Abstract
The spirochete Borrelia burgdorferi causes acute inflammation in mice that resolves with the development of pathogen-specific adaptive immunity. B. burgdorferi lipoproteins activate innate immune cells via Toll-like receptor 2 (TLR2), but TLR2-deficient mice are not resistant to B. burgdorferi-induced disease, suggesting the involvement of other TLRs or non-TLR mechanisms in the induction of acute inflammation. For this study, we used mice that were deficient in the intracellular adapter molecule myeloid differentiation antigen 88 (MyD88), which is required for all TLR-induced inflammatory responses, to determine whether the interruption of this pathway would alter B. burgdorferi-induced disease. Infected MyD88−/− mice developed carditis and arthritis, similar to the disease in wild-type (WT) mice analyzed at its peak (days 14 and 28) and during regression (day 45). MyD88−/− macrophages produced tumor necrosis factor alpha only when spirochetes were opsonized, suggesting a role for B. burgdorferi-specific antibody in disease expression. MyD88−/− mice produced stronger pathogen-specific Th2-dependent immunoglobulin G1 (IgG1) responses than did WT mice, and their IgM titers remained significantly elevated through 90 days of infection. Despite specific antibodies, the pathogen burden was 250-fold higher in MyD88−/− mice than in WT mice 45 days after infection; by 90 days of infection, the pathogen burden had diminished substantially in MyD88−/− mice, but it was still elevated compared to that in WT mice. The elevated pathogen burden may be explained in part by the finding that MyD88−/− peritoneal macrophages could ingest spirochetes but degraded them more slowly than WT macrophages. Our results show that MyD88-dependent signaling pathways are not required for B. burgdorferi-induced inflammation but are necessary for the efficient control of the pathogen burden by phagocytes.
Infection of humans with the Lyme disease spirochete, Borrelia burgdorferi, results in a characteristic pattern of skin lesions, arthritis, carditis, and neurologic abnormalities that can resolve over time despite the incomplete eradication of the pathogen (12). In the murine model of Lyme borreliosis, spirochetes inoculated into the skin disseminate within days to infect all organ systems, but disease is primarily manifested in the joints and heart (8). The severity of inflammation peaks at these sites about 2 to 4 weeks after infection and then regresses in the presence of B. burgdorferi-specific adaptive immune responses. For this animal model, disease is believed to reflect the innate immune response to spirochetes because histopathology reveals mainly neutrophils and macrophages within inflamed joints and hearts, respectively, and arises in the absence of adaptive immunity (11, 26, 33, 35). B. burgdorferi infections of severe combined immunodeficiency (SCID) and rag-deficient mice, which lack both T and B cells (11, 26, 33, 35), or of mice deficient in B cells alone (13, 26) result in higher pathogen burdens but do not have altered disease incidence. In contrast to the disease in immunocompetent mice, however, neither carditis nor arthritis regresses in mice that are deficient in B and T cells (11). The passive transfer of B. burgdorferi-specific antibodies has been shown to both reduce the pathogen burden and attenuate arthritis, and CD4+ T-helper 1 (Th1) cells facilitate carditis resolution when B cells are present (9, 13, 25). Thus, our current understanding of murine Lyme borreliosis implicates innate immunity in the pathogenesis of the disease and adaptive immunity in its resolution.
B. burgdorferi lipoproteins are potent inflammatory stimuli, and immune responses to these molecules are thought to drive disease activity (43). Recent seminal studies have demonstrated that B. burgdorferi lipoproteins activate innate immune cells through the pattern recognition molecule Toll-like receptor 2 (TLR2) (4, 17). The TLR family consists of 10 members that allow innate immune cells to respond to a variety of pathogen-associated molecular patterns, including the presence of lipopolysaccharide, lipoproteins, peptidoglycan, double-stranded RNA, unmethylated CpG DNA, and flagellin (2). The interaction of TLRs with their target motifs initiates a cascade of intracellular signaling events that culminate in the activation of NF-κB and the production of proinflammatory cytokines, chemokines, and costimulatory molecules that are important for host defense. In the case of B. burgdorferi lipoproteins, the induction of inflammation via TLR2 is facilitated by other TLRs, notably TLR1 (3). In vitro, the TLR2 function is essential for innate immune cell activation by viable spirochetes and purified lipoproteins (4, 17). In vivo, however, the absence of TLR2 does not prevent mice from developing acute arthritis after infection with B. burgdorferi (43). By inference, other TLRs or non-TLR mechanisms may signal innate immune cell activation in response to B. burgdorferi during an infection.
A proximal signaling pathway that utilizes the adapter protein myeloid differentiation marker 88 (MyD88) is employed by all TLRs and is critical for downstream signaling events that lead to inflammatory cytokine secretion (36). The majority of TLRs, including TLR2, appear to depend solely upon MyD88 for cell activation (20). Two exceptions are TLR3 and TLR4, which respond to viral double-stranded RNA and lipopolysaccharide, respectively. Studies with mice that have a targeted disruption of the MyD88 gene (MyD88−/− mice) have revealed that TLR3 and TLR4 utilize both MyD88-dependent and MyD88-independent signaling pathways to initiate specific cellular effector functions. In contrast to the MyD88-dependent pathway, which activates cells to produce proinflammatory cytokines, the MyD88-independent pathway leads to the activation of interferon (IFN) regulatory factor 3 and IFN-inducible genes and does not lead to inflammatory cytokine (tumor necrosis factor alpha [TNF-α]) release (21).
Immunization and infection studies using MyD88−/− mice have revealed that MyD88 is critical for the activation of innate immunity and host defense (36, 39). Macrophages and dendritic cells from MyD88−/− mice fail to produce inflammatory cytokines (TNF-α, interleukin-12 [IL-12], and IL-6) in response to a variety of pathogen-associated molecular patterns. MyD88−/− mice are resistant to lipopolysaccharide-induced endotoxin shock (20) and are unable to produce antigen-specific Th1 cell responses or Th1-associated immunoglobulin G (IgG) isotypes after immunization with ovalbumin in TLR-stimulating adjuvants such as complete Freund's adjuvant (36). Immunization with ovalbumin in alum can elicit Th2 responses, indicating that priming for Th2 responses is intact. MyD88−/− mice exhibit an increased susceptibility to infections with intracellular pathogens, due in part to a deficiency in IL-12 production by dendritic cells (34, 37). MyD88−/− mice also exhibit early lethality after infection with gram-positive organisms such as Staphylococcus aureus, which are recognized preferentially by TLR2, and to a lesser extent, by other TLRs (38).
