Abstract
Type I interferons (IFNs) are a family of pro-inflammatory cytokines that are essential for anti-viral immunity but whose overexpression is associated with several autoimmune disorders. Here, we asked how chronic IFN overexpression regulates the activity of different cell types and how this contributes to immune dysfunction during IFN-associated inflammatory diseases. We show that in mice that chronically overproduce type I IFNs due to loss of the DNA exonuclease Trex1, inflammatory disease completely depends on IFNαR signaling in T cells. Although IFNs directly inhibited the proliferation and activation of Foxp3+ regulatory T cells, this was neither required nor sufficient for development of inflammatory disease. Rather, chronic IFN expression directly promoted the expansion and activation of effector T cells, and disease development was completely dependent on IFNαR signaling in these cells. Thus, chronic IFN expression can drive inflammatory disease via its direct effects on effector, but not regulatory, T cells.
Keywords: interferon, IBD, Trex1, autoimmunity
Introduction
The type I interferons (IFNs) are a family of pro-inflammatory cytokines that are essential for anti-viral immunity in both mice and humans. Type I IFNs can be expressed by nearly all nucleated cells, and their expression is triggered during viral infection after activation of cytoplasmic sensors of viral DNA/RNA. In addition, several cell types including plasmacytoid dendritic cells secrete large amounts of type I IFNs following ligation of the nucleic acid binding toll-like receptors, TLR7 and TLR9. Signaling through the ubiquitously expressed type I IFN receptor (IFNαR) induces expression of hundreds of IFN-stimulated genes (ISGs) in responding cells that help establish what is known as the “antiviral” state.
Although essential for proper anti-viral immunity, excessive type I IFN production has been associated with a variety of organ-specific and systemic autoimmune disorders (1). This is best exemplified in systemic lupus erythematosus (SLE), in which patients accumulate autoantibodies against DNA, RNA and other nuclear and nucleolar antigens. Indeed, SLE is associated with a type I IFN gene expression “signature” indicated by over-expression of numerous ISGs in peripheral blood leukocytes of SLE patients (2, 3). Current models of SLE development posit that defects in the handling and degradation of cellular nucleic acids result in the activation of TLR7, TLR9 and/or the cytoplasmic nucleic acid sensors, leading to chronic type I IFN production and loss of tolerance to nuclear antigens. Consistent with this model, mutations in proteins responsible for the clearance and degradation of apoptotic cells or the processing of cytoplasmic DNA lead to development of autoimmunity in various mouse models, and have been associated with development of SLE in humans (4). Moreover, development of SLE, colitis and type I diabetes have all been reported in individuals receiving type I IFN therapeutically, supporting a causative role for type I IFNs in autoimmune pathogenesis (5, 6).
The immunomodulatory effects of type I IFNs are incredibly complex, and several studies have suggested that type I IFNs may exert opposing effects based on a number of contextual factors. For example, IFNs can have both anti- and pro-proliferative effects depending on the cell type on which they act. IFNs inhibit the proliferation of infected cells to help prevent viral dissemination (7), and we recently showed that IFNs directly inhibit the proliferation of CD4+Foxp3+ regulatory T (Treg) cells during acute viral infection to allow the development of optimal antiviral T cell responses during actute infection with lymphocytic choriomeningitis virus (LCMV) (8). By contrast, IFNs can have activating and pro-proliferative effects on cell types important for viral clearance: they directly activate the cytotoxic function of (NK) cells (9), enhance antigen-presentation and production of pro-inflammatory cytokines by dendritic cells (9, 10), and are required for the clonal expansion of virus-specific CD4+ and CD8+ T cells during LCMV infection (11, 12). These effects can even vary based on the activation status of a given cell type. For example, whereas type I IFNs exert STAT1-dependent anti-proliferative effects on naïve CD4+ and CD8+ T cells, they can have pro-proliferative effects on antigen-activated T cells that down-regulate STAT1 expression (11–14). Additionally, two recent studies demonstrated that IFNs can have opposing pro- and anti-inflammatory effects based on the extent and duration of their expression during acute and chronic infection, respectively (15, 16). Whereas IFNs were pro-inflammatory and promoted viral clearance when expressed acutely, they were immunosuppressive and delayed viral clearance when their expression was prolonged during chronic infection.
Although IFNs are known to affect the function of many different cell types, the importance of each of these effects varies considerably in different inflammatory contexts. For example, IFNαR signaling in dendritic cells is required for their maturation and their ability to present antigen and induce CD4+ and CD8+ T cell responses in certain tumor and vaccine models (17, 18). Additionally, Treg cells from healthy controls showed defective suppression in vitro in the presence of APCs from SLE patients, and this was linked to their production of IFNα (19). By contrast, during infection with vaccinia virus, IFNαR signaling is required primarily in NK cells but not dendritic cells for efficient viral clearance (20), while IFNαR signaling in macrophages is a major mediator of lesion formation in a murine model of atherosclerosis (21).
Despite the clear association between overproduction of type I IFNs and development of autoimmunity, the importance of type I IFN signaling in different cell types for disease development has remained unclear. Using a well-established model of inflammatory bowel disease, we show that immunoregulation is impaired in mice that chronically overproduce type I IFNs due to loss of the DNA exonuclease Trex1. Inflammatory disease in this system completely depended on type I IFN signaling in T cells. Although IFN overexpression directly inhibited Treg cell proliferation and activation, this inhibition was not required for the onset of inflammatory disease. Rather, chronic IFN expression directly promoted the expansion of effector T (Teff) cells, and inflammatory disease was completely dependent on IFNαR signaling in Foxp3− effector T cells. Thus, chronic IFN expression can drive inflammatory disease independent of its effects on Treg cells by promoting the expansion and pro-inflammatory function of effector T cells.
Materials and Methods
Mice
C57BL/6J (B6) were purchased from The Jackson Laboratory. Rag2+/−Trex1+/− mice were provided by Daniel Stetson (University of Washington) and bred to generate Rag2−/−Trex1+/− and Rag2−/−Trex1−/− mice. Foxp3GFP were provided by A. Rudensky (Memorial Sloan-Kettering Cancer Center). Ifnar1−/− mice were provided by K. Murali-Krishna (Emory University) and crossed to Foxp3GFP mice. All mice were housed and bred at the Benaroya Research Institute (Seattle, WA), and all experiments were performed in accordance within the guidelines of the Benaroya Research Institute Animal Care and Use Committee.
