Abstract
The process of lymphopoiesis begins in the bone marrow (BM) and requires multiple cellular intermediates. For T cell production, lymphoid progenitors exit the BM and home to the thymus where maturation and selection ensues. These processes are dependent on a number of factors including chemokines and adhesion molecules. While the β2 integrin CD11a plays an important role in the migration of lymphocytes to lymph nodes, the role of CD11a in T cell development is largely undefined. Our studies now show that in CD11a−/− mice, thymic cellularity was decreased and early T cell development was partially impaired. Remarkably, CD11a was critical for generation of common lymphoid progenitors (CLPs) and lymphoid-primed multipotent progenitors (LMPPs). However, in intact CD11a−/− mice, peripheral B and T cell subsets were only modestly altered suggesting compensatory mechanisms were operating. In contrast, competitive BM reconstitution assays revealed an essential role for CD11a in the generation of thymocytes and mature T and B cells. This defect was linked to the requirement for CD11a in the development of CLPs. Furthermore, our results identified CLPs, and not LMPPs, as the requisite CD11a-dependent precursor for lymphocyte development. Thus, these findings established a key role for CD11a in lymphopoiesis.
Introduction
Hematopoietic stem cells (HSCs) are responsible for the continuous maintenance of immune cells during adult life. These rare self-renewing cells first appear within the fetal liver of embryonic mice at E11.5 (1). HSCs begin to traffic into the bone marrow (BM) shortly prior to birth (2). Within the adult BM, murine HSCs are commonly referred to as “LSKs” since they lack expression of all lineage markers (Lin−) but instead express the markers Sca-1 and c-kit (CD117) (3). This LSK subset is heterogeneous in nature. LSKs that express the cytokine receptor Flt3 (CD135) have lost self-renewing capacity and are called multipotent progenitors (MPPs) as they still possess multi-lineage potential (4, 5). MPPs can then give rise to early lymphoid progenitors (ELPs) and lymphoid-primed multipotent progenitors (LMPPs) which can express RAG genes as well as the interleukin-7 receptor α (IL-7Rα) indicating the earliest point of commitment towards the lymphoid lineages (6–9). Further downstream from LSKs are the common lymphoid progenitors (CLPs) (10). Although initially thought to only give rise to cells within the lymphoid lineages, recent data suggest that CLPs are also heterogeneous and some fraction of this population still possesses myeloid potential (11, 12). From the BM, T cell progenitors enter the circulation and home to the thymus (13). Although the identity of the thymic settling progenitor (TSP) is still open to debate, studies show that either LMPPs or CLPs can give rise to T cells in the thymus (14, 15).
In some ways, the homing and traversing of progenitors through thymic endothelium parallels the sequence of events required for entry of lymphocytes into lymph nodes. Numerous studies have suggested that entry into the thymus is tightly regulated, and homing and adhesion molecules, including PSGL-1 and the chemokine receptors CCR7 and CCR9, have been implicated in thymic settling and T cell development in vivo (16–20). In addition, in short-term adoptive transfer experiments, Scimone et.al have shown that antibody blockade of CD18 (the β2 integrin chain) results in reduced migration of progenitors into the thymus (21). Since the β2 integrin family consists of four members defined by distinct α-chains pairing with β2 (CD11a-d), the contribution of each α integrin to T cell development remains unknown.
CD11a is the α chain of the αLβ2 integrin (also known as leukocyte function associated antigen 1 [LFA-1]). While CD11a plays a critical role in the homing of lymphocytes across high endothelial venules to enter lymph nodes (22), the role of CD11a in T lymphopoiesis is not clearly defined. A number of earlier studies examined whether expression of CD11a affected thymocyte binding to thymic epithelium to influence thymocyte maturation; a consensus function for CD11a was not identified. Affinity modulation of CD11a is also important for its function, and it is possible that this may be regulated during T cell development (23). In one study, blocking CD11a in fetal thymic organ cultures resulted in the inhibition of double positive (CD4+CD8+) thymocyte development (24) while in another report, anti-CD11a inhibited T cell development from fetal liver progenitors but not from fetal thymus progenitors (25). Treating mice from birth with anti-CD11a, but not anti-β2 mAb, resulted in a selective loss of mature CD8 thymocytes (26) while blocking CD11a, or its ligand ICAM-1, inhibited intrathymic CD4 T cell migration and antigen recognition (27). In the past, two groups used different lines of CD11a-deficient mice to examine the role of this molecule in productive immune responses (28, 29). While one of these studies reported normal numbers of thymocytes and T cells (28), detailed analyses of individual subsets were not performed. In the second, the authors concluded that T cell selection was intact based on their analysis of TCR transgenic T cells deficient in CD11a (29). Thus, while some of the evidence points to a role for CD11a in T cell development, additional investigation is required to define the developmental stage(s) at which CD11a might operate in vivo.
In the current study, we utilized CD11a-deficient mice to define the role of this β2 integrin during lymphocyte development. We examined the generation of various BM progenitor subsets and analyzed intrathymic T cell differentiation in CD11a−/− mice and in mixed chimeric mice generated from wild type (WT) and CD11a−/− BM cells. Intact CD11a−/− mice exhibited noticeable but relatively minor defects in T cell development despite major defects in lymphoid progenitor generation in the BM. In mixed BM chimeras, CD11a−/− cells exhibited a severe defect in their potential to generate thymocytes and peripheral T and B cells. This defect was likely due to the nearly complete block in CLP development, even in a setting where upstream LSK development was normal. Overall, our results demonstrate a novel role for CD11a in the generation of lymphoid progenitors in the BM that is essential to normal lymphocyte development.
Materials and Methods
Mice
CD11a−/− mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME) and further maintained in the UConn Health, Center for Comparative Medicine. C57BL/6 mice (both CD45.2 and CD45.1) were purchased from the NCI-Charles River Laboratories (Frederick, MD). All animal protocols were carried out in accordance with National Institutes of Health guidelines and approved by the UConn Health Institutional Animal Care and Use Committee.
Tissue preparation
Spleen, thymus and BM were isolated from WT, CD11a−/− or mixed chimeric mice. Single cell suspensions of spleen and thymus were prepared by crushing each of the organs through 40μm cell strainers (BD Biosciences). RBCs in the spleen were lysed before preparing samples for staining. BM was isolated from the femur and tibia of one leg by rapid centrifugation at ~6000 RPM and suspended in RPMI.
