-5, Table 2). In the MNI coordinates, the reach-specific electrode sites were localized along the IPS (Fig 6, Table 3).
Figure 6.
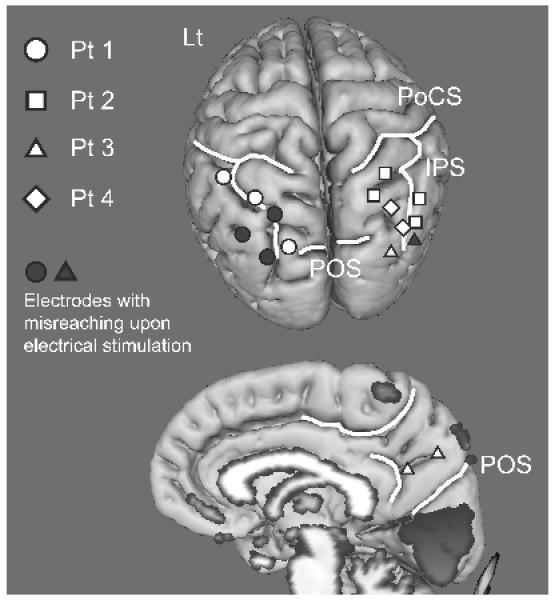
Location of reach-specific electrode sites in all patients shown in the MNI space.
Six circles, four squares, four triangles and two diamonds denote active areas in Patient 1, 2, 3 and 4, respectively. Those are mostly located in the middle to posterior part of the PPC along the IPS in each hemisphere and also in the mesial parietal area. Electrodes with stimulation effect for reaching (i.e., misreaching) are colored in grey (PoCS: postcentral sulcus, IPS: intraparietal sulcus, POS: parieto-occipital sulcus)
Table 3.
Areas active for reach-specific BP on the MNI coordinates in each patient
| Patient | Subregions * | Labels of electrodes |
Stereotaxic coordinates (MNI space) (mm) |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 1 | SPL | B2 | −20 | −80 | 50 |
| SPL | B6 | −24 | −68 | 60 | |
| Peri-IPS | A20 | −50 | −48 | 56 | |
| Peri-IPS | B3 | −30 | −82 | 42 | |
| Peri-IPS | B11 | −36 | −60 | 62 | |
| IPL | B8 | −40 | −72 | 48 | |
| 2 | SPL | A5 | 18 | −68 | 68 |
| SPL | A10 | 24 | −60 | 72 | |
| Peri-IPS | A2 | 38 | −74 | 50 | |
| Peri-IPS | A8 | 38 | −66 | 58 | |
| 3 | Peri-IPS | B3 | 26 | −86 | 42 |
| Peri-IPS | B8 | 36 | −78 | 42 | |
| Precuneus | C6 | 2 | −64 | 48 | |
| Precuneus | C13 | 2 | −52 | 36 | |
| 4 | SPL | A5 | 26 | −72 | 60 |
| Peri-IPS | A4 | 32 | −76 | 50 | |
3.1.2. Temporal profile of reach-related cortical activities
The onset time of reach-specific BP is shown in Table 4. BPs in the peri-IPS (mean = −960 ms, SD = 380 ms) started earlier than in the SPL (mean = −400 ms, SD = 230 ms) (Mann-Whitney test, p < 0.05). BPs in the IPL (−1250 ms, in Patient 1) and the precuneus (mean = −800 ms, in Patient 3) also had a tendency to start earlier than in the SPL (Figs. 2-5, Table 4).
Table 4.
Spatio-temporal profile of reach-specific BPs and cortical areas that induced misreaching upon electrical stimulation in each patient
| Patient | 1 | 2 | 3 | 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subregions | PreC* | SPL | Peri-IPS | IPL | PreC* | SPL | Peri-IPS | PreC* | Peri-IPS | PCu | SPL | Peri-IPS |
| Onset time (msec) / Electrodes |
−350/A12 | −200/B2 | −1150/B3 | −1250/B8 | −50/B10 | −450/A10 | −900/A2 | −600/A3 | −1100/B3 | −800/C6 | −750/A5 | −1450/A4 |
| −200/B6 | −1200/B11 | −400/A5 | −400/A8 | −1100/B8 | −800/C13 | |||||||
| −400/A20 | ||||||||||||
|
| ||||||||||||
| Misreaching** | − | B1-B6 | B3-B8 | B3-B8 | - | n.a. | n.a. | - | B8-B9 | - | n.a. | n.a. |
PreC: precentral gyrus, SPL: superior parietal lobule, IPS: intraparietal sulcus, IPL: inferior parietal lobule, n.a.: not available.
= for a comparison with those at precentral area, representative values are shown.
