Abstract
Treponema denticola and its major outer sheath protein (Msp) induce actin reorganization in fibroblasts. We adapted a barbed-end labeling/imaging assay to monitor Msp-induced subcortical actin filament assembly in neutrophils and fibroblasts. Msp, at an actin-reorganizing concentration, inhibited migration of these dissimilar cell types, whose cytoskeletal functions in locomotion and phagocytosis are crucial for immunity and healing of peripheral infections.
Many pathogenic bacteria are known to induce cytoskeletal reorganization in target host cells, which they contact directly or indirectly through their shed outer membrane components or secreted exotoxins (see reference 8 for review). In most in vitro studies of actin dynamics following the exposure of cultured mammalian cells to bacteria or their virulence determinants, cytoskeletal reorganization is assessed by either qualitative observation of the distribution of fluorescent markers for specific cytoskeletal proteins or by measuring changes in their fluorescence intensity by fluorescence photometry. For example, our laboratory has shown previously that a spirochetal periodontal pathogen, Treponema denticola, or an extract of its outer membrane causes the disruption of stress fibers in cultured oral epithelial cells and gingival fibroblasts and a reduction in the fluorescence intensity of fluorescein isothiocyanate (FITC)-phalloidin-stained actin filaments (2, 17). Cell shrinkage, aberrations in volume regulation, and diminished adhesion to the extracellular matrix (ECM) were affected concomitantly.
Among T. denticola's putative virulence factors, its major outer sheath protein (Msp) has achieved notoriety for its immunogenicity, its ability to bind human matrix proteins, its pore-forming capacity, and its toxicity for some types of human cells in culture (3-6). Our laboratory has recently reported two unprecedented findings that connect Msp's impact on actin dynamics to its cytopathogenic effects on crucial physiologic pathways of fibroblasts: (i) Msp uncouples store-operated Ca2+ channels of fibroblasts, apparently conformationally, by promoting actin assembly subjacent to the plasma membrane (16); and (ii) Msp-induced subcortical actin filament assembly inhibits β1 integrin affinity modulation that normally accompanies the engagement of extracellular collagen (10). Presumably, some other pathogenic bacteria that cause substantial reorganization of the host cell cytoskeleton may likewise perturb store-operated calcium fluxes and receptor-mediated ECM binding affinity.
Given the potential of Msp and other bacterial virulence proteins to disrupt vital physiologic functions of host cells through their impact on ctyoskeletal dynamics, we sought a more-sensitive, real-time approach to measure de novo actin assembly in host cells exposed to Msp. We chose two types of target cells: fibroblasts, the major stromal cells that maintain the homeostasis of the ECM of the gingiva; and neutrophils, the key inflammatory cells that protect the gingiva from local infections arising from the adjacent treponeme-rich microflora. Actin cycling is essential for locomotion and phagocytosis in both of these cell types. The specific purpose of this study was to determine whether Msp does, indeed, induce de novo subcortical actin filament assembly in dissimilar target cells, using a more dynamic assay, and whether subcortical actin assembly is contemporaneous with actin filament disassembly elsewhere in the cell and with impaired locomotion. To test the hypothesis, we applied an assay that was developed to quantify free barbed-end labeling in neutrophils (7) and adapted it for use in fibroblasts.
Neutrophils are the fastest translocating cells in peripheral tissues. Their crawling motility and phagocytosis of bacteria require highly regulated cycling of actin subunits between monomeric and polymeric pools, and they undergo rapid, polarized actin assembly in response to chemoattractants. To accommodate technical challenges imposed by neutrophils being terminally differentiated and refractory to transfection and microinjection, Glogauer and coworkers introduced a modified assay based on the incorporation of exogenous rhodamine actin at the fast-growing barbed ends of actin filaments following controlled partial permeabilization of the plasma membrane (7). The effect of T. denticola Msp on actin assembly in human peripheral blood neutrophils was determined as follows.