Flow cytometry and cell sorting
For surface staining, cells were incubated at 4°C for 30 minutes in staining buffer (HBSS, 2% FBS) with the following directly conjugated antibodies for murine proteins (from Biolegend unless otherwise specified): anti-CD4 (RM4-5), -CD8 (53-6.7, eBioscience), -CD45RB (C363.16A, eBioscience), -CD25 (PC61.5), -CD44 (IM7), -CXCR3 (CXCR3-173), -IFNAR1 (MAR1-5A3), -CD69 (H1.2F3, BD). For intracellular staining, cells were surface stained as described, washed and permeabilized for 20 minutes with eBioscience Fix/Perm buffer at 4°C. Cells were stained for 30 minutes at 4°C with anti-Foxp3 (FKJ-16s; eBioscience), anti-IFN-γ (XMG1; eBioscience) and anti-Ki-67 (B56; BD Biosciences) in PermWash staining medium (eBioscience). For intracellular cytokine staining following restimulation, cells were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) in 96-well U-bottomed plates (Costar, Cambridge, MA) with 10μg/mL monensin in 0.2ml of complete RPMI (RPMI plus 2.05mM L-glutamine, 10% (vol/vol) fetal calf serum, 50units/l of penicillin, 50μg/mL of streptomycin, 50μg/mL gentamycin, 1mM sodium pyruvate, 1mM HEPES, 50μM β-mercaptoethanol) for 5 hours at 37°C, 5%CO2 prior to staining. Data were acquired on LSRII flow cytometers (BD Biosciences) and analyzed using FlowJo software (Treestar). For cell sorting experiments, cells were isolated from spleen and peripheral lymph nodes and enriched for CD4+ cells using CD4 Dynabeads (Invitrogen), stained for desired cell surface markers, and isolated using a FACS Aria (BD Biosciences). The purity of FACS-sorted cells was >95%.
Colitis induction
CD4+CD25hi Treg cells were FACS sorted from spleens and peripheral lymph nodes of CD45.2+ B6 or Ifnar1−/− mice. CD4+Foxp3GFP−CD25−CD45RBhi naïve T cells were FACS sorted from spleens and peripheral lymph nodes of CD45.1+ Foxp3GFP or CD45.1+ Foxp3GFPIfnar1−/− mice. Rag2−/−Trex1+/− or Rag2−/−Trex1−/− mice (8–12 weeks old) were then injected intravenously with 1x105 naïve T cells and 2x105 Treg cells of the indicated genotype. Mice were weighed just prior to T cell transfer (time 0) and 1–2 times per week thereafter. Percent weight change was calculated as: (weight at time X – weight at time 0) / (weight at time 0). All mice in each experiment were sacrificed when any individual mice showed clinical signs of severe disease or 20 percent weight loss.
Cell isolation
Cell suspensions were prepared from spleen and peripheral lymph nodes by tissue disruption with glass slides and filtered thru a 40-μM filter. After dissection and removal of Peyer's patches, intestinal intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) were isolated as follows. The intestinal epithelium was stripped, as previously described (22, 23), and the remaining intestinal pieces were washed three times in CMF solution (HBSS, Ca2+ and Mg2+ free, 100 mM HEPES (Sigma-Aldrich), 250 mM sodium bicarbonate (Fisher Scientific, Pittsburgh, PA), and 2% FBS (HyClone, Logan, UT)). The tissue was then placed into a flask containing CMF with 1 mM DTT and shaken for 20 min at 37°C. The IEL containing supernatant was removed and transferred into 50-ml centrifuge tubes and pelleted by centrifugation. The cells were resuspended in 44% Percoll (Sigma-Aldrich) and layered onto a 67% Percoll cushion in a 15-ml polycarbonate centrifuge tube. The tubes were centrifuged (2800 rpm) for 20 min at room temperature. The IEL were removed from the 44/67% Percoll interface and washed with RPMI 1640. For LPL isolation, immediately following incubation with DTT, HBSS with 0.5M EDTA was added to the intestinal tissue pieces and was incubated an additional 30 min on ice. Intestinal pieces were washed with RPMI and added to 50 ml of RPMI plus 100 μl 0.5 M MgCl2, 100 μl 0.5 M CaCl2, and 150 U/ml collagenase (Roche). Samples were stirred at 37°C for 1 h, and the released cells were then filtered through nitex. Cells isolated from the lamina propria were pelleted, resuspended in 44% Percoll (GE Healthcare) in RPMI, layered over 67% Percoll, and spun at 2,800 rpm for 20 min. Lymphocytes were isolated from the interface and used for subsequent flow cytometry analyses.
Histology and colitis scoring
Colon sections were immersion fixed in 10% neutral buffered formalin, paraffin embedded, cut into 5 μm sections, and stained with hematoxylin and eosin by the Benaroya Research Institute Histology Core. Sections were scored semiquantitatively from 0 to 4 for colitis severity in a blinded fashion (24). A grade of 0 was given when there were no changes observed. Changes typically associated with other grades are as follows: grade 1, minimal scattered mucosal inflammatory cell infiltrates, with or without minimal epithelial hyperplasia; grade 2, mild scattered to diffuse inflammatory cell infiltrates, sometimes extending into the submucosa and associated with erosions, with minimal to mild epithelial hyperplasia and minimal to mild mucin depletion from goblet cells; grade 3, mild to moderate inflammatory cell infiltrates that were sometimes transmural, often associated with ulceration, with moderate epithelial hyperplasia and mucin depletion; grade 4, marked inflammatory cell infiltrates that were often transmural and associated with ulceration, with marked epithelial hyperplasia and mucin depletion; and grade 5, marked transmural inflammation with severe ulceration and loss of intestinal glands.
Enumeration of lymphocytes
Absolute numbers of lymphocytes in various tissues was determined using Polybead polystyrene nonfluorescent microspheres (15 um, Polysciences, Inc.). Briefly, 100ul of the cell suspension to be counted was mixed with 100ul of a fixed concentration (CB) of Polybeads (one drop of Polybeads per ml of PBS) in a FACS tube. Without washing, the samples were acquired on a FACS Calibur (BD) and were quantified using the appropriate gates. Beads and lymphocytes were identified by their distinct forward- and side-scatter characteristics. The ratio of lymphocyte gate events (nL) to bead gate events (nB) was determined and used to calculate the concentration (C) of the original cell suspension as follows: C = (nL / nB) * CB
Statistics
All data are presented as the mean values ± SEM. Statistical significance was determined by one-way ANOVA with Tukey post-test, two-tailed unpaired t-test, or linear regression as indicated in figure legends. Statistical significance was established at the levels of *, p<0.05; **, p<0.005; ***, p<0.0001.
