Abstract
Human and animal studies indicate that reward function is modulated by the circadian clock that governs our daily sleep/wake rhythm. For example, a robust circadian rhythm exists in positive affect, which is lower in the morning hours and peaks in the afternoon. A handful of functional neuroimaging studies suggest that systematic diurnal variation exists in brain activity related to other functions, but no published human studies have examined daily variation in the neural processing of reward. In the present study, we attempt to advance this literature by using functional neuroimaging methods to examine time-of-day changes in the responsivity of the reward circuit. Using a within-person design and a functional magnetic resonance imaging (fMRI) monetary reward task, we compared morning and afternoon reward-related brain activation in a sample of healthy young adults within 24 h. Region of interest analyses focused on the striatum, and we hypothesized greater reward activation in the afternoon, concordant with the circadian peak in positive affect. Results were consistent with our hypothesis. Additionally, we counterbalanced the order of morning and afternoon scans in order to explore the short-term stability of the neural response. Whole-brain analyses showed a markedly higher reactivity to reward throughout the brain in the first scan relative to the second scan, consistent with habituation to the monetary reward stimuli. However, these effects did not appear to explain the time-of-day findings. In summary, we report the first preliminary evidence of circadian variation in the neural processing of reward. These findings have both methodological and theoretical implications.
Keywords: Circadian, Reward, Brain, Sleep, fMRI
1. Introduction
Human and animal studies indicate that reward function is modulated by the circadian clock that governs our daily sleep/wake rhythm. Both positive affect, an experiential phenomenon related to activation of the reward system, and psychophysiologically assessed reward activation show clear 24-h rhythms, and these rhythms vary according to circadian timing (Boivin et al., 1997; Murray et al., 2009). The pattern of these rhythms—levels are lowest close to wake-up time, then rise to a peak in the late afternoon or evening before beginning to fall—roughly parallels the core body temperature rhythm. Furthermore, these rhythms are paralleled by the diurnal patterns of reward-related behaviors (e.g., socializing and alcohol consumption) (Arfken, 1988; Hasler et al., 2008). Rodent studies also support the circadian modulation of reward-related behavior and its underlying physiology within the reward circuit. Drug-seeking behavior, responsiveness to drugs of abuse, expression of the dopamine transporter, and the expression of circadian genes throughout the mesolimbic dopaminergic pathway all show 24-hour rhythms (Sleipness et al., 2007; Webb et al., 2009). However, no published human studies have examined daily variation in the neural processing of reward.
A handful of functional neuroimaging studies suggest that systematic diurnal variation exists in brain activity related to other functions, including time-of-day variations in the activity of the motor cortex (Tamm et al., 2009; Peres et al., 2011), in hypothalamic and brainstem activation related to maintaining attention (Schmidt et al., 2009), and in brain reactivity to cognitive interference (Schmidt et al., 2012). In addition, several studies have compared brain glucose metabolism during morning and evening wakefulness in healthy adults, adults with major depression, and adults with primary insomnia (Buysse et al., 2004; Germain et al., 2007; Hasler et al., 2012). In all three studies, distinct diurnal patterns of relative glucose metabolism emerged in regions relevant to reward function, including the medial prefrontal cortex, anterior cingulate cortex, and striatum. Notably, increased evening activity within striatal regions associated with reward processing was a common thread across all three studies.
In the present pilot study, we take the first steps in attempting to advance this literature by using functional neuroimaging methods to examine time-of-day changes in the responsivity of the reward circuit. Using a within-person design and a functional magnetic resonance imaging (fMRI) monetary reward task, we compared morning and afternoon reward-related brain activation in a sample of healthy young adults within 24 hours. The within-person design provides greater statistical power than between-person designs more typically employed in fMRI studies, and it is also powerful in minimizing the contribution of individual differences, by allowing each participant to serve as his/her own control. We focused on the striatum, hypothesizing greater reward activation in the afternoon, consistent with the circadian peak in positive affect (Boivin et al., 1997; Murray et al., 2009). Secondarily, we performed a preliminary exploration of the test-retest reliability of our reward paradigm, albeit confounded within time-of-day. Given the dearth of test-retest reliability data on fMRI reward tasks re-administered less than 7 days apart (Fliessbach et al., 2010; Plichta et al., 2012) and the importance of understanding the reliability of widely used fMRI techniques, we counterbalanced the order of the AM and PM scans in order to examine the short-term stability of striatal response. Additionally, this approach allowed us to detect any potential effects of task habituation.