Native Msp complex was isolated and enriched from modified new oral spirochete (NOS) broth cultures of T. denticola type strain ATCC 35405 cells as reported by Egli et al. (3), modified in our laboratory by Wang et al. (16). The preparation required sequential deoxycholate and n-octyl polyoxyethylene extraction, ultracentrifugation, autoproteolysis of the extract, concentration by ultrafiltration, extensive washing in 10 mM Tris (pH 8.0) and distilled H2O, ultracentrifugation, and extensive dialysis. It yielded a protease- and peptidase-free aqueous solution that was enriched for a high-molecular-weight complex of Msp that reacted in Western blots with anti-Msp antibodies and that could be dissociated into 53-kDa Msp monomers by boiling (16). The cytoskeleton- and calcium flux-perturbing activities of the solution were inhibited by either heating at 60°C for 30 min or by absorption with anti-Msp antibodies (10, 16). Aliquots of Msp solution were frozen until used in experiments with the neutrophils.
Ten milliliters of blood was taken from a consenting subject, and neutrophils were isolated. Isolation of neutrophils involved the use of 1-Step Polymorph Prep (Accurate Chemical & Scientific Corporation, Westbury, N.Y.) and centrifugation for 30 min at 1,139 × g (7). For the permeabilization step, the neutrophils were washed using Hanks calcium-free buffer and resuspended in Hanks buffer containing 1.1 mM calcium. The cells were then exposed to Msp (20 μg/ml) in Hanks buffer or Msp-free vehicle (control) for 0- to 10-min intervals in a 37°C shaker bath. Prior to staining, the cells were incubated for 10 s with a 4% octyl glucoside-containing permeabilization buffer, and permeabilization was stopped by dilution in a buffer containing EGTA, β-mercaptoethanol, ATP, and Mg2+ and K+ salts, as described previously (7). Except for experiments aimed at colocalization of fluorescent markers, the cells were stained separately for analysis with rhodamine actin (Cytoskeleton, Denver, Colo.) or Alexa Fluor 488 phalloidin (Molecular Probes, Eugene, Oreg.). Cells were incubated with 0.23 μM rhodamine actin in the permeabilization buffer for 2 min at 37°C and subsequently fixed with 3.7% formaldehyde. Cells were incubated with 0.165 μM (5 U/ml) Alexa Fluor 488 phalloidin in phosphate-buffered saline (PBS) for 20 min at ambient temperature (7). The neutrophil suspensions were divided to provide samples for flow cytometry and fluorescence microscopy preparations. For flow cytometry, cell-associated Alexa Fluor 488 or rhodamine fluorescence was measured with a FACScan flow cytometer as described previously (13). Briefly, single-cell suspensions of neutrophils were analyzed and appropriately gated on the forward versus side scatter plot. A total of 10,000 cells were counted in the neutrophil gate. Fluorescence values were expressed as the mean fluorescence intensity (MFI) of the Msp-treated cell sample divided by the MFI of the control cell sample.
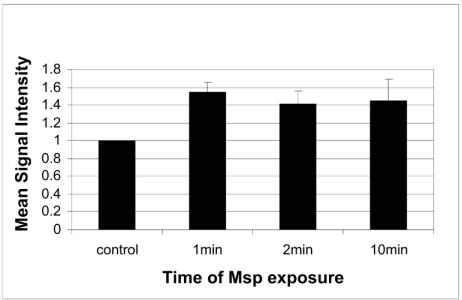
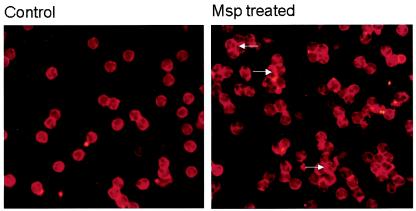
In neutrophils, very brief cell exposure to Msp, for only 1 min, induced a significant increase in actin filaments, as seen in the flow cytometry data for Alexa phalloidin-stained cells (mean increase in fluorescence intensity above control value = 55% ± 0.06%, P < 0.05, n = 3 independent experiments; Fig. 1). There was also an increase in the average rhodamine actin fluorescence in the neutrophils treated with Msp for 1 min (average increase above control value = 20% ± 0.07%, n = 2 experiments). Many of the neutrophils exposed to Msp for 1 min and examined by fluorescence microscopy showed a much brighter band of rhodamine fluorescence in the cell periphery than the fluorescence of cells exposed to the vehicle control (Fig. 2). Together, these data demonstrate that T. denticola Msp induces de novo subcortical actin assembly in human neutrophils.