Antibodies and flow cytometry
Monoclonal antibodies specific for the following antigens were purchased from Biolegend, eBioscience and BD Biosciences: CD4, CD8, CD3, GL3 (TCRγδ), CD19, CD44, CD25, NK1.1, CD11a, CD11b, CD11c, Gr-1, Ter119, Flt3, Sca-1, CD27, c-kit, CD127, Ly6G. Viability was analyzed using LIVE/DEAD cell stain (Invitrogen). Cells were stained with antibodies for 20–30 minutes at 4°C, washed with FACS buffer (PBS/0.2% BSA/0.01% NaN3) and fixed using 2% paraformaldehyde/ PBS (Electron Microscopy Sciences). Fluorescence was measured using an LSRII (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR). Whole BM was analyzed for progenitor populations that were negative for lineage markers (Lin-; CD11b, CD11c, Ter119, NK1.1, CD3, CD19, Gr-1). Fluorescence minus one (FMO) controls were used as indicated in the figure legends, either as the negative controls in order to detect expression of various cell surface markers, or to assist in gating of certain populations.
Progenitor isolation and in vitro culture
BM cells isolated from WT and CD11a−/− mice were depleted of lineage positive cells (CD4, CD8, CD19, CD11b, CD11c, NK1.1, Gr-1, Ter119) using MACS beads (Miltenyi) before sorting on a FACS Aria II (BD Biosciences). Progenitors were defined as Lin− Sca-1+c-kit+. Sorted cells were then seeded on either OP9 or OP9-DL1 cells (generously provided by Dr. Zuniga-Pflucker, University of Toronto, Ontario, Canada) in vitro for the indicated times in the presence of 5ng/ml Flt-3L and 1ng/ml IL-7 (30).
Mixed bone marrow chimeras
Whole BM was isolated from WT (CD45.2+/CD45.1+) and CD11a−/− (CD45.2+) donor mice. Cells were then mixed in either a 1:1 or 1:3 (WT:CD11a−/−) ratio, and a total of 2–3 million BM cells were injected i.v. via the tail vein into WT (CD45.1+) hosts that had been lethally irradiated (1000 rads). Lymphoid tissues were harvested after 13–24 weeks to allow for full reconstitution of the immune compartment.
Statistical analysis
Statistical significance was analyzed by Student's t-test or ANOVA using Prism 5 (Graphpad Software), with a probability of p < 0.05 considered to be significant.
Results
Alterations in peripheral cellularity in CD11a-deficient mice
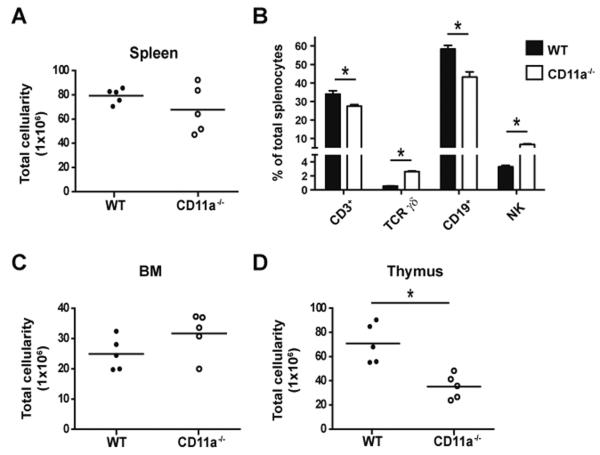
In the course of our previous studies examining the role of CD11a in the T cell response to infection (31), we observed several interesting effects of CD11a deficiency on the lymphoid compartment of uninfected controls. In naïve CD11a−/− mice, there was no significant decrease in the overall cellularity of the spleen compared to WT controls despite some variation in the total size (Fig. 1A). However, the percentage and total numbers of CD3+ T cells and CD19+ B cells were reduced in the spleens of CD11a−/− mice (Fig. 1B and Table IA). The T cell defect was largely accounted for by a loss of CD4 T cells (Table IA). We also noted a significant increase in the percentages of NK cells and TCR γδ T cells in CD11a−/− spleens, although only the latter exhibited increased total cell numbers (Fig. 1B and Table IA). These changes suggested a potential requirement for CD11a in lymphocyte development and prompted us to examine the primary lymphoid tissues responsible for B and T cell generation, the BM and thymus, respectively. No difference in the total number of BM cells in CD11a−/− versus WT mice was noted (Fig. 1C) but there was a consistent 2-fold decrease in the total cellularity of the thymus in CD11a−/− mice (Fig. 1D). Therefore, considering the reduction in peripheral T cells and the reduction in overall thymus size, we hypothesized that CD11a was required for the optimal generation of T cells.
Figure 1. CD11a−/− mice have a dramatically smaller thymi but only modest alterations in peripheral lymphocytes.
(A) Graph shows total cellularity of spleens from adult WT (filled circles) and CD11a−/− (open circles) mice. (B) Mean frequencies of the indicated leukocyte subsets within the spleen are shown for either WT (black bar) or CD11a−/− (white bar) mice. Error bars represent SEM with 5 mice per group. Total number of cells in the BM (C) and thymus (D) are shown. * p < 0.05. Data are representative of 4 individual experiments. Ages of mice used in these experiments ranged from 8–12 weeks.
Table I.
Absolute numbers of lymphocytes and progenitors in the spleen and thymus of WT and CD11a−/−mice.
| A. Spleen | |||
|---|---|---|---|
| Population | WT | CD11a−/− | p value |
| CD3 | 22.70±0.8 | 16.82±1.9 | 0.03 |
| CD3+TCRγδ−CD4+ | 13.45±0.6 | 6.31±0.9 | 0.006 |
| CD3+TCRγδ−CD8+ | 8.39±0.3 | 7.91±0.9 | NS |
| TCRγδ | 0.56±0.0 | 2.72±0.2 | <0.0001 |
| NK | 3.37±0.2 | 3.19±0.2 | NS |
| CD19 | 45.40±3.7 | 22.09±1.2 | 0.001 |
| B. Thymus | |||
|---|---|---|---|
| Population | WT | CD11a−/− | p value |
| CD4−CD8− (DN) | 3.088±0.4 | 1.673±0.8 | 0.03 |
| DN1 | 0.150±0.03 | 0.0375±0.8 | 0.005 |
| DN2 | 0.0175±0.01 | 0.0150±0.8 | NS |
| DN3 | 0.975±0.2 | 1.028±0.8 | NS |
| DN4 | 1.950±0.29 | 0.5925±0.08 | 0.004 |
| ETP | 0.006±0.001 | 0.002±0.0003 | 0.02 |
| c-kit+ DN2 | 0.015±0.005 | 0.006±0.0006 | 0.09 |
| DP | 67.87±10.76 | 31.87±3.697 | 0.0195 |
| CD4 SP | 5.12±0.73 | 1.31±0.23 | 0.0015 |
| CD8 SP | 1.31±0.13 | 0.35±0.07 | 0.0006 |
| TCRγδ | 0.136±0.014 | 0.184±0.028 | NS |
(A) Total cell numbers (x106) and statistical comparison of indicated lymphocyte subsets within the spleen of WT and CD11a−/− mice. (B) Total cell numbers of thymocytes from WT and CD11a−/− mice. DN (CD3−TCRγδ−/−CD4−CD8−) cells were separated based on CD44 and CD25 expression to identify DN1-4 subsets or by c-kit and CD25 expression to identify ETPs and c-kit+DN2 subsets. CD3+TCRγδ−/− cells were further subdivided into CD4+ (CD4 SP) and CD8+ (CD8 SP) thymocytes. p values greater than 0.05 are noted as NS, not significant. Data is inclusive of 5 mice per group. Mice ranged in age from 8–12 weeks. Two individual experiments were performed.
