Abstract
Stem cell-based engineering strategies for tendons have yet to yield a normal functional tissue, due in part to a need for tenogenic factors. Additionally, the ability to evaluate differentiation has been challenged by a lack of markers for differentiation. We propose to inform tendon regeneration with developmental cues involved in normal tissue formation and with phenotypic markers that are characteristic of differentiating tendon progenitor cells (TPCs). Mechanical forces, fibroblast growth factor (FGF)-4 and transforming growth factor (TGF)-β2 are implicated in embryonic tendon development, yet the isolated effects of these factors on differentiating TPCs are unknown. Additionally, developmental mechanisms vary between limb and axial tendons, suggesting the respective cell types are programmed to respond uniquely to exogenous factors. To characterize developmental cues and benchmarks for differentiation toward limb vs. axial phenotypes, we dynamically loaded and treated TPCs with growth factors and assessed gene expression profiles as a function of developmental stage and anatomical origin. Based on scleraxis expression, TGFβ2 was tenogenic for TPCs at all stages, while loading was for late-stage cells only, and FGF4 had no effect despite regulation of other genes. When factors were combined, TGF 2 continued to be tenogenic, while FGF4 appeared anti-tenogenic. Various treatments elicited distinct responses by axial vs. limb TPCs of specific stages. These results identified tenogenic factors, suggest tendon engineering strategies should be customized for tissues by anatomical origin, and provide stage-specific gene expression profiles of limb and axial TPCs as benchmarks with which to monitor tenogenic differentiation of stem cells.
Keywords: Tendon, Differentiation, Embryonic, Mechanical loading, Growth factor, Development
Introduction
Tendons are critical to musculoskeletal function, but their poor healing ability and the limitations of surgical repair have motivated stem cell-based strategies to engineer living tissue replacements. Efforts to differentiate stem cells toward a tendon lineage have been challenged by a paucity of tenogenic cues as well as a lack of phenotypic benchmarks to assess lineage commitment. Previous strategies to tenogenically differentiate stem cells have integrated soluble factors involved in adult tendon wound healing (Moreau et al., 2005; Raghavan et al., 2012), despite their association with abnormal mechanical properties, matrix content and organization (Carpenter et al., 1998). Mechanical loading has also been used (Altman et al., 2002; Kuo and Tuan, 2008), but has yet to generate functional tendons. Our objectives are to inform tendon tissue engineering with embryonic factors involved in normal tendon formation as such factors may enable stem cells to recapitulate a developmental response, and to identify phenotypic benchmarks with which to assess tenogenic differentiation.
In vivo characterizations report differences between limb and axial tendon developmental programs. Tendon progenitor cells (TPCs) express scleraxis (Scx), a bHLH transcription factor (Schweitzer et al., 2001), from embryonic day (E) 9.5 and into adulthood in mice (Maeda et al., 2011; Pryce et al., 2009). Scx-expressing cells in the axis arise from the dorsolateral sclerotome, proximal to the myotome (Brent et al., 2003). In contrast, Scx-expressing cells in the limb arise from dorsal and ventral regions of the superficial limb mesenchyme and lack compartmentalization (Schweitzer et al., 2001; Tozer and Duprez, 2005). Interestingly, limb tendons begin developing normally in the absence of muscle, but degenerate at later embryonic stages (Kardon, 1998; Kieny and Chevallier, 1979), while no muscle-independent phase is reported for axial tendons. Based on these developmental differences, we asked whether a tendon engineering strategy should be customized for anatomically specific tissues.
The mechanical strain environment for developing tendons is largely uncharacterized, but muscle-driven movements beginning at E14 (Kodama and Sekiguchi, 1984) suggest the tissues experience mechanical stimulation during differentiation. This has not been examined with TPCs, but mechanical loading has been shown to influence tendon marker gene expression in MSCs (Altman et al., 2002; Doroski et al., 2010; Kuo and Tuan, 2008; Subramony et al., 2013). A role for mechanical forces in regulating tendon development is also suggested by the degeneration of tendon when muscle is paralyzed during embryonic chick development (Mikic et al., 2000). However, paralysis also results in adjacent muscle and cartilage degeneration (Hall and Herring, 1990), which likely alters tissue-specific paracrine signaling during tendon development. For example, precluding limb muscle formation in embryonic chicks abolished expression of the muscle-derived factor FGF4 and resulted in loss of Scx expression, while grafting mFGF4/RCAS-expressing cells into the muscleless limbs rescued Scx expression (Edom-Vovard et al., 2002). Taken together, it appears muscle influences on tendon development are both physical and soluble, suggesting a successful strategy to direct stem cell tenogenesis will require both.
Both FGF4 and TGF 2 have been implicated in tendon development. Disrupted FGF signaling with SU5402 inhibited Scx expression in progenitor cells of chick and murine embryonic axial and limb tendons (Brent et al., 2005; Brent and Tabin, 2004), while exogenous FGF4 treatment induced Scx expression in vivo at early (murine E10 and chick HH18) and late developmental stages (chick HH36-39) (Brent et al., 2005; Edom-Vovard et al., 2002). TGF 2 is expressed in embryonic chick and murine limb tendons (Kuo et al., 2008; Pryce et al., 2009), and disruption of its expression in TGFβ2−/− mutant mice leads to tendon deficiencies (Pryce et al., 2009). Additionally, TGFβ2 treatment upregulated Scx expression in C3H10T1/2 mesenchymal progenitor cells. Therefore, FGF4 and TGFβ2 appear to be important tenogenic factors, though their effects on TPCs in isolation have not been investigated.