= misreaching was elicited by cortical stimulation at a pair of adjacent electrodes
3.2. Effect of electrical stimulation of PPC on task performance
Cortical functional mapping disclosed motor and sensory areas along the central sulcus in the two patients studied (Patient 1 and 3). Additionally, 20 out of 28 electrodes placed on the PPC in Patient 1 and all 33 electrodes in Patient 3 were stimulated.
In Patient 1, stimulation at two pairs (B1-B6, B3-B8) on the left PPC caused misreaching with the right hand to the target (a cup) placed in the right peripheral visual field (Fig. 7A). By contrast, stimulation of these electrodes neither elicited positive symptoms (e.g., muscle twitch, paresthesia, phosphene) nor interfered with finger tapping, shoulder abduction or visual field. When B1-B6 was stimulated, the hand incorrectly reached out to the left away from the target (Fig. 8). The misreaching was reproducible in two trials. One out of two trials was judged as hypermetric. When B3-B8 was stimulated, reaching was similarly impaired all three times. In contrast to misreaching in the peripheral visual field, the patient could reach the target placed in the central visual field without difficulty during stimulation. During sham stimulation with no electric current while the test was performed in otherwise the same way, the patient could reach the target placed in the right peripheral visual field. The patient did not notice any visual, somatosensory or motor symptoms.
Figure 7.
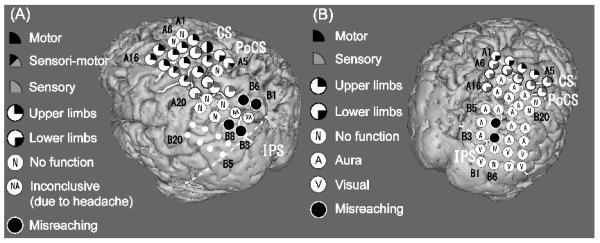
Location of electrodes which caused misreaching upon electrical stimulation in Patient 1 (A) and Patient 3 (B).
Black circles indicate electrodes where cortical stimulation caused misreaching to an object in the contralateral peripheral visual field. Other mapping results are also shown (see the symbols in the insets). Some of these electrode pairs that caused misreaching by electrical stimulation (B1-B6, B3-B8 in Patient 1 and B8-B9 in Patient 3) showed reach-specific BP (B3, B6 and B8 in Patient 1 and B8 in Patient 3). The dashed line indicates the anterior border of the occipital lobe on the lateral surface. (Compare with Fig. 2 and Fig. 4, respectively)
Figure 8.
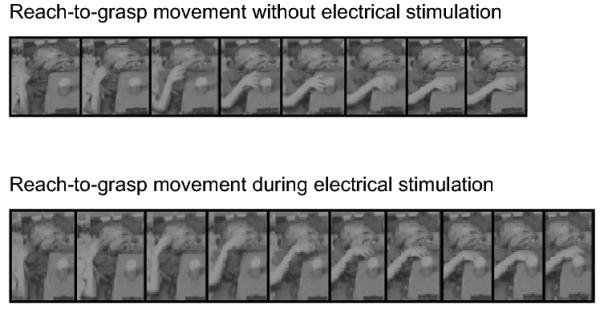
Reaching behaviors with or without cortical electrical stimulation of the left PPC (B1-B6) in Patient 1.
Patient 1 was instructed to reach-to-grasp the object located in the right peripheral visual field with the right hand. Top: Reach-to-grasp movement is successfully performed without electrical stimulation. bottom: The end point of reach shifts leftward from the object during electrical stimulation.
Photos are sequentially arranged every 140 ms.
Patient 3, upon stimulation at one pair (B8-B9) on the right PPC, subjectively felt a difficulty in reaching with the left hand to the target placed in the left peripheral visual field (Fig. 7B). Objectively reaching was possible but slow and imprecise. In addition, stimulation at C6 and C13 (not shown in Fig. 7B because of their location at the mesial parietal lobe) caused drop of the left arm, which was similar to one of the patient’s ictal semiologies. These electrodes also showed the reach-specific BP. Stimulation of the electrode pair (B8-B9) in the PPC, which caused misreaching, neither elicited positive symptoms nor interfered with finger tapping, shoulder abduction, visual field and reaching to the target in the central visual field.
In both patients, stimulation did not elicit misreaching to the central visual field in any electrodes investigated with electrical stimulation in the PPC.
3.3. Epileptogenic foci and surgical resection
In Patient 1, the presumed epileptogenic lesion, which could not be covered by subdural electrodes due to the presence of the large cortical vein, was located in the anterior part of the left precuneus and SPL. The lesion was finally resected while the areas showing reach-specific BP were preserved. In other three patients (Patient 2, 3, and 4), interictal irritative and ictal onset zones were identified in the right parietal lobe, including the areas generating reach-specific BP. They underwent resection of a part of the parietal lobe including the areas where the reach-specific BPs were observed. No patients, however, developed disturbance in hand movement after surgery.