Expression of CD11a on thymic progenitors
In order to examine the role of CD11a in the step-wise process of T cell development in primary lymphoid organs, we first determined CD11a expression levels on progenitor populations in the adult thymus. Individual subsets of double negative (DN; CD3−CD4−CD8−) thymocytes expressed varying levels of CD11a. Interestingly, early thymic progenitors (ETPs; c-kit+ DN1 cells (32)) expressed high levels of CD11a followed by an apparent downregulation of CD11a expression at the DN2 and DN3 stages. CD11a was then upregulated between the DN3 and DN4 stages and again within the double positive (DP) stage corresponding with the acquisition of CD3 expression (Fig. 2A and 2B). Ultimately, there were only slight differences in the mean fluorescence intensity (MFI) of CD11a expression between CD3 DP, CD4 SP, CD8 SP, and TCR γδ T cells in the thymus (Fig. 2B). Overall, our analysis indicated that CD11a expression was regulated during thymocyte maturation suggesting a potential functional relationship between CD11a expression and T cell development.
Figure 2. Expression of CD11a on T cell precursors within the thymus.
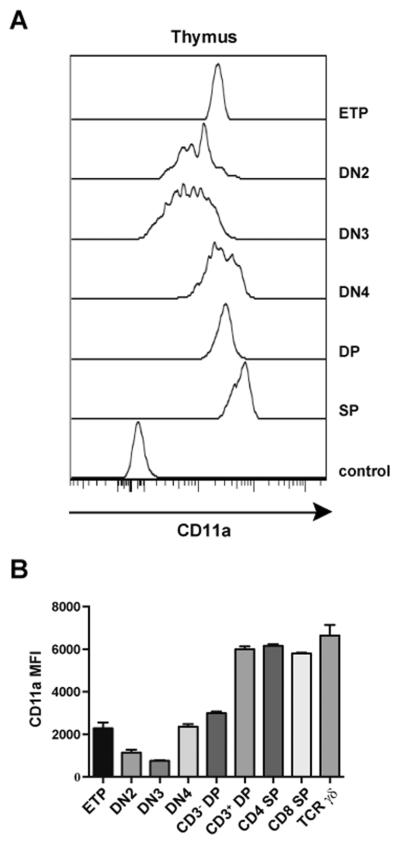
Thymocytes from WT B6 mice were examined for CD11a expression on the indicated populations using flow cytometry. DN (CD3−TCRγδ−CD4−CD8−) stages were separated based on CD44 and CD25 expression (DN3–4). Early thymic progenitors (ETP) were defined as a c-kit+CD25− DN subset while DN2 were identified as the c-kit+CD25+DN subset. Comparison of CD11a on CD3−DP (CD3−CD4+CD8+), CD3+DP (CD3+CD4+CD8+) and total DP (CD4+CD8+) are shown in comparison to the following mature single positive (SP) T cells: CD4 SP (CD3+TCRγδ−CD4+), CD8 SP (CD3+TCRγδ−CD8+) and TCR γδ (CD3+TCRγδ+). (A) Data are shown as representative histogram plots comparing CD11a expression of developing thymocytes to the FMO control of all thymocytes (bottom histogram). (B) Bar graphs show MFI of CD11a expression in the indicated thymocyte subsets analyzed in 3 mice. Error bars represent SEM.
Differentiation of thymocytes is not dependent on CD11a expression
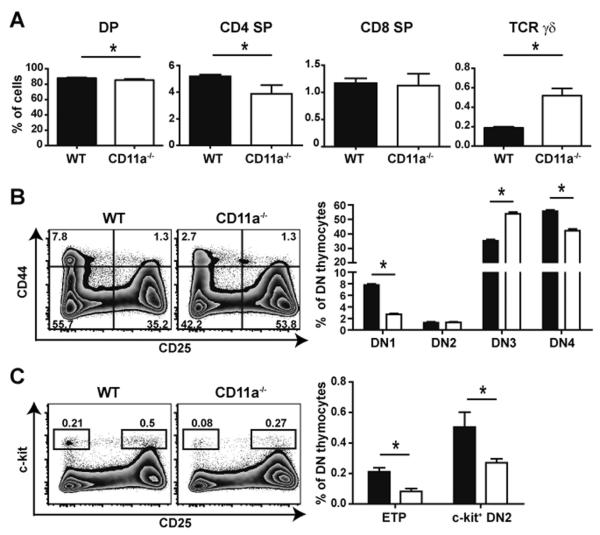
Since all T cell progenitors expressed CD11a, we determined if the 2- to 3-fold decrease in overall thymus size in CD11a-deficient mice was the result of a block at a particular checkpoint during thymocyte maturation. In the CD11a−/− thymus, a minimal but statistically significant reduction in the percentage of DP cells was observed (p=0.0037). Analysis of single positive thymocytes indicated a modest decrease in the frequency of CD4 SP cells (p=0.0053) accompanied by an increased frequency of TCR γδ T cells in the CD11a−/− thymus (Fig. 3A). These relative trends observed in thymic CD4 SP and TCR γδ T cell populations were maintained in the periphery of CD11a−/− mice (data not shown and Table IA). Examination of the DN subsets revealed that the frequency of DN1 thymocytes, ETPs, and the small subset of c-kit+ DN2 cells were all significantly reduced in CD11a−/− mice (Fig. 3B, C). The total number of cells in the DN1 and ETP subsets were also significantly reduced (~3-fold) in the CD11a-deficient thymus (Table 1B). Despite these reductions, the increased frequency of the DN3 subset and equivalent numbers of the DN2 and DN3 cells in the absence of CD11a suggested that perhaps compensatory mechanisms were operating at these early developmental stages. Nevertheless, the most noteworthy outcome of CD11a deficiency was an overall decrease in thymus cellularity.