FIG. 1.
Relative Alexa Fluor 488 phalloidin fluorescence intensity of Msp-treated and control neutrophils. The neutrophils were exposed to Msp (20 μg/ml) for a duration of 1, 2, or 10 min. Mean (± standard error) signal intensity for fluorescence was measured by flow cytometry and normalized to the mean control value of 1.
FIG. 2.
Fluorescence photomicrograph of control (left panel) and Msp-treated (right panel) neutrophils after permeabilization and incubation with rhodamine actin. The cells were treated with Msp for 1 min. Intense rhodamine fluorescence developed in the subcortical area of Msp-treated cells (arrows).
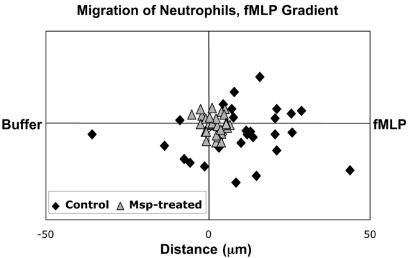
The effect of Msp-induced actin assembly on neutrophil migration was tested in three independent chemotaxis experiments using a single concentration/time protocol. Neutrophils were isolated from the marrow of murine long bones, enriched by Percoll gradient, washed, and suspended in Hanks Ca2+-Mg2+ buffer (7). The cells were incubated with either Msp at a concentration shown to promote actin reorganization (20 μg/ml) or Msp-free Hanks buffer for 30 min, deposited onto the midline of a coverslip, and incubated at 37°C for 30 min; total Msp exposure was for 60 min. The coverslip was rinsed three times with Hanks Ca2+-Mg2+ buffer and carefully centered and mounted onto a Zigmond chamber. Eighty microliters of formyl-Met-Leu-Phe (fMLP; 10−6 M) was added to the left well, and 80 μl of Hanks buffer (1:10 solution) was added to the right well to develop an fMLP gradient. Fields with polarized neutrophils were photographed every minute for 15 min, and the mean distance and speed of neutrophil migration were calculated. Under these specific conditions, pretreatment of neutrophils with Msp inhibited their chemotactic migration (Fig. 3). The speed of the control cells was twice as fast as that of Msp-pretreated cells (4.5 ± 0.6 versus 2.1 ± 0.7 μm/min, respectively; P < 0.01, n = 3 experiments), and the distance covered was sixfold greater for the control neutrophils (17.3 ± 4.8 versus 2.8 ± 0.07 μm, P < 0.01).
FIG. 3.
Example of migration of control (⧫) and Msp-pretreated (▴) neutrophils in an fMLP gradient during a typical chemotaxis experiment. The Msp-pretreated cells remained close to the origin, while the control cells migrated further and faster, most in the direction of higher fMLP concentration (see the text for data from three independent experiments).
Fibroblasts rely on actin cycling at focal complexes during crawling locomotion and for phagocytosis to remodel collagen under physiologic conditions. The permeabilization and buffering conditions of the neutrophil barbed-end labeling assay needed to be modified for use in fibroblasts. For these experiments, we used Rat-2 fibroblasts, which are actin filament rich, readily cultivated, and similar to human gingival fibroblasts in many of the cytoskeletal responses that have been tested (1, 10). Rat-2 cells were subcultured biweekly in T-25 flasks. The cells were grown to a density of 104 cells/well for 48 h at 37°C in a CO2 incubator. The medium (alpha minimal essential medium [α-MEM] with 100-U/ml penicillin G, 50-μg/ml gentamicin, and 10% fetal bovine serum) was changed once during this period. After obtaining 90% confluence, the medium was aspirated and the cells were washed twice with prewarmed PBS (pH 7.4). The fibroblasts were incubated with Msp (20 μg/ml) in α-MEM without serum at 37°C in a CO2 incubator for 1 h. Control cells were treated with α-MEM containing an equivalent volume of distilled water to that used to dilute the Msp. The fibroblasts were washed gently twice with warm PBS.
