Abstract
Oncolytic viruses (OVs) constitute a promising class of cancer therapeutics which exploit validated genetic pathways known to be deregulated in many cancers. To overcome an immune response and to enhance its potential use to treat primary and metastatic tumors, a method for liposomal encapsulation of adenovirus has been developed. The encapsulation of adenovirus in non-toxic anionic lecithin-cholesterol-PEG liposomes ranging from 140–180nm in diameter have been prepared by self-assembly around the viral capsid. The encapsulated viruses retain their ability to infect cancer cells. Furthermore, an immunoprecipitation (IP) technique has shown to be a fast and effective method to extract non-encapsulated viruses and homogenize the liposomes remaining in solution. 78% of adenovirus plaque forming units were encapsulated and retained infectivity after IP processing. Additionally, encapsulated viruses have shown enhanced transfection efficiency up to 4× higher compared to non-encapsulated Ads. Extracting non-encapsulated viruses from solution may prevent an adverse in vivo immune response and may enhance treatment for multiple administrations.
Keywords: Adenovirus, Drug Delivery, Gene Therapy, Liposome, Nanoparticle, Phospholipid
Introduction
Oncolytic replication–selective viruses (OVs) can be directed at several mechanisms of action and exploit genetic pathways known to be deregulated in many cancers [1]. Viral cancer gene therapy holds great promise due to the approach which takes advantage of the virus’ ability to specifically replicate within cancer cells to levels that are many logs higher than the input dose, lyse the infected cell and subsequently spread to adjacent cells [2–4]. Attributable to their therapeutic potential, OVs have been rapidly translated into human clinical trials in patients with advanced cancer [5–9] where their safety has been demonstrated. A number of oncolytic viruses have shown clinical therapeutic activity such as GM-CSF-expressing vaccinia (JX-594; Jennerex Inc.), HSV (Oncovex; Biovex Inc.), and ONYX-015 (Adenovirus, Shanghai Sunway Biotech Co., Ltd) [7, 8, 10]. Replication-selective adenoviruses (Ads) possess a number of advantages [4, 11, 12]. Human Ads are well characterized and are associated with relatively mild diseases, their genomes can be easily manipulated, and they can be produced at high titers [4, 13, 14]. Following positive initial pre-clinical studies of ONYX-015 which has a deletion of the viral E1b-55k gene, clinical trials were conducted in head and neck, gastrointestinal, ovarian, brain, pancreatic and breast cancer as well as oral dysplasia using local injections [8–10, 15–27]. Following ONYX-015 administration, studies demonstrated that the therapy was well-tolerated by intratumoral, intraperitoneal, intravenous, and intraarterial administration, however the therapy lacked adequate potency to be applicable as a single therapeutic agent. Hedjran et al. demonstrated that TAV-255, an E1A enhancer deletion vector, possessed enhanced potent oncolytic activity and tumor selectivity [28]. In addition, OVs can be further enhanced by inserting therapeutic genes such as GM-CSF to improve efficacy [29, 30]. GM-CSF expressing vectors are effective in generating systemic immunity against a number of poorly immunogenic tumors causing long-lasting antitumor immunity [31]. Despite these advances, the utility of OVs for treatment of metastatic disease is limited by restricted distribution of the virus to the tumors cells due to 1) rapid clearance by the reticuloendothelial (RE) system in the liver and 2) neutralization by antibodies.
OVs are promising agents to combine with nanoparticle delivery approaches because of their ability to escape immune recognition. In systemic delivery, targeting with nanoparticles may focus the viral load to the primary tumor cells as well as metastatic tumors to ensure a productive initial infection. Conventionally, encapsulation of negatively charged Ads in positively charged, cationic liposomes or particles have been used to overcome rapid clearance from the circulation to evade the immune barrier [32]. Despite the promising in vitro results, cationic liposomal encapsulation in vivo have been hindered by toxicities, low tissue specificity, and poor serum stability due to incompatibility with the abundance of negatively charged macromolecules present in the physiological environment [33]. To overcome these shortcomings, anionic liposomes may be used for encapsulation of Ad. Zhong et al. reported a calcium-induced phase change method to encapsulate adenovirus5 (Ad5) into anionic liposomes and showed that anionic encapsulation in liposomes enhanced the transfection efficiency of Ad5, significantly improved gene expression in murine airway tissues when delivered in vivo by intratracheal instillation, and provided protection from neutralizing antibodies [34, 35]. Gene expression of apical cells infected with Ads encapsulated in anionic liposomes was 6-fold higher compared to naked virus [34]. Zhong et al. used a negatively charged cholesterol derivant, cholesteryl-hemisuccinate (CHEMS), egg phosphatidylcholine (PC) and cholesterol for liposomal encapsulation. In addition, the research group has shown that folate targeting may be used as a targeting ligand for airway epithelial cells [36].
With the aim to enhance the delivery of viral vectors, a non-toxic material, refined lecithin, a mixture of phosphatidylcholine, phosphatidylethanolamine, inositol phosphatides, and other phospholipids as well as cholesterol and polyethylene glycol-2000 (PEG2000) were employed to encapsulate Ad5. Additionally, an immunoprecipitation technique was implemented to extract non-encapsulated adenovirus in solution to assure that all viruses are protected from immune recognition. The encapsulation efficacy, transfection efficiency and effectiveness to protect against neutralizing antibodies in serum were evaluated for the anionic liposome-adenovirus complex.
Materials and methods
Cell culture
HEK293A human embryonic kidney cells and A549 human lung carcinoma cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured with Dulbecco’s-modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin Streptomycin Glutamine (PSG).
Synthesis of Lecithin liposomes using lipid film hydration method
Refined Lecithin (Alfa Aesar, Ward Hill, VA) or DOTAP (Avanti polar lipids, Alabaster, Al), Cholesterol (Sigma-Aldrich, St. Louis, MO), and DSPE-PEG(2000)carboxylic acid (Avanti polar lipids, Alabaster, Al) suspended in chloroform at 10mg/ml were mixed in 7ml amber vials at a 2:1:0.1mM ratio, respectively. The mixtures were placed under vacuum overnight resulting in dry lipid films. The films were hydrated drop wise while vortexing with 400µl of adenovirus-CMV-GFP (Baylor College of Medicine, Houston, TX) suspended in phosphate buffered saline (1× PBS) at 5×1010 viral particles/ml (vp/ml). For empty liposomes, lipid films were hydrated dropwise with 400µl of 1× PBS. A small magnetic stirring rod was added, and the hydrated films were stirred for 30 min at 4°C. The samples were subsequently transferred to eppendorf tubes and placed in an ultrasonic water bath (Fisher Scientific, Model FS11011) for 10min at 4°C. The suspension was stored at 4°C until ready for use resulting in lecithin:cholesterol:PEG-adenovirus liposomes (Lec_Ad), empty lecithin:cholesterol:PEG liposomes (empty_Lec), and DOTAP:cholesterol:PEG0-adenovirus liposomes (DOTAP_Ad).
Immunoprecipitation of non-encapsulated adenovirus
12.5µl of 1µg/ml anti-hexon IgG (Thermo Scientific, Rockford, IL) was added to 20 µl of Lec_Ad, DOTAP_Ad, or empty_Lec and vortexed at 4°C for 1 h. 25µl of 2µm nonporous superparamagnetic Protein G beads (New England BioLabs) were washed with 1ml of 0.1M sodium biphosphate, resuspended in 80 µl and added to the sample containing anti-hexon IgG mixed with Lec_Ad, DOTAP_Ad, or empty_Lec. The mixture was vortexed for 1 h at 4°C. A magnet was used to pellet the magnetic beads, and the supernatant was transferred to a clean, sterile tube. From herein, lecithin:Chol:DSPE-PEG2000 or DOTAP:Chol:DSPE-PEG2000 liposomes following immunoprecipitaiton processing using magnetic beads will be defined as Lec_Ad +IP, DOTAP_Ad +IP, and empty_Lec +IP. Samples were used for liposome-cell transfection experiments on the same day of preparation.