In contrast to intracellular pathogens, for which cell-mediated immunity is critical for defense, relatively little is known about the role of MyD88-dependent immunity in the host response to extracellular pathogens such as B. burgdorferi, for which specific antibodies rather than T cells are required for protective immunity and pathogen control. For this study, we sought to determine whether TLR responses to spirochete components comprised the main factor responsible for the development of disease in B. burgdorferi-infected mice and the extent to which the absence of MyD88-dependent immune responses impaired host resistance to this pathogen. Toward this end, we used MyD88-deficient mice to examine whether MyD88-dependent immune mechanisms are essential for the development of arthritis and carditis and for their resolution after a B. burgdorferi infection.
MATERIALS AND METHODS
Mice.
C57BL/6J × 129/SvJ (B6129F2) MyD88−/− mice were the kind gift of Ruslan Medzhitov, Yale University School of Medicine (36). Age- and sex-matched B6129F2 mice were purchased from Jackson Laboratory (Bar Harbor, Maine) for use as controls. Mice were housed in microisolator cages and provided with autoclaved food, water, and bedding to reduce opportunistic infections, according to the Yale University institutional animal care and use guidelines. In addition, all MyD88−/− mice were administered the broad-spectrum antibiotic sulfatrim (0.25 mg/ml in drinking water) as a prophylaxis against infection. This antibiotic, which has no effect on B. burgdorferi, was continued throughout the experimental period in both MyD88−/− and wild-type (WT) mice.
Spirochetes.
Low-passage-number, cloned B. burgdorferi strain N40 spirochetes were expanded in modified Barbour-Stoenner-Kelly II medium (6) and enumerated with a Petroff-Hausser counting chamber by dark-field microscopy before inoculation into mice. Mice were inoculated intradermally in the shoulder region with 104 cloned N40 spirochetes in 100 μl of Barbour-Stoenner-Kelly II medium.
Cytokine-specific ELISAs.
Resting macrophages were harvested by peritoneal lavage of MyD88−/− and WT mice with 10 ml of ice-cold Ca2+- and Mg2+-free phosphate-buffered saline (PBS) and then were washed and resuspended in α-minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) without antibiotics. Cells were divided into aliquots at 2 × 106/ml in a 24-well plate and stimulated for 24 h with viable spirochetes at a ratio of macrophages to spirochetes of 1:100. In some cases, spirochetes were first opsonized with immune sera from 45-day B. burgdorferi-infected WT mice. Culture supernatants were harvested after 72 h and analyzed by cytokine-specific enzyme-linked immunosorbent assays (ELISAs) using a capture antibody, a biotinylated anti-mouse TNF-α monoclonal antibody (MAb), and a recombinant murine TNF-α standard (R&D Systems, Minneapolis, Minn.).
For analysis of the CD4+-T-cell production of IL-4 and IFN-γ, splenocytes were isolated from MyD88−/− and WT mice, and the CD4+-T-cell population was enriched by negative selection with biotinylated anti-mouse CD8, CD19, and Mac1 MAbs (Pharmingen) and anti-biotin antibody-coated magnetic microbeads (Miltenyi Biotec, Auburn, Calif.). CD4+ T cells were resuspended in Click's medium supplemented with 10% FBS, 5 × 10−5 M 2-mercaptoethanol, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and divided into triplicate aliquots at 4 × 105 cells/well in 96-well microtiter plates. T cells were stimulated with titrating amounts of B. burgdorferi lysate in the presence of 106 mitomycin C-treated splenocytes. Culture supernatants were harvested after 72 h of incubation, and cytokines were quantified by ELISAs using cytokine-specific capture and detection antibodies and recombinant cytokine standards (Pierce Biotech, Rockford, Ill.).
Intracellular cytokine analysis.
Splenocytes were isolated from individual mice and processed for intracellular cytokine staining. Briefly, after red blood cell lysis, 2 × 106 splenocytes were resuspended in 200 μl of Dulbecco's MEM supplemented with 10% FBS, 5 × 10−5 M 2-mercaptoethanol, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 100 μg of l-glutamine/ml. Triplicate wells were stimulated for 12 h with 35 μg of B. burgdorferi lysate/ml, with 1 μl of Golgi plug (Pharmingen)/ml added during the final 4 h of incubation. Cells were harvested, washed, and stained with a phycoerythrin-labeled anti-CD4 MAb and an allophycocyanin-labeled anti-Thy1.2 MAb. After fixation in 2% formaldehyde and permeabilization with 0.5% saponin in PBS, cells were stained with a fluorescein isothiocyanate-labeled anti-IFN-γ antibody. Intracellular cytokine staining was assessed by flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, Calif.).
Histopathology.
Bilateral tibiotarsal joints and hearts were processed and stained with hematoxylin and eosin by routine histologic techniques (10). The joints were scored for arthritis severity on a scale of 0 (negative) to 3 (severe) in a blinded fashion as described previously (10). Carditis was considered active when acute inflammatory cell infiltrates were seen in the heart base tissues (5).
Measurement of B. burgdorferi-specific antibodies.
B. burgdorferi-specific antibody end-point titers were determined by an ELISA, as described above, using microtiter plates (ICN Biomedicals, Aurora, Ohio) that were previously coated with B. burgdorferi lysate (3 μg/well). Bound antibodies were labeled with alkaline phosphatase-conjugated goat anti-mouse IgM, IgG, or IgG1, IgG2a, IgG2b, and IgG3 and were detected with a Vector Elite visualization kit (Vector Laboratories, Burlingame, Calif.) used according to the manufacturer's protocol.
For immunoblot analysis, B. burgdorferi proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose. After being blocked with Tris-buffered saline (TBS) containing 0.5% Tween 20 (TBS-Tween), membranes were incubated with sera from 14- or 45-day B. burgdorferi-infected mice, diluted 1:100 and 1:400, respectively, in TBS-Tween. Where indicated, individual lanes of the gel were incubated with outer surface protein A (OspA) MAb VIIIC3.78, mouse antisera to decorin binding protein A (DbpA)-N40 (a gift of MedImmune Corp., Gaithersburg, Md.), or rabbit antisera to a recombinant glutathione transferase-OspC fusion protein in order to mark the location of specific B. burgdorferi antigens. Bound B. burgdorferi antibodies were detected by using an alkaline phosphatase-conjugated goat antibody specific for mouse IgM or IgG or for rabbit IgG (Vector Laboratories) or an alkaline phosphatase-conjugated anti-rabbit IgG and were visualized with 5-bromo-4-chloro-3-indoyl phosphatase p-toluidine and nitroblue tetrazolium substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.).