To permeabilize and to simultaneously pulse the fibroblasts with rhodamine actin monomers, the cells were exposed for 30 s to 100 μl of OG buffer: 10 μl of 4% n-octyl glucopyranoside diluted in 90 μl of PHEM buffer, pH 6.9, containing 6 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 2.5 mM HEPES, 1 mM EGTA, 0.2 mM MgCl2, 0.1 mM ATP, and 0.23 mM rhodamine actin. Permeabilization of the fibroblasts was stopped by adding 300 μl of buffer B (5 mM β-mercaptoethanol, 1 mM Tris base, 1 mM EGTA, 2 mM MgCl2, 10 mM KCl, 5 mM ATP, pH 7.4) for 2 min at 37°C. The buffer was removed and the cells were fixed with 3.7% formaldehyde for 10 min. The cells were washed with PBS four times. Two hundred microliters of 0.1% Triton X-100 was added to the wells; after 5 min, the cells were washed three times. Alexa Fluor 488 phalloidin was added to the wells for 20 min, and the cells were then washed three times. The chamber and gasket were removed, and the slide was covered with antifade mounting fluid and a coverslip. Fluorescence microscopy at a magnification of ×40 (Zeiss Universal Research Microscope, Germany) was used to produce images of cells stained with rhodamine actin (excitation light BP546/12, emission filter LP 590, FT580 chromatic beam splitter) and Alexa Fluor 488 phalloidin (exitation light 420, emission filter BP520-560, FT510 chromatic beam splitter). The images obtained from the two channels were merged with Photoshop software.
NIH Image software was used to analyze localized mean pixel intensity of the cells (n = 30 per group) for both rhodamine actin and Alexa Fluor 488 phalloidin staining. The images were acquired with a Nikon Coolpix 990 digital camera at 2 s of exposure. Data were collected for 90 images, and data analysis was performed in Microsoft Excel.
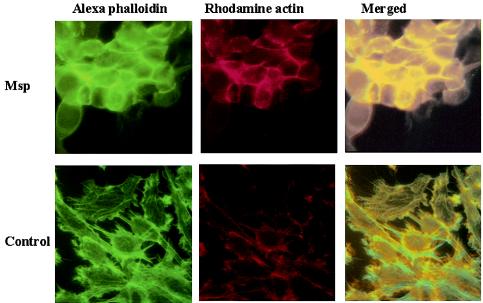
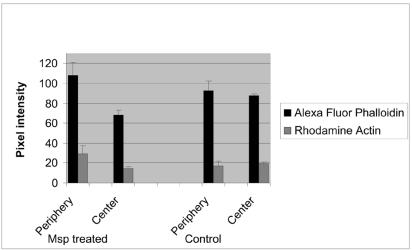
Rat-2 fibroblasts exposed to Msp yielded a significant increase in both Alexa Fluor 488 and rhodamine mean pixel intensity above control values at the cell periphery and a concomitant decrease in Alexa Fluor 488 intensity in the stress fiber-rich central region of the cells (P < 0.05, Fig. 4 and 5). Dual-labeled fluorescent images of Msp-treated fibroblasts showed an intense band of subcortical rhodamine actin fluorescence colocalized with Alexa Fluor phalloidin-stained filaments along the periphery of the cells, and detected contemporaneous stress fiber disruption toward the center of the cells (Fig. 4). Msp-treated cells became oval in shape. In contrast, the control vehicle-treated cells maintained their stellate shape, and colocalized fluorescence was limited to discreet focal complexes at the plasma membrane. In Msp-treated cells, there was a significant decrease in the ratio of centrally distributed Alexa Fluor phalloidin fluorescence intensity to subcortical fluorescence intensity (P < 0.001, Fig. 5), indicating that disassembly of actin filaments toward the center of the cell occurred contemporaneously with de novo subcortical actin filament assembly. The concomitant increase in peripheral rhodamine actin fluorescence intensity in the Msp-treated cells relative to the vehicle-treated cells (P < 0.05) confirmed that exposure of fibroblasts to Msp led to elongation of actin filaments at their fast-growing barbed end. A previous study by our laboratory had found a significant reduction in the proportion of FITC-phalloidin fluorescence intensity associated with stress fibers in the ventral third relative to the dorsal third of human gingival fibroblasts upon 60 min of exposure to T. denticola whole cells (17).