2. Methods
2.1. Participants and procedures
These data come from a pilot study designed to examine daily changes in reward-related brain function using fMRI. The study was approved by the University of Pittsburgh Institutional Review Board, and all participants provided written, informed consent.
Participants included 11 healthy young adults (7 females), including five participants recruited from an ongoing study using fMRI to study the impact of sleep deprivation on affect regulation. All participants were free of major medical, sleep, or psychiatric disorders, based on a clinical interview and several standard sleep-related instruments, including the Pittsburgh Sleep Quality Index (PSQI; (Buysse et al., 1989)) and a daily sleep diary, which included items on bedtime, lights out (the time participants closed their eyes with their intention to fall asleep), sleep onset, sleep offset, sleep latency (interval from lights out until sleep onset), and wakefulness after sleep onset (amount of wakefulness between sleep onset and sleep offset). The diary was completed for a mean (±SD) of 7.50 ± 2.83 days per participant. No participants reported clinically-significant sleep disturbance based on the PSQI (PSQI < 6 for all participants) and mean sleep diary was consistent with this conclusion (see Table 1). Participants also completed the Composite Scale of Morningness (CSM; (Smith et al., 1989)) to assess individual differences in preferred sleep-wake timing (i.e., chronotype or morningness-eveningness). None of the participants reported an extreme chronotype based on the CSM. See Table 1 for demographic information.
Table 1.
Demographics and sleep data (n = 11 unless otherwise indicated)
| Age | 21.51 ± 1.72 years, range = 19–24 |
| Sex | 4 males/7 females |
| Race | 6 Caucasian, 3 African-American, 1 Asian-Pacific, 1 refused to provide info |
| PSQI | 1.82 ± 1.17, range = 0–4 |
| CSM | 34.20 ± 4.86, range = 28–42 |
| Sleep diary (n=10) | |
| Sleep onset (clock time) | 1:01± 0:54, range = 23:44–2:31 |
| SOL (min) | 8.04±1.66, range = 0.00–15.00 |
| WASO (min) | 2.90±0.88, range = 0.00–6.63 |
| Sleep offset (clock time) | 8:36± 0:49, range = 7:50–10:05 |
| TST (h) | 7.53±0.97, range = 5.25–8.33 |
Notes: PSQI = Pittsburgh Sleep Quality Index; CSM = Composite Scale of Morningness; SOL = Sleep onset latency; WASO = Wake after sleep onset; TST = Total sleep time
The study included two fMRI scans.1 Following the baseline assessment and a week on a stable self-selected sleep-wake schedule, participants underwent the two scans: one during the morning (on average, 1.56 h after habitual waketime; range = −0.10–3.80 h), and one during the afternoon (on average, 8.23 h after waketime; range = 6.62–10.80 h1).
The order of these scans was counter-balanced, such that six participants completed the morning scan first, and the afternoon scan later that day, and five participants completed the afternoon scan first, and the morning scan the next morning. All six of the AM–PM order participants completed both scans on the same day. Four of the five PM–AM order participants completed scans on consecutive days. The sole exception completed the PM scan as scheduled, but rescheduled the AM scan 13 days later.
On average, AM scans occurred at 10:11 (range = 7:32 – 11:47) and PM scans occurred at 16:51 (range = 15:06 – 18:38), with a mean difference in timing between AM and PM scans equal to 11 h and 40 min (range = 5.50 – 18.82 h2). Participants were asked to avoid naps, caffeine and alcohol use on scan days. Participants completed a guessing task with monetary reward during both the morning and afternoon scans.
2.3. fMRI monetary reward task
To probe patterns of neural activity in response to monetary reward, we used a card guessing fMRI paradigm. The block design paradigm consists of pseudorandom presentation of trials wherein participants played a card guessing game and received either positive or negative (i.e., win or loss) feedback for each trial. Participants were told that their performance on the game would determine the monetary reward received at the end of the study, earning $1 for each “correct” guess and losing $0.50 for each “incorrect” guess. Participants were unaware of the fixed outcome probabilities associated with each block until the entire study protocol was completed, at which time they were debriefed and compensated $10 for each completion of the reward task.