Viral titer determination
To quantify the virus titer, a plaque forming assay was performed as described by Clontech Adeno-X Expression System (Mountain View, CA). Briefly, HEK293A cells were plated overnight at 1× 106 cells/well on 6-well tissue culture plates pre-coated with Collagen (Biocoat, Falcon). 1/10 serial dilutions of Ad stock or Lec_Ad +IP were prepared and 10µl aliquots were added to cells. The cells were infected for 6 hours, overlaid with agar, and monitored for plaque formation. At day 11, plaques were stained with 0.1%v/v MTT (Sigma) for 3 hrs at 37°C and plaq ue forming units per ml (pfu/ml) was determined. The number of isolated plaques were counted, and the following formula was used to determine the titer (pfu/ml) of the viral stock and the titer of encapsulated adenovirus after immunoprecipitation (Lec_Ad + IP). , where DF= dilution factor, and V= volume of diluted virus added to the well. At least two different wells with different serial dilutions were counted to ensure consistency. The viral particles per ml (vp/ml) was provided by the manufacturer (Baylor College of Medicine, Houston, TX). The vp/ml of Ad stock and Lec_Ad +IP was 5×1012 and 8.8×109 vp/ml, respectively.
Characterization of liposomes
Liposomes were characterized by dynamic light scattering using Malvern Zetasizer nano series (Model Zen3600, Malvern Instruments, Inc) to measure the intensity weighted mean hydrodynamic diameter, zeta potential (ξ), and polydispersity.
Serum-Induced particle aggregation
Human serum (Innovative Research, Novi, MI) was incubated in 96-well plates at 37 °C and 5% CO2 for 1 hr before use for decomplementation. Lec_Ad or DOTAP_Ad liposomes were incubated in whole serum at a 1:1 v/v ratio. The final concentration of Lec_Ad and DOTAP_Ad was 1.4mg lipid/ml and 1.3mg lipid/ml, respectively. All samples contained a final viral particle concentration of 5 × 1010 vp/ml. Samples were incubated in 50% human serum and aggregation was monitored for 24hrs by measurement of absorbance at 560 nm at 37 °C using a Tecan Infinite M200 microplate reader.
Transfection efficiency of Adenovirus
A549 cells were plated overnight at 1 × 105 cells/chamber (1.7 cm2/chamber) (BD Falcon, Bedford, MA) for fluorescence microscopy experiments or at 1 × 104 cells/well in a 96-well plate (Greiner bio-one, Germany) for fluorescence spectroscopy experiments; cultures were incubated at 37°C and 5% CO2 in DMEM media supplemented with 10% FBS, 1%PSG. Ad, Lec-Ad, DOTAP-Ad, and Lec-empty samples before and after immunoprecipitation (−IP, +IP, respectively) were added to cells at a multiplicity of infection (MOI) ranging from 0.43 to 43 and incubated for 48 hours at 37°C and 5% CO2. For fluorescence microscopy analysis, cells were washed two times in 1× PBS and fixed with 2% paraformaldehyde. The slides were sealed with ProLong Gold Antifade reagent (Invitrogen) and imaged using a Zeiss Axio Examiner.Z1 microscope (AlexaFluor488 filter). For fluorescence spectroscopy analysis, cells were re-suspended in 100µl of 1× PBS and fluorescence intensities were measured using a Tecan Infinite M200 microplate reader at an excitation λ of 480 nm and an emission λ of 520 nm.
Neutralization Assay of Encapsulated Ad5
Blood was collected from 129/sv mice containing high neutralizing antibodies due to repeated i.t injection of Ad5. Blood was collected in EDTA vacutainers, centrifuged at 25,000rpm for 15 min and plasma was collected. Plasma was stored at −80°C until ready for use. A549 cells were plated at 10,000 cells/well in a 96-well plate overnight. Ad5, Lec_Ad5, or Lec_Ad5 after immunoprecipitation of non-encapsulated viruses (Lec_Ad5 +IP) were incubated with anti-adenovirus whole antiserum for 1 hour at 37°C diluted to 1/16× followed by ½ serial dilutions. Plasma was first decomplemented for 30min at 56°C. 10µl of plasma was added to cells at corresponding concentrations up to 1/256 followed by addition of Ad, Lec_Ad, or Lec_Ad +IP. Samples were incubated with cells for 24 hours at 37°C and 5% CO2. Cells were re-suspended in 100µl of 1× PBS and fluorescence intensities were measured using a Tecan Infinite M200 microplate reader at an excitation λ of 480 nm and an emission λ of 520 nm.
Folate Targeting
Lecithin:Cholesterol:DSPE-PEG2000/folate liposomes encapsulating Ad5 were prepared as mentioned above. The total moles of DSPE-PEG2000 and DSPE-PEG2000-folate was kept constant (0.1mM); however, the ratio between the two was varied. These ratios consisted of DSPE-PEG2000 to DSPE-PEG2000-folate at 0:0.1mM, 0.01:0.09mM, 0.03:0.07mM, 0.05:0.05mM, 0.07:0.03mM, and 0.1:0mM, respectively.
Results
Viral determination after Immunoprecipitation
A plaque forming assay was employed to determine the viral titer before and after encapsulation. The number of plaques was counted at two different dilutions for Ad stock and Lec_Ad +IP, as shown in Table 1. The stock adenovirus titer was an average of 5.3 × 109 pfu/ml, and the viral titer diluted to the same concentration of samples after IP processing was an average of 9.4 × 106 pfu/ml. Since encapsulated virus is diluted during IP processing (Lec_Ad+IP), the titer of naked Ad is calculated as pfu/ml at +IP concentration. (The dilution factor for IP processing is 1/562.6). The viral titer for Lec_Ad +IP samples was determined to be an average of 7.3 × 106pfu/ml. Therefore, 78% of adenovirus plaque forming units were encapsulated and retained infectivity after IP processing. The data is consistent with a high encapsulation of Ad after hydration and IP processing.
Table 1. PFU assay.
The number of isolated plaques were counted, and the following formula was used to determine the titer (pfu/ml) of the viral stock and the titer of encapsulated adenovirus after immunoprecipitation (IP). pfu/ml= plaques÷(DF×V), where DF= dilution factor, and V= volume of diluted virus added to the well. At least two different wells with different serial dilutions were counted to ensure consistency. Percentage of infective virus after IP is Lec+IP/Ad stock at 1/562.6 concentration. % of retained infectivity of Ad stock +IP is not calculated since pfu was not quantified for Ad+IP.