FIG. 4.
Fluorescence photomicrographs of individual and merged images showing colocalized rhodamine actin and Alexa phalloidin in Msp-treated and vehicle-treated (control) Rat-2 fibroblasts.
FIG. 5.
Distribution of Alexa fluor 488 and rhodamine fluorescence intensity between central and peripheral (subcortical) areas of Msp-treated and vehicle-treated control Rat-2 fibroblasts. Msp treatment increased the mean (± standard error) subcortical actin filament and actin barbed end fluorescence intensity (n = 3 experiments).
We investigated the effect of Msp treatment on the migration of fibroblasts across a type 1 collagen substratum. Msp-pretreated (20 μg/ml for 1 h, followed by washing) and vehicle-treated Rat-2 cells were grown to confluence. A sterile needle was drawn across the monolayer to create a wound. The migration of cells at the periphery to close the wound was observed hourly for 14 h. Msp pretreatment had a significant inhibitory effect on the migration of the fibroblasts. Migration of the control cells covered the wound gap 50% faster than that of the Msp-pretreated cells (8.4 ± 0.2 h versus 12.2 ± 0.1 h, respectively, P < 0.01, n = 3 experiments).
Chronic inflammation is a hallmark of prolonged bacterial infections of peripheral tissues like the periodontium, which is exposed to a complex microflora associated with subgingival biofilms. In essence, the pathogenesis of the lesion creates a chronic wound that persists as long as the infection is not controlled. Cells like neutrophils that migrate to contain the spread of the microorganisms and cells like fibroblasts that migrate to remodel and heal the inflamed connective tissue rely on tightly regulated cytoskeletal dynamics for translocation and phagocytosis. Our findings indicate that the major outer sheath protein of T. denticola, one of the most prevalent species that colonizes the gingival interface of biofilms (9, 11), which penetrates the gingiva during advanced infections (12) and which is a member of the mixed bacterial complexes most associated with progressive periodontitis (14, 15), induces de novo subcortical actin filament assembly and inhibits locomotion in both neutrophils and fibroblasts under the limited range of in vitro conditions tested.
Our understanding of the complex interactive signal transduction pathways by which pathogenic bacteria exploit the host cell cytoskeleton will be advanced greatly by developing dynamic assays for measuring assembly and disassembly of cytoskeletal components like actin filaments. A key step in the remodeling of the actin cytoskeleton is the initiation of actin nucleation sites also known as free barbed ends. It is at these nucleation sites that actin polymerization occurs in all cells. The most common method of assessing actin polymerization involves quantification of filamentous actin within cells before and after a given stimulus. The assay we describe here allows us to assess not only the level of filamentous actin but also the intracellular location of the initiation of actin filament assembly. This is crucial when trying to identify the precise effects and the mechanism of action of bacterial adhesins and toxins on mammalian cells. The technique we describe here also allows for real-time assessment of actin assembly dynamics and identifies areas of “new” actin assembly. The adaptation of this quantitative barbed-end labeling assay for actin filament assembly will allow us and other investigators to relate actin dynamics to a wider range of protective and wound healing functions of host cells that respond to infections by treponemes and other infectious agents that target the cytoskeleton.