Results
IFNαR signaling in T cells is required for immune dysfunction in Trex1−/− mice
To assess how dysregulated overproduction of type IFNs drives inflammatory disease, we used mice lacking Trex1, a 3’→5’ cytoplasmic DNA exonuclease that is ubiquitously expressed and functions to degrade endogenous retroelements and other cytoplasmic DNA (25, 26). Trex1 is a critical negative regulator of the IFN-stimulatory DNA (ISD) response, and mutations in Trex1 cause an accumulation of endogenous cytoplasmic DNA that triggers type I IFN production, inflammation and autoimmunity in mice and humans (25–28). Autoimmunity in Trex1−/− mice is entirely dependent on lymphocyte function and type I IFN expression, as Trex1−/−Rag2−/− and Trex1−/−Ifnar1−/− double-deficient animals are completely protected from disease development (26, 29). Importantly, although Rag2−/−Trex1−/− mice remain healthy, they display chronically elevated type I IFN production as early as 8 days after birth (29).
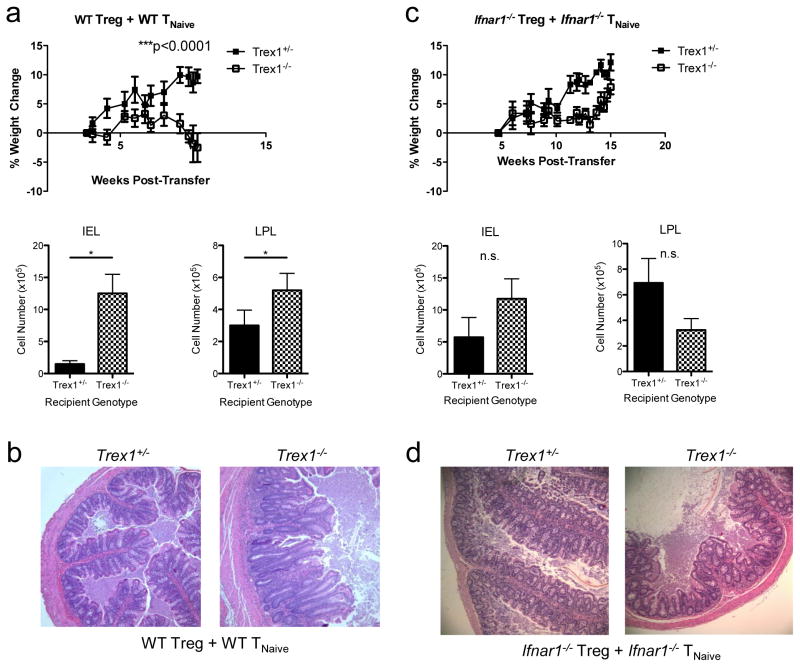
We used a well-described T cell transfer model of colitis to determine out how type I IFN overproduction in Trex1−/− mice affects different cell types in vivo. In this model, adoptive transfer of CD4+Foxp3−CD45RBhi naive T cells (TNaive) into Rag2−/− hosts induces inflammatory colitis and wasting disease that can be prevented by co-transfer of purified CD4+CD25+ Treg cells (24, 30, 31). To determine how overproduction of type I IFNs impacts immune regulation in this system, we sorted CD4+Foxp3GFP−CD45RBhi naïve T cells from CD45.1+Foxp3GFP mice and CD4+CD25+ Treg cells from CD45.2+ C57BL/6 (WT) mice and co-transferred them into either Rag2−/−Trex1+/− or Rag2−/−Trex1−/− recipients. As expected, Rag2−/−Trex1+/− animals given WT TNaive cells and WT Treg cells remained healthy, gained weight and showed no external signs of colitis development. By contrast, Rag2−/−Trex1−/− recipients exhibited clinical signs of colitis, including hunching, diarrhea, and rectal inflammation, and gained significantly less weight than Rag2−/−Trex1+/− recipients as early as 4 weeks post-transfer (Fig 1a; not shown). Moreover, Rag2−/−Trex1−/− recipients developed significant colonic inflammation, with higher numbers of intraepithelial (IEL) and lamina propria lymphocytes (LPL) in the colon compared to Rag2−/−Trex1+/− recipients (Fig 1a). Consistent with this, histological analysis of colons showed leukocytic infiltrate in the LP, depletion of goblet cells, and moderate epithelial cell hyperplasia, as well as disrupted colonic architecture in Rag2−/−Trex1−/− mice compared to Rag2−/−Trex1+/− mice (Fig 1b). Altogether, these data clearly demonstrate that normal immunoregulation is impaired in Trex1-deficient recipients.
Figure 1. IFNαR signaling in T cells is required for immune dysfunction in Trex1−/− mice.
a) Top: percent weight change in Rag2−/−Trex1+/−(“Trex1+/−“, black squares) and Rag2−/−Trex1−/−(“Trex1−/−“, open squares) mice at various time points after co-transfer of WT CD45.2+CD4+CD25+ Treg and WT CD45.1+CD4+Foxp3GFP−CD45RBhi TNaive cells. Bottom: absolute number of intraepithelial (IEL) and lamina propria lymphocytes (LPL) in the colons of Rag2−/−Trex1+/− (black) and Rag2−/−Trex1−/−(checkered) recipient mice at time of sacrifice. b) Representative H&E staining of cross-sections of intermediate to distal colon from Rag2−/−Trex1+/− and Rag2−/−Trex1−/− recipients of WT Treg and WT TNaive cells at time of sacrifice. c) Top: percent weight change in Rag2−/−Trex1+/−(“Trex1+/−“, black squares) and Rag2−/−Trex1−/−(“Trex1−/−“, open squares) mice at various time points after co-transfer of Ifnar1−/− CD45.2+CD4+CD25+ Treg and Ifnar1−/− CD45.1+CD4+Foxp3GFP−CD45RBhi TNaive cells. Bottom: absolute number of intraepithelial (IEL) and lamina propria lymphocytes (LPL) in the colons of Rag2−/−Trex1+/− (black) and Rag2−/−Trex1−/−(checkered) recipient mice at time of sacrifice. d) Representative H&E staining of cross-sections of intermediate to distal colon from Rag2−/−Trex1+/− and Rag2−/−Trex1−/− recipients of Ifnar1−/− Treg and Ifnar1−/− TNaive cells at time of sacrifice. Statistical significance was determined using unpaired two-tailed Student’s t-test. Data are representative of two independent experiments with 3–4 mice per group. *, p<0.05; **, p<0.005; ***, p<0.0001.