During each trial of this task, participants are given 3 s to guess, via button press, whether the value of a visually presented card would be higher or lower than 5 (index and middle finger, respectively). After a choice was made, the numerical value of the card was presented for 500 ms and followed by appropriate feedback (green, upward arrow for win; red, downward arrow for lose) for an additional 500 ms. Upon receiving positive feedback, subjects were required to respond via button press to collect the money for that trial (i.e., consummatory behavior). An inter-trial crosshair was then presented for 3 s, for a total trial length of 7 s. Each block consisted of five trials, with three “win” blocks each of predominantly positive feedback (80% correct) and three “lose” blocks of predominantly negative feedback (80% incorrect), interleaved with three control blocks. During control (‘neutral’) blocks, participants were instructed to simply make alternating button presses during the presentation of an ‘x’ (3 s), which is followed by an asterisk (500 ms) and a yellow circle (500 ms), and then a crosshair (3 s). Each block was preceded by a 2-s instruction of “Guess Number” (for positive or negative feedback blocks) or “Press Button” (for control blocks), resulting in a total block length of 37 s and a total task length of less than 6 min.
2.3.1. fMRI data acquisition
Neuroimaging data were collected using a 3.0 Tesla Siemens TIM Trio MRI scanner at the Magnetic Resonance Research Center at the University of Pittsburgh Medical Center. Visual stimuli were presented by projecting images onto a rear projection screen at the subject’s chest and viewed through a mirror attached to the head coil. Stimulus presentation and registration of responses were controlled by a Windows-based computer running E-prime (Psychology Software Tools, Pittsburgh, PA). Participants’ button press responses and reaction times were recorded using an RF-shielded response box and cable connected to the computer. Structural T1-weighted volumetric scans were obtained during the first scanning session using a magnetization prepared gradient echo (MP-RAGE) sequence: 192 1.0-mm axial slices; flip angle 9°; field of view=256×192 mm; TR=2200 ms; TE=3.29 ms; matrix=256×192. BOLD images were acquired with a gradient echo EPI sequence with 39 3-mm axial slices; flip angle 90°; field of view=205mm; TR=2000 ms; TE=25 ms; matrix=64×64.
2.4. Subjective positive affect
Just before each scan, participants completed the state version of the Positive Affect and Negative Affect Schedule (PANAS; Watson et al., 1988) to provide a subjective assessment of reward function.
2.5. Monitoring of wakefulness and subjective sleepiness
Participants were monitored for wakefulness by having an Applied Sciences Laboratory (Bedford, MA) eye-tracker camera trained on the participant’s left eye. While the scan was being collected, the participant’s eye was continually monitored by study staff. In the event of eye-lid closures > 5 s, participants were prompted by a click or by speaking over the intercom. Few participants required any such prompts—one participant was prompted twice during a scan session, two other participants were each prompted once, and the remaining participants required no prompting.
Participants also completed a self-reported measure of sleepiness via 5-point Likert scale (ranging from 1 ‘Not at all’ to 5 ‘Extremely’) immediately following the reward task.
2.6. Data analysis
Clinical data were analyzed using SPSS (v20). Neuroimaging data were preprocessed and analyzed using SPM8. Preprocessing steps included realignment each participant’s data to the first volume in the time series to correct for head motion, coregistration of the realigned image with the subject’s anatomical image, segmentation to restrict analyses to gray matter, normalization to the standard Montreal Neurological Institute (MNI) template, and spatial smoothing with a Gaussian kernel of 6-mm full-width at half-maximum.