| Sample | dilution factor (DF) |
plaques counted |
calculated pfu/ml |
vp/ml | calculated pfu/ml, diluted to +IP conc) |
% retained after IP |
|---|---|---|---|---|---|---|
| Ad stock | 1E-06 | 56 | 5.6E+09 | 5.0E+12 | 9.9E+06 | ------ |
| Ad stock | 1E-05 | 501 | 5.0E+09 | 5.0E+12 | 8.8E+06 | ------ |
| Lec_Ad+IP | 1E-03 | 75 | 7.5E+06 | 8.8E+09 | 7.5E+06 | 80% |
| Lec_Ad+IP | 1E-02 | 700 | 7.0E+06 | 8.8E+09 | 7.0E+06 | 75% |
Characterization
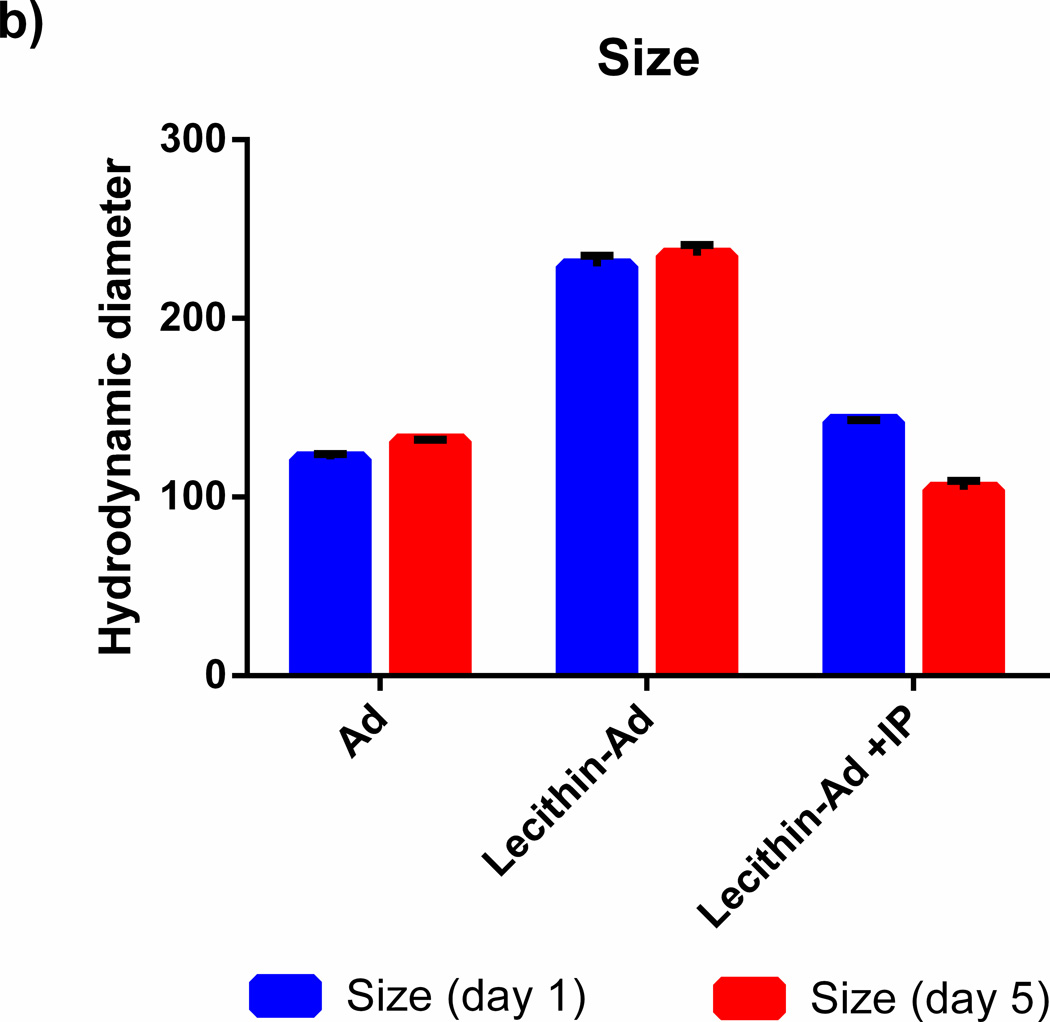
Adenovirus encapsulated in anionic lecithin and cationic DOTAP liposomes were characterized using dynamic light scattering and electrophoretic light scattering as shown in Table 2. The hydrodynamic diameter of naked Ad5 was 123nm with a negative charge of −21mV. After encapsulation in lecithin or DOTAP liposomes, the charge was −59mV and +44mV, respectively. Before immunoprecipitation (IP), lecithin-adenovirus complexes (Lecithin-Ad) were 180nm in size with a high polydispersity index (PDI = 0.7). After IP, the size was reduced to 143nm and the liposomes became more monodispersed (PDI = 0.3). An accurate charge measurement for Lec_Ad +IP, empty Lec_Ad +IP, and DOTAP_Ad +IP samples was not obtained due to the sample being too dilute for an accurate zeta potential measurement. The IP step was incorporated in order to extract non-encapsulated viruses from solution. In addition to an extraction method, the IP technique also served as a homogenization step. For proof of concept, the size of empty lecithin liposomes was measured before and after IP. The size of empty liposomes was reduced from 738nm to 138nm, and the polydispersity was reduced from 0.6 to 0.4. The hydrodynamic diameter and PDI measurements for Ad +IP is not shown due to poor dynamic light scattering measurements that result in a low correlation coefficient. These results are consistent with Ad being effectively extracted from solution and the count being too low for an accurate measurement.
Table 2. Characterization of Ad encapsulation in liposomes.
Size and polydispersity of Ad, Lecithin-Ad, DOTAP-Ad, and empty Lecithin, before and after IP. The measurements shown are the averages taken from three different samples prepared on different days.
| Sample | Hydrodynamic Diameter (d.nm) | Polydispersity Index (PDI) |
|---|---|---|
| Adenovirus | 123 ± 6 | 0.1 |
| Lecithin-Ad –IP | 180 ± 26 | 0.7 |
| Lecithin-Ad +IP | 143 ± 4 | 0.3 |
| DOTAP-Ad –IP | 342 ± 2 | 0.3 |
| DOTAP-Ad +IP | 301 ± 2 | 0.3 |
| empty Lecithin -IP | 738 ± | 0.6 |
| empty Lecithin +IP | 38 138 ± 3 | 0.4 |
Serum-induced particle aggregation
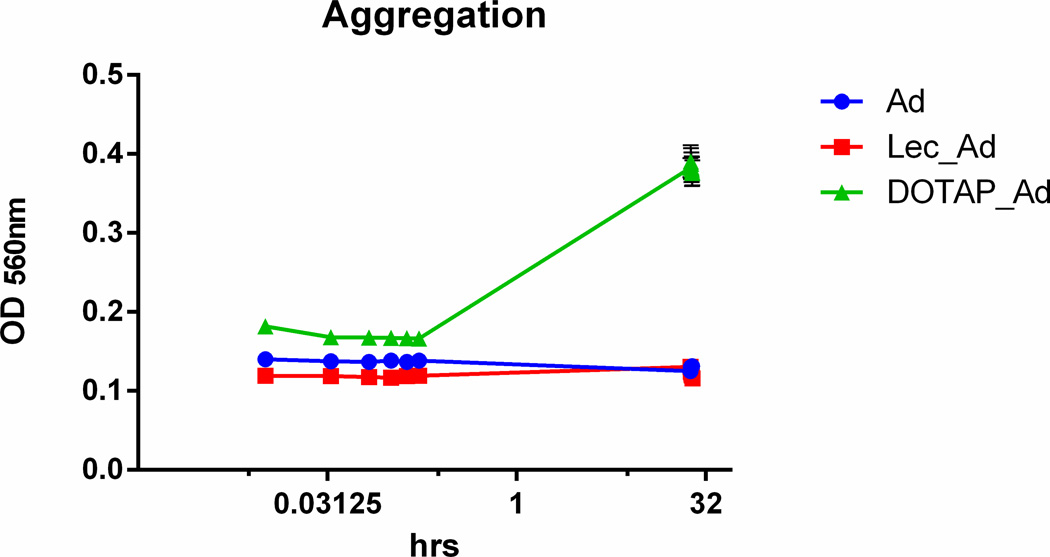
Serum stability of anionic Lec_Ad liposome complexes and cationic DOTAP_Ad liposome complexes was assessed in healthy human serum at 1:1 v/v as shown in Figure 1. The final viral particle concentration was 2.5 × 1010vp/ml for all samples. The final lipid concentration for Lec_Ad and DOTAP_Ad liposome formulations were 1.4mg lipid/ml and 1.3mg lipid/ml, respectively. There are abundant negatively charged serum components present in serum which caused cationic DOTAP_Ad complexes to aggregate over time; conversely, Ad5 encapsulated in anionic lecithin liposomes did not aggregate.