Trex1-deficiency results in the dysregulation of many cellular processes, such as the clearance of extranuclear DNA, activation of the ISD response, and production of type I IFNs. To determine if overproduction of IFNs and its corresponding effects on T cells were responsible for the inflammatory disease that developed in Trex1-deficient mice, we co-transferred sorted CD45.1+ TNaive and CD45.2+ Treg cells from Ifnar1−/− mice that lack the ability to signal through the type I IFN receptor into Rag2−/−Trex1+/− or Rag2−/−Trex1−/− mice. Importantly, Ifnar1−/− naïve T cells are just as capable of inducing colitis in Rag-deficient mice as WT naïve T cells (32). Interestingly, neither Rag2−/−Trex1+/− nor Rag2−/−Trex1−/− recipients developed colitis, showing comparable weight gain, similar IEL and LPL numbers, and histologically normal colons (Fig 1c, 1d). Thus, although IFNs are capable of acting on many different innate immune cell types in these recipient animals, their direct effects on the transferred T cells specifically were required for the impaired immune regulation observed during colitis induction in Trex1−/− mice.
IFNs directly inhibit Treg cell and promote effector T cell proliferation and activation
In our colitis system, type I IFNs could be driving inflammatory disease in Trex1-deficient mice through their effects on Treg cells, effector T cells, or both. By co-transferring different combinations of WT and Ifnar1−/− Treg and naïve T cells into Rag2−/−Trex1−/− mice, this system also allows us to determine how IFNs directly impact effector and/or Treg cell activation and function and how this contributes to the development of inflammatory disease.
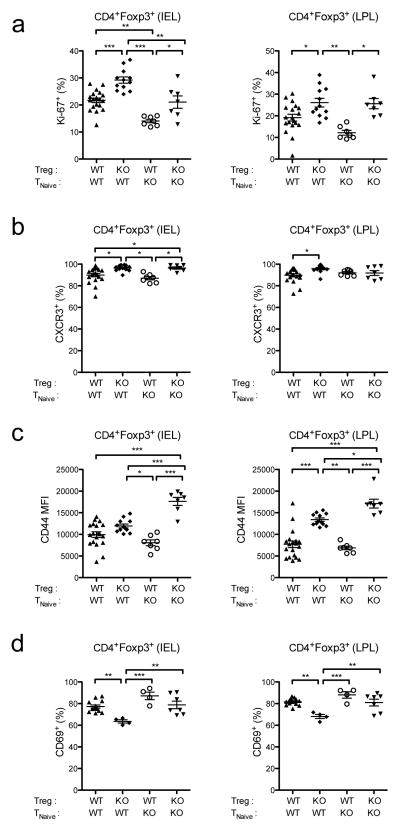
We have previously shown that IFNs have direct anti-proliferative effects on Treg cells during acute viral infection, and this was especially pronounced in the intestinal mucosa (8). Consistent with this, CD45.2+Ifnar1−/− Treg cells transferred into Rag2−/−Trex1−/− mice consistently showed enhanced proliferation in the IEL and LPL of the colon compared to transferred CD45.2+ WT Treg cells, as measured by the percentage of cells expressing the cell cycle-associated nuclear antigen Ki-67 (Fig 2a). This was true in the presence of either WT or Ifnar1−/− effector T cells, indicating that IFNs directly inhibit Treg cell proliferation in the mucosa. Moreover, Ifnar1−/− Treg cells also displayed a more activated phenotype than WT Treg cells in the IEL and LPL, with a greater proportion expressing the chemokine receptor CXCR3 and elevated expression of the activation marker CD44 (Fig 2b, 2c). By contrast, Ifnar1−/− Treg cells expressed lower levels of CD69 than WT Treg cells in the gut, consistent with the ability of type I IFNs to induce CD69 expression in T cells (33, 34). Thus, similar to what we observed during acute viral infection, chronic IFN expression in Trex1−/− mice appears to directly inhibit Treg cell proliferation and activation.
Figure 2. Type I IFNs directly inhibit Treg cell proliferation and activation in Trex1−/− mice.
Summary of Ki-67 (a), CXCR3 (b), CD44 (c), and CD69 (d) expression by CD45.2+CD4+Foxp3+ Treg cells in the IEL (left) and LPL (right) in the colons of Rag2−/−Trex1−/− mice receiving the indicated combinations of CD45.2+ WT or Ifnar1−/− (knockout, “KO”) Treg and CD45.1+ TNaive cells. Data are summarized from 8 independent experiments with 3–4 mice per group. Statistical significance was determined using one- way ANOVA with Tukey post-test. *, p<0.05; **, p<0.005; ***, p<0.0001.
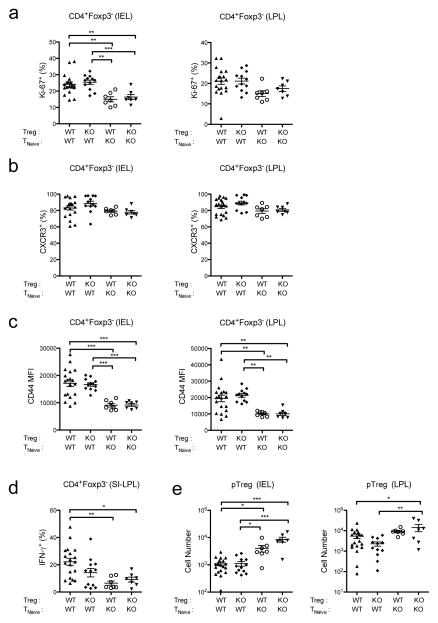
In contrast to their inhibitory effects on Treg cells, IFNs can deliver pro-survival signals to antigen-activated CD4+ and CD8+ effector T cells, and are actually required for the expansion of virus-specific T cells during LCMV infection (11, 35). Consistent with this, CD45.1+Ifnar1−/− effector T cells transferred into Rag2−/−Trex1−/− mice proliferated at significantly lower levels than transferred WT cells in the IEL, and to a lesser degree in the LPL (Fig 3a). Moreover, CD45.1+Ifnar1−/− effector T cells appeared significantly less activated than CD45.1+ WT effector T cells by several measures: although CXCR3 expression did not differ significantly between WT and Ifnar1−/− effector T cells, Ifnar1−/− effector T cells expressed significantly lower levels of the activation marker CD44 and produced significantly less IFN-γ upon restimulation with PMA and ionomycin (Fig 3c, 3d). This decline in proliferation and activation among Ifnar1−/− effector T cells was evident regardless of the presence of either WT or Ifnar1−/− Treg cells. No differences in the frequency of IL-17A or IL-10-producing effector T cells were observed between WT and Ifnar1−/− effector T cells (data not shown). However, because our donor naïve T cell and Treg cell populations were congenically marked, we were able to determine if any CD4+Foxp3gfp− naïve T cells transferred into Rag2−/−Trex1−/− recipients upregulated expression of Foxp3 and differentiated into peripheral Treg (pTreg) cells. Interestingly, the number of CD4+Foxp3+ pTreg cells arising from donor CD45.1+Ifnar1−/− naïve T cells was significantly higher than those arising from CD45.1+ WT naïve T cells, particularly in the IEL compartment (Fig 3e). Altogether, these data indicate that type I IFN signaling directly promotes effector T cell proliferation and pro-inflammatory activation, while inhibiting the generation of anti-inflammatory pTreg cells.