Preprocessed data were analyzed using a two-level random-effects procedure within a general linear modeling (GLM) framework. For each participant and scan, the main effect of task at each voxel was calculated using a t-statistic, generating a 3D statistical image for the contrast of interest (win – control). Movement parameters from the realignment stage were entered as six covariates of no interest to control for participant movement. These first level contrast images then included in second level region of interest (ROI) and whole-brain analyses. We conducted two sets of analyses. Our primary analyses used a SPM’s flexible factorial model for comparing AM to PM scans (a within-person factor assessing diurnal variation) while including a between-person factor to control for scan order. The second exploratory analysis used a paired t-test to compare the first and second scan, independent of time of day (assessing task habituation/potentiation effects). In both sets of analyses we examined the win-control contrast within the a priori defined striatal ROI. In addition, we conducted whole-brain analysis to confirm the involvement of the striatum and to explore other regions sensitive to the time-of-day effect. We selected the win-control contrast given our particular interest in positive affect and reward processes unconfounded by negative affect. We additionally analyzed the win-loss contrast within the striatal ROI, as some investigators argue this contrast is superior for isolating a specific reward effect. The striatal mask was anatomically defined combining the nucleus accumbens, caudate head, and putamen regions from WFU PickAtlas (v3.0.3). We applied a height threshold of p<0.05 and corrected cluster-level threshold of p<0.05 (based on 1000 Monte Carlo simulations in AlphaSim). We used the AlphaSim program 3dFWHM to calculate smoothness for the AlphaSim simulations, obtaining a FWHM=7 mm. The AlphaSim simulations resulted in a minimum extent of 110 voxels for the VS ROI and 720 voxels for the whole-brain analyses.
3. Results
3.1. AM-PM differences in subjective positive affect
A total of nine participants completed the PANAS before both AM and PM scans. Positive affect (PANAS) did not significantly differ between AM and PM assessments [mean (± SD)=24.78 ± 9.61 and 25.44 ± 9.15, respectively; t (8) −0.44, p=0.67, d=0.31].
3.2. AM-PM differences in neural response to reward
Consistent with our hypothesis, ROI analyses revealed greater activation to win > neutral within the ventral striatum (137 voxels, t=5.19, pcorrected <0.05, peak voxel [12 12 −2]) during the PM scan relative to the AM scan (Fig. 1A). No clusters within the striatum showed greater activation during the AM scan.
Fig. 1.
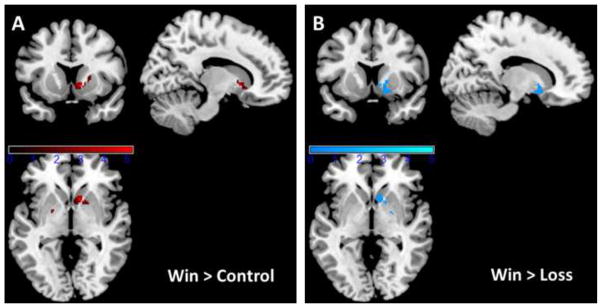
Time of day changes in the neural response to monetary reward. Results are based on a paired t-test run in SPM8 and masked for the region of interest in striatum. Overlapping clusters in the ventral striatum showed greater reactivity to win vs. control (A) and to win vs. loss (B) during the PM scan compared with the AM scan. Results are thresholded at pcorrected < 0.05 using extent computed with AlphaSim to adjust for Type I error.
We ran several follow-up analyses to better understand our ROI analyses. First, we re-conducted the ROI analysis using the win > loss contrast. This analysis identified a 141-voxel striatal cluster that overlapped with the cluster identified in the win > control contrast (Fig. 1B), encompassing the same peak voxel (t=3.31, pcorrected <0.05, peak voxel [18 8 −10]). Second, given that the temporal interval between the scans was larger in the PM-AM group than in the AM-PM group, we re-ran the analyses with scan interval as a covariate and found no change in our findings.
Our exploratory whole-brain analyses revealed different patterns of relative activation across AM and PM scans, and the PM>AM results included a cluster encompassing the same peak voxel [12 12 −2] identified in the ROI analysis (see Supplement).
3.2.1. AM-PM differences in measures related to attention and/or vigilance
We investigated potential AM-PM differences in reaction times during the initial guess of whether the number was less than or more than 5. Participants did not evidence any AM-PM differences in reaction time during the task [mean (± SD)=697.70 ± 125.01 and 711.86 ± 176.39 ms, respectively; t (10)= −0.35, p=0.74].