Quantitative PCR.
DNA was extracted from the urinary bladders of individual mice by the use of a DNeasy tissue kit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions and then were resuspended in 50 μl of double-distilled H2O (ddH2O). Real-time PCRs for B. burgdorferi ospA, flaB, and the eukaryotic β-actin gene were performed with primers and probes selected by Primer Express software (PE Biosystems, Foster City, Calif.). The 5′ ospA primer (5′-TGAAGGCGTAAAAGCTGACAAA-3′) and the 3′ ospA primer (5′-TTCTGTTGATGACTTGTCTTTGGAA-3′) were synthesized to amplify a 139-bp fragment of ospA. The internal oligonucleotide ospA probe (5′-CAATTTTGAACGATCTACGTCAAACCACACTTGA-3′) was labeled at the 5′ end with the reporter dye 6-carboxyfluorescein (FAM) and at the 3′ end with the quencher dye 5-carboxytetramethylrhodamine (TAMRA). flaB was amplified with the following primers and probe: 5′ primer, 5′-AGCTGAAGAGCTTGGAATGC-3′; 3′ primer, 5′-AACAGCAATTGCCTCATCCT-3′; probe, 6-FAM-CACCAGCATCACTAGCTGGATCAC-TAMRA. The 5′ β-actin primer (5′-ATCCTGGCCTCACTGTCCAC-3′) and the 3′ β-actin primer (5′-GGGCCGGACTCATCGTACT-3) were synthesized to amplify a 68-bp fragment of β-actin. The β-actin internal probe (5′-TCCAGCAGATGTGGATCAGCAAGCATA-3′) was labeled as described above for the ospA probe. Two microliters of isolated DNA was amplified in a 50-μl final volume containing 10× buffer, 5 mM MgCl2, 200 μM deoxynucleoside triphosphates, a 9 μM concentration of each primer, and 1 U of Taq polymerase (Life Technologies, Gaithersburg, Md.). Amplification was performed in an iCycler (Bio-Rad, Hercules, Calif.) for 50 cycles, with a 60°C annealing temperature. Copy numbers for mouse β-actin and B. burgdorferi flaB and ospA were calculated with iCycler software, and the B. burgdorferi gene results were normalized to the starting copy number of the β-actin gene for each sample.
Macrophage uptake of B. burgdorferi spirochetes.
Resident peritoneal macrophages were isolated from MyD88−/− and WT mice by peritoneal lavage with ice-cold Ca2+- and Mg2+-free PBS. Cells were plated at 2 × 105 cells/12-mm-wide Chromerge-cleaned coverslip in α-MEM supplemented with 10% heat-inactivated FBS (MEM-10% FBS), 100 U of penicillin/ml, and 100 μg of streptomycin/ml in a 24-well plate. After an overnight incubation at 37°C to allow adherence, cells were rinsed in antibiotic-free MEM-10% FBS and B. burgdorferi was added at a ratio of spirochetes to macrophages of 100:1 or 10:1. Coverslips were incubated on ice for 5 min to allow for B. burgdorferi attachment and then were warmed to 37°C for 10 min to allow phagocytosis to proceed. All coverslips were then immersed in ddH2O five times for 1 s each to eliminate extracellular spirochetes (28). Cultures were incubated at 37°C in MEM-10% FBS without antibiotics until fixation at chase times of 0, 20, 60, and 180 min. After methanol fixation, cells were labeled with a polyclonal rabbit anti-B. burgdorferi antiserum (a kind gift of Fred Kantor, Yale University) and a rat anti-mouse LAMP-1 antibody (Development Studies Hybridoma Bank, Iowa City, Iowa) and were visualized with tetramethyl rhodamine isocyanate-labeled F(ab′)2 goat anti-rabbit IgG and fluorescein isothiocyanate-labeled F(ab′)2 goat anti-mouse IgG (Biosource, Camarillo, Calif.), respectively. Coverslips were mounted in Moviol (Calbiochem, San Diego, Calif.) and examined on a Zeiss LSM 510 confocal imaging system mounted on a Zeiss Axiovert 10 microscope, using multitracking to prevent bleeding between fluorescence channels. For each time point, 100 cells associated with spirochetes were examined and the number of cells with elongated spirochetes was recorded.
RESULTS
B. burgdorferi-infected mice develop arthritis and carditis in the absence of MyD88.
We compared the kinetics of disease expression in B. burgdorferi-infected MyD88−/− and WT mice over a time period comprising peak disease (days 14 to 28) and disease regression (day 45). Surprisingly, MyD88−/− mice developed acute arthritis and carditis that evolved and regressed within the same time frame observed for WT mice (Table 1). Histopathology revealed cellular infiltrates in the joints and hearts of B. burgdorferi-infected MyD88−/− mice that were indistinguishable from those seen in infected WT animals (data not shown). The remarkable similarity in disease severity and kinetics in the absence of MyD88 demonstrates that MyD88-dependent signaling is not required for B. burgdorferi-induced disease, even though innate immune cells comprise the inflammatory infiltrate.
TABLE 1.
B. burgdorferi-infected MyD88−/− mice develop disease and are persistently spirochetemic
| No. of days after infectione | Arthritis severitya
|
No. of mice with active carditis/totalb
|
No. of mice with spirochetes in blood cultures/totalc
|
|||
|---|---|---|---|---|---|---|
| WT | MyD88−/− | WT | MyD88−/− | WT | MyD88−/− | |
| 14 | 0 ± 0 | 0.2 ± 0.1 | 4/5 | 3/5 | 1/5 | 4/5 |
| 17 | 0 ± 0 | 0.3 ± 0.1 | 0/4 | 5/5d | 1/4 | 5/5 |
| 28 | 1.6 ± 0.2 | 1.6 ± 0.3 | 0/5 | 1/5 | 0/5 | 4/5 |
| 45 | 1.2 ± 0.3 | 0.7 ± 0.3 | 0/5 | 0/5 | 0/5 | 4/5 |
Arthritis severity is based on the degree of leukocyte infiltration on a scale of 0 to 3. The data represent the means of scores ± standard errors of the means for the most severely affected joint. There was no statistical difference in arthritis severity among mouse groups at any time point.
Carditis is reported as the number of mice with acute inflammation at the base of the heart. With the exception of the 17-day time point there was no statistical difference in the presence of carditis among mouse groups.
The presence of spirochetes in the blood was determined by dark-field microscopy after 14 days of culture in BSK II medium at 33°C.