Figure 3. CD11a−/− mice exhibit reduced frequencies of thymic ETPs but do not have an additional developmental block in differentiation.
(A) Thymi of WT and CD11a−/− mice were stained with antibodies against cell surface markers to identify immature and mature thymocytes by flow cytometry. Bar graphs show a comparison of DP (CD3−CD4+CD8+), CD4 SP (CD3+TCRγδ−CD4+CD8−), CD8 SP (CD3+TCRγδ−CD8+CD4−) and TCR γδ T cells (CD3+TCRγδ+) in either WT (black bars) or CD11a−/− mice (white bars). Representative zebra plots show differences between the WT and CD11a−/− thymus in regards to DN subsets using CD44 versus CD25 expression (B) or ETP and DN2 subsets using c-kit versus CD25 expression (C). Bar graphs show mean frequencies of indicated DN thymocyte subsets. Error bars represent SEM with 5 mice per group that ranged in age from 8–12 weeks. * p < 0.05. Data are representative of 3 individual experiments with 4–5 mice in each group.
CD11a is critical for lymphoid progenitor development in the BM
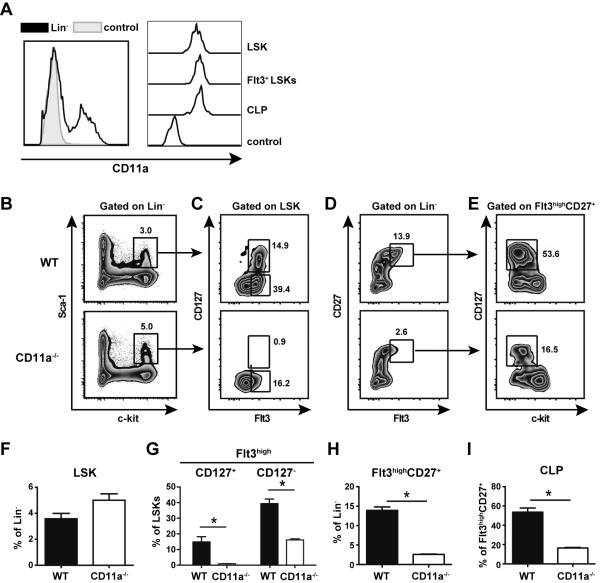
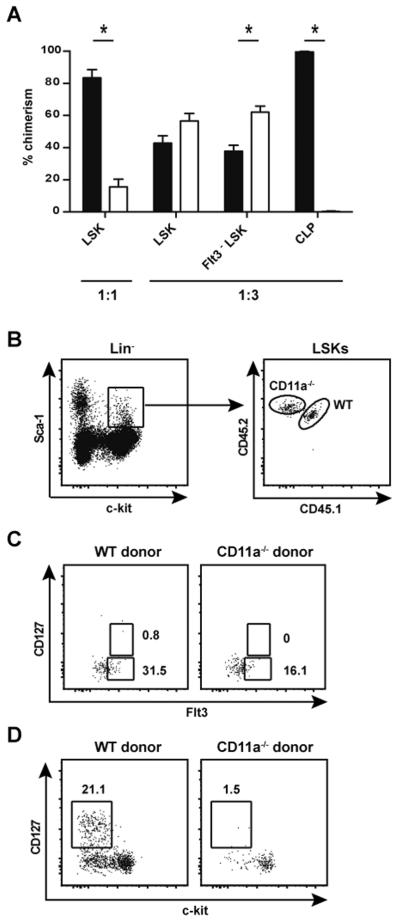
Given the defects observed in the ETP population, we compared lymphoid progenitor populations in the BM of WT and CD11a−/− mice. First, we examined CD11a expression on WT progenitors and observed that on average only ~40% of total Lin− cells expressed CD11a (Fig. 4A, left panel). However, the earliest BM progenitors (LSKs, Flt3+LSKs and CLPs) were found within the CD11a+ population, and their CD11a expression levels were comparable (Fig. 4A, right panel). CD11a−/− mice had normal frequencies of total Lin− BM cells (data not shown). There was a trend toward increased percentages of LSKs in CD11a−/− mice compared to WT mice, although this was not statistically significant (Fig. 4B, F, p=0.06). Interestingly, further analysis revealed a severe defect in the progression of LSKs to subsequent hematopoietic intermediates in the absence of CD11a. Within the LSK subset, expression of Flt3 identifies MPPs and LMPPs (4, 5, 9), and Flt3+LSKs were dramatically influenced by the absence of CD11a, such that both the CD127+ and CD127− fractions were significantly reduced in CD11a−/−mice (Fig. 4C, G). In addition, using a gating strategy previously reported by Saran and colleagues (33), we discovered an overall decrease in the Flt3highCD27+ population in CD11a−/− mice (Fig. 4D, H). Moreover, the development of CD127+ CLPs, a subset of the Flt3highCD27+ population was severely impaired in CD11a−/− mice (Fig. 4E, I). The frequency of CLPs can also be assessed using an alternative gating strategy whereby Lin−Sca-1loc-kitlo cells that also express CD127 are defined as CLPs (see Supplemental Fig. 1). Not only did we note a distinct reduction of Sca-1loc-kitlo cells in CD11a-deficient BM, but we also found a substantial reduction in the frequency of CD127+ cells (CLPs) within that gate. Thus, this second/alternative gating strategy indicated a significant reduction in the CLP population of CD11a−/− mice as compared to the WT control mice, consistent with the data shown in Fig. 4E, I. As both CLPs and LMPPs are believed to be able to enter the circulation and seed the thymus (34), the impaired development of these populations in the BM may explain the reduced number of ETP cells in the thymus of CD11a−/− mice.
Figure 4. CD11a−/− mice have normal frequencies of LSKs but severe depletion of downstream T cell progenitors in the BM.