4. Discussion
4.1. Cortical areas active in reaching
BPs are slow field potentials that precede self-initiated movements, and are usually observed in the central area (Kornhuber et al., 1965, Shibasaki et al. , 1980). A recent study, however, reported that complex movement tasks, such as tool-use pantomimes, showed BPs on scalp EEG recording with more posterior distribution than conventional simple movement tasks (Wheaton et al. , 2005). Based on the results of human epicortical recording, BPs with simple movement tasks are generated in the primary and non-primary motor areas, i.e., SMA proper, pre-SMA, lateral premotor area, and frontal eye field (Ikeda et al., 1992, Yazawa et al., 2000, Yamamoto et al. , 2004). In contrast, BP has not been investigated directly from the human parietal cortex.
In the present study, BP associated with reach-to-grasp movements was for the first time recorded directly from the human PPCs. BPs specific for reaching with the contralateral hand (i.e., reach-specific BPs) and also with the ipsilateral hand (with smaller amplitude) were recorded mainly in the middle to posterior part of the PPC along the IPS, and also in the more dorsal part of the SPL (>5 mm from the IPS), precuneus and IPL. In two patients, cortical electrical stimulation indicated that the reach-specific areas thus identified were crucial for reaching movements because it caused misreaching to the contralesional visual field.
These findings are consistent with the results of the previous human functional imaging studies for visually-guided reaching or pointing and with those of the lesion studies of optic ataxia (Perenin et al., 1988, Kawashima et al., 1995, Kertzman et al., 1997, Inoue et al., 1998, Desmurget et al., 2001, Simon et al., 2002, Astafiev et al., 2003, Connolly et al., 2003, Grefkes et al., 2004, Karnath et al., 2005, Prado et al., 2005, Beurze et al., 2007, Filimon et al., 2007). Those human functional imaging studies provide spatial resolution that is comparable to the intracranial spacing of subdural electrode of 1cm. In these techniques, however, the temporal resolution is limited compared with electrophysiological studies (Shibasaki, 2008). In the present study, a combined approach of direct epicortical potential recording and direct cortical stimulation provided incomparable information in space and time.
With regard to the active area in the reaching task, the present findings are essentially consistent with the previous experimental studies. Homologous areas in the PPC between monkey and human brains have not been fully understood, but the locations of reach-specific BPs were also supported by neurophysiological studies in monkeys. Neurons responding to reaching movements have been identified in the PPC, including the medial intraparietal area (MIP), V6A, areas 5, 7a and 7m (Kalaska et al., 1983, Colby et al., 1991, MacKay, 1992, Johnson et al., 1996, Ferraina et al., 1997, Battaglia-Mayer et al., 2000, Fattori et al., 2001, Ferraina et al., 2001, Buneo et al., 2002, Galletti et al., 2003). Although the experimental paradigm employed in the present study contained a grasping phase, the obtained results were not exactly the same as those in the studies for grasping movements. The anterior part of the IPS was more crucial for grasping movements than the posterior part (Grafton et al. , 1996, Culham et al. , 2003). It is possibly explained by the fact that the task used in the present study was not time-locked to the grasping movement or the hand pre-shaping because the EMG onset of the corresponding deltoid muscle was adopted as the fiducial point of data analysis. Therefore, the present results are interpreted to be preferentially related to the reaching movements, but we still could consider the contribution of grasping component to reach-specific BP. Signal averaging was done relative to the onset of EMG activity reporting the onset of reaching. However, neural activity preparing for the grasp could have occurred in parallel and even simultaneously with neural activity preparing for the reach.
As discussed above, a number of studies have demonstrated the importance of the IPS for reaching, but our method cannot record activities directly from the IPS because the subdural electrodes are placed almost always on the gyral convexities. However, reach-specific BPs were found around the IPS in all four patients of the present study. The polarity of reach-specific BPs in the SPL was all negative while peri-IPS activities were either positive or negative among patients. Previous studies for BPs at/around the frontal eye field and SMA suggested that the cortical potentials of smaller amplitude and positive polarity might partially reflect activities from the sulcal bank (Ikeda et al. , 1995, Yamamoto et al., 2004). Thus, the peri-IPS activities may simply arise from these areas on the convexity, but it may partially represent those from the IPS wall when the solid angle of the current source is open for the electrodes.
The PPC is also involved in saccade and visuo-spatial attention, where the related areas overlap with those related to the pointing movements (Simon et al., 2002, Astafiev et al., 2003). In the present study, as the patients were asked to fixate the target and avoid eye movements during task performance, the effect of an attentional shift and eye movements should be small. However, our results may include overlapped areas between reaching and saccade or attention because the present study employed simple movements as control tasks and did not control attention or saccade.