We also investigated AM-PM differences in participants’ self-reported sleepiness following the reward task. The PM sleepy ratings [mean (± SD)=2.45 ± 1.29] were significantly higher (t=−2.67, p =0.02) than AM sleepy ratings [mean (± SD) =1.73 ± 0.79].
3.3. Exploratory analyses of first scan-second scan differences in neural response to reward
Based on ROI analyses, no clusters within the striatum showed relatively greater activation during the first or second scan.
Our whole brain analyses revealed relatively greater reward reactivity during the first scan in widespread regions throughout the brain, including a 7692-voxel cluster encompassing bilateral medial frontal areas with a peak in the dorsal medial PFC (t=9.29, pcorrected < 0.05, peak voxel [−10 62 20]), a 2580-voxel cluster encompassing portions of the right temporal and parietal lobes (t=5.25, pcorrected < 0.05, peak voxel [54 −72 20]), a 1652-voxel cluster encompassing bilateral precuneus, inferior parietal lobule, and occipital lobe (t=4.76, pcorrected = 0.051, peak voxel [−44 −82 8]), a 1525-voxel cluster encompassing the bilateral precuneus, left superior parietal lobule, and left posterior cingulate (t=3.47, pcorrected < 0.05, peak voxel [2 −36 24]), and a 736-voxel cluster encompassing portions of the left middle temporal lobe, parahippocampal gyrus, fusiform gyrus, lingual gyrus, and hippocampus (t=5.67, pcorrected < 0.05, peak voxel [−24 −40 −6]).
In contrast, no clusters from whole-brain analyses exhibited greater reward reactivity in the second scan relative to the first scan at the whole-brain level.
Participants did not evidence any first scan-second scan differences in reaction time during the task [mean (± SD) = 729.81 ± 149.20 and 679.76 ± 152.36 ms, respectively; t (10) = 1.32, p = 0.22].
4. Discussion
Our novel design examined within-person changes in neural response to monetary reward according to time-of-day in a sample of 11 healthy young adults. As hypothesized, we found preliminary evidence of elevated reactivity to reward in the ventral striatum in the afternoon relative to the morning, consistent with circadian modulation of the neural processing of reward. Our counterbalanced scan order also allowed us to explore order or habituation effects over timeframes less than 24 h, and we found preliminary evidence of markedly higher reactivity to reward throughout the brain in the first scan relative to the second scan, consistent with habituation to the monetary reward stimuli. No clusters exhibited greater reactivity to reward in the second scan relative to the first scan. Importantly, we employed both experimental (counterbalanced order) and statistical (covariate for scan order) controls in our time-of-day analyses, indicating that our finding is not an artifact of the order or habituation effects. While our findings must be interpreted with caution until replicated with a larger sample, they suggest that the neural processing of reward shows time-of-day changes consistent with those seen in other reward-related processes, and both the apparent time-of-day and scan order effects have methodological implications for neuroimaging studies of the reward circuit.
Our finding that the ventral striatum responded more strongly to monetary reward in the afternoon relative to the morning is consistent with well-documented circadian patterns in positive affectivity (Clark et al., 1989; Boivin et al., 1997; Hasler et al., 2008; Murray et al., 2009), which tends to be at low levels in the early morning before rising to a peak in the late afternoon or early evening. While positive affect and reward responsivity are overlapping, but not synonymous processes, our findings suggest that neural correlates of the hedonic experience associated with receiving a monetary reward show a similar daily pattern to that observed in positive affect in other studies. Furthermore, a psychophysiological marker of reward activation—specifically changes in heart rate in response a simple motor task with monetary reward—showed a circadian pattern parallel to that observed in positive affect (Murray, 2009). Our block-design task does not allow us to distinguish between anticipatory and consummatory aspects of the reward response, and future studies should include event-related designs capable of parsing these “wanting” and “liking” facets. In addition, our study measured diurnal differences between AM and PM, and did not include a physiological marker of circadian timing nor the controlled light/dark and sleep/wake conditions required to determine if these diurnal patterns (i.e., time-of-day differences without certain endogenous rhythmicity) are bona fide circadian effects (i.e., the rhythm has an endogenous basis). Thus, while it is intriguing to consider whether these findings reflect circadian influence, it will be critical for future studies to test this hypothesis directly and to investigate whether this pattern of findings reflects circadian influence or other factors that may change across the course of the day, such as alertness or sleep homeostasis. Nonetheless, taken together with this prior literature and animal studies (e.g., (Sleipness et al., 2007; Murray et al., 2009; Webb et al., 2009), our findings offer preliminary evidence that circadian rhythms in reward function exist at multiple levels of analysis.
