Abstract
The major burden of influenza morbidity resides within the elderly population. The challenge managing influenza-associated illness in the elderly is the decline of immune function, where mechanisms leading to immunological senescence have not been elucidated. To better represent the immune environment, we investigated clinical morbidity and immune function during sequential homologous and heterologous H1N1 influenza infection in an aged ferret model. Our findings demonstrated experimentally that aged ferrets had significant morbidity during monosubtypic heterologous 2° challenge with significant weight loss and respiratory symptoms. Furthermore, increased clinical morbidity was associated with slower and shorter hemagglutinin antibody generation and attenuated type 1 T-cell gene responses in peripheral blood. These results revealed dampened immune activation during sequential influenza infection in aged ferrets. With the presence of an aged model, dissecting clinical morbidity, viral dynamics and immune response during influenza infection will aid the development of future prophylactics such as age specific influenza vaccines.
Keywords: Influenza A H1N1, Pandemic influenza, Influenza, Heterologous Immunity, Aging, Immunological Senescence, Ferret Model, Humoral Response, T-Cells
Introduction
The elderly influenza disease rate in humans carries a heavy burden on healthcare systems and perpetuates virus circulation in the general population. During the 2012-2013 season, persons aged ≥ 65 years accounted for ∼50% of all influenza-related hospitalizations in the US (Centers for Disease Control and Prevention (CDC), 2013). Since the host immune system deteriorates significantly during aging, the inability to generate effective, broad-spectrum immune memory following infection or vaccination contributes to increased influenza burden among the elderly (Bridges et al., 2000; Castle, 2000; Dao et al., 2010; Thompson et al., 2003). Aging influences various facets of the immune response, but T-cell populations are prominently affected due to thymus involution limiting naïve T-cell production (Aw and Palmer, 2011; Buchholz et al., 2011; Castle, 2000). Post-thymic homeostatic T-cell proliferation compensates for production deficit (Aw and Palmer, 2011; Buchholz et al., 2011), but long-term T-cell replication leads to cell-intrinsic dysfunction highlighted by progressive loss of repertoire diversity and weakened responses (Buchholz et al., 2011). This directly influences cell-mediated T-cell responses, most evident during viral infection (Buchholz et al., 2011; Deng et al., 2004; Effros et al., 2003), while indirectly affecting humoral responses (Eaton et al., 2004). Together with high rates of antigenic change of circulating virus population, this process puts the elderly at greater risk of recurring influenza infection (Bridges et al., 2000).
Age-related immune dysregulation modestly impacts disease severity in animal models of primary influenza infection (Guo et al., 2012; Josset et al., 2012; Muto et al., 2012; Pica et al., 2012). Heterosubtypic influenza A immune memory is severely impaired in aged animals (Bender and Small, 1993; Decman et al., 2010), although the elderly's sensitivity to monosubtypic antigenic change is unclear. To address this question, we investigated clinical morbidity, viral dynamics, and subsequent immune responses to sequential influenza A H1N1 infection for the first time in an aged ferret model. Here we report dampened immunity in aged ferrets upon monosubtypic heterologous 2° challenge associated with diminished antibody production and altered T-cell responses in peripheral blood. These findings help elucidate the immune dynamics which contribute to elderly influenza susceptibility and put forth the aged ferret model for further study of aging immunity and influenza.
Results
Aged ferrets develop more severe disease than adults during heterologous monosubtypic 2° challenge
Ferrets closely mimic the clinical manifestations of influenza infection in humans as shown previously (Banner and Kelvin, 2012; Belser et al., 2011; Huang et al., 2011; Huang et al., 2013; Rowe et al., 2010). Naïve adult (4-6 months old) and aged (≥ 4 years old) male ferrets were placed into groups for either homologous or heterologous H1N1 sequential infection studies. The homologous sequentially infected group was first infected intranasally with pandemic 2009 H1N1 strain A/Mexico/4108/2009 (Mex/4108) as the 1° infection after 46 days the animals were then infected with pandemic 2009 H1N1 A/California/07/2009 (Cal/07) as 2° challenge. The heterologous 1° infection - 2° challenge group was infected with seasonal H1N1 A/Brisbane/59/2007 (Bris/59) and then subsequently infected with Mex/4108 Day 39 post 1° infection. Animals were intranasally infected with the indicated virus strain at 106 EID50 and clinical signs were monitored for 14 days post-infection/challenge (temperature, weight change, nasal discharge (clear discharge or color dry mucus / exudate), sneezing, and lethargy).