4.2. Temporal profile and functional properties of reach-related cortical activities
Among the present patients, reach-specific BPs in the peri-IPS area and its neighboring IPL as well as the precuneus started earlier with respect to the movement onset as compared with those in the dorsal part of the SPL. In the frontal area, it is generally accepted that the early components of BPs start first in the pre-SMA and the late components occur afterwards mainly in the primary motor cortex and SMA proper (Shibasaki et al., 2006, Shibasaki, 2011). The temporal profile would indicate the serial signal processing from internal triggering of movement, movement selection and preparation to movement initiation and execution. Similarly, the peri-IPS including the adjacent IPL and the precuneus might contribute to the planning, initiation and execution, whereas the SPL is mainly involved in the movement initiation and execution.
The PPC is considered to be involved in planning and execution of reach. Electrophysiological studies in monkey suggested that the above mentioned areas in the PPC are implicated in the reaching preparation and execution. Some investigators refer to MIP and V6A as the parietal reach region (PRR) (Andersen et al. , 2002, Cohen et al. , 2002), where the neurons are active during reach planning and execution (Johnson et al., 1996, Snyder et al. , 1997). Human fMRI experiments, which employed reaching or pointing task with delay between target presentation and movement execution in order to focus on planning phase of action, also revealed that the medial IPS and the precuneus are involved in reaching preparation (Astafiev et al., 2003, Connolly et al., 2003, Medendorp et al. , 2005, Beurze et al., 2007). These areas are also activated during reaching movement (Astafiev et al., 2003). Human neuropsychological studies of virtual lesion induced by TMS and actual lesions of the patients with optic ataxia pointed to an important role of the PPC in on-line adjustment of movements (Desmurget et al., 1999, Pisella et al., 2000, Grea et al. , 2002). Indeed, online-correction of hand trajectory has been regarded as one of the important functions of the parietofrontal network in monkey (Georgopoulos et al. , 1983, Archambault et al. , 2011). Electrophysiological studies in monkey and human fMRI studies by means of similar paradigm also suggested that the medial intraparietal cortex in both species were crucial for the transformation of visual coordinates into motor commands and for the on-line control of goal-directed movements (Eskandar et al. , 2002, Grefkes et al., 2004). Regarding the dorsal SPL where reach-specific BPs with later onset were observed in this study, human lesion studies for optic ataxia suggested that the SPL, as well as the IPS, was a common lesion site (Perenin et al., 1988, Karnath et al., 2005). The previous study of reaching in monkeys revealed that neuronal properties gradually shifted from response in delayed period to that related to actual movement along the rostro-caudal axis between the dorsal premotor and primary motor areas in the frontal lobe. Similarly, in the parietal lobe, activities during delay period were found more frequently in the ventral part of the MIP, whereas those during movement were distributed more uniformly in both the dorsal area 5 and MIP (Johnson et al., 1996). It has been established that the frontal and parietal reach-related areas are uniquely connected as follows: the areas located more posteriorly in the parietal cortex are linked more strongly to the rostral premotor area and vice versa. (Johnson et al., 1996, Marconi et al. , 2001). Indeed, this mirror-symmetric organizational framework across the central sulcus has been recently demonstrated by cortico-cortical evoked potentials in humans (Matsumoto et al., 2011). Other studies suggested that the MIP coded reach-related activity in the eye-centered reference frame, while area 5 did so in the hand-centered reference frame, which is possibly estimated by hand-target vector subtraction in eye-centered coordinate (Batista et al. , 1999, Buneo et al., 2002, Buneo et al. , 2006). These results would support the temporal profile in the present study starting from the peri-IPS areas and precuneus and shifting to the dorsal SPL.
Localization of BP in the PPC associated with reaching with the ipsilateral hand overlapped with that of the contralateral hand, although it was smaller in amplitude and area in the ipsilateral hand reaching than in the contralateral. In contrast, no clear pre-movement activities were observed in the precentral areas in the ipsilateral reaching task. This finding is in conformity with the results of fMRI studies of reach planning suggesting bilateral involvement of parietal cortex with the contralateral hand preference (Beurze et al., 2007).
4.3. Clinical implication for optic ataxia
Optic ataxia, a deficit of visually-guided hand movements, was originally reported by Balint (Balint, 1909) in association with other attentional impairments in a patient with bilateral PPC lesions. It has also been found in isolation, preferentially to the contralesional peripheral hemivisual field (Garcin et al., 1967, Rondot et al., 1977, Auerbach et al., 1981, Jeannerod, 1986, Perenin et al., 1988, Jakobson et al., 1991, Roy et al., 2004, Karnath et al., 2005, Khan et al., 2005).