We aimed to characterize the tenogenicity of developmental factors and define benchmarks for axial and limb TPC differentiation in vitro by profiling phenotypic responses of TPCs at each developmental stage in response to TGFβ2, FGF4, loading and their combinations. We hypothesized that tendon developmental factors would be tenogenic in vitro and that TPCs would respond to treatments differentially as a function of developmental stage and anatomical origin. We harvested limb and axial TPCs from Scx-green fluorescent protein (GFP) transgenic mice from different stages of embryonic and neonatal development, and mechanically loaded or treated cells with TGFβ2 or FGF4, and analyzed the expression of tendon marker genes. Distinct gene response profiles of TPCs were dependent on treatment, developmental stage and anatomical origin. These results characterized the tenogenicity of developmental factors, provided phenotypic signatures as benchmarks for differentiation, and demonstrated a need to customize tendon engineering strategies for tissues by anatomical origin.
Methods
Materials were from Invitrogen (Carlsbad, CA) unless otherwise specified.
TPC Harvest
Postnatal day (P) 7 and pregnant Scx-GFP mice (Pryce et al., 2007) were sacrificed by CO2 asphyxiation and decapitation with Tufts University IACUC approval. Embryos were harvested on embryonic days (E) 13-17 (Theiler, 1989). Limbs and torsos were skinned (E15 and older), cleaned of organs and cervical tissues, and washed in phosphate buffered saline (PBS) without MgCl2/CaCl2. Limb and axial tissue (with tail) were digested in 1% type II collagenase in PBS at 37°C for 45 min while shaking at 200 RPM, and neutralized with growth medium (GM; high glucose Dulbecco's Modified Eagle Medium (DMEM), 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S)). Digests were strained through a 40-μm cell strainer, pelleted, washed in PBS and resuspended in GM. Cells were grown to 80% confluency, trypsinized and sorted by GFP signal using a MoFlo Legacy cell sorter (Beckman Coulter, Brea, CA) to isolate TPCs. TPCs were expanded to passage 1-2 for experimentation. At least 3 independent cell pools from different embryos were harvested for each stage and anatomical origin.
Mechanical Stimulation and Growth Factor Treatment
TPCs were seeded at 2×104 cells/cm2 on Col I-coated Uniflex® culture plates (Flexcell International, Hillsborough, NC) for loading, or on tissue culture plastic (TCP) plates for growth factor treatment. To examine effects of combined treatments, E16.5 limb TPCs were seeded on Uniflex plates. On day (D) 0 (after 48 h in GM), medium was replaced with basal (control) medium (BM; DMEM, 1% FBS, 1% P/S). Cells were uniaxially loaded with 1% sinusoidal tensile strain at 0.5 Hz for 1 h/day (2 h/day for E16.5 limb TPCs), and/or supplemented with 100 ng/mL rhFGF4 and/or 1 ng/mL rhTGFβ2 (PeproTech, Rocky Hill, NJ). Because mechanical strains experienced by embryonic tendon during development are unknown, strains used this study were based on those shown previously to elicit a gene expression response in progenitor cells (Kuo and Tuan, 2008; Subramony et al., 2013). The loading frequency of 0.5 Hz was based on the frequency of limb muscle motor unit bursts detected in day 7 embryonic chicks by electromyographic recordings (Bekoff et al., 1975). Medium was changed every two days. Cells were harvested on D0, 1, 3 and 5 without loading or treatment on their harvest day.
Quantitative Polymerase Chain Reaction (qPCR)
Samples were homogenized in TRIzol reagent and total RNA-isolated and reverse-transcribed using the Superscript III First Strand Synthesis kit. QPCR was performed with Brilliant II SYBR Green qPCR master mix on a Stratagene Mx3000P multiplex qPCR system (Agilent, Wilmington, DE). Mouse-specific primer pairs were designed, optimized and efficiency-corrected (Yuan et al., 2008) for tendon marker and housekeeping genes (Table 1). Fold-change was calculated by 2−ΔCT and baseline gene expression by 2−ΔCT (on D0 prior to treatments). Means and standard errors were calculated for the three independent cell pools for each anatomical origin, developmental stage and condition.
Table 1.
| Gene | Forward | Reverse | Accession No. |
|---|---|---|---|
| Scleraxis | CCTCAGCAACCAGAGAAAGTTGAGCA | GCCATCACCCGCCTGTCCATC | NM_198885.3 |
| Tenomodulin | ATGAGCAATGGGTGGTCCCGC | ACAGACACGGCGGCAGTAACG | NM_022322.2 |
| Collagen 1α2 | ACCTCCTGGCAACCCTGGAACAA | TCTGGCACCTGTAGCACCAGCA | NM_007743.2 |
| Elastin | CTTTGGACTTTCTCCCATTTATCC | GGTCCCCAGAAGATCACTTTCTC | NM_007925.3 |
| TGFβ2 | GAGTGGCCGAGCAGCGGATT | CCCAGGTTCCTGTCTTTGTGGTGAA | NM_009367.3 |
| 18S | TCAACTTTCGATGGTAGTCGCCGT | TCCTTGGATGTGGTAGCCGTTTCT | X00686.1 |
Statistical Analysis
To evaluate baseline gene expression across developmental stage, gene expression was normalized to 18S, and compared using ANOVA with Tukey's post hoc test, p<0.05. The effect of a treatment (loading or growth factor) on gene expression was normalized to matching (stage and anatomical location) non-treated controls, expressed as a fold-change, and evaluated using a ratio t-test, p<0.05. To evaluate the effect of a single treatment (loading or growth factor) over time (D0, 1, 3, or 5) for a given developmental stage, gene expression was normalized to D0, and compared using ANOVA with Tukey's post hoc test, p<0.05. To compare the effects of combined treatments (loading, growth factor, and growth factor+loading) on E16.5 limb TPCs, gene expression was normalized to non-treated controls, and compared using ANOVA with Tukey's post hoc test, p<0.05. For comparisons of gene expression by anatomical origin, the fold-change values for a given stage were analyzed by unpaired t-test, p<0.05.