Statistically significant compared to WT (P = 0.0079 by Fisher's exact test).
The 14-day and 45-day results are from a single experiment, whereas the 17-day and 28-day results are from two separate experiments.
B. burgdorferi-infected MyD88−/− mice are persistently spirochetemic and fail to limit pathogen burden in the first 45 days of infection.
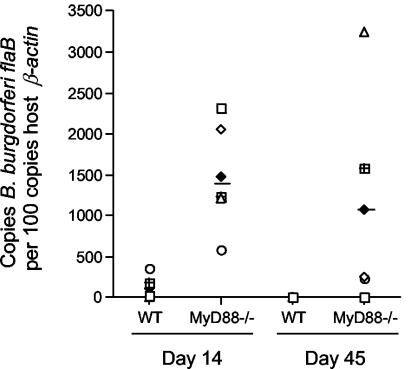
The culturing of blood at the time of mouse sacrifice revealed the presence of spirochetes at this site in MyD88−/− mice at every time point examined, including day 45 of infection (Table 1). In WT mice, however, the hematogenous phase of B. burgdorferi infection was more limited, with spirochetes detected in the blood only within the first 17 days of infection. This persistent spirochetemia suggested that the pathogen burden had increased in MyD88−/− mice even though the disease was not significantly different. We therefore used real-time PCR to quantify the B. burgdorferi DNA in mouse urinary bladders, a nondiseased site reflective of disseminated infection in which the pathogen burden is less likely to be influenced by local inflammation (Fig. 1). After 14 days of infection, the mean number of ospA DNA copies per 100 copies of β-actin DNA in MyD88−/− mouse urinary bladders was 11 times that for WT mice (2,045 versus 187; P = 0.0276), and by day 45, the mean level was 259 times higher than that for WT mice (4,920 versus 19; P = 0.0079). Similar results were obtained by real-time PCR for flaB (Fig. 1), confirming that chromosomal as well as plasmid DNA levels were increased in MyD88-deficient mice. Whereas the pathogen burden in WT mice declined significantly between 14 and 45 days of infection (P = 0.0317 by the Mann-Whitney test), there was no difference in the spirochete numbers in MyD88−/− mice at these two time points (P = 0.4206 by the Mann-Whitney test). Thus, the absence of MyD88-dependent immunity permits excessive pathogen growth during the first 45 days of infection.
FIG. 1.
Enhanced pathogen burden in B. burgdorferi-infected MyD88−/− mice. B. burgdorferi flaB DNA was quantified in the urinary bladder of individual mice by real-time PCR. Each data point represents the result from an individual mouse normalized to the amount of host β-actin. At both 14 and 45 days after infection, urinary bladders from MyD88−/− mice harbored significantly more DNA than those from WT mice (13-fold increase at day 14 [P = 0.0079 by the Mann-Whitney test] and 267-fold increase at day 45 [P = 0.0317 by the Mann-Whitney test]).
Peritoneal macrophages from MyD88−/− mice can ingest but are slower to degrade B. burgdorferi.
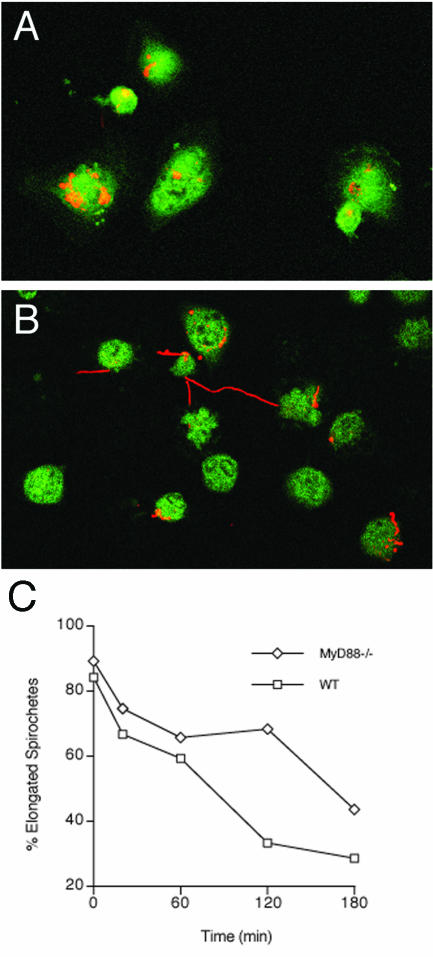
The elevated pathogen burden in B. burgdorferi-infected MyD88−/− mice may indicate that macrophages require TLR signaling to eliminate spirochetes efficiently. TLR recognition of microbial ligands can occur at the cell surface or in endosomal compartments. During phagocytosis, ingested material enters phagosomes, where TLRs can interact with microbial motifs (40). The details of TLR recruitment to phagosomes and the signaling of phagocytosis are not yet fully understood. We used confocal microscopy to examine in vitro the binding and uptake of unopsonized cultured B. burgdorferi organisms by peritoneal macrophages from uninfected MyD88−/− and WT mice (Fig. 2). Several spirochete-to-macrophage ratios were used, although even the lowest ratio (10:1) still exceeds the in vivo situation, in which spirochetes are believed to be comparatively few in number. Macrophages have been shown to rapidly and efficiently ingest spirochetes in the presence or absence of specific antibodies (28). Ingested spirochetes arrive in lysosomes, where they are degraded, with a half-life (t1/2) of 20 min (27). In macrophages from MyD88−/− and WT mice, the numbers of cells binding B. burgdorferi were similar, and binding occurred with kinetics equivalent to those that were previously reported (28). However, the morphology of ingested spirochetes was significantly different in macrophages from MyD88−/− mice. In the absence of MyD88 and at low spirochete-to-macrophage ratios, the spirochetes exhibited a persistence of elongated morphology, suggesting a deficit in the degradation of spirochetes (Fig. 2C). At chase times of 2 and 3 h, many elongated spirochetes were observed in association with MyD88−/− macrophages (Fig. 2B and C), whereas at the same time points, the majority of spirochetes in WT cells were compact (Fig. 2A). A higher spirochete-to-macrophage ratio (100:1) or the use of an opsonizing serum (data not shown) negated this effect. These findings suggest that in the absence of opsonization, MyD88-dependent TLR signaling is necessary for the efficient degradation of viable B. burgdorferi cells when the spirochete-to-macrophage ratio is low, as might be expected early in infection.
FIG. 2.