Figure 1. Serum stability of anionic and cationic liposomes.
Aggregation of Ad, Lec_Ad, and DOTAP_Ad was monitored over 24 hrs in 50% human serum by measurement of absorbance at 560 nm at 37 °C. All samples had a final viral particle concentration of 2.5×1010vp/ml, where Lec_Ad and DOTAP_Ad complexes had a final lipid concentration of 1.4mg lipid/ml and 1.3mg lipid/ml, respectively. Aggregation of particles in serum is observed for DOTAP while lecithin liposomes were stable over time.
Transfection Efficiency
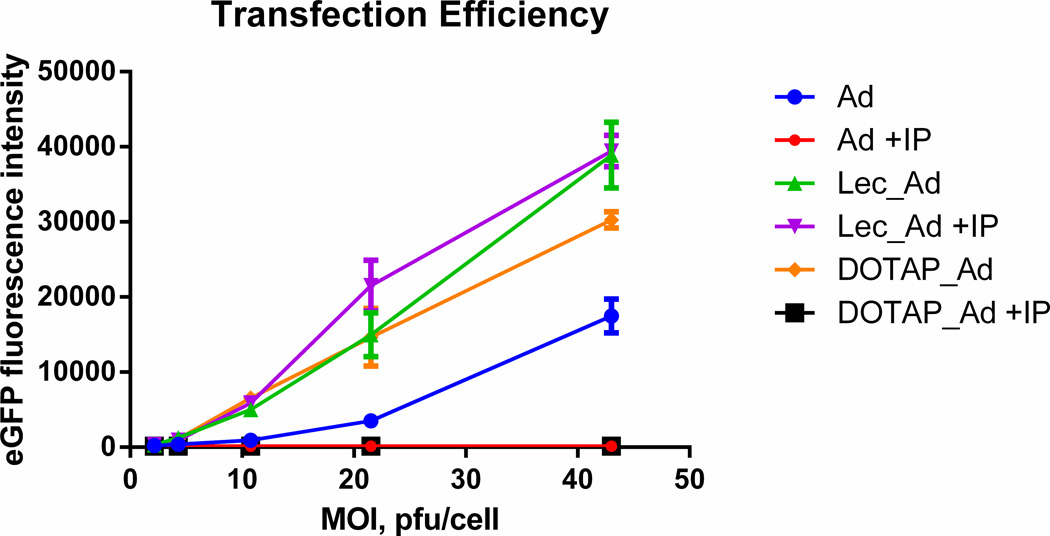
A549 cells strongly express the Coxsackie virus and Adenovirus Receptor (CAR) which enables entry of Ad5 into the cells, and these cells are high permissible to infection. A549 cells were transfected with Ad5, Lec_Ad5, or DOTAP_Ad5 before and after immunoprecipitation (IP) at MOI 2.2, 4.3, 10.75, 21.5 and 43pfu/ml as shown in Fig 2. After IP of naked adenovirus (Ad +IP, red circles), viral protein expression (eGFP) was reduced to zero which showed that the IP method is effective in extracting non-encapsulated viruses. At a wide range of tested MOIs, the transfection efficiency of Ad5 was enhanced when encapsulated in anionic lecithin-Chol-PEG-Ad liposomes (Lec_Ad +IP, purple triangles) compared to naked Ad, DOTAP_Ad, and Lec_Ad before IP. Lec_Ad +IP was the most effective at transfecting cells most likely due to reduction of size and homogenization after immunoprecipitation. In addition, anionic liposomes may enter through an alternative endocytotic pathway which may be more effective and more abundant than the CAR receptor. Before IP, Ad5 encapsulated in DOTAP was effective (DOTAP_Ad, orange diamonds); however, after IP (DOTAP_Ad +IP, black circles), viral protein expression was reduced to background signal only. This suggests that Ad encapsulated in DOTAP is not fully encapsulated or that the IP step is disrupting the cationic liposome layers due to some charge interactions but not affecting anionic liposomes.
Figure 2. Transfection efficiency at a wide range of MOIs.
A549 cells were transfected with Ad, lecithin-Ad (Lec_Ad), and DOTAP_Ad, before and after immunoprecipitation (IP) at MOIs 2.2, 4.3, 10.75, 21.5, and 43 pfu/cell. Lec_Ad +IP (purple) had the highest transfection efficiency at a wide-range of MOIs in comparison to naked Ad, DOTAP_Ad, and Lec_Ad before IP most likely due to reduction of size and homogenization after immunoprecipitation. DOTAP_Ad +IP (black) showed no signal suggesting that Ad was not fully encapsulated or IP is disrupting liposome layers due to charge interactions. Ad+IP (red) and DOTAP_Ad +IP showed no signal and data points are overlaid.
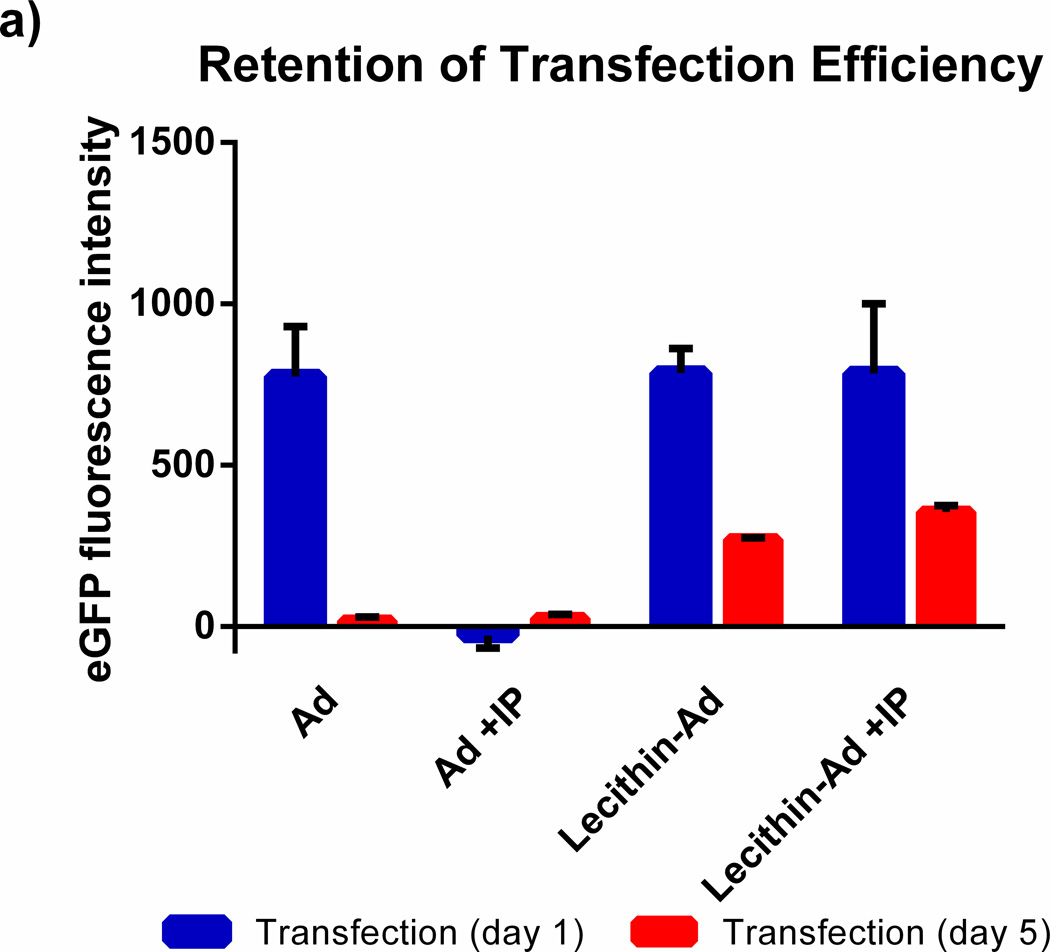
The retention of transfection efficiency and size stability of naked Ad5 and Ad5 encapsulated in lecithin liposomes was evaluated at day 1 and 5 as shown in Figure 3. When Ad5 is stored in PBS at 4°C for 5 days, the vi rus loses its complete ability to transfect. However, when Ad5 is encapsulated in lecithin liposomes and stored at 4°C, substantial transfection efficiency is retained. In addition, the size distribution of the liposomes before IP remains the same over several days and the size of liposomes after IP is stabilized after several days however the polydispersity is slightly increased from PDI= 0.3 to 0.4.