(A) BM was isolated from femurs and tibias of adult WT B6 mice and assessed for the expression of CD11a in hematopoietic progenitor cells. Representative histograms show CD11a expression in total lineage negative (Lin−) cells compared to the FMO control of Lin− BM cells (left panel). CD11a expression on individual populations of lymphoid progenitors compared to the negative control is shown on the right. Gating strategy for progenitors was as follows: (LSK, Lin−Sca-1+c-kit+; Flt3highLSKs; CLP, Linc-kitlo Sca-1lo Flt3high CD127+). (B–E) BM was isolated from WT and CD11a−/− mice to compare frequencies of the T cell progenitor populations. Representative zebra plots show LSK (Lin−c-kit+Sca-1+) gating from Lin− cells (B); LSKs were further subdivided into CD127+Flt3+ and CD127−Flt3+ cells (C). Lin− BM cells that expressed Flt3 and CD27 (D) were subsequent gated to identify CLPs (Lin− Flt3+CD27+CD127+c-kitlo/−) (E). The frequencies of each population is shown in the bar graphs (F–I). * p < 0.05 Five animals per group were analyzed in three individual experiments.
Cell intrinsic CD11a expression is required for optimal T cell development in vitro
Next, we asked if the reduced development of T cells in CD11a−/− mice was the result of an intrinsic defect of CD11a-deficient progenitor cells to efficiently generate T cells. Thus, to compare the developmental potential of WT and CD11a−/− BM cells, we utilized the in vitro OP9-DL1 stromal cell co-culture system (35). Equal numbers of LSK BM cells were sorted from either WT or CD11a−/− mice and co-cultured with OP9-DL1 stromal cells for various times. On day 9 after seeding, substantially reduced numbers of Thy1.2+ T cells were generated from CD11a−/− BM cells as compared to WT (Fig. 5A, B). By day 12, total Thy1.2+ frequencies were still reduced in cultures seeded with CD11a−/− LSKs (Fig. 5B). Interestingly, at this time point we also observed an increased frequency of DN2 (Thy1.2+CD44+CD25+) cells in the CD11a-deficient progenitor cultures suggesting that perhaps in WT progenitors CD11a enhanced the developmental progression of thymocytes beyond the DN2 stage in vitro (Fig. 5C, D). However, by day 15 of culture, this difference was no longer present despite a continued reduction in the total percentage of Thy1.2+ cells in CD11a−/−-derived cells (Fig. 5B, D). Thus, these results indicated that CD11a expression by BM progenitors regulated T cell developmental potential, especially at the early stages.
Figure 5. CD11a−/− LSKs exhibit a reduced potential to generate T cells in vitro.
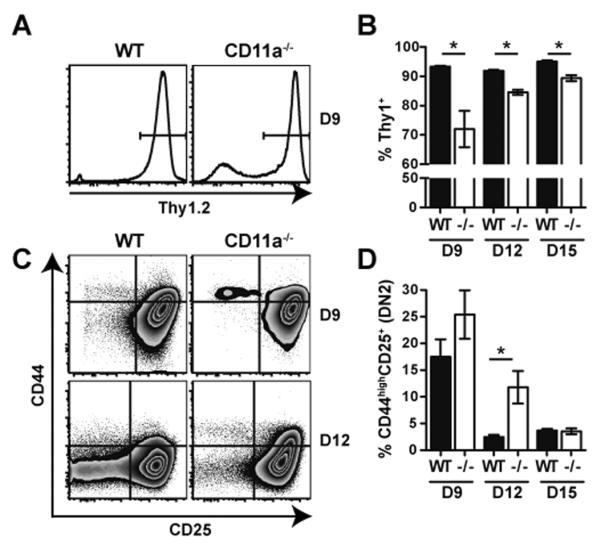
Equal numbers of LSKs sorted from WT and CD11a−/− BM were co-cultured with OP9-DL1 stromal cells for a total of 15 days to assess the generation of Thy1.2+Gr-1−T cells. (A) Representative histogram plot shows the gating of total Thy1.2+ population within cultures. (C) Representative zebra plots show differences in DN subsets using CD44 versus CD25 expression within the Thy1.2+ Gr-1− population after 9 or 12 days in vitro. Bar graphs in B and D depict the mean frequencies of the total Thy1.2+Gr-1− population and the CD44+CD25+ DN2 subset, respectively, over time. Data are representative of two individual experiments. * p < 0.05.
Competitive BM reconstitution reveals an obligate requirement for CD11a in lymphoid development
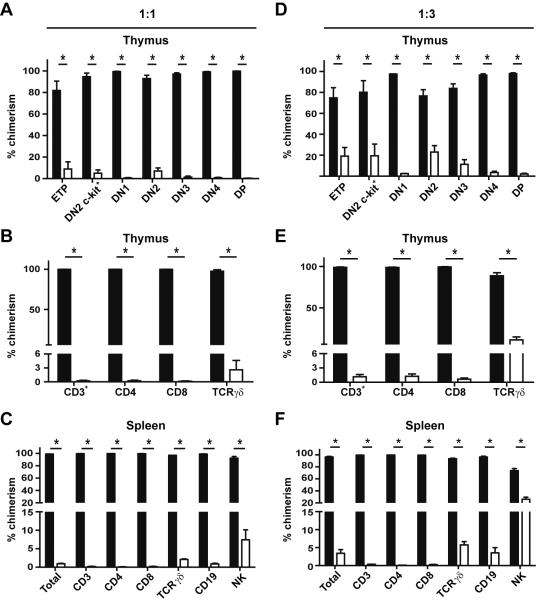
The results thus far suggested that cell intrinsic expression of CD11a was important for optimal T cell development. However, despite dramatic alterations in BM precursors in CD11a−/− mice, overall T cell development was only marginally affected. To test whether this finding was due to compensatory mechanisms available in the complete absence of CD11a, we generated mixed BM chimeric mice to determine the competitive fitness of CD11a−/− progenitors to give rise to mature T cells. We transplanted equal ratios (1:1) of unfractionated BM cells from WT and CD11a−/− donor mice into irradiated WT hosts, using congenic expression of CD45 to differentiate each donor population from the recipient. After allowing >12 weeks for full reconstitution to take place, chimerism was assessed in the spleen and thymus of recipient mice. Chimerism was determined by first gating on each cellular subset of interest and then further separating the cells based on congenic marker expression. Surprisingly, CD11a−/− BM was virtually unable to generate ETPs, mature thymocytes or mature peripheral T and B cells, while NK cell development was impaired to a lesser degree (Fig. 6A–C). Similar trends were observed in chimeric hosts that had been allowed to reconstitute for a longer period of time (24 weeks; data not shown).
Figure 6. BM cells from CD11a−/− donors are unable to reconstitute multiple lymphocytic subsets within the thymus and periphery of mixed chimeras.