During both 1° strain infections aged animals exhibited clinical morbidity that was modestly more pronounced than in adults, with greater weight loss (7-8% peak loss) and more frequent production of mucus/exudate. Sneezing was also observed more frequently in our aged cohorts, but sneezing incidence rates in aged ferrets (50%) were consistent with previously reported rates in adults during sH1N1 or H1N1pdm infection (Huang et al., 2011). Upon homologous 2° challenge with Cal/07, neither age group exhibited clinical symptoms except for sporadic detection of clear nasal discharge and sneezing (Figure 1A). In contrast, during heterologous 2° challenge with Mex/4108, adult ferrets showed mild clinical morbidity whereas the aged animals developed greater illness with >6% peak weight loss, sneezing, prominent nasal discharge (yellow-brown color exudate or dry mucus), and lethargy.
Figure 1. Aged ferrets exhibited increased clinical morbidity during heterologous 2° challenge.

Clinical sign monitoring of aged (> 4 years old) and adult (4-6 months old) ferrets during homologous [1°: A/Mexico/4108/2009 (H1N1); 2°: A/California/07/2009 (H1N1)] (A) or heterologous [1°: A/Brisbane/59/2007 (H1N1); 2°: A/Mexico/4108/2009 (H1N1)] monosubtypic sequential infections (B). All infections at 106 EID50. Temperature and weight were recorded daily following 1° infection until day 5 post-infection (pi), then at days 7, 10, and 14 pi. Temperature and weight were recorded daily after 2° challenge for 14 days. Both are reported as a percentage relative to the average preinfection level calculated from days 0 and −1. Animals were also evaluated daily for nasal discharge, sneezing, and inactivity level, and the highest percentages (and fractions [number of ferrets displaying symptoms/total number of ferrets tested]) of infected ferrets displaying symptoms are shown. The physical inactivity index measures the degree to which ferrets respond to environmental stimuli, with the basal level being 1.000. Viral titres were measured in nasal washes collected from aged and adult ferrets at days 3 and 7 after 1° infection and 2° challenge and determined by titration on MDCK cells and reported as TCID50/mL (C).
Having detected disease differences between aged and adult ferrets during sequential influenza infection, we next examined if morbidity was associated with viral burden in nasal washes. Live virus titres were measured at Day 3 and 7. No significant differences in viral burden/clearance were detected between the age groups at any time-point tested. Virus was undetectable by Day 3 following homologous 2° challenge in both age groups. Mex/4108 virus titres were dramatically reduced at Day 3 post-heterologous 2° challenge when compared to 1° infection (∼ 100-fold), consistent with previous reports (Fang et al., 2012) (Figure 1C). Interestingly, aged animals still developed severe clinical morbidity during heterologous 2° challenge (Figure 1B) despite reduced viral titres.
Antibody production in aged ferrets is delayed and not sustained at levels equivalent to adults
The increased disease severity in aged ferrets during heterologous 2° challenge prompted us to investigate possible causes of the disease disparity between aged and adult animals. Roles for humoral (Fang et al., 2012) and cell-mediated immunity (Guo et al., 2011; Tu et al., 2010) have been identified in heterologous influenza A H1N1 rechallenge models. First we investigated humoral responses in our cohort by assaying sera taken from the ferrets at designated time points for haemagglutination inhibition (HI) against Bris/59 or Mex/4108 viruses. Aged ferrets failed to maintain antibody titres at the same levels as adults following either 1° Bris/59 or Mex/4108 infection. By Day 28 post Mex/4108 1° infection, aged ferrets had significantly reduced haemagglutinin antibody levels compared to adults which remained significantly lower through the 2° infection with Cal/07 (Figure 2A). Aged ferrets were also slower to mount an initial humoral response to the Bris/59 virus during 1° infection, as adults had generated antibodies toward Bris/59 by Day 7 post infection whereas aged animals had undetectable levels (Figure 2B). Similar antibody responses were seen between adult and aged ferrets during the 2° challenge with the Mex/4108 virus. Together, these results show that aged ferrets respond differently (slower and less) compared to adult ferrets in respect to protective antibody generation which may be dependent on the virus strain and the sequence of insult.