Results
Baseline Gene Expression Comparison
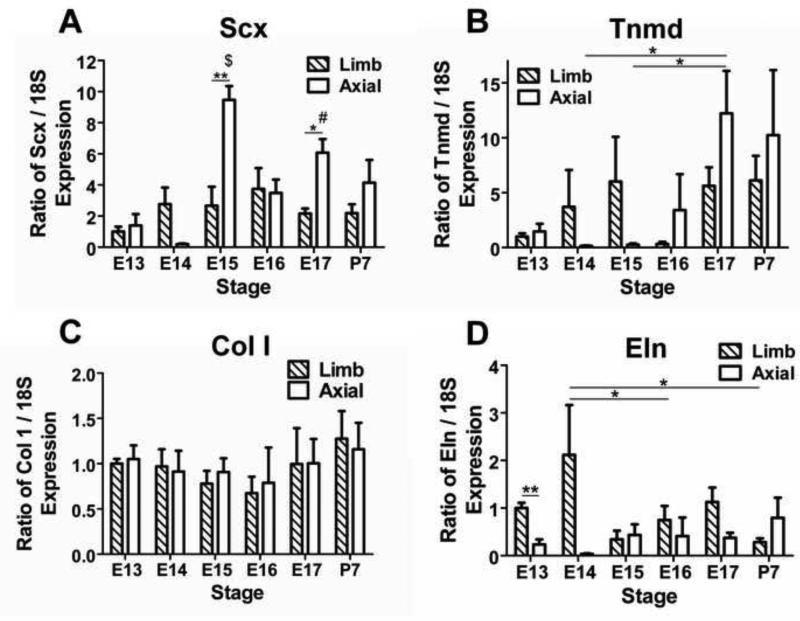
Baseline expression of Scx did not significantly vary between different stage limb TPCs (p ≥0.53; Fig 1A). Axial TPC Scx expression was highest at E15, which was significantly higher than all other stages of axial TPCs except E17 (p<0.05; Fig. 1A). Baseline Scx expression was higher in axial than limb TPCs at E15 (p<0.01) and E17 (p<0.05; Fig. 1A). Baseline tenomodulin (Tnmd; late tendon marker) expression did not significantly vary between different stage limb TPCs (p ≥0.37), while Tnmd expression in axial TPCs was higher at E17 than E14-15 (p<0.05; Fig. 1B). Col I baseline expression did not vary significantly by developmental stage for either limb (p ≥0.87) or axial TPCs (p ≥0.94) or by anatomical origin for any stage (p ≥0.56; Fig. 1C). For limb TPCs, Eln baseline expression was higher at E14 than at E16 and P7 (p<0.05), while axial TPC expression did not vary by developmental stage (p ≥0.47; Fig. 1D). At E13, baseline Eln expression was higher in limb than in axial TPCs (p<0.01; Fig. 1D).
Figure 1.
Baseline gene expression by TPCs at each stage normalized to E13 limb expression. (A) Baseline Scx expression in limb TPCs did not vary between different stages, but in axial TPCs was higher at E15 than all stages except E17 ($ p<0.05), and higher at E17 than at E13 and 14 (# p<0.05). At E15 and 17, baseline Scx expression was higher in axial than in limb TPCs (* p<0.05, ** p<0.01). (B) Baseline Tnmd expression did not vary by developmental stage in limb TPCs, but in axial TPCs was higher at E17 than at E14 and 15 (* p<0.05). (C) Baseline Col I expression did not vary by either developmental stage or anatomical origin. (D) Baseline Eln expression in limb TPCs was higher at E14 than at E16 and P7 (* p<0.05). At E13, baseline Eln expression was higher in limb than in axial TPCs (** p<0.01). Data are mean ± SEM for 3 independent pools of TPCs plated on tissue culture plastic.
Effects of Mechanical Loading
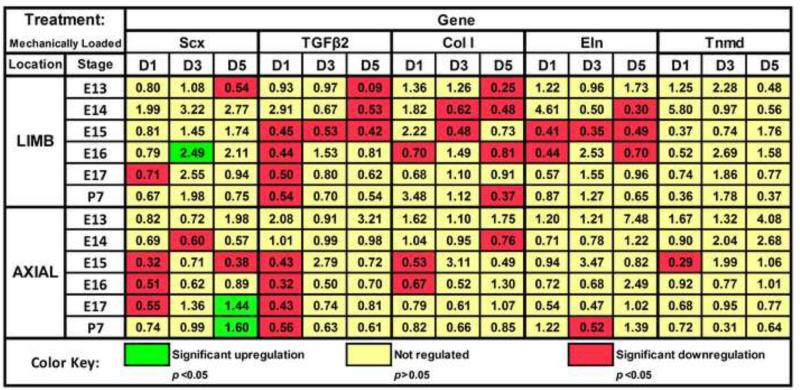
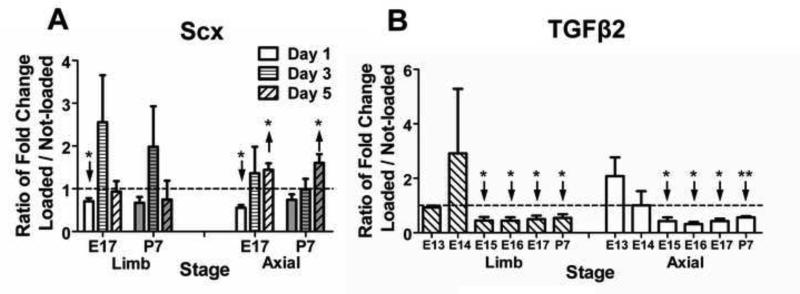
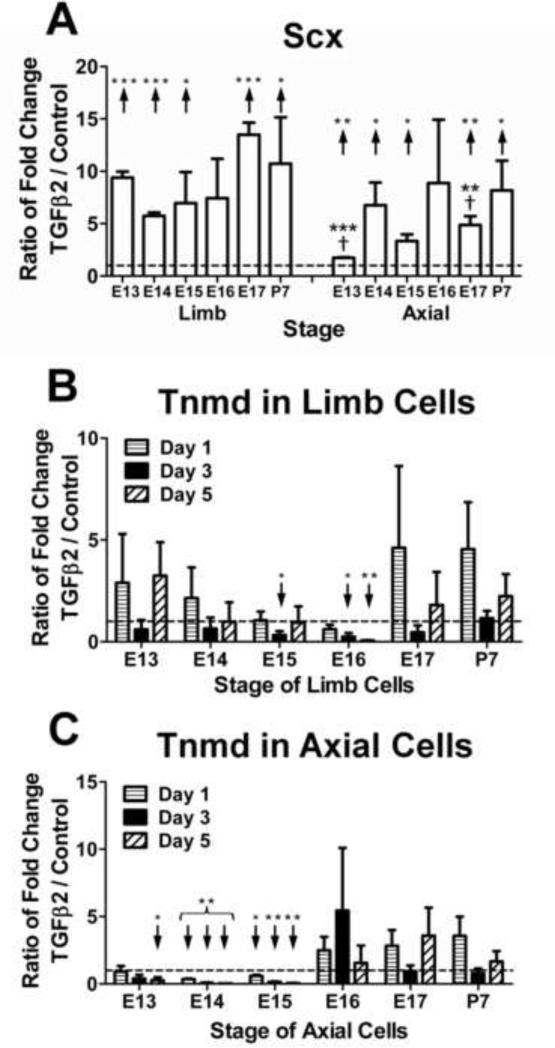
Mechanoregulation of Scx expression varied by anatomical origin and developmental stage (Fig. 2). For instance, on D1, loading downregulated Scx expression in both E17 limb and axial cells (p<0.05) while P7 cells were unaffected (p ≥0.09; Fig. 3A). However, by D5, loading upregulated Scx expression in E17 and P7 axial TPCs (Fig. 3A; p<0.05) whereas limb cells were no longer affected (p=0.17). E16 TPCs demonstrated anatomically dependent