Unfractionated BM cells from WT and CD11a−/− mice were mixed in either equal (1:1) ratios or at a ratio of 1:3 (WT:CD11a−/−) before transplantation into lethally irradiated WT hosts. Reconstitution was allowed to take place for 13–24 weeks before hosts were assessed for chimerism using CD45.1 and CD45.2 congenic markers in 1:1 chimeras (A–C) and 1:3 chimeras (D–F). (A, D) Thymocytes in chimeric hosts were assessed for the presence of either WT-derived cells (black bars) or CD11a−/−-derived cells (white bars). Lin− (CD3−TCRγδ−CD4−CD8−) cells were separated based on CD44 and CD25 expression to identify DN1–4 subsets or by c-kit and CD25 expression to identify ETPs and the c-kit+ DN2 subset. (B, E) Chimerism was also determined in total mature T cells (CD3+) and individual CD3+ T cell subsets (CD4, CD8, TCR γδ) in the thymus. (C, F) Bar graphs show the contribution of WT-derived cells (black bars) or CD11a−/− derived cells (white bars) to total lymphocytes (total), NK cells, CD19+ B cells, and various CD3+ T cell subsets within the spleens of both sets of chimeras. Data are representative of 4–5 mice per ratio. Three individual experiments were performed. * p <0.05.
To determine if it was possible to offset the defect observed in the 1:1 mixed chimeras, we generated mixed chimeras by transferring 3-fold more CD11a−/− BM cells than WT BM cells into irradiated WT recipients (1:3 ratio; Fig. 6D–F). Remarkably, the defects in the development of ETPs, as well as DP thymocytes, remained severe, although limited recovery of ETPs and DN subsets was noted (Fig. 6D). Mature subsets of thymocytes were also nearly absent while significant but impaired development of TCR γδ T cells occurred (Fig. 6E). T and B cell populations in the spleen were greatly reduced with some recovery noted for TCR γδ T cells and NK cells (Fig. 6F). Thus, when placed in a competitive environment with WT BM cells, CD11a−/− progenitors were effectively outcompeted indicating a critical role for CD11a in promoting lymphocyte development.
CD11a is essential for development of lymphoid progenitors
Lastly, we directly addressed our hypothesis that CD11a expression was intrinsically critical for the generation of developing hematopoietic intermediates within the BM compartment. To this end, we analyzed the BM from reconstituted mice receiving either a 1:1 or 1:3 ratio of WT and CD11a−/− progenitors. In contrast to the results in intact CD11a−/− mice (Fig. 4B, F), there was a marked deficiency in the contribution of CD11a−/− cells to the LSK compartment in 1:1 chimeric mice (Fig. 7A), indicating a requirement for CD11a in the generation or maintenance of this progenitor pool. Interestingly, the defect in LSK generation was overcome in the 1:3 mixed chimeras, where equal or greater numbers of LSKs and Flt3−LSKs (HSCs) of CD11a−/− origin were present as compared to their WT counterparts (Fig. 7A and 7B). Despite the presence of a normal LSK compartment, CLP development was essentially abrogated (Fig. 7D). This finding indicated a developmental block in the transition from the self-renewing Flt3−LSK into downstream progenitors that lack the capacity to self-renew. Of note, we were unable to detect a discernable population of Flt3highCD127+LSKs in the chimeric mice derived from either donor (Fig. 7C), suggesting that irradiation resulted in the loss of the niche required for development of this LMPP subset. This finding also implied that Flt3highCD127+LSKs were not essential for lymphocyte development since T and B cells developed normally from WT cells in the chimeras. A small, but identifiable population of Flt3+CD127− LSKs was derived from both CD11a-deficient and WT bone marrow donors, although the frequency of the cells was reduced ~2-fold in the absence of CD11a expression (Fig. 7C).
Figure 7. CD11a−/− LSKs are unable to generate CLPs in irradiated chimeric hosts.
Chimerism was assessed in the BM of the WT:CD11a−/− chimeras at 13–24 weeks after reconstitution. (A) Bar graphs show contribution of WT donors (black bars) or CD11a−/− donors (white bars) to the BM LSK population in either 1:1 or 1:3 chimeras. Flt3− LSKs and CLPs in 1:3 chimeras are also shown. (B) Representative dot plot shows gating and equal chimerism of the LSK population in 1:3 chimeras. (C) LSKs (as shown in B, right panel) were further subdivided to identify Flt3+CD127+ and Flt3+CD127− cells. Dot plots show the absence of the Flt3+CD127+ cells from both WT and CD11a−/− donors in 1:3 chimeras. (D) Lin−CD27+Flt3+ BM cells of 1:3 chimeras were gated as in Figure 4D and separated based on congenic markers to identify WT and CD11a−/− donor populations (not shown). Representative dot plot shows CD127+c-kitlo/− CLPs within each gated donor population. Five chimeras per ratio of donors were used in 2 individual experiments. * p < 0.05.
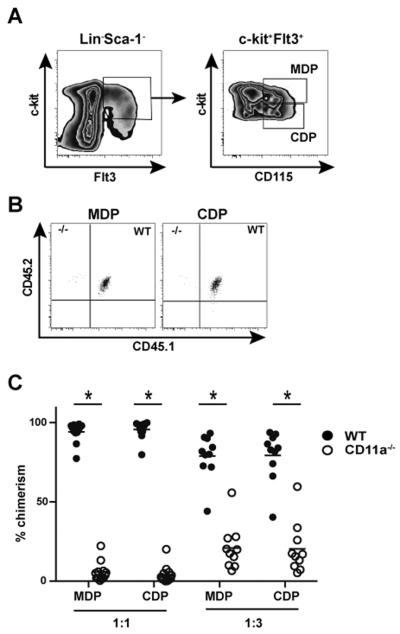
Given that innate myeloid cells also originate from HSCs in the BM, we wondered if hematopoietic intermediates required for myelopoiesis were similarly compromised in the absence of CD11a expression. Thus, we analyzed chimeric mice to examine macrophage and DC progenitor (MDP) and common DC precursor (CDP) populations using a previously illustrated gating strategy (36). Briefly, MDPs are identified based on the expression of Lin−cKithiCD115+ Flt3+ (37), while the CDPs, which are downstream of MDPs and can only give rise to cDCs and pDCs, have an identical phenotype except for lower overall expression of c-kit (i.e. c-kitlo) (Fig. 8A) (36, 38). Interestingly, MDPs and CDPs derived from CD11a−/− BM were significantly reduced in both the 1:1 and 1:3 chimeras (Figure 8B, C), suggesting an aberration in myeloid development downstream of LSKs in the absence of CD11a expression. Overall, these results suggested that in addition to acting as a crucial element of controlling lymphoid progenitor development, CD11a expression also contributed to myeloid progenitor development.