Figure 2. Antibody production in aged ferrets was delayed and not maintained at adult levels.
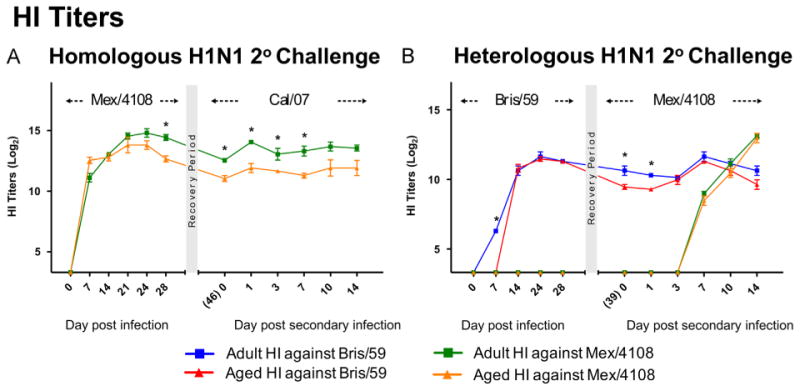
Antibody titres against live A/Mexico/4108/2009 (H1N1) and A/Brisbane/59/2007 (H1N1) viruses were determined by HI assay for aged and adult ferret sera collected at the indicateddays post-1° infection and 2° challenge,. Error bars represent standard errors of the means. *: p < 0.05 by Student's t test comparing infected adult and aged ferrets.
Peripheral type 1 T-cell gene responses to heterologous monosubtypic 2° challenge are attenuated in aged ferrets
We next investigated other peripheral immune responses to complement the humoral immunity evaluation. Host gene expression analysis for the molecular dissection of circulating immune cell regulation (Figure 3) was performed on in-life peripheral blood samples taken from animals throughout the infection time course. In our analysis we included gene sets for cell mediated immunity, inflammatory cytokines and T cell regulation. Strikingly, we detected rapid increases in CD4 and CD8 mRNA expression during heterologous 2° challenge in adult but not aged ferrets, with peak differences in CD4 (2-fold) and CD8 (3-fold) expression detected at Day 1 post- 2° challenge (p2°) (Figure 3B i, ii). A similar expression profile was detected for CD28 (T-cell activation cell surface marker) with significantly higher expression in adults. (Figure 3B iii). Together, these profiles suggested rapid mobilization of adult T-cells to peripheral blood during heterologous challenge which was diminished in the aged. Moreover, we also detected a trend of reduced CD19 (B-lymphocyte maker) expression in aged ferrets (Figure 3B iv), consistent with impaired humoral responses as suggested by our HI data. In contrast, similar innate response gene profiles (CXCL8, CXCL10, TLR3) were detected in both groups with mostly no changes in gene regulation throughout the time course except for acute upregulation of CXCL8 and CXCL10 during heterologous 2° challenge at Day 1 p2° (Figure 3 v-vii) (2 fold and 10-20 fold increase, respectively).
Figure 3. Type 1 T-cell gene responses detected in peripheral blood of adult but not aged ferrets upon heterologous 2° challenge.

Gene expression analysis from peripheral blood total RNA collected from aged and adult ferrets at days 0, 3, 7, and 14 during 1° infection and days 0, 1, 3, 7 during 2° challenge. Expression levels of CD4 (i), CD8 (ii), CD28 (iii), CD19 (iv), CXCL8 (v), CXCL10 (vi), TLR3 (vii), TBX21 (viii), GZMA (ix), TNFα (x), IFNγ (xi), and CD44 (xii) mRNA were determined by qRT-PCR, normalized to housekeeping gene β-Actin and expressed as fold change difference in expression relative to Day 0 adult basal levels. Three samples from each time point were collected per group. Error bars represent standard errors of the means. *: p < 0.05 by Student's t test comparing infected adult and aged ferrets.