Figure 3. Type I IFNs directly promote effector T cell proliferation and activation in Trex1−/− mice.
Summary of Ki-67 (a), CXCR3 (b), and CD44 (c) expression by CD45.1+CD4+Foxp3− effector T (Teff) cells in the IEL (left) and LPL (right) in the colons of Rag2−/−Trex1−/− mice receiving the indicated combinations of CD45.2+ WT or Ifnar1−/− (knockout, “KO”) Treg and CD45.1+ TNaive cells. d) Summary of IFN-γ expression by CD45.1+CD4+Foxp3− Teff cells in the small intestinal lamina propria of Rag2−/−Trex1−/− mice receiving the indicated combinations of CD45.2+ WT or Ifnar1−/− (knockout, “KO”) Treg and CD45.1+ TNaive cells. IFN-γ expression was determined by intracellular cytokine staining after 5-h stimulation of small intestine lamina propria lymphocytes (SI- LPL) with PMA and ionomycin. e) Summary of absolute numbers of CD45.1+CD4+ Foxp3gfp+ peripheral Treg (pTreg) cells in the IEL (left) and LPL (right) in the colons of Rag2−/−Trex1−/− mice receiving the indicated combinations of CD45.2+ WT or Ifnar1−/− (knockout, “KO”) Treg and CD45.1+ TNaive cells. Data are summarized from 8 independent experiments with 3–4 mice per group. Statistical significance was determined using one-way ANOVA with Tukey post-test. *, p<0.05; **, p<0.005; ***, p<0.0001.
IFN signaling in effector, but not regulatory, T cells is required for colitis development in Trex1−/− recipients
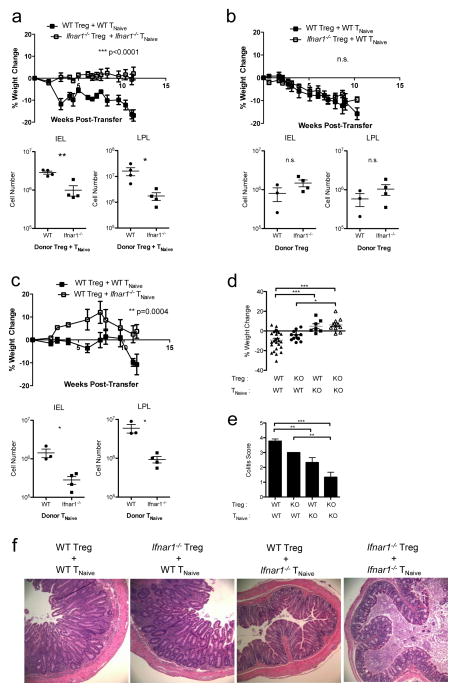
Given that IFNs both inhibited Treg cells and activated effector T cells, we next asked whether IFNs’ effects on Treg cells and/or naïve T cells was responsible for the loss of immunoregulation observed during colitis development in Trex1-deficient recipients. To test this, we assessed disease development in Rag2−/−Trex1−/− mice given different combinations of WT and Ifnar1−/− Treg and TNaive cells. Consistent with our previous findings, Rag2−/−Trex1−/− mice receiving WT TNaive and WT Treg cells developed severe colitis while those receiving Ifnar1−/− TNaive and Ifnar1−/− Treg cells were completely protected from disease, as measured by weight loss, lymphocytic infiltrates in the colon, and histological analysis of colon cross-sections (Fig 4a, 4d, 4e).
Figure 4. IFNαR signaling in effector, but not regulatory, T cells is required for colitis development in Trex1−/− mice.
a–c) Top: percent weight change in Rag2−/−Trex1− /− mice at various time points after co-transfer of WT Treg + WT TNaive cells (black squares) or co-transfer of: (a) Ifnar1−/− Treg + Ifnar1−/− TNaive cells (open squares); (b) Ifnar1−/− Treg + WT TNaive cells (open squares); or (c) WT Treg + Ifnar1−/− TNaive cells (open squares). Bottom: absolute number of intraepithelial (IEL) and lamina propria lymphocytes (LPL) in the colons of the indicated Rag2−/−Trex1−/− mice at time of sacrifice. Data are representative of 2–3 independent experiments with 3–4 mice per group. d) summary of the final percent weight change at time of sacrifice in Rag2−/−Trex1−/− mice receiving the indicated WT or Ifnar1−/− (knockout, “KO”) Treg and TNaive cells. e) summary of colitis scores based on histological analysis of colon cross-sections from Rag2−/−Trex1−/− mice receiving the indicated WT or Ifnar1−/− (knockout, “KO”) Treg and TNaive cells. f) Representative H&E staining of cross-sections of intermediate to distal colon from Rag2−/−Trex1−/− recipients of the indicated Treg and TNaive cells. (d, e) Data are summarized from 8 independent experiments with 3–4 mice per group. Statistical significance was determined using unpaired two-tailed Student’s t-test (a–c) or one-way ANOVA with Tukey post-test (d, e). *, p<0.05; **, p<0.005; ***, p<0.0001.
We next asked whether IFNαR signaling in Treg cells, specifically, was responsible for the loss of Treg suppressive function in Trex1-deficient mice. Surprisingly, all Rag2−/−Trex1−/− mice developed colitis regardless of whether they received WT or Ifnar1−/− Treg cells in combination with WT TNaive cells (Fig 4b), indicating that loss of immunoregulation in Trex1-deficient mice does not depend on direct type I IFN signaling in Treg cells. Transfer of WT TNaive cells with either WT or Ifnar1−/− Treg cells induced weight loss with similar kinetics and magnitude, with no difference in the numbers of IEL or LPL (Fig 4b, 4d). Histological analysis of colon cross-sections displayed similar levels of epithelial cell hyperplasia and disruption of crypt architecture in Rag2−/−Trex1−/− mice that received WT or Ifnar1−/− Treg cells. However, there was slightly more severe goblet cell depletion, leukocytic infiltrate, and ulceration in mice that received WT Treg cells compared to those that received Ifnar1−/− Treg cells, resulting in colitis scores that trended higher in mice that received WT Treg cells, though this did not reach statistical significance. Thus, despite the ability of type I IFNs to directly inhibit Treg cell activation and function in vivo, WT and Ifnar1−/− Treg cells do not differ significantly in their ability to suppress colon inflammation in Trex1-deficient mice.