Figure 3. Infective Retention of encapsulated Ad5.
A549 cells were transfected with Ad or lecithin-Ad before and after immunoprecipitation (IP) at MOI 4.3. Samples were stored at 4°C and added to cells at day 1 or 5. Ad loses its ability to transfect cells after 5 days whereas Ad encapsulated in lecithin retains some infectivity. The sizes of Ad, lecithin-Ad, and lecithin-Ad + IP are shown at day 1 and 5 (black circle, red triangle, respectively). There is no significant change in the size of encapsulated Ad after 5 days.
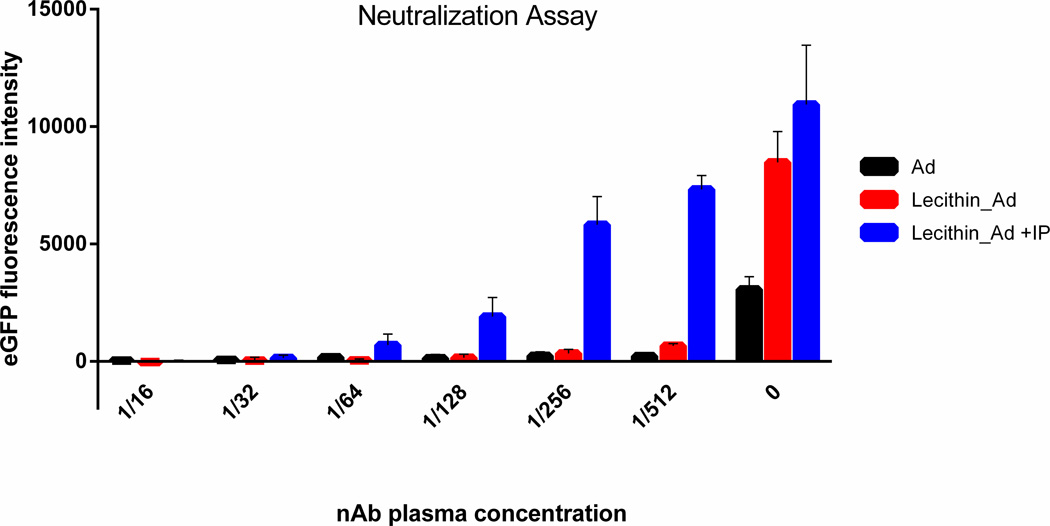
Neutralization Assay
Mice were inoculated with adenovirus every three days for three weeks and terminally bled. Plasma which contained a high neutralizing antibody (nAb) titer was collected, decomplemented, and diluted to 1:16 followed by ½ dilutions up to 1:512. Plasma concentrations higher than 1:16 showed high toxicity resulting in high cell death therefore plasma samples were diluted to at least 1:16 (data not shown). Plasma was added to cells followed by addition of Ad, Lecithin_Ad, or Lecithin_Ad +IP. As shown in Figure 4, naked Ad was neutralized at all plasma concentrations. Lecithin_Ad before immunoprecipitation was neutralized even at more dilute concentrations (1:512). This suggests that not all viral particles were fully coated before IP. With non-encapsulated Ad, the neutralizing plasma had a nearly complete inhibitory effect even at a dilution of 1:512. However, incubation of the neutralizing plasma with Ad encapsulated in lecithin after IP (Lecithin_Ad +IP) at this concentration had a more limited effect on the transfection efficiency where >95% inhibition was achieved for Ad incubated with 1:512 plasma whereas only 33% of viral expression was lost for Lecithin_Ad +IP at the same concentration. This suggests that Lecithin_Ad +IP is protected from neutralizing antibodies however, protection from neutralization of Lecithin-Ad +IP is directly relative to the concentration of circulating antibodies in plasma.
Figure 4. Neutralization Assay.
Ad (black), Lecithin_Ad (red), or Lecithin_Ad +IP (blue) were incubated with mouse plasma containing a high titer of neutralization antibodies and added to A549 cells at MOI 4.3. Mice were inoculated with Ad5 weekly three weeks prior to plasma collection and plasma was diluted to 1/16, 1/32, 1/64, 1/256, 1/512, and 0. GFP fluorescence intensity was measured at 48hrs post-infection. Lecithin_Ad +IP shows protection from nAb neutralization especially at more dilute concentrations.
Folate Targeting
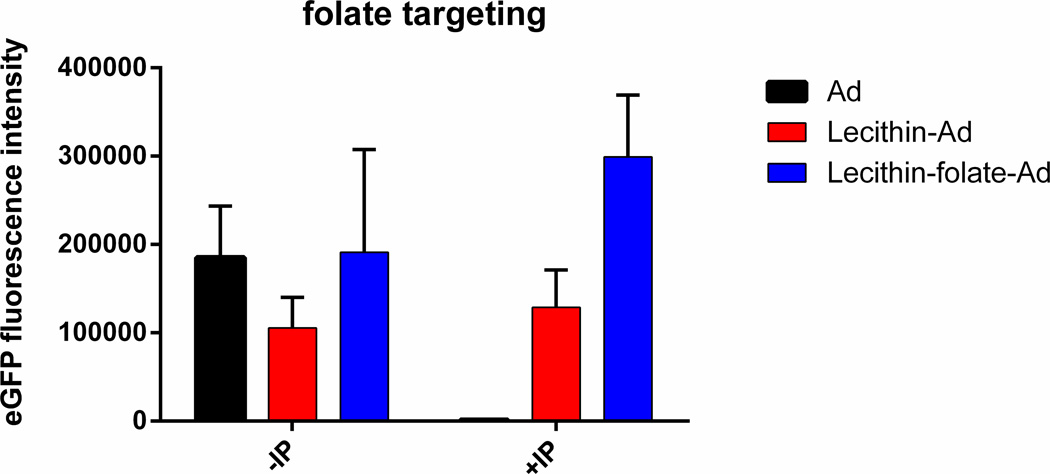
DSPE-PEG-folate was incorporated into lecithin-Ad5 liposomes during the dry lipid film step at a concentration of 0.03mM DSPE-PEG-folate and 0.07mM DSPE-PEG. The folate receptor is known to be overexpressed in many cancer cells. Specifically, 74% of adenocarcinomas (NSCLC subtype) exhibit positive folate receptor α expression which makes folate an attractive targeting ligand for lung cancer. A549 cells were transfected with Ad, lecithin_Ad, and lecithin-folate-Ad before and after IP. At all folate concentrations of 0.01 - 0.1mM before IP, all liposomes displayed large size and large size distributions (PDI =0.5–0.9). After IP, all lecithin-folate-Ad complexes were reduced in size and PDI. Some aggregation was observed at folate concentration higher than 0.03mM (PDI >0.3); therefore, for cell experiments, a molar concentration of 0.03mM DSPE-PEG-folate was employed. Incorporation of folate led to an increase in transfection efficacy after immunoprecipitation, as shown in Figure 5. This effect may be due to the synergy of reduced size of the liposome following IP processing and targeting.