Reduced degradation of B. burgdorferi by MyD88−/− macrophages. Adherent macrophages were exposed to spirochetes at a 1:10 ratio of macrophages to spirochetes, dipped in ddH2O to eliminate extracellular spirochetes, and incubated in fresh medium until fixation at 0, 20, 60, 120, and 180 min as described in Materials and Methods. Monolayers were then fixed and double labeled for spirochetes (red) and the lysosomal marker LAMP-1 (green). Images were recorded on a Zeiss LSM 510 confocal imaging system using a 63× objective. (A) WT macrophages at 2 h. (B) MyD88−/− macrophages at 2 h. (C) Percentages of elongated B. burgdorferi spirochetes associated with macrophages as a function of time. Note the protracted presence of elongated spirochetes in MyD88−/− macrophages. The results are representative of two experiments.
Opsonization of spirochetes is required for stimulation of TNF-α production from MyD88−/− mouse macrophages.
To determine whether MyD88−/− macrophages could be activated to produce inflammatory cytokines after the ingestion of B. burgdorferi, we exposed macrophages in vitro to opsonized or unopsonized spirochetes for 24 h and then quantified the level of TNF-α in the culture supernatants by ELISA (Table 2). As expected, WT macrophages produced TNF-α when exposed to B. burgdorferi and the level increased when the spirochetes were opsonized. In contrast, TNF-α was detected in the culture supernatants of MyD88−/− macrophages only after exposure to opsonized B. burgdorferi. Thus, even though MyD88−/− macrophages can ingest B. burgdorferi in the absence of opsonizing Ab, opsonization is required for inflammatory cytokine production.
TABLE 2.
MyD88−/− macrophages produce TNF-α after exposure to opsonized, but not unopsonized, B. burgdorferi
| Stimulus | TNF-α level (pg/ml)a
|
|
|---|---|---|
| WT mice | MyD88−/− mice | |
| None | <100 | <100 |
| Unopsonized B. burgdorferi | 2,510 ± 43 | <100 |
| Opsonized B. burgdorferi | 4,550 ± 411 | 2,750 ± 64b |
Values are means of triplicate samples ± standard errors of the means.
Significantly different (P = 0.01) from the value for WT samples, as calculated by Student's t test.
B. burgdorferi-infected MyD88−/− mice exhibit a shift in the humoral immune response toward Th2-associated Ab isotypes.
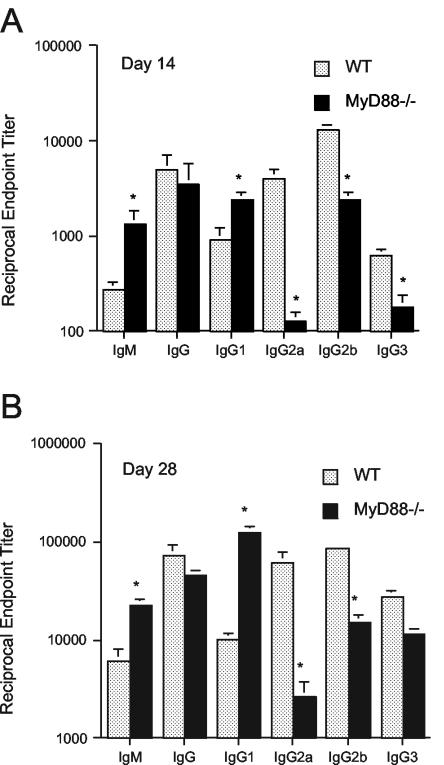
MyD88−/− mice have a defect in the activation of antigen-specific Th1 responses after immunization with a conventional antigen or after infection with some intracellular pathogens (34, 36). Th2 responses are intact, however, and MyD88−/− mice produce antibody isotypes (IgG1) that are characteristic of Th2-driven B-cell isotype switching (36). After infection with the extracellular pathogen B. burgdorferi, we observed that MyD88−/− mice had significantly higher B. burgdorferi-specific IgM end-point titers and a trend toward lower B. burgdorferi-specific IgG titers than WT mice (Fig. 3). The B. burgdorferi-specific IgG1 (a Th2-associated isotype) level was elevated in comparison to that in WT mice, but MyD88−/− mice were still able to produce B. burgdorferi-specific IgG2a (a Th1-associated isotype).
FIG. 3.
MyD88−/− mice exhibit a shift toward Th2-associated B. burgdorferi-specific antibody isotype responses. B. burgdorferi-specific antibody titers in the sera of WT and MyD88−/− mice infected for 14 days (A) or 28 days (B) were measured by isotype-specific ELISA. *, the value for MyD88−/− mouse sera differed significantly from the WT value for the indicated antibody class and isotype (P value range, 0.0007 to 0.05, by the Mann-Whitney test).
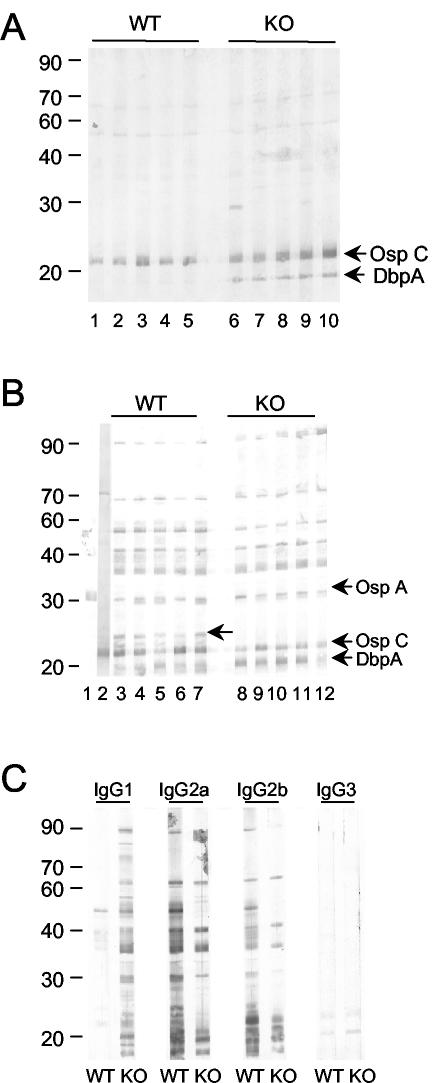
In an immunoblot analysis, B. burgdorferi-specific IgM from MyD88−/− mice exhibited more reactivity to proteins migrating in the location of OspC and DbpA than IgM from WT mice (Fig. 4A). Little difference in reactivities was noted for an IgG immunoblot, with sera from MyD88−/− and WT mice binding to a similar array of B. burgdorferi proteins; however, one ∼26-kDa protein appeared to be exclusively targeted by WT, but not MyD88−/−, immune sera (Fig. 4B). The ∼26-kDa protein could not be detected with OspE, OspF, OspC, or DbpA antisera (data not shown), and its identity is not yet known. The immunoblot reactivities of IgG isotypes (Fig. 4C) revealed that an antibody of at least one isotype in MyD88−/− mice recognized the majority of proteins detected by WT immune sera and that the deficiency in IgG2a and IgG2b responses was compensated for by IgG1 responses.