Since direct inhibition of Treg cells by type I IFNs was not necessary for the failed immunoregulation observed in Trex1−/− recipient mice, we next determined how type I IFN signaling in effector T cells contributes to disease development. For this, we transferred either WT or Ifnar1−/− TNaive cells together with WT Treg cells into Rag2−/−Trex1−/− mice and monitored disease. Whereas recipients of WT naïve T cells developed colitis as expected, recipients of Ifnar1−/− naïve T cells were protected from wasting disease, showing no significant weight loss (Fig 4c, 4d). Additionally, recipients of Ifnar1−/− naïve T cells were partially protected from colon inflammation, with lower numbers of IEL and LPL in the colon and significantly lower colitis scores (Fig 4c, 4e). Histological analysis revealed lower numbers of leukocytic infiltrates in the LP and less goblet cell depletion in the colons of Rag2−/−Trex1−/− that received Ifnar1−/− naïve T cells, although there was still evidence of moderate epithelial cell hyperplasia (Fig 4f).
Taken together, these results indicate that Rag2−/−Trex1−/− recipients of Ifnar1−/− naïve T cells were protected to the greatest degree from weight loss and colitis (Fig 4d, 4e). By contrast, recipients of WT naïve T cells developed similarly severe weight loss and colitis, regardless of the presence of WT or Ifnar1−/− Treg cells (Fig 4d, 4e). Thus, IFNs appear to induce chronic inflammatory disease in Trex1-deficient mice primarily through their direct effects on effector T cells.
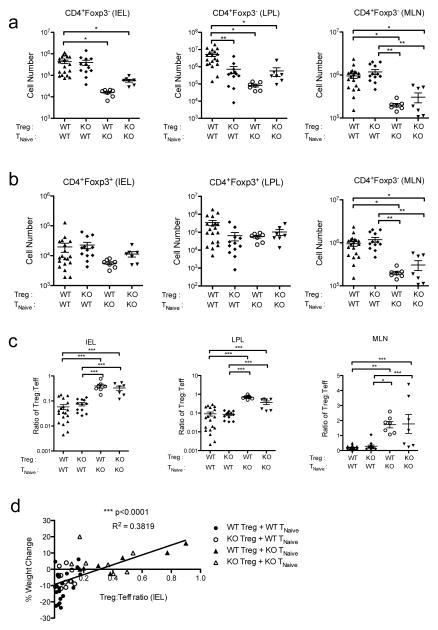
IFN signaling in effector T cells directly promotes effector T cell abundance
To understand why IFNs’ effects on effector, but not Treg, cells were required for colitis development, we examined the absolute numbers of Treg and effector T cells in Rag2−/−Trex1−/− mice that received different combinations of WT and Ifnar1−/− naïve and Treg cells. Consistent with their higher levels of proliferation (Fig 3a), WT effector T cells were present at roughly 10-fold higher levels in the IEL and LPL of the colon compared to Ifnar1−/− effector T cells (Fig 5a). Interestingly, WT effector T cells were also increased in number in the mesenteric lymph node, suggesting that Ifnar1−/− effector T cells are impaired in their priming and expansion in lymphoid tissues. By contrast, despite their lower levels of proliferation and activation (Fig 2), WT Treg cells were not significantly reduced in number compared to Ifnar1−/− Treg cells in either the mesenteric lymph node or in the colonic IEL or LPL (Fig 5b). Accordingly, the ratio of Treg cells to effector T cells in the IEL and LPL of the colon was significantly lower in recipients of WT naïve T cells than in recipients of Ifnar1−/− naïve T cells, due primarily to the numbers of effector T cells in these mice (Fig 5c). The ratio of Treg to effector T cells correlated significantly with percent weight loss (Fig 5d), with the lowest Treg to effector T cell ratios associated with the most severe weight loss, suggesting that the ability of type I IFNs to promote effector T cell population expansion is the primary factor contributing to disease development in recipients of WT cells.
Figure 5. Type I IFNs directly promote effector T cell expansion in Trex1−/− mice.
a, b) Summary of the absolute number of CD45.1+CD4+Foxp3− effector T cells (Teff) (a) and CD45.2+CD4+Foxp3+ Treg cells (b) in the mesenteric lymph node (MLN) (right), and IEL (left) and LPL (middle) of the colons of Rag2−/−Trex1−/− mice receiving the indicated combinations of CD45.2+ WT or Ifnar1−/− (knockout, “KO”) Treg and CD45.1+ TNaive cells. c) Summary of the ratio of Treg to Teff cells in the MLN (right) and IEL (left) and LPL (middle) of the colons of Rag2−/−Trex1−/− mice receiving the indicated combinations of CD45.2+ WT or Ifnar1−/− (knockout, “KO”) Treg and CD45.1+ TNaive cells. d) Correlation plot comparing the Treg:Teff ratio in the IEL of the colon to the percent weight change at time of sacrifice in Rag2−/−Trex1−/− mice receiving the indicated combinations of WT or Ifnar1−/− (knockout, “KO”) Treg and TNaive cells. Data are summarized from 8 independent experiments 3–4 mice per group. Statistical significance was determined using one-way ANOVA with Tukey post-test (a–c) or by linear regression slope test. *, p<0.05; **, p<0.005; ***, p<0.0001.
Discussion
The effects of type I IFNs are incredibly complex and vary based on a number of contextual factors, such as the cell type acted on, its activation status, and the timing and extent of IFN expression. Although inflammatory disease in Trex1-deficient mice is known to depend on IFNαR signaling on hematopoietic cells (29), it has been unclear what cell type is the major mediator of inflammatory disease. IFNs are known to have pro-inflammatory effects on NK cells, macrophages, and dendritic cells, in addition to their effects on T cells, and the importance of each of each of these effects varies considerably in different inflammatory contexts. Here, we found that IFNαR signaling in T cells, and not innate immune cells, was the major driver of disease in a model of chronic inflammatory bowel disease in Trex1-deficient mice. That is, in the presence of Ifnar1−/− Treg and Ifnar1−/− effector T cells, IFN-responsive myeloid and NK cells were not sufficient to drive colitis in Trex1-deficient mice, as these mice were completely protected from disease. Although IFNs directly activated effector T cells and inhibited Treg cells, only their effects on effector T cells were required for the onset of inflammatory disease.