Circadian rhythms in reward function have both theoretical and methodological implications. At the theoretical level, such rhythms would inform our mechanistic understanding of the role of circadian disturbance in mood and substance use disorders. The rhythms in reward function may contribute to diurnal mood variation reported by some individuals with depression, in which the distress experienced is typically greatest early in the day (Leibenluft et al., 1992; Murray, 2007; Wirz-Justice, 2008). Likewise, the afternoon/evening peak in ventral striatum reward responsivity may contribute to the typically greater substance use during that time of day (Arfken, 1988). Although circadian-based hypotheses of mood disorders have been discussed in the literature for decades (e.g., Wehr et al., 1979), past findings have not converged on any pathophysiological mechanisms. Nevertheless, interest in circadian contributions to mood and substance use disorders has increased dramatically in recent years. This increased interest is driven in part by the identification of the molecular clock and the recognition that this clock is operating in cells throughout the brain and periphery (Mohawk et al., 2012). Notably, circadian genes are rhythmic in neural tissues outside the central clock in the hypothalamus (the suprachiasmatic nucleus, or SCN), including brain regions related to reward processing. Desynchrony between the SCN and reward-related brain regions, or between cortical and subcortical elements of the reward circuit, may contribute to the dysregulated reward processing intrinsic to mood and substance use disorders. Our findings are the first step in documenting that the responsiveness of the ventral striatum to reward follows a predictable rhythm, and future studies might use more frequent sampling of a broader range of brain areas to look for evidence of desynchrony in mood-disordered individuals.
Even if the effects are not strictly circadian, time-of-day differences in the neural response to reward also have methodological implications. Our findings suggest that it is important to consider time-of-day effects when scheduling scan sessions, or at least to include the time-of-day of the scan as a covariate when analyzing data from reward or affective fMRI tasks. Furthermore, ostensible circadian changes in the neural response to reward also suggest that it is important to consider individual differences in circadian phase, sometimes referred to as chronotype, which fall along a continuum from morning-types to evening-types. Indeed, we recently reported that chronotypes differ in their neural responses to a variant of the monetary reward task used in this study (Hasler et al., 2013). The present study excluded extreme chronotypes for this very reason, but such a practice is rare outside the sleep and circadian literature. Notably, given changes in chronotype across the lifespan (Roenneberg et al., 2003), any studies comparing adolescents or young adults (who tend towards eveningness) to older adults (who tend towards morningness) would be confounded by chronotype without proper design considerations.
Although they were exploratory analyses, we found a marked reduction in neural response to reward from the first scan to the second scan, regardless of time-of-day, suggesting that habituation to the monetary reward is occurring on this time scale. The affected regions have all been implicated in reward-relevant processes, including medial frontal regions associated with evaluating the relative value of reward and reward-directed behavior (Haber and Knutson, 2010), superior and middle temporal regions bordering on the parietal cortex that have been linked to social processing (Korn et al., 2012) and salience facilitating value-based decision-making (Kahnt and Tobler, 2013), and a cluster encompassing parts of the precuneus and inferior parietal lobule associated with self-referential cognition and future planning (Buckner et al., 2008). Past studies of test-retest reliability of similar fMRI reward tasks have reported mixed findings, although the most carefully controlled of these reported good re-test reliability of the monetary incentive task (MID)—a widely used monetary reward task—when repeated approximately 14 days later (Plichta et al., 2012). Notably, that study reported a mild enhancement effect at the second administration—increased activation in ventral striatum—a pattern that we did not replicate on our <24-h time scale. We speculate that this lack of replication may be due in part to the differences in length of delay—the apparent habituation effect might only appear when short intervals between scans are used—as well to differences between the MID (which emphasizes reward anticipation) and the current task (which includes both reward anticipation and reward outcome). Importantly, our counterbalanced design should minimize the impact of this apparent habituation effect on our time-of-day finding (i.e., greater striatal activation to reward in the evening), a conclusion buttressed by the fact that none of the observed clusters exhibiting habituation encompassed the right ventral striatum. Finally, the increased response during the first scan could relate to a larger stress response (resulting in the release of cortisol or alpha-amylase) resulting from the first time undergoing a scanning procedure.