FIG. 4.
Western blot reactivities of sera from B. burgdorferi-infected MyD88−/− and WT mice. Sera from individual mice infected for 28 days were assessed for reactivity to B. burgdorferi proteins by immunoblotting of B. burgdorferi lysates. (A) IgM reactivities of WT (lanes 1 to 5) and MyD88−/− mouse sera (lanes 6 to 10). Arrows mark the locations of OspC and DbpA, which were assessed by the use of antisera for these proteins. (B) IgG reactivities of WT (lanes 3 to 7) and MyD88−/− mouse sera (lanes 8 to 12). Lanes 1 and 2 depict the locations of OspA and OspC, respectively, as assessed by the use of OspA MAb VIIIC3.78 and an antiserum to recombinant OspC. The ∼30-kDa protein bound by antibodies in both WT and MyD88−/− sera migrates below OspA. The unlabeled arrow marks the location of a protein targeted by the WT but not the MyD88−/− immune serum. (C) Isotype-specific immunoblot of B. burgdorferi lysates using sera pooled from individual WT and MyD88−/− mice. KO, MyD88−/− mice.
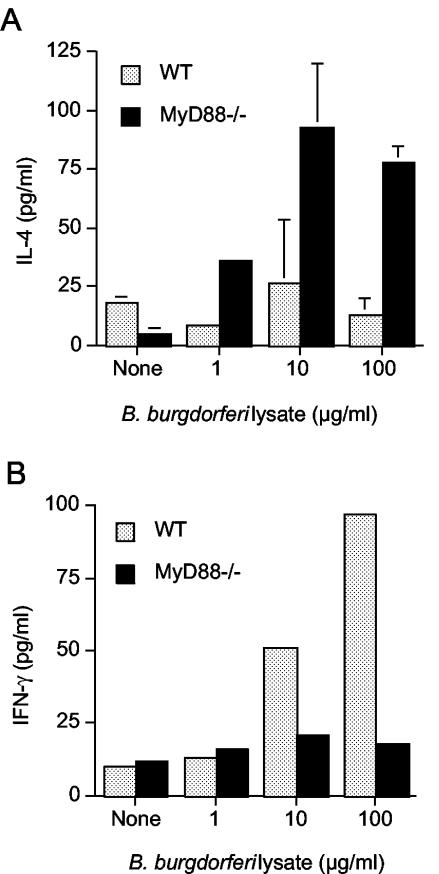
As expected, CD4+ T cells from MyD88−/− mice produced more IL-4 than WT cells after in vitro restimulation with B. burgdorferi lysate (Fig. 5A). We could not, however, detect IFN-γ in the culture supernatants of MyD88−/− T cells (Fig. 5B), even though the presence of B. burgdorferi-specific IgG2a suggested that some priming of Th1 cells had occurred. By intracellular cytokine analysis, we detected small numbers of CD4+ T cells from infected WT and MyD88−/− mice that produced IFN-γ in response to B. burgdorferi stimulation (1.09% versus 0.37%, respectively; unstimulated controls, <0.03% [data not shown]). Taken in the context of the IgG isotype response, these results suggest that infection can prime B. burgdorferi-specific CD4+ Th1 cells in the absence of MyD88-dependent TLR signaling, in contrast to the lack of antigen-specific IgG2a and Th1 cell priming after immunization with conventional antigen (36). This finding is supported by a recent report in which MyD88−/− mice infected with an attenuated ActA-deficient strain of Listeria monocytogenes developed antigen-specific CD4+ Th1 responses, albeit at reduced levels compared to similarly infected WT mice (41).
FIG. 5.
Splenic CD4+-T-cell production of IL-4 and IFN-γ. CD4+ T cells were purified from the spleens of infected WT and MyD88−/− mice and stimulated in vitro with B. burgdorferi lysates. After 72 h, supernatants were harvested and the amounts of IL-4 (A) and IFN-γ (B) were analyzed by cytokine-specific ELISAs. The results are reported as the means of triplicate samples ± standard errors of the means (A) or of duplicate samples (B). The minimum level of cytokine detection was 30 pg/ml for IL-4 and 30 pg/ml for IFN-γ.
B. burgdorferi-specific antibody reduces pathogen burden in MyD88−/− mice.
To assess the effects of MyD88 deficiency in the chronic phase of B. burgdorferi infection, we analyzed MyD88−/− and WT mice after 90 days of infection. Although the pathogen burden at this stage had decreased substantially, MyD88−/− mice continued to harbor more spirochetes than WT mice, with a mean number of flaB DNA copies per 105 copies of β-actin DNA of 204 for MyD88−/− mice, compared to 0.1 for WT mice (P = 0.0079 by the Mann-Whitney test). B. burgdorferi-specific IgG titers were equivalent between the mouse groups, but IgM levels were significantly higher in MyD88−/− mice (mean reciprocal end-point titer of 25,600 versus 4,480; P = 0.0172 by the two-tailed, unpaired Student t test). Arthritis and carditis had resolved completely in both mouse groups (data not shown).