Figure 5. Folate Targeting.
A549 cells were transfected with Ad, lecithin-Ad, or lecithin-folate-Ad before and after IP at an MOI of 4.3. A) Fluorescent images of cells 48 hrs post infection (p.i). B) Average mean green fluorescence of three images 48 hrs p.i. Lecithin-folate-Ad +IP showed an increase in eGFP expression. Statistical analysis 2-way ANOVA, p-value = 0.0059
Discussion
Various adenoviral vectors are promising agents with considerable potential as gene therapeutics for a broad range of diseases. Cancer-specific immune stimulation by cytokine-expressing oncolytic Ads hold great promise. Several cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, IL-12, IL-18, and IL-24 have shown therapeutic potency and antitumoral effects [37, 38]. Systemic administration of immunostimulatory cytokines have shown systemic toxicity which is dose and schedule dependent [39]. Cytokine-expressing oncolytic Ads provide a sustained cytokine release which can elicit an antitumor immune response which may lead to reduced toxicity [37]. The underlying principle of this approach is that OV replication of Ads in tumor tissue will induce tumor cell death and release tumor antigens. The released antigens would then lead to tumor-specific T cells activation and generate a persistent and systemic antitumor response [29].
For the treatment of metastatic cancer, systemic delivery of the virus is required to reach metastatic sites. Despite promising pre-clinical results of Ads as selective cancer therapeutics, Ads have shown limited efficacy in the clinic. Intravenous administration has shown to result in sequestration in the liver due to macrophage uptake and pre-existing neutralizing antibodies [4, 40]. This is due to recognition of surface viral proteins, primarily directed against the hexon protein [41, 42]. Viral protein modifications have shown limited success due to a reduced titer yield [43], impairment of intracellular vector particle trafficking, loss of infectivity, and requirement of retargeting after modification [44–46]. Coating the virus with phospholipids or polymer has shown the ability to evade the immune system and increase the delivery to the tumor locale [47, 48].
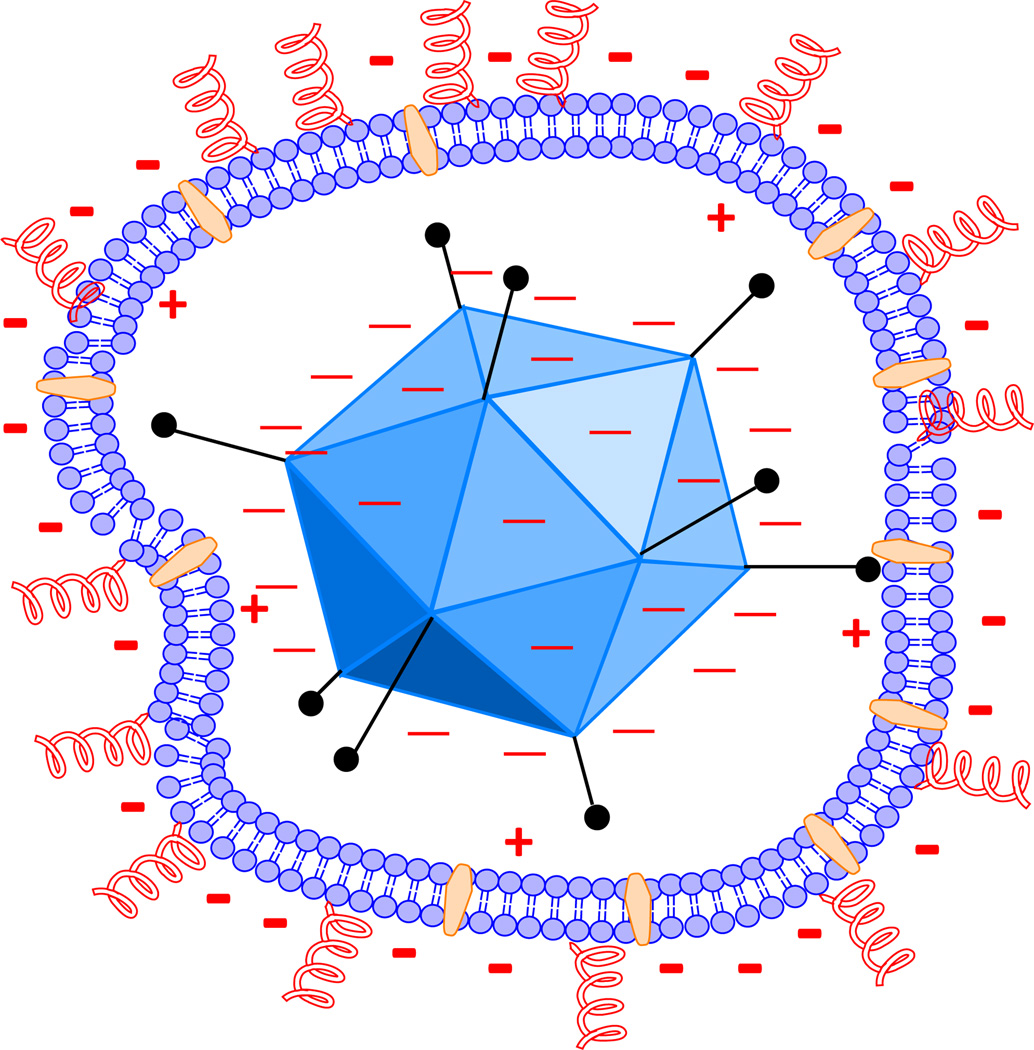
Cationic liposomes and cationic polymers exhibit high transfection efficiencies however are substantially toxic following administration [49–51]. A new approach described in this manuscript consists of encapsulation of adenovirus in a non-toxic anionic lecithin-cholesterol-PEG/folate liposome. PEGylation has shown to increase the circulation time in vivo, hence having the potential to extend the interaction time between the encapsulated virus and the tumor [52, 53]. Lecithin is a combination of naturally-occurring phospholipids which mainly consists of phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylinositol (PI). The proposed liposome-Ad5 structure which is energetically favorable is that zwitterionic and cationic phospholipids interact with the negatively charged adenovirus capsid thereby confining them to the inner leaflet of the liposome as shown in Figure 6. Conversely, the negatively charged phospholipids such as inositol phosphatides localize on the outer leaflet of the liposome due to electrostatic interactions. Cholesterol is energetically favorable in the interstitial sites of the leaflet and liposomal stability and cargo retention has shown to be dependent on their cholesterol content [54]. PEG2000 chains localize on the surface of the liposome complex and PEGylation is known to increase the circulation half-life during systemic delivery [55]. This study has shown that the lecithin liposome-Ad5 complexes result in enhancement of transfection efficiency, are protected from anti-hexon antibodies during IP processing, and are protected from neutralizing antibodies in human serum in a concentration-dependent manner when compared to naked Ad5.
Figure 6. Electrostatic interactions of phospholipids with Ad5.
Lecithin is composed of zwitterionic and anionic phospholipids. A proposed structure is shown. Zwitterionic phospholipids are shown interacting with the negatively charged adenovirus. The assembly of negatively charged phospholipids such as inositol phosphatides is shown on the outer leaflet of the liposome which is energetically more favorable due to electrostatic interactions. Cationic and zwitterionic phospholipids are shown on the inner leaflet, interacting with adenovirus. Cholesterol is shown in between the lipid bilayer and PEG2000 chains are shown on the surface of the liposome complex illustrated in red.