In contrast to consistent demonstrations of higher positive affect in the late afternoon and evening relative to morning hours (Clark et al., 1989; Boivin et al., 1997; Hasler et al., 2008; Murray et al., 2009; Hasler et al., 2012), we did not observe a statistically significant difference in self-reported positive affect assessed prior to the AM and PM scans. However, our sample size gave us limited power to detect effects on subjective mood, and the medium effect size of this finding (d = 0.31) indicates that this difference would be significant with a larger sample. Furthermore, the assessments may not have been spaced sufficiently far apart in order to detect the typical diurnal increase in positive affect, which starts in the early morning hours and peaks in the late afternoon. Notably, self-reported sleepiness was relatively greater after the afternoon scan, suggesting that the time of the afternoon scan may have fallen into the afternoon “circadian dip” that characterizes circadian rhythms in alertness (Monk et al., 1996), and which could potentially apply to positive affect (and reward function more broadly). (In contrast, we did not find evidence of behavioral differences in alertness based on the comparison of reaction time during the AM and PM administrations of the task.) Based on this assumption, we speculate that our time-of-day effects on reward may have been larger if the PM scan had been scheduled sufficiently later to occur after the “circadian dip”.
In conclusion, the present findings provide the first preliminary evidence that the neural correlates of reward function exhibit a pattern of daily changes consistent with other documented circadian rhythms in positive affect and a psychophysiological marker of reward activation (Murray et al., 2009). Definitive demonstration that our time-of-day effects reflect endogenous circadian timing will require physiological circadian measures and experimental protocols capable of disentangling circadian effects from other factors that change thoughout the day (e.g., homeostatic sleep drive). Nevertheless, our study joins a small contingent of neuroimaging studies that have consistently documented time-of-day effects on neural responsivity in areas throughout the brain (Schmidt et al., 2009; Tamm et al., 2009; Peres et al., 2011; Schmidt et al., 2012). Although the within-person design enhanced statistical power, given the small sample size, replication with a larger sample will be essential. Follow-up studies will also need to address the challenge of identifying or developing reward tasks with greater test-retest reliability when re-administered within the same 24-h period. Such efforts are worthwhile, as definitive evidence of circadian rhythms in the neural processing of reward would importantly inform mechanistic understanding of the role of disturbed circadian rhythms in mood and substance use disorders. More broadly, we assert that neuroimaging studies of all stripes should intentionally investigate time-of-day effects, given their important methodological and theoretical implications.
Supplementary Material
HIGHLIGHTS.
Reward function, including positive affect, is modulated by the circadian clock
No neuroimaging studies have examined time-of-day changes in reward function
Using fMRI, we compared AM-PM changes in striatal response to monetary reward
As predicted, striatal response to reward was higher in the afternoon
In addition, whole brain findings suggest habituation from first to second scan
Acknowledgments
The authors would like to thank the participants who participated in this study. The authors would also like to thank Denise Duryea and the staff of the University of Pittsburgh Magnetic Resonance Research Center for their invaluable technical assistance. This work was supported by a neuroimaging pilot award from the Department of Psychiatry at the University of Pittsburgh and grants from the National Institutes of Health (K01 MH077106 (Franzen); K01 DA 032557 (Hasler)).
Footnotes
These were the first and second scans under normal sleep conditions. Two of the five participants recruited from the sleep deprivation study had already completed a fMRI scan and reward task under sleep-deprived conditions a respective 7 and 12 days beforehand. Removing these two participants from the analyses did not result in substantive changes to the findings reported in section 3.3 (all three clusters remained significant after correction for multiple comparisons).