A possible reason as to why optic ataxia is usually found in the peripheral visual field may be that the spatial resolution is lower in the peripheral retina and thus the symptoms easily become apparent. This idea is supported by the findings that patients with optic ataxia and normal subjects under TMS of the PPC present impairment of hand control even in the central visual field especially for the function of on-line adjustment of movements (Desmurget et al., 1999, Pisella et al., 2000). Another explanation is that distinct networks could work in reaching the foveal and peripheral targets. Recent fMRI study suggested that reaching the peripheral visual field activates wider areas in the premotor area and the PPC including parieto-occipital junction compared with the foveal target (Prado et al., 2005). In the present study, electrical stimulation of the PPC caused misreaching to the contralateral visual field, but not to the central visual field. To our knowledge, the present study is the first demonstration of misreaching similar to optic ataxia by direct cortical stimulation of the human PPC. However, there was one report that described arrest of visually-guided reaching followed by upward arm drift during cortical stimulation at the PPC (Dijkerman et al. , 2009). By contrast, the present BP study of reaching employed a reaching task to the target placed in the central vision, but not in the peripheral vision. In spite of the different targeting space between BP recording and cortical stimulation, the electrodes that caused misreaching by cortical stimulation, for example B3 and B8 in Fig.7A, overlapped with the reach-specific electrode sites. In Patient 1 and 3 who were investigated by cortical stimulation, four out of six electrodes where misreaching was caused by stimulation also showed reach-specific BP. In four out of ten electrodes with reach-specific BP, stimulation caused misreaching. These results might support the former explanation for peripheral visual field preference of optic ataxia. Alternatively, in case of the latter explanation, we could not detect a difference between the central and peripheral vision in the present study because BPs were only recorded for reaching to the central target but not to the peripheral target and because a comparison was made indirectly between two different techniques - BP and electrical stimulation.
No visual field defect was detected in the present stimulation study. However, since the confrontation test is known to be insensitive especially for the peripheral visual field (Kerr et al. , 2010), possible participation of visual field defect, visual agnosia or inattention in the generation of misreaching could not be completely excluded in the present study.
Reach-specific cortical areas were resected as a part of epileptogenic region in three patients (Patient 2, 3 and 4). However, none of them developed optic ataxia postoperatively. It could be explained by 1) a compensatory mechanism that might have taken place in the resected areas, 2) that the resected areas might not have been critical for the essential function, or 3) that the relevant areas might not have been totally resected. A similar observation has been reported in the negative motor area in the frontal lobe in humans. A negative motor area is defined as an area showing difficulty of even simple movement execution during cortical electrical stimulation while consciousness is completely preserved; such areas are especially found in the premotor cortices (Luders et al. , 1995). After resection of a part of these areas, patients showed no or only transient deficits of a skilled movement (Mikuni et al. , 2006). The authors postulated that dysfunction was most likely compensated after surgery when it was partly resected.
From the view point of clinical neuroscience, the results of the present study may reflect distorted functional anatomy because of the pathological lesion. However, it would be clinically useful that functional mapping of reaching could be made in an individual level in order to prevent postoperative optic ataxia. More precise information on the size and/or position of lesion is needed to predict the prognosis of the surgical treatment as to whether a deficit is transient or permanent.
Highlights.
Epicortical recording of Bereitschaftspotential (BP) for reaching movement provided the spatio-temporal profile of cortical activity related to reaching movement.
Cortical electrical stimulation of the posterior parietal cortex (PPC) showing reach-specific BP provoked misreaching to the peripheral visual field.
Combined epicortical BP recording and direct cortical stimulation demonstrated that the PPC plays a crucial role in visually-guided reaching.