responses to loading, upregulating Scx expression in limb cells on D3 (p<0.05) but downregulating expression in E16 axial cells at all time points significantly (p<0.05) or trend-wise (Fig. 2). For both limb and axial TPCs, a stage-dependent trend was observed for mechanoregulation of TGFβ2 on D1 (Fig. 3B), where loading downregulated expression in E15 and later stage cells (p<0.05) but not earlier (p ≥0.09).
Figure 2.
Effects of mechanical loading on TPC gene expression. Numbers in colored boxes indicate the fold-change of loaded normalized to matching (same stage and anatomical location) control samples on a given day. Green indicates significant upregulation (p<0.05) of a gene, red indicates downregulation (p<0.05) of a gene, and yellow indicates the fold-change was not significantly regulated
Figure 3.
Effects of mechanical loading on TPC expression of Scx and TGFβ2. Dashed horizontal line = 1 indicates no regulation by loading. (A) On D1, loading downregulated Scx expression in E17 limb and axial cells but not in P7 limb or axial cells. After 5 days of loading, Scx was upregulated in E17 and P7 axial but not limb TPCs; (B) On D1, loading downregulated TGFβ2 expression in E15 and later stages of limb and axial TPCs but not in E13 and 14 limb or axial TPCs. Data are mean ± SEM fold-change of loaded samples normalized to matching (same stage and anatomical location) not-loaded controls for 3 independent cell pools. ↑ or ↓ indicates significant up- or downregulation, respectively.
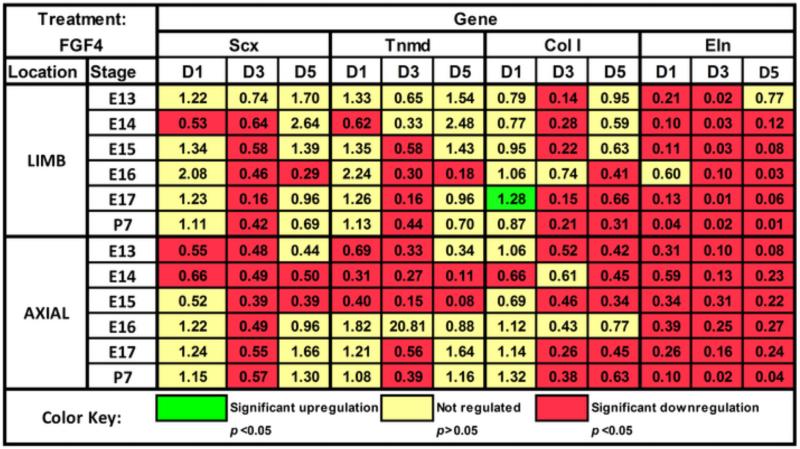
Effects of FGF4
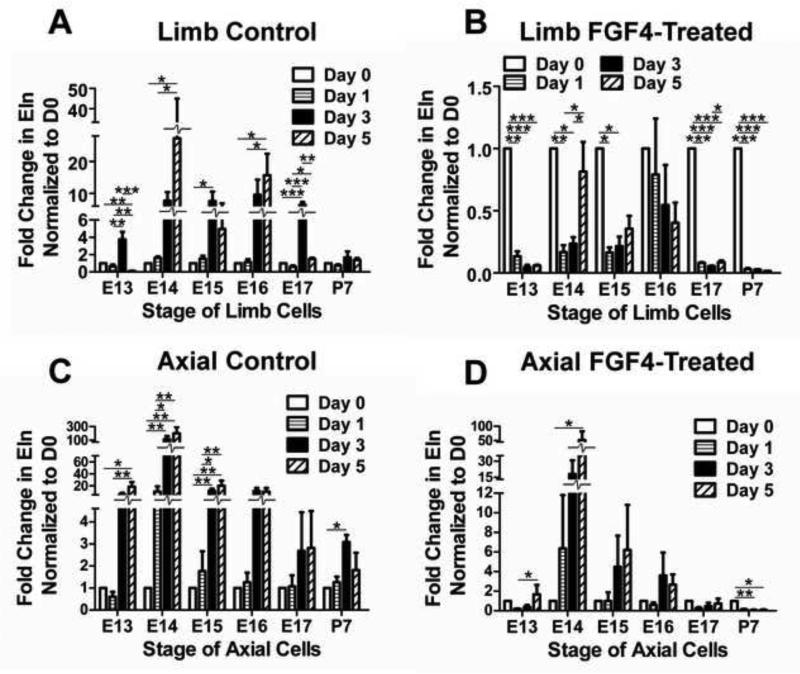
FGF4 seemed to have similar regulatory effects on TPCs as a function of anatomical origin and developmental stage, regulating Scx and Tnmd in similar patterns in limb and axial cells over different developmental stages, and downregulating Col I and Eln expression (Fig. 4). Interestingly, Eln expression increased with time from D0 in embryonic limb cells in control culture (Fig. 5A), but FGF4 reversed this trend and downregulated Eln expression over time for all stages (p<0.05) except E16, which trended down (p=0.44; Fig. 5B). In axial cells, Eln expression also increased significantly or trend-wise over time in control culture (p<0.05; Fig. 5C), but in contrast to limb cells continued to trend up from D0 with FGF4 treatment, with significant increases in E13-14 TPCs (p<0.05; Fig. 5D). Significant increases in expression in control culture were not typically observed for other genes, and only FGF4 treatment induced a more elongated and spindly cell morphology (not shown).