Given the role of type 1 T-cell responses in heterologous immunity (Guo et al., 2011; Tu et al., 2010), we further investigated circulating T-cell population effector status by measuring TBX21 (transcription factor expressed in Th1-commited CD4+ T-cells and CD8+ T-cells) (Sullivan et al., 2003; Szabo et al., 2000), GZMA (cytotoxic T-cell effector molecule) (Anthony et al., 2010), as well as IFNγ and TNFα (type 1 cytokines) (Grivennikov et al., 2005; Xu et al., 2004) levels (Figure 3 viii-xi). As above, TBX21 and TNFα mRNA expression declined early (Day 1 p2°) during heterologous 2° challenge in aged ferrets while remaining stable in adult ferrets (TBX21: 2 fold) (TNFα: 2 fold) (Figure 3B viii, x). Furthermore, peak GZMA mRNA expression was reduced in aged ferrets (Figure 3 ix). Together, these findings suggest a potential age-related decrease in type 1 T-cell responses specific to heterologous 2° challenge which may have contributed to disease (Bender and Small, 1993; Decman et al., 2010).
Discussion
Our study revealed increased disease severity in aged ferrets during sequential heterologous H1N1 influenza infection which was associated with altered aged T-cell responses and antibody production (Figure 4). Our data suggested modulation of T-cell function during vaccination as a potential target for improving elderly immune memory against influenza, and recommends the aged ferret influenza model for the development of immunomodulatory influenza therapeutics and vaccines. Moreover, antibody titres in aged ferrets were not sustained at the same levels observed in adults which may also be a consequence of dampened T cell responses and should be further explored. The elderly have the highest influenza-related hospitalization rates placing stress on healthcare systems and increasing virus exposure to other members of the community such as hospital works and other hospitalized patients. Better management of influenza infection in the older population is urgently needed. Boosting elderly T-cell responses may be a valuable therapeutic target for broadening of immune memory.
Figure 4. Elderly/aged ferrets (> 4 years) had diminished peripheral T cell responses, decreased haemagglutinin antibody production, and significant weight loss following seasonal H1N1 primary-pandemic 2009 H1N1 heterologous 2° challenge.

Adult (4-6 months) and aged (> 4 years) ferrets were infected with seasonal H1N1 (sH1N1) influenza virus (A/Brisbane/59/2007) then 2°-challenged with pandemic 2009 H1N1 virus (H1N1pdm) (A/Mexico/4108/2009) 4 – 5 weeks following 1°-infection. Animals were observed for a 14 period following secondary infection where body weight, temperature, and clinical symptoms were observed and blood was sampled for immune correlate (RNA) and haemagglutinin antibody profiling. Aged animals had significantly more weight loss during both 1°-infection and 2°-challenge, and the aged animals were unable to recover to original body weight following H1N1pdm challenge. Upper respiratory tract clinical symptoms were more severe in aged ferrets during both 1°-infection and 2°-challenge and aged had increased inactivity during challenge with 2° H1N1pdm. During H1N1pdm 2°-challenge, aged animals had an associated decrease in peripheral T cell markers (CD4, CD8 and CD28). As well, a marked lag in haemagglutinin antibody production was observed in the aged animals following sH1N1 1°-infection, as antibodies were not detected until Day 14 following infection compared to Day 7 in the adults. These antibody levels were also decreased significantly in the aged ferrets compared to the adults over long term.
The analysis of clinical symptoms in aged versus adult ferrets were highly insightful and recapitulated the clinical disease course observed in elderly humans as well as other animal studies. Here we found that aged ferrets did not recover to their original weight following sequential heterologous H1N1 influenza infection (Figure 4), a finding consistent with observations of long-term disability in the elderly following repeated influenza exposure (McElhaney, 2005). Clinical signs in our aged ferret model were also in agreement with previous animal studies where the disease severity of aged animal was modestly increased during 1° infection (Guo et al., 2012; Josset et al., 2012; Muto et al., 2012; Pica et al., 2012) but importantly, heterologous monosubtypic 1° infection – 2° challenge caused severe morbidity in aged ferrets similar to previous rechallenge mouse studies (Bender and Small, 1993; Decman et al., 2010). Our work together with previously published studies has begun to suggest a profile of the aged clinical following subsequent influenza infection.