Acknowledgements
This work was supported by The Research Grant for Nervous and Mental Disorders (22A-6) from the Ministry of Health and Welfare, The Research Grant from the Japan Epilepsy Research Foundation (2009-2010) and Grants-in-Aid for Scientific Research (C) (23591275 and 23591273) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Footnotes
Author disclosure: none declared
References
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Archambault PS, Ferrari-Toniolo S, Battaglia-Mayer A. Online control of hand trajectory and evolution of motor intention in the parietofrontal system. J Neurosci. 2011;31:742–52. doi: 10.1523/JNEUROSCI.2623-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–99. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SH, Alexander MP. Pure agraphia and unilateral optic ataxia associated with a left superior parietal lobule lesion. J Neurol Neurosurg Psychiatry. 1981;44:430–2. doi: 10.1136/jnnp.44.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint R. Seelenlahmung des “Schauens”, optische Ataxie, raumliche Stoning der Aufmerksamkeit. Monatschrift fur Psychiatrie und Neurologic. 1909;25:5–81. [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–60. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferraina S, Mitsuda T, Marconi B, Genovesio A, Onorati P, et al. Early coding of reaching in the parietooccipital cortex. J Neurophysiol. 2000;83:2374–91. doi: 10.1152/jn.2000.83.4.2374. [DOI] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol. 2007;97:188–99. doi: 10.1152/jn.00456.2006. [DOI] [PubMed] [Google Scholar]
- Blangero A, Gaveau V, Luaute J, Rode G, Salemme R, Guinard M, et al. A hand and a field effect in on-line motor control in unilateral optic ataxia. Cortex. 2008;44:560–8. doi: 10.1016/j.cortex.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature. 2002;416:632–6. doi: 10.1038/416632a. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–62. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia. 1991;29:517–37. doi: 10.1016/0028-3932(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a ‘parietal reach region’ in the human brain. Exp Brain Res. 2003;153:140–5. doi: 10.1007/s00221-003-1587-1. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–9. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Malfait N, Ostry DJ, Paus T. Stimulation of the posterior parietal cortex interferes with arm trajectory adjustments during the learning of new dynamics. J Neurosci. 2004;24:9971–6. doi: 10.1523/JNEUROSCI.2833-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2:563–7. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grea H, Grethe JS, Prablanc C, Alexander GE, Grafton ST. Functional anatomy of nonvisual feedback loops during reaching: a positron emission tomography study. J Neurosci. 2001;21:2919–28. doi: 10.1523/JNEUROSCI.21-08-02919.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkerman HC, Meekes J, Ter Horst A, Spetgens WP, de Haan EH, Leijten FS. Stimulation of the parietal cortex affects reaching in a patient with epilepsy. Neurology. 2009;73:2130. doi: 10.1212/WNL.0b013e3181c67999. [DOI] [PubMed] [Google Scholar]
- Eskandar EN, Assad JA. Distinct nature of directional signals among parietal cortical areas during visual guidance. J Neurophysiol. 2002;88:1777–90. doi: 10.1152/jn.2002.88.4.1777. [DOI] [PubMed] [Google Scholar]
- Fattori P, Gamberini M, Kutz DF, Galletti C. ‘Arm-reaching’ neurons in the parietal area V6A of the macaque monkey. Eur J Neurosci. 2001;13:2309–13. doi: 10.1046/j.0953-816x.2001.01618.x. [DOI] [PubMed] [Google Scholar]
- Ferraina S, Battaglia-Mayer A, Genovesio A, Marconi B, Onorati P, Caminiti R. Early coding of visuomanual coordination during reaching in parietal area PEc. J Neurophysiol. 2001;85:462–7. doi: 10.1152/jn.2001.85.1.462. [DOI] [PubMed] [Google Scholar]
- Ferraina S, Johnson PB, Garasto MR, Battaglia-Mayer A, Ercolani L, Bianchi L, et al. Combination of hand and gaze signals during reaching: activity in parietal area 7 m of the monkey. J Neurophysiol. 1997;77:1034–8. doi: 10.1152/jn.1997.77.2.1034. [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI. Human cortical representations for reaching: mirror neurons for execution, observation, and imagery. Neuroimage. 2007;37:1315–28. doi: 10.1016/j.neuroimage.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P. Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp Brain Res. 2003;153:158–70. doi: 10.1007/s00221-003-1589-z. [DOI] [PubMed] [Google Scholar]
- Garcin R, Rondot P, de Recondo J. [Optic ataxia localized in 2 left homonymous visual hemifields (clinical study with film presentation)] Rev Neurol (Paris) 1967;116:707–14. [PubMed] [Google Scholar]
- Gemba H, Matsuura-Nakao K, Matsuzaki R. Preparative activities in posterior parietal cortex for self-paced movement in monkeys. Neurosci Lett. 2004;357:68–72. doi: 10.1016/j.neulet.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. Interruption of motor cortical discharge subserving aimed arm movements. Exp Brain Res. 1983;49:327–40. doi: 10.1007/BF00238775. [DOI] [PubMed] [Google Scholar]