Figure 4.
Effects of FGF4 on TPC gene expression. Numbers in colored boxes indicate the fold-change of treated normalized to matching (same stage and anatomical location) control samples on a given day. Green indicates significant upregulation (p<0.05) of a gene, red indicates downregulation (p<0.05) of a gene, and yellow indicates the fold-change was not significantly regulated.
Figure 5.
Eln expression over time (D0-5) in TPCs in control or FGF4-supplemented medium. (A) Limb TPC Eln expression in control culture increased over time starting from D0 for all embryonic stages. (B) Limb TPC Eln expression decreased over time with FGF4 treatment for all stages except E16. (C) Axial TPC Eln expression in control culture increased over time starting from D0 significantly or trend-wise; (D) Axial TPC Eln expression increased over time with FGF4 treatment in E13 and 14 cells, and decreased over time in P7 cells. Data are mean ± SEM of fold-change in expression from D0 for 3 independent cell pools; * p<0.05, ** p<0.01, *** p<0.001.
Effects of TGFβ2
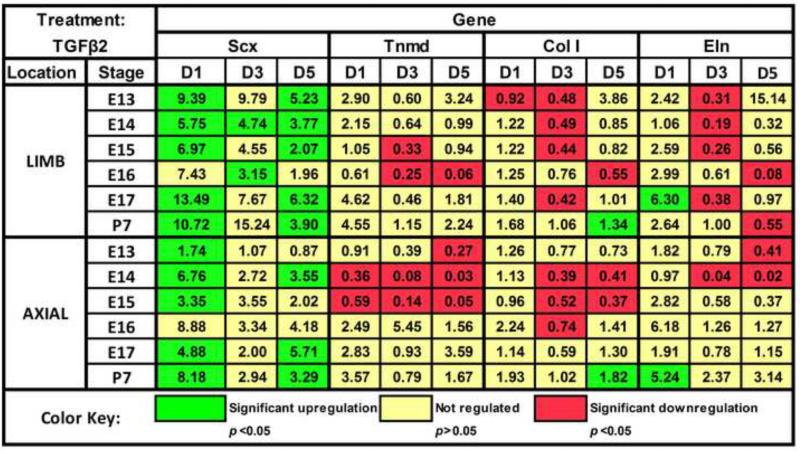
TGFβ2 upregulated Scx expression in both limb and axial TPCs of all stages, and matrix gene expression at late stages (Fig. 6). On D1, TGFβ2 upregulated Scx expression at all stages (p<0.05) except E16, interestingly, which trended up in both limb and axial cells (p ≥0.10; Fig. 7A). TGFβ2 also upregulated Col I expression in P7 TPCs (p<0.05) but not in embryonic cells on D5 (Fig. 6). While TGFβ2 upregulated Eln expression in both E17 limb and P7 axial cells on D1 (p<0.05), differential regulation of Eln by origin was also observed. Over time, TGFβ2 regulation of P7 limb Eln expression trended down, reaching significance on D5 (p<0.05), while regulation of P7 axial expression continued to trend up. Anatomically dependent differences were also observed with Tnmd expression in limb (not regulated) vs. axial cells (downregulated) at early stages (Fig. 7B,C).
Figure 6.
Effects of TGFβ2 on TPC gene expression. Numbers in colored boxes indicate the fold-change of treated normalized to matching (same stage and anatomical location) control samples on a given day. Green indicates significant upregulation (p<0.05) of a gene, red indicates downregulation (p<0.05) of a gene, and yellow indicates the fold-change was not significantly regulated.
Figure 7.