Acknowledgments
This study was supported by CIHR operating grants MOP-5619 (R.P.E.) and MOP-53136 (M.G.) and a CIHR Group grant. M.A. was supported by a University of Toronto Open Fellowship, A.H. and J.L. were supported by CIHR summer studentships, and A.P.B.S. was supported by a CIHR strategic training fellowship (STP-52877).
We thank D. A. Grove and W. Lee for technical assistance.
Editor: J. B. Bliska
REFERENCES
- 1.Arora, P. D., L. Silvestri, B. Ganss, J. Sodek, and C. A. G. McCulloch. 2000. Mechanism of cyclosporin-induced inhibition of intracellular collagen degradation. J. Biol. Chem. 276:14100-14109. [DOI] [PubMed] [Google Scholar]
- 2.DeFilippo, A. B., R. P. Ellen, and C. A. G. McCulloch. 1995. Induction of cytoskeletal rearrangements and loss of volume regulation in epithelial cells by Treponema denticola. Arch. Oral Biol. 40:199-207. [DOI] [PubMed] [Google Scholar]
- 3.Egli, C., W. K. Leung, K.-H. Müller, R. E. W. Hancock, and B. C. McBride. 1993. Pore-forming properties of the major 53-kilodalton surface antigen from the outer sheath of Treponema denticola. Infect. Immun. 61:1694-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenno, J. C., P. M. Hannam, W. K. Leung, M. Tamura, V.-J. Uitto, and B. C. McBride. 1998. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect. Immun. 66:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenno, J. C., and B. C. McBride. 1998. Virulence factors of oral treponemes. Anaerobe 4:1-17. [DOI] [PubMed] [Google Scholar]
- 6.Fenno, J. C., K.-H. Müller, and B. C. McBride. 1996. Sequence analysis, expression, and binding activity of recombinant major outer sheath protein (Msp) of Treponema denticola. J. Bacteriol. 178:2489-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glogauer, M., J. Hartwig, and T. Stossel. 2000. Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils. J. Cell Biol. 150:785-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruenheid, S., and B. B. Finlay. 2003. Microbial pathogenesis and cytoskeletal function. Nature 422:775-781. [DOI] [PubMed] [Google Scholar]
- 9.Moter, A., C. Hoenig, B.-K. Choi, B. Riep, and U. B. Göbel. 1998. Molecular epidemiology of oral treponemes associated with periodontal disease. J. Clin. Microbiol. 36:1399-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paes Batista da Silva, A., W. Lee, E. Bajenova, C. A. G. McCulloch, and R. P. Ellen. 2004. The major outer sheath protein of Treponema denticola inhibits the binding step of collagen phagocytosis in fibroblasts. Cell. Microbiol. 6:485-498. [DOI] [PubMed] [Google Scholar]
- 11.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, B. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riviere, G. R., K. S. Weisz, D. F. Adams, and D. D. Thomas. 1991. Pathogen-related oral spirochetes from dental plaque are invasive. Infect. Immun. 59:3377-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal, G., W. Lee, P. D. Arora, M. McKee, G. Downey, and C. A. G. McCulloch. 2001. Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J. Cell Sci. 114:119-129. [DOI] [PubMed] [Google Scholar]
- 14.Socransky, S. S., A. D. Haffajee, M. S. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 15.Socransky, S. S., C. Smith, and A. D. Haffajee. 2002. Subgingival microbial profiles in refractory periodontal disease. J. Clin. Periodontol. 29:260-268. [DOI] [PubMed] [Google Scholar]
- 16.Wang, Q., K. S. Ko, A. Kapus, C. A. G. McCulloch, and R. P. Ellen. 2001. A spirochete surface protein uncouples store-operated calcium channels in fibroblasts. J. Biol. Chem. 276:23056-23064. [DOI] [PubMed] [Google Scholar]
- 17.Yang, P. F., M. Song, D. A. Grove, and R. P. Ellen. 1998. Filamentous actin disruption and diminished inositol phosphate response in gingival fibroblasts caused by Treponema denticola. Infect. Immun. 66:696-702. [DOI] [PMC free article] [PubMed] [Google Scholar]