Including the 13 intervening days of the aforementioned participant that rescheduled their AM scan would raise the maximum scan time difference to 304.32 h. Dropping this participant from the AM–PM comparison (Section 3.2) resulted in findings that were in the same direction, albeit somewhat weaker (the VS cluster in the PM>AM contrast had the same peak voxel with a t-value = 4.49 and cluster size below the AlphaSim threshold at 52 voxels).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arfken CL. Temporal pattern of alcohol consumption in the United States. Alcoholism: Clinical and Experimental Research. 1988;12:137–142. doi: 10.1111/j.1530-0277.1988.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Totterdell P, Waterhouse JM. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombau H, Kupfer DJ, Moore RY. Regional brain glucose metabolism during morning and Evening wakefulness in humans: Preliminary findings. Sleep. 2004;27:245–254. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- Fliessbach K, Rohe T, Linder NS, Trautner P, Elger CE, Weber B. Retest reliability of reward-related BOLD signals. NeuroImage. 2010;50:1168–1176. doi: 10.1016/j.neuroimage.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Meltzer CC, Wood A, Kupfer DJ, Moore RY, Buysse DJ. Diurnal variation in regional brain glucose metabolism in depression. Biological Psychiatry. 2007;62:438–445. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, James JA, Bi W, Buysse DJ. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. Journal of Sleep Research. 2012;94:215–226. doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Mehl MR, Bootzin RR, Vazire S. Preliminary evidence of diurnal rhythms in everyday behaviors associated with positive affect. Journal of Research in Personality. 2008;42:1537–1546. [Google Scholar]
- Hasler BP, Sitnick SL, Shaw DS, Forbes EE. Preliminary evidence that an altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging. 2013;214:357–364. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Tobler PN. Salience signals in the right temporoparietal junction facilitate value-based decisions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:863–869. doi: 10.1523/JNEUROSCI.3531-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn CW, Prehn K, Park SQ, Walter H, Heekeren HR. Positively biased processing of self-relevant social feedback. The Journal of Neuroscience. 2012;32:16832–16844. doi: 10.1523/JNEUROSCI.3016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Noonan BM, Wehr TA. Diurnal variation: reliability of measurement and relationship to typical and atypical symptoms of depression. Journal of Affective Disorders. 1992;26:199–204. doi: 10.1016/0165-0327(92)90016-y. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annual Review of Neuroscience. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, 3rd, Kupfer DJ. Circadian determinants of the postlunch dip in performance. Chronobiology International. 1996;13:123–133. doi: 10.3109/07420529609037076. [DOI] [PubMed] [Google Scholar]
- Murray G. Diurnal mood variation in depression: A signal of disturbed circadian function? Journal of Affective Disorders. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Peres I, Vetter C, Blautzik J, Reiser M, Poppel E, Meindl T, Roenneberg T, Gutyrchik E. Chronotype predicts activity patterns in the neural underpinnings of the motor system during the day. Chronobiology International. 2011;28:883–889. doi: 10.3109/07420528.2011.619084. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, Gerdes AB, Sauer C, Tost H, Esslinger C, Colman P, Wilson F, Kirsch P, Meyer-Lindenberg A. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage. 2012;60:1746–1758. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A, Gais S, Schabus M, Desseilles M, Dang-Vu TT, Salmon E, Balteau E, Degueldre C, Luxen A, Maquet P, Cajochen C, Peigneux P. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–519. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Leclercq Y, Sterpenich V, Vandewalle G, Phillips C, Berthomier P, Berthomier C, Tinguely G, Gais S, Schabus M, Desseilles M, Dang-Vu T, Salmon E, Degueldre C, Balteau E, Luxen A, Cajochen C, Maquet P, Collette F. Circadian preference modulates the neural substrate of conflict processing across the day. PloS One. 2012;7:e29658. doi: 10.1371/journal.pone.0029658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Research. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Tamm AS, Lagerquist O, Ley AL, Collins DF. Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. Journal of Biological Rhythms. 2009;24:211–224. doi: 10.1177/0748730409334135. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive affect and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. Journal of Biological Rhythms. 2009;24:465–476. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A, Goodwin FK, Duncan W, Gillin JC. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206:710–713. doi: 10.1126/science.227056. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues in Clinical Neuroscience. 2008;10:337–343. doi: 10.31887/DCNS.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