- Glover S, Miall RC, Rushworth MF. Parietal rTMS disrupts the initiation but not the execution of on-line adjustments to a perturbation of object size. J Cogn Neurosci. 2005;17:124–36. doi: 10.1162/0898929052880066. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Woods RP, Arbib MA. Functional anatomy of pointing and grasping in humans. Cereb Cortex. 1996;6:226–37. doi: 10.1093/cercor/6.2.226. [DOI] [PubMed] [Google Scholar]
- Grea H, Pisella L, Rossetti Y, Desmurget M, Tilikete C, Grafton S, et al. A lesion of the posterior parietal cortex disrupts on-line adjustments during aiming movements. Neuropsychologia. 2002;40:2471–80. doi: 10.1016/s0028-3932(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Ritzl A, Zilles K, Fink GR. Human medial intraparietal cortex subserves visuomotor coordinate transformation. Neuroimage. 2004;23:1494–506. doi: 10.1016/j.neuroimage.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Hinkley LB, Nagarajan SS, Dalal SS, Guggisberg AG, Disbrow EA. Cortical temporal dynamics of visually guided behavior. Cereb Cortex. 2011;21:519–29. doi: 10.1093/cercor/bhq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Lüders HO, Burgess RC, Shibasaki H. Movement-related potentials recorded from supplementary motor area and primary motor area. Role of supplementary motor area in voluntary movements. Brain. 1992;115(Pt 4):1017–43. doi: 10.1093/brain/115.4.1017. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Lüders HO, Shibasaki H, Collura TF, Burgess RC, Morris HH, 3rd, et al. Movement-related potentials associated with bilateral simultaneous and unilateral movements recorded from human supplementary motor area. Electroencephalogr Clin Neurophysiol. 1995;95:323–34. doi: 10.1016/0013-4694(95)00086-e. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Goto R, Koyama M, et al. PET study of pointing with visual feedback of moving hands. J Neurophysiol. 1998;79:117–25. doi: 10.1152/jn.1998.79.1.117. [DOI] [PubMed] [Google Scholar]
- Jakobson LS, Archibald YM, Carey DP, Goodale MA. A kinematic analysis of reaching and grasping movements in a patient recovering from optic ataxia. Neuropsychologia. 1991;29:803–9. doi: 10.1016/0028-3932(91)90073-h. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Mechanisms of visuomotor coordination: a study in normal and brain-damaged subjects. Neuropsychologia. 1986;24:41–78. doi: 10.1016/0028-3932(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–19. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Caminiti R, Georgopoulos AP. Cortical mechanisms related to the direction of two-dimensional arm movements: relations in parietal area 5 and comparison with motor cortex. Exp Brain Res. 1983;51:247–60. doi: 10.1007/BF00237200. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Perenin MT. Cortical control of visually guided reaching: evidence from patients with optic ataxia. Cereb Cortex. 2005;15:1561–9. doi: 10.1093/cercor/bhi034. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Roland PE, O’Sullivan BT. Functional anatomy of reaching and visuomotor learning: a positron emission tomography study. Cereb Cortex. 1995;5:111–22. doi: 10.1093/cercor/5.2.111. [DOI] [PubMed] [Google Scholar]
- Kerr NM, Chew SS, Eady EK, Gamble GD, Danesh-Meyer HV. Diagnostic accuracy of confrontation visual field tests. Neurology. 2010;74:1184–90. doi: 10.1212/WNL.0b013e3181d90017. [DOI] [PubMed] [Google Scholar]
- Kertzman C, Schwarz U, Zeffiro TA, Hallett M. The role of posterior parietal cortex in visually guided reaching movements in humans. Exp Brain Res. 1997;114:170–83. doi: 10.1007/pl00005617. [DOI] [PubMed] [Google Scholar]
- Khan AZ, Pisella L, Vighetto A, Cotton F, Luaute J, Boisson D, et al. Optic ataxia errors depend on remapped, not viewed, target location. Nat Neurosci. 2005;8:418–20. doi: 10.1038/nn1425. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH, Deecke L. [Changes in the Brain Potential in Voluntary Movements and Passive Movements in Man: Readiness Potential and Reafferent Potentials] Pflugers Arch Gesamte Physiol Menschen Tiere. 1965;284:1–17. [PubMed] [Google Scholar]
- Kunieda T, Ikeda A, Ohara S, Matsumoto R, Taki W, Hashimoto N, et al. Role of lateral non-primary motor cortex in humans as revealed by epicortical recording of Bereitschaftspotentials. Exp Brain Res. 2004;156:135–48. doi: 10.1007/s00221-003-1769-x. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Dinner DS, Morris HH, Hahn JF, Friedman L, et al. Commentary: chronic intracranial recording and stimulation with subdural electrodes. In: Engel J,J, editor. Surgical treatment of the epilepsies. Raven; New York: 1987. pp. 297–321. [Google Scholar]
- Lüders HO, Dinner DS, Morris HH, Wyllie E, Comair YG. Cortical electrical stimulation in humans. The negative motor areas. Adv Neurol. 1995;67:115–29. [PubMed] [Google Scholar]
- MacDonald PA, Paus T. The role of parietal cortex in awareness of self-generated movements: a transcranial magnetic stimulation study. Cereb Cortex. 2003;13:962–7. doi: 10.1093/cercor/13.9.962. [DOI] [PubMed] [Google Scholar]