Along with a more severe clinical profile, aged ferrets had an attenuated peripheral humoral and T cell immune response compared to adults and previously modeled immune responses following influenza infection (Cheng et al., 2013; Huang et al., 2011; Jang et al., 2013; Kelvin et al., 2014; O'Donnell et al., 2012; Paquette et al., 2014). We found aged ferrets to have a significant decrease in sustained HA antibody production following 1° and continued into secondary 2° pandemic H1N1 infection which may indicate a factor in the increased susceptibility to sequential influenza infections in the elderly. Also, aged ferrets were markedly slower to mount an initial humoral response to the Bris/59 virus during 1° infection where antibody levels remained undetected 7 days following infection which may play a role in prolonged disease in our aged ferrets. Conversely, we found that the 1° Mex/4108 infection did not show differential HA antibody generation. It is possible that higher viral titres detected during Mex/4108 versus Bris/59 1° infection (∼100-fold at Day 3) may have masked humoral deficiencies in the aged cohort. Alternatively, these disparate data sets may indicate influenza strain-specific immune responses in aged ferrets which could be further investigated. A protective role for cross-reactive antibodies has been identified during sequential influenza A H1N1 infection (Fang et al., 2012) and it is possible in aged animals that lower antibody titres may have contributed to the increased disease severity observed during heterologous 2° challenge.
Interestingly, peripheral T cell responses in the aged ferrets also appeared to be dampened as determined by real-time RT-PCR. Specifically, CD4, CD8, and CD28 were significantly more robust in the adults compared to the aged during 1° Bris/59 – 2° Mex/4108 infection combination. Previous reports in humans and other mammals have suggested T-cell responses are impaired in the elderly (Aw and Palmer, 2011; Buchholz et al., 2011). Similarly, we detected attenuated type 1 T-cell gene responses in aged ferret peripheral blood which may indicate intrinsic differences in the aged ferret T-cell population. Reduced antibody titres in aged ferrets may also have been a consequence of impaired T-cell function (Eaton et al., 2004; Haynes et al., 2005). Together, these observations suggest altered T-cell populations and/or activity in aged ferrets may have limited the breadth of immune memory following 1° infection, recommending additional investigation of the aged ferret T-cell population. Reduced T-cell proliferation and interleukin-2 production in response to antigen have been linked to immune decline in aged mice (Decman et al., 2010; Deng et al., 2004; Haynes et al., 2005) and these should be investigated further in aged ferrets. Aging has also been associated with declines in T-cell repertoire diversity (Buchholz et al., 2011; Naylor et al., 2005; Yager et al., 2008) which may be attributable in part to reduced naïve T-cell production (Aw and Palmer, 2011; Buchholz et al., 2011; Castle, 2000) and gradual deletion of low-avidity T-cell clones as peripheral T-cell expansion is increasingly biased towards high-avidity clones with age (Rudd et al., 2011). Loss of clonal diversity in aged mice has been shown to impair heterosubtypic immunity (Yager et al., 2008) and may have similarly contributed to the weaker memory responses detected in aged ferrets during heterologous 2° infection. Accordingly, changes in the T-cell repertoire during ferret aging should also be the focus of future work. Meanwhile, our data provides indication that boosting T-cell responses to antigen during vaccination of the elderly may help limit susceptibility to severe influenza infection. Studies in human cells ex vivo and in mice have shown that increasing inflammatory cytokine production by antigen presenting cells improves aged T-cell responses to antigen (Behzad et al., 2012; Jones et al., 2010). Accordingly, the aged ferret model described here may serve as a valuable tool for the future development of such immune boosting therapies. Further study of aged T-cell function during sequential influenza infection in ferrets may also reveal more direct therapeutic targets.