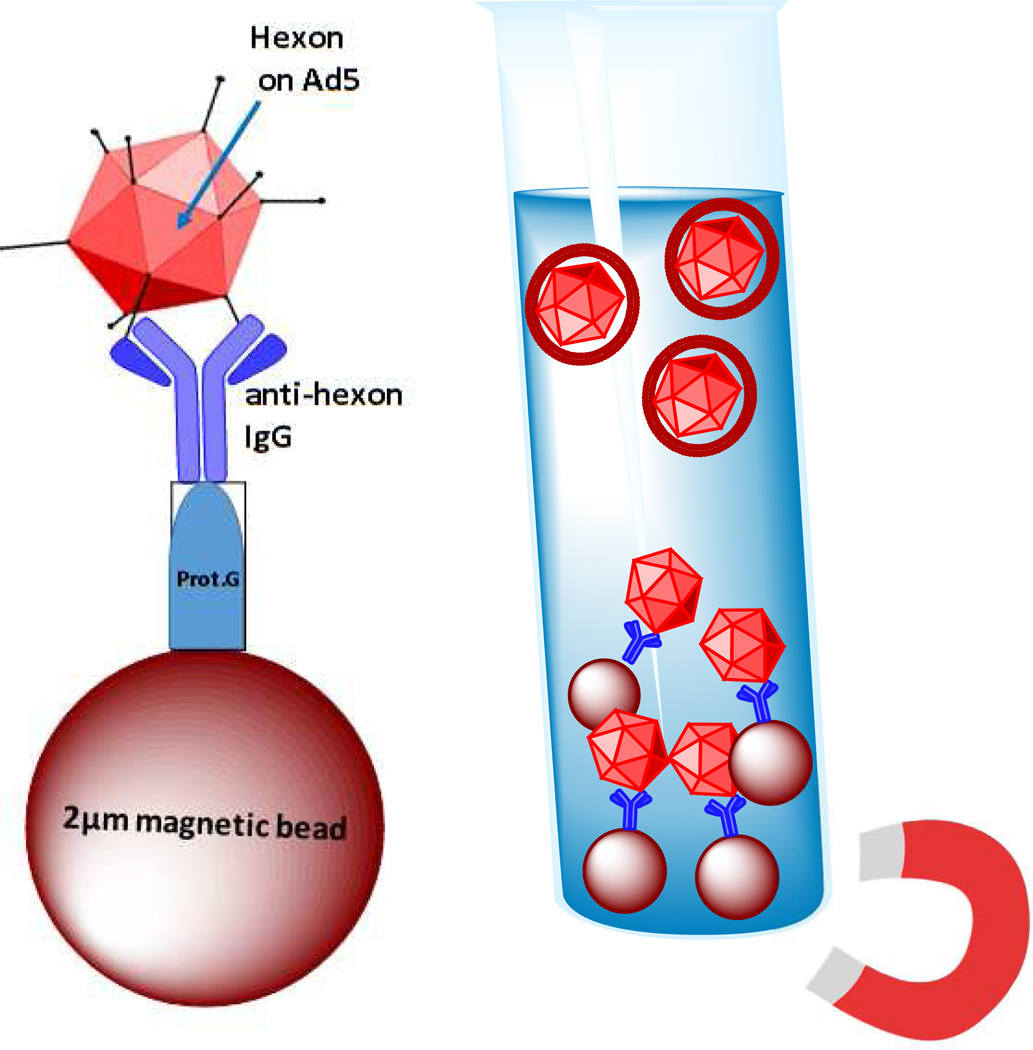
In addition, an immunoprecipitation technique was implemented to extract non-encapsulated viruses from solution in order to ensure evasion from the immune system during the initial injection. After encapsulation in lecithin:Chol:DSPE-PEG or DOTAP:Chol:DSPE-PEG liposomes, complexes were incubated with anti-hexon IgG followed by incubation with 2µm magnetic Protein G beads to extract non-encapsulated viruses using a magnet as shown in Figure 7. Complete encapsulation reduces the risk of an adverse immune response when administered at a higher dose and reduces clearance from the bloodstream due to neutralizing antibodies. These studies have shown that the method described results in particle size reduction, full coating of the virus, effective incorporation of targeting, and evasion of neutralizing antibodies when encapsulated in anionic liposomes in a serum concentration-dependent manner; it is noted that evasion of neutralizing antibodies in-vivo may require additional optimization. Partial neutralization of Lecithin-Ad5 +IP was observed at high plasma concentrations which may be due to leakage of Ad5 from liposomes in the presence of plasma components at high concentrations or exposed viral capsids which leads to recognition by neutralizing antibodies in plasma. A higher cholesterol and PEG content may be evaluated to improve liposomal stability.
Figure 7. Immunoprecipitaiton (IP) of non-encapsulated adenoviruses.
IP technique extracts non-encapsulated viral particles from solution. After liposomal encapsulation, the adenovirus-liposome complex was incubated with anti-hexon IgG. Non-encapsulated viruses bound to anti-hexon IgG were extracted using Protein G magnetic beads. The technique also reduces size of complexes and homogenizes the sample due to incubation with 2µm magnetic beads.
This study has shown effective transfection efficiency even without targeting. Folate targeting further enhanced uptake in folate-expressing cells. Bearing in mind that the fiber and penton viral proteins and masked after encapsulation, it is speculated that lecithin-Ad liposomes are entering via an alternative pathway which do not utilize CAR. A better comprehension of the cellular uptake of the adenovirus lipoplexes may allow for the development of an improved viral gene delivery platform. Further investigations of circulation time and tumor accumulation after intratumoral and intravenous administration is needed to assess the efficacy of the platform in vivo.
Conclusion
In summary, encapsulation of viral vectors has the potential to enhance viral gene therapy by masking viruses from clearance and by extending the circulation time. Cationic liposomal encapsulation have had limited success in vivo due to high toxicities, low tissue specificity, and poor serum stability due to incompatibility with the abundance of negatively charged macromolecules present in the physiological environment. In this study, the encapsulation of adenovirus in 140–180nm anionic liposomes has been prepared by self-assembly of lecithin, cholesterol, and DSPE-PEG2000 around the viral capsid. Lecithin consists of a variety of phospholipids including phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol with a net negative charge. The encapsulated viruses in lecithin liposomes have shown to retain and enhance their transfection efficiency in cancer cells. Furthermore, an immunoprecipitation (IP) technique has been implemented as a fast and effective method to extract non-encapsulated viruses and to homogenize the liposomes remaining in solution. In this study, a stable, non-toxic encapsulation method has been designed to enhance the delivery of adenovirus to cancer cells.