DISCUSSION
In the murine model of Lyme borreliosis, disease is believed to be caused by the activation of innate immune cells by proinflammatory spirochete components because both arthritis and carditis can occur in B. burgdorferi-infected mice in the absence of adaptive immunity. B. burgdorferi lipoproteins alone are sufficient to incite inflammation, as has been demonstrated in vitro by the release of IL-6, nitrous oxide, and TNF-α by macrophages after exposure to purified lipoproteins (17, 24, 31) and in vivo by the accumulation of neutrophils at the site of lipoprotein inoculation (30). The seminal works of Hirschfeld (18) and Aliprantis (4) revealed that the pattern recognition molecule TLR2 is required for B. burgdorferi lipoprotein-mediated inflammation, but in vivo, the absence of TLR2 does not eliminate disease (43). The observation that macrophages from TLR2−/− mice can produce IL-6 and nitrous oxide in response to B. burgdorferi lysates, but not in response to purified lipoproteins (43), suggested that nonlipoprotein components, perhaps unmethylated CpG DNA or flagellin, may contribute to disease through the activation of other TLRs. Using mice deficient in MyD88, the common adapter molecule utilized by all TLRs to initiate inflammatory cytokine secretion, we showed in this work that acute inflammation still develops in hearts and joints even though innate immune cells cannot respond to pathogen motifs through MyD88-dependent mechanisms. Moreover, despite the timely development of B. burgdorferi-specific antibody responses, the pathogen burden in urinary bladders remained elevated during the disease resolution phase of infection. In vitro, we showed that MyD88−/− macrophages are less efficient than WT macrophages at clearing ingested spirochetes at low spirochete-to-macrophage ratios, and they produce TNF-α only when exposed to opsonized spirochetes. These findings suggest that other pathways for innate immune cell activation, such as the ingestion of opsonized spirochetes, contribute to the development of acute inflammation and disease in B. burgdorferi-infected MyD88−/− mice and that pathogen proliferation in the absence of MyD88 is due to impaired early clearance of B. burgdorferi by phagocytes.
The prevailing notion has been that adaptive immunity is not required for B. burgdorferi-induced disease because innate immune cells comprise the cellular infiltrates in diseased tissues and because pathology occurs in SCID and B-cell-deficient mice. Yet the host-pathogen relationship is fundamentally changed when mice are unable to produce antibodies: antibody-mediated selective pressure on B. burgdorferi is not present and the population of spirochetes within the antibody-deficient host is likely different from that found in an infected immunocompetent host. Microarray analysis of lipoprotein gene expression during the course of B. burgdorferi infection of normal mice revealed that mRNAs for 116 of 137 genes could be detected at 10 days, but by infection day 30, after the induction of adaptive immunity, mRNAs for fewer than 40 genes remained (23). A similar reduction in select gene expression was noted in a separate study in which the levels of transcription were quantified by real-time reverse transcription-PCR (19). The inability to detect mRNAs for specific lipoprotein genes may be due to selective antibody-mediated elimination of spirochete populations that express the lipoprotein, as has been shown by the disappearance of OspC mRNA after the passive transfer of OspC antibodies to SCID mice (22). Thus, lipoprotein-mediated inflammation may be dominant in a B. burgdorferi-infected antibody-deficient host, but it may be only one factor that gives rise to disease in an immunocompetent host. The observation that innate immune cells still infiltrate hearts and joints in B. burgdorferi-infected MyD88−/− mice confirms that a TLR interaction with B. burgdorferi components is not necessary for the induction of disease.
The severity of murine Lyme arthritis is determined by the genetic background, with C57BL/6 and 129 mouse strains being relatively resistant to disease compared to C3H/HeN mice (7). Mapping studies have shown that the genes that regulate arthritis severity are found on several chromosomes (4, 5, and 11 in the mouse) and do not include those involved in adaptive immunity (32, 42). The pattern of arthritis susceptibility and resistance is preserved in SCID mice and is determined by genes outside the major histocompatibility locus (15, 16). Surprisingly, pathogen burden does not appear to correlate with disease severity, which we confirmed in this study. The mildly inflamed joints of C57BL/6 mice have been shown to harbor equivalent numbers of spirochetes as those of more severely arthritic C3H/HeN mice (14, 44). The induction of inflammation may be more closely related to the amount of target antigen expressed by spirochetes and the threshold level for host cell activation than to the spirochete burden per se. Our study utilized MyD88−/− mice from the disease-resistant C57BL/6 × 129 background, so a substantial attenuation of arthritis might not have been apparent even though both WT and MyD88−/− mice developed moderately robust joint infiltrates (scoring as high as 1.6 on a scale of 0 to 3). Our findings instead showed that arthritis development in disease-resistant mice does not require MyD88-dependent TLR signaling. Other pathways for innate immune cell activation, such as Fc receptor uptake of IgG-opsonized spirochetes, may contribute to arthritis. We did not observe enhanced joint swelling, as was seen for B. burgdorferi-infected TLR2−/− mice (43). It is still possible that the more severe arthritis seen in susceptible mouse strains is influenced by MyD88-dependent immune responses.
The kinetics of disease evolution and regression were essentially identical between MyD88−/− and WT mice. Disease regression requires intact adaptive immune responses, and MyD88−/− mice developed significant titers of B. burgdorferi-specific antibody. An interesting finding in our study was that the titers of B. burgdorferi-specific IgM were significantly elevated in MyD88−/− mice infected for 90 days, even though the IgG titers were equivalent to those of WT mice. It has been shown previously that antibodies that arise in the absence of T-cell help are sufficient to protect mice against B. burgdorferi infection and to resolve arthritis. Although the relative contribution of IgM versus IgG to disease regression has not been assessed, our results suggest that in the absence of MyD88-dependent immunity, B. burgdorferi-specific IgM may be critical for the effective elimination of host-adapted spirochetes, even when Fc receptor-mediated uptake is presumably intact.
Phagocytes are crucial to the initial control of the pathogen burden, prior to the development of adaptive immunity. We show that in the absence of MyD88, peritoneal macrophages are able to internalize spirochetes but degrade them more slowly. Members of our laboratory have previously shown in vitro that macrophages rapidly ingest and kill B. burgdorferi organisms in large numbers and do not require spirochete opsonization or the participation of Fc receptors for these responses (27-29). In vitro experiments utilize cultured B. burgdorferi spirochetes, which express abundant amounts of lipoproteins, and much of their recognition by macrophages may be mediated through TLR2. The spirochetes that enter the cells in phagosomes have the potential to interact with TLRs (40) before their subsequent degradation in the lysosomes (27, 28). Our study suggests that TLR recognition of spirochetes within phagosomes may be critical for their efficient degradation. This concept is supported by a recent study demonstrating that the phagocytosis of bacteria and phagolysosomal fusion are defective in the absence of TLR signaling (24a). In that study, the uptake of bacterial pathogens such as S. aureus, Escherichia coli, and Salmonella enterica serovar Typhimurium by MyD88−/− macrophages was delayed compared to that by WT cells even though the phagocytosis of apoptotic cells was equivalent between the two types of cells. In addition, the maturation of bacterium-containing phagosomes was delayed in MyD88−/− macrophages, with a slower fusion of phagosomes and lysosomes observed than that for WT cells. This may possibly result in a slower degradation of ingested bacteria in MyD88−/− macrophages and give rise to the elevated B. burgdorferi DNA levels in MyD88−/− mouse tissues.