Influenza disease rates are increased among the elderly due in part to a failure in generating broad, long-lasting immunity following influenza exposure (Bridges et al., 2000; Castle, 2000; Centers for Disease Control and Prevention (CDC), 2013; Dao et al., 2010; Thompson et al., 2003). New approaches to improve elderly immune responses and immune memory are needed, yet aged animal models for the study of influenza infection and immunity are limited (Bender and Small, 1993). Our data puts forth the aged ferret influenza model and showed that aged ferrets failed to mount an equivalent immune response as adults to monosubtypic heterologous 2° challenge which was associated with altered peripheral T-cell responses, decreased antibody production, and increased morbidity.
Materials and Methods
Ethics Statement
Animal work was performed in strict accordance with the Canadian Council of Animal Care (CCAC) guidelines. The animal use protocol was approved by the Animal Care Committee (ACC) of the University Health Network (UHN) where UHN has certification with the Animals for Research Act (Permit Number: #0045 and #0085 of the Ontario Ministry of Agriculture, Food and Rural Affairs) and follows NIH guidelines (OLAW #A5408-01). Infections and subsequent sample collection were performed under 5% isofluorane anesthesia in an effort to minimize suffering.
Animal Infections and Clinical Monitoring
Ferrets were bred and housed in an on-site specific-pathogen-free facility (UHN, Toronto, ON, Canada). Adult ferrets were defined as 4-6 months of age, while aged ferrets were defined as > 4 years of age (all male). All ferrets were tested for the presence of antibodies against circulating influenza A and B virus strains by HI and shown to be seronegative prior to infection. Infection experiments were conducted with H1N1pdm strains A/Mexico/4108/2009 (H1N1) and A/California/07/2009 (H1N1) or sH1N1 strain A/Brisbane/59/2007 (H1N1). All viruses were provided by the Centers for Disease Control and Prevention ([CDC], Atlanta, GA, USA). Viral stocks were propagated in embryonated eggs for no more than one passage, stored in liquid nitrogen, and thawed prior to use. All 1° infections and 2° challenges were performed at 106 EID50. Infections and daily clinical sign monitoring were performed as previously described (Huang et al., 2012).
Viral and Humoral Kinetics
Nasal washes were collected at days 3 and 7 post-infection/challenge and viral titres were determined by titration over Madin-Darby Canine Kidney (MDCK) cells and TCID50/mL calculation using the Reed-Muench method (Huang et al., 2012). In-life bleeds were performed at designated time-points for isolation of sera and determination of antibody titres against Bris/59 and Mex/4108 haemaggultinin by HI assay, as previously described (Huang et al., 2012).
Peripheral Blood Gene Expression Analysis
In-life bleeds were performed at designated time-points for isolation of peripheral blood total cellular RNA. Blood was collected in PAXgene Blood RNA tubes and stored at -80°C until extraction by PAXgene Blood RNA Kit (PreAnalytiX, Hombrechtikon, Switzerland), as per manufacturer's instructions. Purified RNA was reverse transcribed using ImProm-II Reverse Transcription System (Promega, Madison, WI, USA) and host gene expression was investigated by qRT-PCR using ABI-Prism 7900HT Sequence Detection Systems (Applied Biosystems, Foster City, CA, USA). Each sample was run in quadruplicate at 5 ng cDNA / reaction well. Host gene expression was normalized to β-actin, and quantified by the relative standard curve method. Primer sequences listed in Table S1.
Statistical Methods
The Student's t-test (α=0.05) was used to ascertain significance for two group comparisons, assuming two-tailed distributions and unequal variances.
Supplementary Material
Research Highlights.