Acknowledgements
This study was funded by the National Institute of Health: CRIN grant 3 R25 CA153915-03S1, NSF Alliance for Graduate Education and the Professoriate (AGEP) grant #0450366 and in part by the NCI Comprehensive Partnerships to Reduce Cancer Health Disparities (CPRCHD) grant #U54CA132384
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breitbach CJ, Reid T, Burke J, Bell JC, Kirn DH. Navigating the clinical development landscape for oncolytic viruses and other cancer therapeutics: No shortcuts on the road to approval. Cytokine & Growth Factor Rev. 2010;21(2–3):85–89. doi: 10.1016/j.cytogfr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Heise CC, Williams AM, Xue S, Propst M, Kirn DH. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59(11):2623–2628. [PubMed] [Google Scholar]
- 3.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8(2):89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2010;18(2):243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9(2):555–561. [PubMed] [Google Scholar]
- 6.DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61(20):7464–7472. [PubMed] [Google Scholar]
- 7.Liu T-C, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Prac Oncol. 2007;4(2):101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 8.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19(2):289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 9.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): Phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62(21):6070–6079. [PubMed] [Google Scholar]
- 10.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6(8):879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 11.Alemany R, Balagué C, Curiel D. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18(7):723. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Curiel DT. Cancer gene therapy. Technol Cancer Res Treat. 2005;4(4):315–330. doi: 10.1177/153303460500400402. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M. Conditionally replicative adenovirus for gastrointestinal cancers. Expert Opin. Biol. Ther. 2004;4(8):1241–1250. doi: 10.1517/14712598.4.8.1241. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Curiel DT. Nonreplicating DNA viral vectors for suicide gene therapy: the adenoviral vectors. Methods Mol Med. 2004;90:61–70. doi: 10.1385/1-59259-429-8:61. [DOI] [PubMed] [Google Scholar]
- 15.Mulvihlll S, Warren R, Venook A, Adler A, Randlev B, Heise C, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8(4):308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 16.Ganly I, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6(5):798–806. [PubMed] [Google Scholar]
- 17.Lamont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann Surg Oncol. 2000;7(8):588–592. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- 18.Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8(10):746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 19.Makower D, Rozenblit A, Kaufman H, Edelman M, Lane ME, Zwiebel J, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9(2):693–702. [PubMed] [Google Scholar]
- 20.Hamid O, Varterasian ML, Wadler S, Hecht JR, Benson A, Galanis E, et al. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21(8):1498–1504. doi: 10.1200/JCO.2003.09.114. [DOI] [PubMed] [Google Scholar]
- 21.Nemunaitis J, Cunningham C, Tong AW, Post L, Netto G, Paulson AS, et al. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther. 2003;10(5):341–352. doi: 10.1038/sj.cgt.7700585. [DOI] [PubMed] [Google Scholar]
- 22.Portella G, Pacelli R, Libertini S, Cella L, Vecchio G, Salvatore M, et al. ONYX-015 enhances radiation-induced death of human anaplastic thyroid carcinoma cells. J Clin Endocrinol Metab. 2003;88(10):5027–5032. doi: 10.1210/jc.2003-030385. [DOI] [PubMed] [Google Scholar]
- 23.Rudin CM, Cohen EEW, Papadimitrakopoulou VA, Silverman S, Recant W, El-Naggar AK, et al. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J Clin Oncol. 2003;21(24):4546–4552. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I–II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12(5):437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- 26.Reid TR, Freeman S, Post L, McCormick F, Sze DY. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005;12(8):673–681. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- 27.Nemunaitis J, Senzer N, Sarmiento S, Zhang YA, Arzaga R, Sands B, et al. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther. 2007;14(11):885–893. doi: 10.1038/sj.cgt.7701080. [DOI] [PubMed] [Google Scholar]
- 28.Hedjran F, Shantanu K, Tony R. Deletion analysis of Ad5 E1a transcriptional control region: impact on tumor-selective expression of E1a and E1b. Cancer Gene Ther. 2011;18(10):717–723. doi: 10.1038/cgt.2011.41. Epub 2011/08/06. [DOI] [PubMed] [Google Scholar]
- 29.Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13(13):1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- 30.Bristol JA, Zhu M, Ji H, Mina M, Xie Y, Clarke L, et al. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Mol Ther. 2003;7(6):755–764. doi: 10.1016/s1525-0016(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 31.Fagerberg J. Granulocyte-macrophage colony-stimulating factor as an adjuvant in tumor immunotherapy. Med Oncol. 1996;13(3):155–160. [PubMed] [Google Scholar]
- 32.Yotnda P, Chen D-H, Chiu W, Piedra PA, Davis A, Templeton NS, et al. Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Mol Ther. 2002;5(3):233–241. doi: 10.1006/mthe.2002.0545. [DOI] [PubMed] [Google Scholar]
- 33.Ishida T, Harashima H, Kiwada H. Liposome clearance. Biosci Rep. 2002;22(2):197–224. doi: 10.1023/a:1020134521778. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Z, Han J, Wan Y, Zhang Z, Sun X. Anionic liposomes enhance and prolong adenovirus-mediated gene expression in airway epithelia in vitro and in vivo. Mol Pharm. 2011;8(3):673–682. doi: 10.1021/mp100404q. [DOI] [PubMed] [Google Scholar]
- 35.Zhong Z, Shi S, Han J, Zhang Z, Sun X. Anionic liposomes increase the efficiency of adenovirus-mediated gene transfer to coxsackie-adenovirus receptor deficient cells. Mol Pharm. 2009;7(1):105–115. doi: 10.1021/mp900151k. [DOI] [PubMed] [Google Scholar]
- 36.Zhong Z, Wan Y, Han J, Shi S, Zhang Z, Sun X. Improvement of adenoviral vector-mediated gene transfer to airway epithelia by folate-modified anionic liposomes. Int J Nanomed. 2011;6:1083. doi: 10.2147/IJN.S19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J-W, Lee J-S, Kim SW, Yun C-O. Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Deliv Rev. 2012;64(8):720–729. doi: 10.1016/j.addr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28(3):109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Lotze MT, Zitvogel L, Campbell R, Robbins PD, Elder E, Haluszczak C, et al. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann N Y Acad Sci. 1996;795(1):440–454. doi: 10.1111/j.1749-6632.1996.tb52715.x. [DOI] [PubMed] [Google Scholar]
- 40.Tao N, Gao G-P, Parr M, Johnston J, Baradet T, Wilson JM, et al. Sequestration of adenoviral vector by kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3(1):28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 41.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132(3):397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174(11):7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 43.Roberts DM, Nanda A, Havenga MJE, Abbink P, Lynch DM, Ewald BA, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441(7090):239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 44.Corjon S, Wortmann A, Engler T, van Rooijen N, Kochanek S, Kreppel F. Targeting of adenovirus vectors to the LRP receptor family with the high-affinity ligand RAP via combined genetic and chemical modification of the pIX capsomere. Mol Ther. 2008;16(11):1813–1824. doi: 10.1038/mt.2008.174. [DOI] [PubMed] [Google Scholar]
- 45.Prill J-M, Espenlaub S, Samen U, Engler T, Schmidt E, Vetrini F, et al. Modifications of adenovirus hexon allow for either hepatocyte detargeting or targeting with potential evasion from kupffer cells. Mol Ther. 2011;19(1):83–92. doi: 10.1038/mt.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campos SK, Barry MA. Comparison of adenovirus fiber, protein IX hexon capsomeres as scaffolds for vector purification and cell targeting. Virology. 2006;349(2):453–462. doi: 10.1016/j.virol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 47.Beer SJ, Matthews CB, Stein CS, Ross BD, Hilfinger JM, Davidson BL. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5(6):740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- 48.Lee SG, Yoon SJ, Kim CD, Kim K, Lim DS, Yeom YI, et al. Enhancement of adenoviral transduction with polycationic liposomes in vivo. Cancer Gene Ther. 2000;7(10) doi: 10.1038/sj.cgt.7700237. [DOI] [PubMed] [Google Scholar]
- 49.Audouy SL, de Leij LMH, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res. 2002;19(11):1599–1605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- 50.Filion MC, Phillips NC. Major limitations in the use of cationic liposomes for DNA delivery. Int J Pharm. 1998;162(1–2):159–170. [Google Scholar]
- 51.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Controlled Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Park R, Shahinian AH, Bading JR, Conti PS. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl Med Biol. 2004;31(1):11–19. doi: 10.1016/j.nucmedbio.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10(21):1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 54.Kirby C, Clarke J, Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J. 1980;186(2):591–598. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta - Biomembranes. 1991;1066(1):29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]