Effects of TGFβ2 on Scx and Tnmd expression in TPCs. (A) On D1, TGFβ2 upregulated Scx expression in both limb and axial TPCs of all developmental stages except E16. TGFβ2 upregulated Scx expression to a higher degree in E13 and E17 limb TPCs than in axial TPCs (†). (B) TGFβ2 downregulated Tnmd expression in E15 and 16 limb TPCs. (C) TGFβ2 downregulated Tnmd expression in E13-15 axial TPCs but caused expression to trend up in cells of later developmental stages. Data are mean ± SEM fold-change in expression of TGFβ2-treated samples normalized to non-treated controls for 3 independent cell pools. ↑ or ↓ indicates significant up- or downregulation, respectively. * p<0.05, ** p<0.01, *** p<0.001
Effects of Combined Treatments
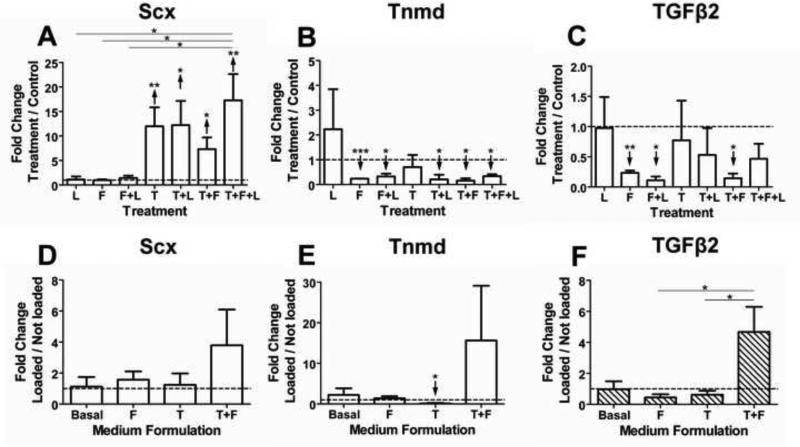
We also examined the effects of combinations of treatments on E16.5 limb TPCs (Fig. 8). Scx was upregulated by all TGFβ2 combinations, but unaffected in other conditions (p ≥0.25; Fig. 8A). Although neither TGFβ2 nor loading individually regulated Tnmd expression (p ≥0.17; Fig. 8B), TGFβ2+loading downregulated Tnmd, as did other treatments (p<0.05). Interestingly, FGF4 alone and in combination with either loading or TGFβ2 downregulated TGFβ2 expression (p<0.05; Fig. 8C), while other treatments had no effect. We also examined the effect of loading as a function of medium formulation (Fig. 8D-F). Loading upregulated Scx, Tnmd and TGFβ2 expression trend-wise in medium containing TGFβ2+FGF4 (p≥0.11; Fig. 8D-F).
Figure 8.
Effects of treatment combinations of mechanical loading (L), FGF4 (F) and TGFβ2 (T) on E16.5 limb TPCs for 3 days. Gene expression was evaluated as a function of treatment, shown as fold-change in gene expression of treated samples normalized to non-loaded and non-treated controls (A-C). To evaluate the effects of loading when coupled with growth factor treatments, gene expression of loaded samples was normalized to not-loaded samples in the same medium formulation (D-F). (A) Scx expression was upregulated by all treatments involving TGFβ2. TGFβ2+FGF4+loading upregulated Scx expression to a significantly higher level than did loading, FGF4, or FGF4+loading. (B) Tnmd expression was downregulated by all treatments except by TGFβ2 or loading. (C) TGFβ2 expression was downregulated by FGF4 alone and when combined with either loading or TGFβ2. (D) Loading enhanced Scx expression in medium containing TGFβ2+FGF4 compared to either growth factor alone. (E) Loading downregulated Tnmd expression in the presence of TGFβ2, but enhanced its expression in medium containing TGFβ2+FGF4. (F) Loading enhanced TGFβ2 expression in medium containing TGFβ2+FGF4 and was significantly different than in medium containing either TGFβ2 or FGF4 alone. Data are mean ± SEM of fold-change of treated normalized to matched control on D3 for 3 independent cell pools; ↑ or ↓ indicates significant up- or downregulation, respectively. * p<0.05, ** p<0.01, *** p<0.001.
Discussion
The success of stem cell-based tendon tissue engineering has been challenged by few known factors to effectively induce and guide tenogenesis, and few known markers with which to assess differentiation. Current approaches typically use adult wound healing factors that encourage an unfavorable scarring repair response in vivo. In contrast, we propose embryonic factors that are essential for normal tendon development will induce a tenogenic regenerative response from stem cells. In this study, we identified embryonic factors tenogenic for TPCs as a function of developmental stage and anatomical origin, and characterized the transcriptional response of TPCs as benchmarks with which to assess tenogenic differentiation. Despite developmental implications for all three factors in vivo, TGFβ2 was the only factor that was tenogenic individually (Fig. 6) and when combined with other factors (Fig. 8A). Interestingly, both synergistic and antagonistic combinations were observed for specific combinations involving FGF4 and loading, despite the lack of tenogenicity of each factor when applied individually (Fig. 8). Finally, distinct gene expression profiles as a function of developmental stage and anatomical origin provided benchmarks with which to characterize progression of lineage commitment and differentiation, and suggested that tissue engineering programs should be customized for axial vs. limb tendons.
To elucidate inherent differences in limb vs. axial TPCs, we characterized baseline tenogenic gene expression as a function of developmental stage, after expansion in vitro to realistically represent cells that would be used in a tissue engineering approach. Significant differences were observed in baseline expression levels of Scx and Eln between limb and axial TPCs (Fig. 1A,D), perhaps because the different microenvironments have started to direct limb vs. axial TPCs to specialize with distinct identities. These differences also suggest that certain axial and limb tendon cell phenotypic characteristics were maintained during culture on TCP, in agreement with a previous report that phenotypic drift of adult tenocytes in vitro does not occur until after four passages (Yao et al., 2006). Notably, TPCs were re-plated on Col I-coated silicone substrates for mechanical loading; we anticipated a change in substrate stiffness or material would affect cell phenotype, as seen with other cells (Crapo and Wang, 2010; Darling et al., 2009; Discher et al., 2005). However, 48 h after re-plating, baseline expression profiles for TPCs on silicone were similar to those on TCP (not shown), suggesting the substrate did not significantly alter phenotypes.
Mechanical loading upregulated Scx expression in E16 limb and E17 and P7 axial TPCs (Figs. 2,3A). In vivo, muscle-driven movements begin at E14, with limb and axial movements becoming more frequent and complex during development (Fox, 1965; Kodama and Sekiguchi, 1984). After E14, it is possible that TPCs become programmed with greater mechanoresponsiveness. For instance, total protein production increased in day 17-18 embryonic chick limb tendon organ cultures in response to cyclic loading (Slack et al., 1984). To date, no other reports have examined the effects of dynamic loading on embryonic TPCs, but in other studies fluid-induced shear maintained Scx expression in adult tenocytes in vitro (Maeda et al., 2011), and treadmill training of adult mice upregulated tendon Scx expression in vivo (Mendias et al., 2012). Our observations of upregulated Scx expression in response to dynamic mechanical loading with later stage TPCs are consistent with these previous studies.