The major burden of influenza morbidity resides within the elderly
We developed an aged ferret model to study influenza and the aged immune system
We found sequential H1N1 infection caused significant morbidity in aged ferrets
Aged ferrets had reduced HA antibody generation and type 1 T-cell responses
The aged ferret model will aid the development of age specific therapeutics
Acknowledgments
This study was supported by Li Ka-Shing Foundation of Canada, Immune Diagnostics & Research, Shantou University Medical College and NIH 1U01AI11598-01 Subaward no. 0038591(123721-3) for financial support in conducting this study. We would also like to thank the UHN Animal Resources Centre (ARC) staff for help with animal care and aging of the animals. All influenza strains (A/California/07/2009 (H1N1), A/Mexico/4108/2009 (H1N1), and A/Brisbane/59/2007 (H1N1)) were obtained through the Influenza Reagent Resource, Influenza Division, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza, CDC, Atlanta, GA, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony DA, Andrews DM, Watt SV, Trapani JA, Smyth MJ. Functional dissection of the granzyme family: cell death and inflammation. Immunological Reviews. 2010;235:73–92. doi: 10.1111/j.0105-2896.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- Aw D, Palmer DB. The origin and implication of thymic involution. Aging and Disease. 2011;2:437–443. [PMC free article] [PubMed] [Google Scholar]
- Banner D, Kelvin AA. The current state of H5N1 vaccines and the use of the ferret model for influenza therapeutic and prophylactic development. Journal of Infection in Developing Countries. 2012;6:465–469. doi: 10.3855/jidc.2666. [DOI] [PubMed] [Google Scholar]
- Behzad H, Huckriede AL, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. Journal of Infectious Diseases. 2012;205:466–473. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Katz JM, Tumpey TM. The ferret as a model organism to study influenza A virus infection. Disease Models & Mechanisms. 2011;4:575–579. doi: 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender BS, Small PAS. Heterotypic immune mice lose protection against influenza virus infection with senescence. Journal of Infectious Diseases. 1993;168:873–880. doi: 10.1093/infdis/168.4.873. [DOI] [PubMed] [Google Scholar]
- Bridges C, Winquist A, Fukuda K, Cox NJ, Singleton J, Strikas R. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and Reports. 2000;49:1–38. [PubMed] [Google Scholar]
- Buchholz VR, Neuenhahn M, Busch DH. CD8+ T cell differentiation in the aging immune system: until the last clone standing. Current Opinion in Immunology. 2011;23:549–554. doi: 10.1016/j.coi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Castle SC. Clinical relevance of age-related immune dysfunction. Clinical Infectious Diseases. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention (CDC) Influenza activity - United States, 2012-13 season and composition of the 2013-14 influenza vaccine. MMWR Morbidity and Mortality Weekly Report. 2013;62:473–479. [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Zengel JR, Suguitan AL, Xu Q, Wang W, Lin J, Jin H. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. Journal of Infectious Diseases. 2013;208:594–602. doi: 10.1093/infdis/jit207. [DOI] [PubMed] [Google Scholar]
- Dao CN, Kamimoto L, Nowell M, Reingold A, Gershman K, Meek J, Arnold KE, Farley M, Ryan P, Lynfield R. Adult hospitalizations for laboratory-positive influenza during the 2005-2006 through 2007-2008 seasons in the United States. Journal of Infectious Diseases. 2010;202:881–888. doi: 10.1086/655904. [DOI] [PubMed] [Google Scholar]
- Decman V, Laidlaw BJ, DiMenna LJ, Abdulla S, Mozdzanowska K, Erikson J, Ertl HC, Wherry EJ. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. Journal of Immunology. 2010;184:5151–5159. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. Journal of Immunology. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. The Journal of Experimental Medicine. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Critical Reviews in Immunology. 2003;23:45–64. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- Fang Y, Banner D, Kelvin AA, Huang SS, Paige CJ, Corfe SA, Kane KP, Bleackley RC, Rowe T, Leon AJ, Kelvin DJ. Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. Journal of Virology. 2012;86:2229–2238. doi: 10.1128/JVI.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Förster I, Clausen BE. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. Journal of Virology. 2011;85:448–455. doi: 10.1128/JVI.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Feng Y, Barnes P, Huang FF, Idell S, Su DM, Shams H. Deletion of FoxN1 in the thymic medullary epithelium reduces peripheral T cell responses to infection and mimics changes of aging. PLoS One. 2012;7:e34681. doi: 10.1371/journal.pone.0034681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. The Journal of Experimental Medicine. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Banner D, Degousee N, Leon AJ, Xu L, Paquette SG, Kanagasabai T, Fang Y, Rubino S, Rubin B, Kelvin DJ, Kelvin AA. Differential pathological and immune responses in newly weaned ferrets are associated with a mild clinical outcome of pandemic 2009 H1N1 infection. Journal of Virology. 2012;86:13187–13201. doi: 10.1128/JVI.01456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Banner D, Fang Y, Ng DC, Kanagasabai T, Kelvin DJ, Kelvin AA. Comparative analyses of pandemic H1N1 and seasonal H1N1, H3N2, and influenza B infections depict distinct clinical pictures in ferrets. PLoS One. 2011;6:e27512. doi: 10.1371/journal.pone.0027512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Lin Z, Banner D, León AJ, Paquette SG, Rubin B, Rubino S, Guan Y, Kelvin DJ, Kelvin AA. Immunity toward H1N1 influenza hemagglutinin of historical and contemporary strains suggests protection and vaccine failure. Scientific Reports. 2013;3:1698. doi: 10.1038/srep01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YH, Byun YH, Lee DH, Lee KH, Lee YJ, Lee YH, Park JK, Song CS, Seong BL. Cold-adapted X-31 live attenuated 2009 pandemic H1N1 influenza vaccine elicits protective immune responses in mice and ferrets. Vaccine. 2013;31:1320–1327. doi: 10.1016/j.vaccine.2012.12.072. [DOI] [PubMed] [Google Scholar]
- Jones SC, Brahmakshatriya V, Huston G, Dibble J, Swain SL. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. Journal of Immunology. 2010;185:6783–6794. doi: 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josset L, Engelmann F, Haberthur K, Kelly S, Park B, Kawoaka Y, García-Sastre A, Katze MG, Messaoudi I. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. Journal of Virology. 2012;86:11115–11127. doi: 10.1128/JVI.01571-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelvin AA, Degousee N, Banner D, Stefanski E, Leon AJ, Angoulvant D, Paquette SG, Huang SS, Danesh A, Robbins CS, Noyan H, Husain M, Lambeau G, Gelb M, Kelvin DJ, Rubin BB. Lack of group X secreted phospholipase A2 increases survival following pandemic H1N1 influenza infection. Virology. 2014:454–455. 78–92. doi: 10.1016/j.virol.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23:S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Muto NA, Sunden Y, Hattori T, Fujikura D, Nakayama Y, Miyazaki T, Maruyama M, Kimura T, Sawa H. Pathological examination of lung tissues in influenza A virus-infected mice. Japanese Journal of Infectious Diseases. 2012;65:383–391. doi: 10.7883/yoken.65.383. [DOI] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. The Journal of Immunology. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- O'Donnell CD, Wright A, Vogel LN, Wei CJ, Nabel GJ, Subbarao K. Effect of priming with H1N1 influenza viruses of variable antigenic distances on challenge with 2009 pandemic H1N1 virus. Journal of Virology. 2012;86:8625–8633. doi: 10.1128/JVI.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette SG, Banner D, Chi LTB, Leon AJ, Xu L, Ran L, Huang SS, Farooqui A, Kelvin DJ, Kelvin AA. Pandemic H1N1 influenza A directly induces a robust and acute inflammatory gene signature in primary human bronchial epithelial cells downstream of membrane fusion. Virology. 2014;448:91–103. doi: 10.1016/j.virol.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Pica N, Langlois RA, Krammer F, Margine I, Palese P. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge. Journal of Virology. 2012;86:10293–10301. doi: 10.1128/JVI.01131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T, León AJ, Crevar CJ, Carter DM, Xu L, Ran L, Fang Y, Cameron CM, Cameron MJ, Banner D, Ng DC, Ran R, Weirback HK, Wiley CA, Kelvin DJ, Ross TM. Modeling host responses in ferrets during A/California/07/2009 influenza infection. Virology. 2010;401:257–265. doi: 10.1016/j.virol.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Žugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor: pMHC interactions. Proceedings of the National Academy of Sciences. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proceedings of the National Academy of Sciences. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, Chan PL, Lam KT, Guan J, Zhang L. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. Journal of Virology. 2010;84:6527–6535. doi: 10.1128/JVI.00519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI. Cutting Edge: Pulmonary immunopathology mediated by antigen-specific expression of TNF-α by antiviral CD8+ T cells. Journal of Immunology. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. The Journal of Experimental Medicine. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