Figure 8. CD11a−/− BM exhibits defective generation of myeloid progenitors in chimeric mice.
In the mice described above, chimerism of myeloid progenitor populations was also determined in the BM of 1:1 and 1:3 chimeras. (A) Representative contour plots show the gating strategy for MDP and CDP identification. Briefly, c-kit+Flt3+ population of Lin−Sca-1− BM cells were further subdivided into c-kithiCD115+ MDPs and c-kitloCD115+ CDPs. (B) Representative dot plots show chimerism of gated MDPs (left panel) and CDPs (right panel) in 1:1 chimeras. The total chimerism of MDP and CDP populations in 1:1 and 1:3 chimeras is shown in C and pooled from 3 individual experiments with a total of 12 mice for 1:1 ratio chimeras and 10 mice for 1:3 ratio chimeras at 13–24 weeks after reconstitution. * p < 0.05.
Discussion
Earlier work suggested that CD11a plays a role in T lymphocyte development but the precise stage(s) of development in which CD11a participated was unclear. During the complex process of adult T cell lymphopoiesis, T cell progenitors from the BM enter the circulation and consequently home to the thymus for successful generation of mature T cells that can populate the periphery. Our results in intact CD11a−/− mice hinted at defects in T cell development based on alterations in ETPs and mature thymic and peripheral T cell subsets. Moreover, in intact CD11a−/− mice, while the overall LSK population in the BM was not affected, CD11a deficiency resulted in a significant decrease in the frequencies of downstream lymphoid progenitor subsets, the Flt3+LSKs (which includes the LMPP subset) and the CLPs. One explanation for this phenomenon is that CD11a expression is critical for proper localization of progenitors within specific BM niches. If this is the case, the absence of CD11a may result in poor survival due to an inability of the progenitors to localize to areas rich in cytokines that promote homeostasis. Alternatively, the absence of CD11a could potentially disrupt interactions between progenitors and BM stromal elements that are needed to drive progressive development. In the absence of these interactions, progenitors could be liberated into the circulation. Previously, others have provided evidence to support this notion using anti-β2 integrin treatment (39, 40). However, these studies showed that blocking of β2 integrins alone was not sufficient to induce mobilization into the blood and rather co-treatment with anti-α4 integrin was required to facilitate their release. Therefore, poor survival or defective generation of LMPPs and CLPs is a more likely scenario that could explain the reduced frequencies of these subsets in CD11a−/− mice. Regardless, limited availability of these two subsets likely leads to reduced numbers of TSPs in the thymus. Indeed, frequencies of ETPs were reduced in the thymus of CD11a−/− mice. However, the possibility remains that the absence of CD11a may also diminish the ability of TSPs to traverse through thymic endothelium when entering the thymus, and a role for β2 integrins in thymic settling of progenitors has previously been suggested (21). Nonetheless, our in vivo and in vitro data suggested that CD11a expression by BM progenitors plays a cell intrinsic role in driving T cell generation. However, we were unable to directly test an additional defect in thymic entry in CD11a−/− mice as two of the candidates of TSPs (LMPP and CLP) were both greatly reduced in the BM of CD11a−/− mice.
By generating mixed BM chimeras, we were able to highlight the requisite role that CD11a played in lymphocyte development. In this setting, CD11a-deficient BM progenitors were forced to compete with WT BM progenitors for niches and survival signals that are key for optimal lymphocyte generation. This competition resulted in the nearly complete inability of CD11a−/− precursors to produce either immature thymocytes or mature T cells and B cells. Although chimerism of TCR γδ T cells was not equivalent between CD11a−/− and WT cells, development of mature TCR γδ T cells appeared to be less dependent on CD11a expression as compared to TCR αβ T cells. Interestingly, NK cells, considered closely related to T cells and also generated from CLPs (10) were reduced but not completely absent in the chimeras. Thus, while most lymphocytes required CD11a during development, certain subsets were nevertheless able to partially overcome this requirement. The underlying defect in lymphocyte development in the BM chimeras was the loss of HSCs and BM lymphoid progenitors. In BM chimeras, and in contrast to intact CD11a−/− mice, CD11a−/− LSKs failed to equally contribute to the total LSK subset in the 1:1 chimeras. In addition, CLP did not develop from CD11a−/− progenitors. The production or maintenance of LSKs did not absolutely require CD11a however since in chimeras where three-fold greater numbers of CD11a−/− BM cells were mixed with WT BM cells and infused into irradiated recipients, CD11a−/− LSK development was slightly greater from CD11a−/− than WT BM. Importantly, even in this case, development of CLPs derived from CD11a−/− BM progenitors was essentially absent. Thus, CD11a was absolutely required for development of CLPs in the BM.
A recent study demonstrated that the absence of only one lymphoid precursor population fails to completely abolish T cell development, indicating that multiple precursor subsets can contribute to T cell development (33). Thus, in the case of CD11a−/− mice, although the frequencies of Flt3highLSKs and CLPs were reduced, both populations together could apparently provide a sufficient source of progenitors for T cell generation in the adult thymus. This could explain why only a mild defect in T cell populations was observed in the periphery of intact CD11a−/− mice. An interesting possibility to be considered in future work is that the requirements for CD11a during hematopoiesis and entry into the thymus during embryonic and neonatal life could be unique from our findings in adult animals. Acquisition of this knowledge, irrespective of whether differential requirements for CD11a adhesion exist, would contribute to advancing understanding of the fundamental mechanisms utilized during fetal and adult hematopoiesis and T cell development.
Of particular interest in the mixed BM chimeric mice was our finding that there was minimal, if any, development of the Flt3highCD127+LSK subset that includes LMPPs, even from WT BM. Although the reason for this phenomenon is not clear, irradiation has previously been reported to cause thymic stromal damage and alterations in the BM microenvironment (41–43). Perhaps such effects lead to the loss of the BM niche required for development of this lymphoid progenitor. In any case, this fortuitous finding indicated that the LMPP subset was not essential to lymphocyte development and was not an essential precursor to CLPs.
Collectively, we have shown that CD11a expression is required for T and B cell development, although compensatory mechanisms could partially overcome this requirement in the absence of competition from WT progenitors. The nearly complete dependence of CLP development on CD11a is unique among known regulators of hematopoietic development and our results provide new insight into this process. Future studies will seek to identify the cellular interactions mediated by CD11a that promote the generation of BM progenitors.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Daqi Xu for assistance in preparing this manuscript.