The variation in stage-dependent effects of mechanical loading between limb vs. axial TPCs was interesting. In vivo, preclusion of muscle progenitors during development affected both limb and axial tendons but the effects were anatomically dependent, as a complete loss of Scx expression in axial TPCs was seen by E10.5, while limb TPCs did not exhibit diminished Scx expression until E13.5 (Brent et al., 2005). However, because skeletal muscle-driven movement is not detected until E14, cells may have been responding to chemical influences. These experiments suggest TPCs begin to differentiate as a function of their particular anatomical microenvironments prior to E14, when muscle-driven physical stimulation begins. Such developmental differences could explain the distinct axial vs. limb TPC transcriptional responses to mechanical cues at subsequent stages.
We also observed that loading downregulated TGFβ2 expression in both axial and limb TPCs of E15 and later (Fig. 3B). TGFβ2 is critical during early tendon development, evident by fewer Scx-expressing limb and axial cells by E12.5 in TGFβ2−/− mutant mice, and diminution or complete loss of tendons by subsequent stages (Pryce et al., 2009). TGFβ2 protein expression in embryonic chick limb tendons has been reported to decrease dramatically from developmental days 13-16 (Kuo et al., 2008), suggesting autocrine TGFβ2 signaling by TPCs is less critical at later developmental stages. Thus, it is possible that in vivo, the initiation of muscle-generated mechanical strain of TPCs reduces autocrine TGFβ2 expression. This does not diminish possible roles for TGFβ2 at later stages of embryonic tendon development via signaling from nearby tissues or through the release of matrix-bound TGFβ2.
During tendon development, muscle-generated forces collaborate with soluble factors to direct cell function. For a tissue engineering strategy, it is important to understand how these cues synergistically direct differentiation. Here, we found that combinatorial effects were not necessarily additive of individual effects (Fig. 8). For example, in the presence of TGFβ2 or FGF4, loading downregulated TGFβ2 and late tendon marker Tnmd expression significantly and trend-wise, but this was reversed when both growth factors were present during loading (Fig. 8E,F). Interestingly, the combination of loading+FGF4+TGFβ2 upregulated Scx expression to the greatest extent, though not statistically higher than other treatments involving TGFβ2 (Fig. 8A). Longer treatment times or combinations of these factors at other concentrations may have elicited significant differences.
FGF4 lacked tenogenicity, having no upregulatory effect on Scx expression and downregulating other genes at most stages (Fig. 4). This was surprising because FGF4 induces and maintains Scx expression during tendon development (Brent and Tabin, 2004; Edom-Vovard et al., 2002). It was also interesting that FGF4 inhibited Eln upregulation in limb cells but failed to reverse this trend in axial cells (Fig. 5), again demonstrating an anatomically dependent TPC response. Notably, FGF4-influenced Scx and Tnmd gene expression patterns were similar between limb and axial cells across stages (Fig. 4). Tnmd is a type II transmembrane protein that is expressed in tendon cells from E13.5 and into adulthood (Brent et al., 2005; Shukunami et al., 2006; Taylor et al., 2009). Scx expression precedes and subsequently overlaps with Tnmd expression in chick somites (Shukunami et al., 2006). Moreover, genetic disruption of Scx expression abrogated Tnmd expression in murine limb tendons (Murchison et al., 2007). Interestingly, matching patterns of Scx and Tnmd expression were not observed with other treatments (Figs. 2,6), suggesting loading and TGFβ2 regulate Scx and Tnmd via different regulatory pathways from FGF4. The deviation in FGF4 effects in our study from in vivo studies may be a function of the developmental stages investigated, as FGF4 regulation of Scx in vivo has been reported as early as E10 (Brent et al., 2005). Additionally, perhaps unidentified critical factors need to couple with FGF4 to effectively influence tenogenesis, or the FGF receptor or transcriptional profile changed during in vitro culture, impairing a tenogenic response by TPCs to FGF4.
TGFβ2 was highly tenogenic, upregulating Scx at all stages, and matrix genes Col I and Eln at late stages (Fig. 6). In vivo, matrix deposition is greater at later stages of tendon and ligament development (Brown et al., 2012; Marturano et al., 2013; McBride et al., 1988). Though effects of TGFβ2 on later embryonic tendon developmental stages have not previously been reported, our results suggest a role for TGFβ2 in promoting this stage-dependent increased production of matrix proteins. Our observations are in agreement with studies demonstrating TGFβ2 treatment upregulated Scx expression in somites and limb buds during early development in vivo and in micromass cultures and C3H10T1/2 cells in vitro (Lorda-Diez et al., 2009; Pryce et al., 2009). In contrast to Scx, Tnmd was differentially regulated by TGFβ2 in an anatomically and stage-dependent manner (Fig. 7B,C), with TGFβ2 downregulating Tnmd expression in axial cells as early as E13, but later in limb cells at E15-16. Interestingly, TGFβ2 upregulated Tnmd expression at later developmental stages in both axial and limb cells. When embryonic chick hindlimbs were electroporated with a retroviral RCAS-cScx vector, Tnmd expression in tendons was only upregulated at HH33 and later stages (Shukunami et al., 2006). Moreover, Tnmd expression is abrogated in limb tendons of TGFβ2−/−/TGFβ3−/− and Scx−/− mutant mice (Murchison et al., 2007; Pryce et al., 2009). Taken together with our results, regulation of Tnmd expression in tenocytes, either by TGFβ2 or Scx itself, appears to be stage-dependent, though the mechanisms remain to be elucidated.