Previous studies have provided conflicting results regarding the impact of type I IFNs on Treg cells and generally have not used experimental systems that directly examined the effects of IFNs on Treg cell homeostasis and function (36–40). Recently, we showed that type I IFNs can directly inhibit Treg cell activation and proliferation both in vitro and in vivo during acute viral infection, and that this transient inhibition is necessary for the generation of optimal antiviral T cell responses (8). Similar to what we observed during acute LCMV infection, chronic IFN expression directly inhibited Treg cell proliferation in the gut, as Ifnar1−/− Treg cells consistently proliferated more and exhibited a more activated phenotype than WT Treg cells in Trex1-deficient mice. These results are in contrast to those of a recent study that demonstrated a direct role for IFN in the maintenance of Treg cells in the mucosa during inflammatory colitis (41). This discrepancy may be due to differences in the mode of IFN induction in the different models used. Whereas Lee et al. treated mice with pegylated IFNα intraperitoneally for several weeks, in Trex1−/− mice elevated expression of IFNβ initiates in non-hematopoietic cells during embryonic development (29, 41, 42). Differences in the timing and extent of type I IFN expression, the IFN subtypes induced, or the sites of IFN production may all contribute to differences in the ways IFNs modulate Treg cell activity in these different systems.
However, unlike what we observed during acute LCMV infection, the inhibition of Treg cell proliferation in Trex1-deficient mice by IFNs did not affect Treg cell numbers in the gut and was not required for the overall immune dysfunction observed in Trex1-deficient mice. Whereas during acute LCMV infection Ifnar1−/− Treg cells were able to suppress antiviral T cell responses better than WT Treg cells, in Trex1-deficient mice Ifnar1−/− Treg cells were no better at suppressing effector T cell expansion or inflammatory colitis than WT Treg cells. This may be due to differences in the way IFNs inhibit Treg cells directly in these two models: while IFNs inhibited both proliferation and accumulation of Treg cells during LCMV infection, they only inhibited Treg cell proliferation without affecting their accumulation in the guts or lymphoid tissues of Trex1-deficient mice, suggesting differences in the survival of Treg cells in these two models. Treg cell numbers may not have been dramatically impacted by IFNs in Trex1-deficient mice due to the lymphopenia present in Rag2−/−Trex1−/− mice. That is, the extensive lymphoproliferation of Treg cells that occurred upon transfer into lymphopenic Rag2−/−Trex1−/− mice may have obscured any potential anti-proliferative or pro-apoptotic effects of IFNs on these cells. Additionally, IL-2 is a critical factor for Treg cell survival that inhibits apoptosis via promotion of Bcl2 and Mcl1 activity and may promote Treg cell survival in Trex1-deficient mice despite the anti-proliferative effects of IFNs (43, 44). Consistent with this, as IL-2 signaling is known to promote expression of the high-affinity IL-2 receptor chain CD25, Treg cells transferred into Rag2−/−Trex1−/− mice had elevated levels of surface CD25 expression compared to those transferred into Rag2−/−Trex1+/− mice, suggesting they received more IL-2 signaling in Trex1-deficient mice (data not shown). Finally, although Trex1-deficient mice receiving Ifnar1−/− naïve T cells with WT Treg cells were protected significantly from colitis, there was still evidence of epithelial cell hyperplasia in their colons that was not present in the colons of mice receiving Ifnar1−/− naïve T cells with Ifnar1−/− Treg cells. Likewise, Trex1-deficient mice receiving Ifnar1−/− Treg cells with WT naïve T cells exhibited slightly, though not significantly, lower colitis scores and weight loss than mice receiving WT Treg cells with WT naïve T cells. Altogether, these data suggest that although Ifnar1−/− Treg cells are unable to completely suppress inflammatory disease in Trex1-deficient mice, they may still protect from some aspects of inflammatory colitis better than WT Treg cells.
Interestingly, despite the effects of IFNs on Treg cells, immune dysfunction in Trex1-deficient recipient mice seemed primarily due to the direct effects of IFNs on effector T cells. WT effector cells were significantly more pro-inflammatory than Ifnar1−/− cells, proliferating at higher levels and producing more IFN-γ, which is a major cytokine involved in the pathogenesis of this colitis model (45). By contrast, we found no difference in IL-17A or IL-10 expression between WT and Ifnar1−/− effector T cells (data not shown), suggesting that IFNs specifically promote Th1 pro-inflammatory function. Interestingly, transferred WT cells were also less likely to differentiate into pTreg cells than Ifnar1−/− cells. Thus, although the tolerogenic environment of the gut is known to support pTreg cell development, IFNs likely promote intestinal inflammation in this model by supporting Th1 differentiation at the expense of pTreg cell development. In addition to being more activated, accumulation of WT effector T cells in the guts of Trex1-deficient mice was significantly higher than that of Ifnar1−/− effector T cells. This may be due to defects in the priming and expansion of Ifnar1−/− T cells in secondary lymphoid tissues, as Ifnar1−/− effector T cells were also present at significantly lower numbers in the mesenteric lymph nodes. The number of effector T cells in the intestine correlated best with disease severity as measured by percent weight loss, suggesting that IFNs’ ability to promote effector T cell proliferation and accumulation is a major contributor to the severity of inflammatory disease that develops in Trex1-deficient mice.
The heightened pro-inflammatory function of WT effector T cells compared to Ifnar1−/− cells may also be due in part to their resistance to Treg cell-mediated suppression. The resistance of effector T cells to Treg-mediated suppression has been described both in patients and in mouse models of many autoimmune disorders, including type I diabetes, multiple sclerosis, and SLE. This often occurs as a result of pro-inflammatory cytokine signaling in effector T cells. For example, Treg cells are able to migrate to the central nervous system during experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, but are unable to suppress effector T cells during active disease due to the production of IL-6 and TNFα, both of which have been implicated in driving effector T cell activation and resistance to suppression (46). A recent study also demonstrated that IL-1/MyD88 signaling in effector T cells is required to overcome suppression by Treg cells (47). Thus, despite the presence of normal numbers of Treg cells, a number of factors in the inflammatory milieu may still circumvent Treg cell-mediated suppression and drive inflammatory disease. Activation of effector T cells by IFNs in Trex1-deficient mice, therefore, may render them resistant to suppression by Treg cells. The longer duration of IFN signaling in Trex1-deficient mice compared to during acute LCMV infection may also make effector T cells more resistant to suppression, even by Ifnar1−/− Treg cells.
Although important for immunity to viral infections, type I IFNs are strongly linked to the development of certain autoimmune diseases such as SLE, and there have been several reports of autoimmune and inflammatory diseases developing in patients receiving type I IFN therapeutically. Our work demonstrating that effector T cells are the major IFN-responsive cell type mediating inflammatory disease in Trex1-deficienct mice has important implications for treatment of SLE and other type I IFN-associated autoimmune diseases. Targeted blockade of IFNαR signaling in effector T cells, for example, may be an attractive therapy for IFN-associated autoimmune diseases that disrupts a central pro-inflammatory axis while avoiding off-target effects on other cell types. Moreover, considering that administration of type I IFNs is a commonly prescribed treatment for chronic infection, cancer, and even multiple sclerosis (48), it will be crucial to understand how type I IFNs regulate different cell types, both directly and indirectly, in these different contexts in order to tailor the effectiveness of this treatment option.