Abbreviations used in this article
- HSC
hematopoietic stem cell
- LMPP
lymphoid primed multipotent progenitor
- CLP
common lymphoid progenitor
- BM
bone marrow
- WT
wild type
- DN
double negative
- SP
single positive
- DP
double positive
- Lin−
Lineage negative
- LSK
Lineage− Sca-1+ c-kit+
- ETP
early thymic progenitor
- MPP
multipotent progenitor
- CDP
common dendritic cell progenitor
- MDP
macrophage and dendritic cell progenitor
- TSP
thymic settling progenitor
Footnotes
This work was supported by USPHS grants AI78289 and AI76457 (to L.L.), T32 AI07080 (to T.O.B.), and American Cancer Society Fellowship PF-11-152-01-LIB (to S.L.C.).
References
- 1.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 2.Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikuta K, Uchida N, Friedman J, Weissman IL. Lymphocyte development from stem cells. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- 4.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 5.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 7.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitnicka E, Brakebusch C, Martensson IL, Svensson M, Agace WW, Sigvardsson M, Buza-Vidas N, Bryder D, Cilio CM, Ahlenius H, Maraskovsky E, Peschon JJ, Jacobsen SE. Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. J Exp Med. 2003;198:1495–1506. doi: 10.1084/jem.20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 11.Balciunaite G, Ceredig R, Massa S, Rolink AG. A B220+ CD117+ CD19-hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35:2019–2030. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 12.Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, Plevritis SK, Dill DL, Weissman IL. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrie HT. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol. 2003;3:859–866. doi: 10.1038/nri1223. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 15.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 16.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, Lipp M, Kondo S, Manley N, Takahama Y. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 18.Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 20.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 23.St-Pierre Y, Hugo P, Legault D, Tremblay P, Potworowski EF. Modulation of integrin-mediated intercellular adhesion during the interaction of thymocytes with stromal cells expressing VLA-4 and LFA-1 ligands. Eur J Immunol. 1996;26:2050–2055. doi: 10.1002/eji.1830260913. [DOI] [PubMed] [Google Scholar]
- 24.Fine JS, Kruisbeek AM. The role of LFA-1/ICAM-1 interactions during murine T lymphocyte development. J Immunol. 1991;147:2852–2859. [PubMed] [Google Scholar]
- 25.Wada K, Kina T, Kawamoto H, Kondo M, Katsura Y. Requirement of cell interactions through adhesion molecules in the early phase of T cell development. Cell Immunol. 1996;170:11–19. doi: 10.1006/cimm.1996.0128. [DOI] [PubMed] [Google Scholar]
- 26.Revilla C, Gonzalez AL, Conde C, Lopez-Hoyos M, Merino J. Treatment with anti-LFA-1 alpha monoclonal antibody selectively interferes with the maturation of CD4− 8+ thymocytes. Immunology. 1997;90:550–556. doi: 10.1046/j.1365-2567.1997.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda Y, Katagiri K, Tomiyama T, Yasuda K, Habiro K, Katakai T, Ikehara S, Matsumoto M, Kinashi T. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat Commun. 2012;3:1098. doi: 10.1038/ncomms2105. [DOI] [PubMed] [Google Scholar]
- 28.Schmits R, Kundig TM, Baker DM, Shumaker G, Simard JJ, Duncan G, Wakeham A, Shahinian A, van der Heiden A, Bachmann MF, Ohashi PS, Mak TW, Hickstein DD. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shier P, Otulakowski G, Ngo K, Panakos J, Chourmouzis E, Christjansen L, Lau CY, Fung-Leung WP. Impaired immune responses toward alloantigens and tumor cells but normal thymic selection in mice deficient in the beta2 integrin leukocyte function-associated antigen-1. J Immunol. 1996;157:5375–5386. [PubMed] [Google Scholar]
- 30.Holmes R, Zuniga-Pflucker JC. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb Protoc. 2009;2009 doi: 10.1101/pdb.prot5156. pdb prot5156. [DOI] [PubMed] [Google Scholar]
- 31.Bose TO, Pham QM, Jellison ER, Mouries J, Ballantyne CM, Lefrancois L. CD11a Regulates Effector CD8 T Cell Differentiation and Central Memory Development in Response to Infection with Listeria monocytogenes. Infect Immun. 2013;81:1140–1151. doi: 10.1128/IAI.00749-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuzaki Y, Gyotoku J, Ogawa M, Nishikawa S, Katsura Y, Gachelin G, Nakauchi H. Characterization of c-kit positive intrathymic stem cells that are restricted to lymphoid differentiation. J Exp Med. 1993;178:1283–1292. doi: 10.1084/jem.178.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saran N, Lyszkiewicz M, Pommerencke J, Witzlau K, Vakilzadeh R, Ballmaier M, von Boehmer H, Krueger A. Multiple extrathymic precursors contribute to T-cell development with different kinetics. Blood. 2010;115:1137–1144. doi: 10.1182/blood-2009-07-230821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlotoff DA, Bhandoola A. Hematopoietic progenitor migration to the adult thymus. Ann N Y Acad Sci. 2011;1217:122–138. doi: 10.1111/j.1749-6632.2010.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 38.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 39.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM, Harlan JM. Synergistic mobilization of hemopoietic progenitor cells using concurrent beta1 and beta2 integrin blockade or beta2-deficient mice. Blood. 2001;97:1282–1288. doi: 10.1182/blood.v97.5.1282. [DOI] [PubMed] [Google Scholar]
- 40.Velders GA, Pruijt JF, Verzaal P, van Os R, van Kooyk Y, Figdor CG, de Kruijf EJ, Willemze R, Fibbe WE. Enhancement of G-CSF-induced stem cell mobilization by antibodies against the beta 2 integrins LFA-1 and Mac-1. Blood. 2002;100:327–333. doi: 10.1182/blood.v100.1.327. [DOI] [PubMed] [Google Scholar]
- 41.Han W, Yu Y, Liu XY. Local signals in stem cell-based bone marrow regeneration. Cell Res. 2006;16:189–195. doi: 10.1038/sj.cr.7310026. [DOI] [PubMed] [Google Scholar]
- 42.Li XM, Hu Z, Jorgenson ML, Wingard JR, Slayton WB. Bone marrow sinusoidal endothelial cells undergo nonapoptotic cell death and are replaced by proliferating sinusoidal cells in situ to maintain the vascular niche following lethal irradiation. Exp Hematol. 2008;36:1143–1156. doi: 10.1016/j.exphem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Zlotoff DA, Zhang SL, De Obaldia ME, Hess PR, Todd SP, Logan TD, Bhandoola A. Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation. Blood. 2011;118:1962–1970. doi: 10.1182/blood-2010-12-324954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.