In this work, TPCs were isolated based on Scx promoter-driven GFP expression. Considering developing ligament progenitors express Scx similarly as TPCs during embryonic development (Schweitzer et al., 2001), our cell harvests likely included ligament progenitor cells. Scx function is yet unknown, but in Scx−/− mice many tendons fail to develop or have disrupted matrix, while ligaments are morphologically normal (Murchison et al., 2007). Tnmd is also expressed in both tendon and ligament cells starting from E13.5 (Brent et al., 2005; Shukunami et al., 2006; Taylor et al., 2009). Genetic disruption of Tnmd in mice results in aberrant tendon collagen fibril diameters and abated tenocyte proliferation, though effects on ligaments were not reported (Docheva et al., 2005). Thus, based on the common expression of Scx and Tnmd, tendons and ligaments may share mechanisms of early formation, but the extent to which mechanisms are shared remains to be elucidated. The similarities may be limited based on the disparate effects that genetic disruptions to Scx and Tnmd have on tendons compared to ligaments. Taken together, ligament progenitor cell responses to treatments may have contributed to our findings, possibly leading to some unexpected results such as a lack of tenogenic response to FGF4.
This work is a first step toward establishing quantitative phenotypic benchmarks of tenogenically differentiating cells in vitro, which have been lacking. This is also a first study to characterize effects of exogenous embryonic factors as tenogenic cues on differentiating TPCs of varying stages of development and anatomical origin. Our results identify developmental tenogenic factors that may be useful for guiding specification of stem cells (such as MSCs or tendon stem cells (Zhang et al., 2011)) toward the tendon lineage, and provide benchmarks with which to assess differentiation. Future studies may examine responses at the protein-level. Our study demonstrates a need to tailor tissue engineering strategies for tissues by anatomical location. Finally, our study provides unique insight into potential roles of TGFβ, FGF and mechanical loading in tendon development. For instance, the unexpected contrast in FGF4 in vitro effects compared to reported in vivo effects deserves further investigation to understand mechanisms of FGF4 activity in TPC differentiation. The synergistic and antagonistic effects of specific factor combinations were intriguing; a deeper understanding may shed important light on in vivo developmental events. Together with this work, such studies may enhance our ability to regulate the tenogenic fate of progenitor cells and significantly advance tendon engineering efforts.
Acknowledgements
We thank Drs. Ronen Schweitzer and Cliff Tabin for Scx-GFP breeder mice, Zachary Schiller, Drs. Joseph Marturano and Nathan Schiele for technical consultation, and Dr. Juan Taboas for mechanical loader designs. We thank funding support by Award Number R03AR061036 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (to C.K.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Stein PS, Hamburger V. Coordinated motor output in the hindlimb of the 7-day chick embryo. Proc Natl Acad Sci U S A. 1975;72:1245–1248. doi: 10.1073/pnas.72.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent AE, Braun T, Tabin CJ. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development. 2005;132:515–528. doi: 10.1242/dev.01605. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131:3885–3896. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- Brown JP, Lind RM, Burzesi AF, Kuo CK. Elastogenic protein expression of a highly elastic murine spinal ligament: the ligamentum flavum. PLoS One. 2012;7:e38475. doi: 10.1371/journal.pone.0038475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- Crapo PM, Wang Y. Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials. 2010;31:1626–1635. doi: 10.1016/j.biomaterials.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling EM, Pritchett PE, Evans BA, Superfine R, Zauscher S, Guilak F. Mechanical properties and gene expression of chondrocytes on micropatterned substrates following dedifferentiation in monolayer. Cell Mol Bioeng. 2009;2:395–404. doi: 10.1007/s12195-009-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Jammey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroski DM, Levenston ME, Temenoff JS. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng Part A. 2010;16:3457–3466. doi: 10.1089/ten.tea.2010.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Fox WM. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Kieny M, Chevallier A. Autonomy of tendon development in the embryonic chick wing. J Embryol Exp Morphol. 1979;49:153–165. [PubMed] [Google Scholar]
- Kodama N, Sekiguchi S. The development of spontaneous body movement in prenatal and perinatal mice. Dev Psychobiol. 1984;17:139–150. doi: 10.1002/dev.420170205. [DOI] [PubMed] [Google Scholar]
- Kuo CK, Petersen BC, Tuan RS. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev Dyn. 2008;237:1477–1489. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- Lorda-Diez CI, Montero JA, Martinez-Cue C, Garcia-Porrero JA, Hurle JM. Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J Biol Chem. 2009;284:29988–29996. doi: 10.1074/jbc.M109.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci U S A. 2013;110:6370–6375. doi: 10.1073/pnas.1300135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DJ, T.R.L., Silver FH. Structural and mechanical assessment of developing chick tendon. Int. J. Bio. Macromol. 1988;10:194–200. [Google Scholar]
- Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012;30:606–612. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000;37:127–133. [PubMed] [Google Scholar]
- Moreau JE, Chen J, Bramono DS, Volloch V, Chernoff H, Vunjak-Novakovic G, Richmond JC, Kaplan DL, Altman GH. Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res. 2005;23:164–174. doi: 10.1016/j.orthres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan SS, Woon CY, Kraus A, Megerle K, Pham H, Chang J. Optimization of human tendon tissue engineering: synergistic effects of growth factors for use in tendon scaffold repopulation. Plast Reconstr Surg. 2012;129:479–489. doi: 10.1097/PRS.0b013e31823aeb94. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Slack C, Flint MH, Thompson BM. The effect of tensional load on isolated embryonic chick tendons in organ culture. Connect Tissue Res. 1984;12:229–247. doi: 10.3109/03008208409013685. [DOI] [PubMed] [Google Scholar]
- Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, Lu HH. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942–1953. doi: 10.1016/j.biomaterials.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Vaughan-Thomas A, Clements DN, Pinchbeck G, Macrory LC, Smith RK, Clegg PD. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord. 2009;10:27. doi: 10.1186/1471-2474-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler K. The House Mouse: Atlas of Embryonic Development, The New York Medical Journal. Springer-Verlag; New York: 1989. [Google Scholar]
- Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- Yao L, Bestwick CS, Bestwick LA, Maffulli N, Aspden RM. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006;12:1843–1849. doi: 10.1089/ten.2006.12.1843. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Wang D, Stewart CN., Jr. Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnol J. 2008;3:112–123. doi: 10.1002/biot.200700169. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li B, Wang JH. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32:6972–6981. doi: 10.1016/j.biomaterials.2011.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]