In addition to its role in TLR activation pathways, MyD88 is an adapter molecule for the IL-1 receptor and IL-18 receptor signaling pathways (1). IL-1 induces acute-phase proteins, other cytokines, and adhesion molecules that facilitate the immune clearance of pathogens. IL-18 synergizes with IL-12 to promote IFN-γ production, NK cell activity, and Th1 cell development. Although they were not examined in this study, impaired effector functions of these cytokines may also contribute to the elevated pathogen burden observed in MyD88−/− mice. Since TLR2−/− mice infected with B. burgdorferi also harbor significantly more spirochetes than WT mice (43), an interruption of MyD88-dependent TLR signaling may play a larger role than impaired IL-1/IL-18 receptor signaling in the reduced spirochete clearance noted in MyD88−/− mice.
In summary, we have shown that even when the signaling pathways of all TLRs have been interrupted, B. burgdorferi can still incite acute inflammation in the MyD88−/− host. Remarkably, the histology is unchanged whether TLR signaling is present or not, and the disease evolves and regresses as it does in WT mice. We postulate that antibody opsonization of B. burgdorferi drives the pathology in MyD88−/− mice. Further studies are in progress to determine the interplay between antibody- and MyD88-dependent immune responses in B. burgdorferi-induced disease.
Acknowledgments
This work was supported by NIH grants AR47048 and AI50604 and by the Arthritis Foundation (L.K.B.), by NIH grants AR10493 and AI43558 (R.R.M.), and by NIH grant AI26815 (S.W.B.).
We thank Jialing Mao, Debbie Beck, and Rita Palmarozza for their excellent technical assistance.
Editor: D. L. Burns
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5-11. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 4.Aliprantis, A. O., R.-B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong, A. L., S. W. Barthold, D. H. Persing, and D. S. Beck. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47:249-258. [DOI] [PubMed] [Google Scholar]
- 6.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 8.Barthold, S. W., M. de Souza, E. Fikrig, and D. H. Persing. 1992. Lyme borreliosis in the laboratory mouse, p. 223-242. In S. E. Schutzer (ed.), Lyme disease: molecular and immunologic approaches. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 9.Barthold, S. W., M. deSouza, and S. Feng. 1996. Serum-mediated resolution of Lyme arthritis in mice. Lab. Investig. 74:57-67. [PubMed] [Google Scholar]
- 10.Barthold, S. W., S. Feng, L. K. Bockenstedt, E. Fikrig, and K. Feen. 1997. Protective and arthritis-resolving activity in serum of mice infected with Borrelia burgdorferi. Clin. Infect. Dis. 25(Suppl. 1):S9-S17. [DOI] [PubMed] [Google Scholar]
- 11.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 12.Bockenstedt, L. K. 2001. Spirochetal disease: syphilis and Lyme disease, p. 688-697. In T. G. Parslow, D. P. Stites, A. I. Terr, and J. B. Imboden (ed.), Medical immunology, 10th ed. McGraw-Hill Co., New York, N.Y.
- 13.Bockenstedt, L. K., I. Kang, C. Chang, D. Persing, A. Hayday, and S. W. Barthold. 2001. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect. Immun. 69:5264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893-901. [DOI] [PubMed] [Google Scholar]
- 15.Brown, C. R., and S. L. Reiner. 2000. Bone-marrow chimeras reveal hemopoietic and nonhemopoietic control of resistance to experimental Lyme arthritis. J. Immunol. 165:1446-1452. [DOI] [PubMed] [Google Scholar]
- 16.Brown, C. R., and S. L. Reiner. 1999. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect. Immun. 67:1967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschfeld, M., C. J. Kirschning, R. Schwander, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 18.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodzic, E., S. Feng, K. J. Freet, and S. W. Barthold. 2003. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 71:5042-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 22.Liang, F. T., M. B. Jacobs, L. C. Bowers, and M. T. Philipp. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 195:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine stimulatory properties. Infect. Immun. 61:3843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Magarian Blander, J., and R. Medzhitov. Regulation of phagosome maturation by signals from Toll-like receptors. Science, in press. [DOI] [PubMed]
- 25.McKisic, M. D., and S. W. Barthold. 2000. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 68:5190-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKisic, M. D., W. L. Redmond, and S. W. Barthold. 2000. T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 165:6096-6099. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery, R. R., and S. E. Malawista. 1996. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect. Immun. 64:2867-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery, R. R., M. H. Nathanson, and S. E. Malawista. 1993. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages: destruction, survival, recovery. J. Immunol. 150:909-915. [PubMed] [Google Scholar]
- 29.Montgomery, R. R., M. H. Nathanson, and S. E. Malawista. 1994. Fc and non Fc-mediated phagocytosis of Borrelia burgdorferi by macrophages. J. Infect. Dis. 170:890-893. [DOI] [PubMed] [Google Scholar]
- 30.Norgard, M. V., B. S. Riley, J. A. Richardson, and J. D. Radolf. 1995. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect. Immun. 63:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radolf, J. D., L. L. Arndt, D. R. Kins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 32.Roper, R. J., J. J. Weis, B. A. McCracken, C. B. Green, Y. Ma, K. S. Weber, D. Fairbairn, R. J. Butterfield, M. R. Potter, J. F. Zachary, R. W. Doerge, and C. Teuscher. 2001. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2:388-397. [DOI] [PubMed] [Google Scholar]
- 33.Ruderman, E. M., J. S. Kerr, S. R. Telford III, A. Spielman, L. H. Glimcher, and E. M. Gravallese. 1995. Early murine Lyme carditis has a macrophage predominance and is independent of major histocompatibility complex class II-CD4+ T cell interactions. J. Infect. Dis. 171:362-370. [DOI] [PubMed] [Google Scholar]
- 34.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 35.Schaible, U. E., S. Gay, C. Museteanu, M. D. Kramer, G. Zimmer, K. Eichmann, U. Museteanu, and M. M. Simon. 1990. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137:811-820. [PMC free article] [PubMed] [Google Scholar]
- 36.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 37.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi, O., K. Hoshino, and S. Akira. 2000. TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi, O., K. Takeda, K. Hoshino, O. Adachi, T. Ogawa, and S. Akira. 2000. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int. Immunol. 12:113-117. [DOI] [PubMed] [Google Scholar]
- 40.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilston, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 41.Way, S. S., T. R. Kollmann, A. M. Hajjar, and C. B. Wilson. 2003. Protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J. Immunol. 171:533-537. [DOI] [PubMed] [Google Scholar]
- 42.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162:948-956. [PubMed] [Google Scholar]
- 43.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 44.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]