Acknowledgments
This work was funded by grants to D.J.C. (AR055695, AI067750, AI085130, HL098067). S.S. is the recipient of a National Cancer Institute training grant from the Department of Immunology at the University of Washington School of Medicine. The authors have no conflicting financial interests.
We wish to thank K. Arumuganathan for assistance in flow cytometry and cell sorting, Dr. Alexander Rudensky for providing Foxp3GFP mice, Pamela Johnson and Mary Beauchamp for assistance with histology, and Sylvia McCarty for administrative assistance.
References
- 1.Baccala R, Kono DH, Theofilopoulos AN. Interferons as pathogenic effectors in autoimmunity. Immunological reviews. 2005;204:9–26. doi: 10.1111/j.0105-2896.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 2.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. The Journal of experimental medicine. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 2009;10:373–379. doi: 10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36:511–518. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- 6.Mavrogiannis C, I, Papanikolaou S, Elefsiniotis IS, Psilopoulos DI, Karameris A, Karvountzis G. Ulcerative colitis associated with interferon treatment for chronic hepatitis C. Journal of hepatology. 2001;34:964–965. doi: 10.1016/s0168-8278(01)00022-8. [DOI] [PubMed] [Google Scholar]
- 7.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava S, Koch MA, Pepper M, Campbell DJ. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. Journal of Experimental Medicine. 2014;160:521. doi: 10.1084/jem.20131556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biron CA. Interferons alpha and beta as immune regulators--a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 10.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. Journal of immunology. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 11.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. Journal of immunology. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 13.Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood. 2006;107:987–993. doi: 10.1182/blood-2005-07-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil MP, Ploquin MJY, Watford WT, Lee SH, Kim K, Wang X, Kanno Y, O'Shea JJ, Biron CA. Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood. 2012;120:3718–3728. doi: 10.1182/blood-2012-05-428672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persistent LCMV infection is controlled by blockade of type I interferon signaling. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. Journal of Experimental Medicine. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. Journal of Experimental Medicine. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis and rheumatism. 2008;58:801–812. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- 20.Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. Journal of immunology. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- 21.Goossens P, Gijbels MJJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJAP, Kalinke U, Weber C, Lutgens E, de Winther MPJ. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr Protoc Immunol Chapter. 2001;3(Unit 3.19–3.19.16) doi: 10.1002/0471142735.im0319s17. [DOI] [PubMed] [Google Scholar]
- 23.Goodman T, Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. The Journal of experimental medicine. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. The Journal of experimental medicine. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131:873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, Daly G, Lindahl T, Barnes DE. Gene-targeted mice lacking the Trex1 (DNase III) 3“-->5” DNA exonuclease develop inflammatory myocarditis. Molecular and cellular biology. 2004;24:6719–6727. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice G, Newman WG, Dean J, Patrick T, Parmar R, Flintoff K, Robins P, Harvey S, Hollis T, O'Hara A, Herrick AL, Bowden AP, Perrino FW, Lindahl T, Barnes DE, Crow YJ. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. American journal of human genetics. 2007;80:811–815. doi: 10.1086/513443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Barber GN, Stetson DB. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. Journal of immunology. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. Journal of immunology. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 32.Kole A, He J, Rivollier A, Silveira DD, Kitamura K, Maloy KJ, Kelsall BL. Type I IFNs regulate effector and regulatory T cell accumulation and anti-inflammatory cytokine production during T cell-mediated colitis. The Journal of Immunology. 2013;191:2771–2779. doi: 10.4049/jimmunol.1301093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. The Journal of experimental medicine. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 35.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. Journal of immunology. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 36.Golding A, Rosen A, Petri M, Akhter E, Andrade F. Interferon-alpha regulates the dynamic balance between human activated regulatory and effector T cells: implications for antiviral and autoimmune responses. Immunology. 2010;131:107–117. doi: 10.1111/j.1365-2567.2010.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mozzillo N, Ascierto P. Reduction of circulating regulatory T cells by intravenous high-dose interferon alfa-2b treatment in melanoma patients. Clin Exp Metastasis. 2012;29:801–805. doi: 10.1007/s10585-012-9504-2. [DOI] [PubMed] [Google Scholar]
- 38.Namdar A, Nikbin B, Ghabaee M, Bayati A, Izad M. Effect of IFN-beta therapy on the frequency and function of CD4(+)CD25(+) regulatory T cells and Foxp3 gene expression in relapsing-remitting multiple sclerosis (RRMS): a preliminary study. Journal of neuroimmunology. 2010;218:120–124. doi: 10.1016/j.jneuroim.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Pace L, Vitale S, Dettori B, Palombi C, La Sorsa V, Belardelli F, Proietti E, Doria G. APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. The Journal of Immunology. 2010;184:5969–5979. doi: 10.4049/jimmunol.0900526. [DOI] [PubMed] [Google Scholar]
- 40.Riley CH, Jensen MK, Brimnes MK, Hasselbalch HC, Bjerrum OW, Straten PT, Svane IM. Increase in circulating CD4+CD25+Foxp3+ T cells in patients with Philadelphia-negative chronic myeloproliferative neoplasms during treatment with IFN-α. Blood. 2011;118:2170–2173. doi: 10.1182/blood-2011-03-340992. [DOI] [PubMed] [Google Scholar]
- 41.Type I interferons maintain Foxp3 expression and T-regulatory cell functions under inflammatory conditions in mice. 2012;143:145–154. doi: 10.1053/j.gastro.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schönefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas AA, Luther RJ, Weaver CT, Dooley J, Gray DHD, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tischner D, Gaggl I, Peschel I, Kaufmann M, Tuzlak S, Drach M, Thuille N, Villunger A, Jan Wiegers G. Defective cell death signalling along the Bcl-2 regulated apoptosis pathway compromises Treg cell development and limits their functionality in mice. J Autoimmun. 2012;38:59–69. doi: 10.1016/j.jaut.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 46.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature medicine. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Signaling through the Adaptor Molecule MyD88 in CD4(+) T Cells Is Required to Overcome Suppression by Regulatory T Cells. 2014;40:78–90. doi: 10.1016/j.immuni.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein D, Laszlo J. Interferon therapy in cancer: from imaginon to interferon. Cancer research. 1986;46:4315–4329. [PubMed] [Google Scholar]