- MacKay WA. Properties of reach-related neuronal activity in cortical area 7A. J Neurophysiol. 1992;67:1335–45. doi: 10.1152/jn.1992.67.5.1335. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, et al. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–27. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M, Ikeda A, Ohara S, Matsumoto R, Yamamoto J, Takayama M, et al. Multisensory convergence at human temporo-parietal junction - epicortical recording of evoked responses. Clin Neurophysiol. 2004;115:1145–60. doi: 10.1016/j.clinph.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Ikeda A, Ohara S, Matsuhashi M, Baba K, Yamane F, et al. Motor-related functional subdivisions of human lateral premotor cortex: epicortical recording in conditional visuomotor task. Clin Neurophysiol. 2003;114:1102–15. doi: 10.1016/s1388-2457(03)00065-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, Ikeda A, Fumuro T, Lapresto E, Mikuni N, et al. Parieto-frontal network in humans studied by cortico-cortical evoked potential. Hum Brain Mapp. 2012;33:2856–72. doi: 10.1002/hbm.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–30. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Crawford JD, Vilis T. Integration of target and effector information in human posterior parietal cortex for the planning of action. J Neurophysiol. 2005;93:954–62. doi: 10.1152/jn.00725.2004. [DOI] [PubMed] [Google Scholar]
- Mikuni N, Ohara S, Ikeda A, Hayashi N, Nishida N, Taki J, et al. Evidence for a wide distribution of negative motor areas in the perirolandic cortex. Clin Neurophysiol. 2006;117:33–40. doi: 10.1016/j.clinph.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Nagamine T, Kajola M, Salmelin R, Shibasaki H, Hari R. Movement-related slow cortical magnetic fields and changes of spontaneous MEG- and EEG-brain rhythms. Electroencephalogr Clin Neurophysiol. 1996;99:274–86. doi: 10.1016/0013-4694(96)95154-8. [DOI] [PubMed] [Google Scholar]
- Neshige R, Lüders H, Friedman L, Shibasaki H. Recording of movement-related potentials from the human cortex. Ann Neurol. 1988;24:439–45. doi: 10.1002/ana.410240313. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernthey C. Atlas of the Cerebral sulci. Thieme Verlag; Stuttgart: 1990. [Google Scholar]
- Perenin MT, Vighetto A. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain. 1988;111(Pt 3):643–74. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, et al. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci. 2000;3:729–36. doi: 10.1038/76694. [DOI] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron. 2005;48:849–58. doi: 10.1016/j.neuron.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Rondot P, de Recondo J, Dumas JL. Visuomotor ataxia. Brain. 1977;100:355–76. doi: 10.1093/brain/100.2.355. [DOI] [PubMed] [Google Scholar]
- Roy AC, Stefanini S, Pavesi G, Gentilucci M. Early movement impairments in a patient recovering from optic ataxia. Neuropsychologia. 2004;42:847–54. doi: 10.1016/j.neuropsychologia.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Shibasaki H. Human brain mapping: hemodynamic response and electrophysiology. Clin Neurophysiol. 2008;119:731–43. doi: 10.1016/j.clinph.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Shibasaki H. Cortical activities associated with voluntary movements and involuntary movements. Clin Neurophysiol. 2012;123:229–43. doi: 10.1016/j.clinph.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Barrett G, Halliday E, Halliday AM. Components of the movement-related cortical potential and their scalp topography. Electroencephalogr Clin Neurophysiol. 1980;49:213–26. doi: 10.1016/0013-4694(80)90216-3. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–56. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–87. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–70. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Adams J. Gaze-dependent deviation in pointing induced by transcranial magnetic stimulation over the human posterior parietal cortex. J Mot Behav. 2005;37:157–63. doi: 10.3200/JMBR.37.2.157-163. [DOI] [PubMed] [Google Scholar]
- Vesia M, Monteon JA, Sergio LE, Crawford JD. Hemispheric asymmetry in memory-guided pointing during single-pulse transcranial magnetic stimulation of human parietal cortex. J Neurophysiol. 2006;96:3016–27. doi: 10.1152/jn.00411.2006. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Yakota S, Hallett M. Posterior parietal negativity preceding self-paced praxis movements. Exp Brain Res. 2005;163:535–9. doi: 10.1007/s00221-005-2314-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Ikeda A, Satow T, Matsuhashi M, Baba K, Yamane F, et al. Human eye fields in the frontal lobe as studied by epicortical recording of movement-related cortical potentials. Brain. 2004;127:873–87. doi: 10.1093/brain/awh110. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Ikeda A, Kunieda T, Ohara S, Mima T, Nagamine T, et al. Human presupplementary motor area is active before voluntary movement: subdural recording of Bereitschaftspotential from medial frontal cortex. Exp Brain Res. 2000;131:165–77. doi: 10.1007/s002219900311. [DOI] [PubMed] [Google Scholar]


