Abstract
Gender differences in psychological processes have been of great interest in a variety of fields. While the majority of research in this area has focused on specific differences in relation to test performance, this study sought to determine the underlying neurofunctional differences observed during working memory, a pivotal cognitive process shown to be predictive of academic achievement and intelligence. Using the BrainMap database, we performed a meta-analysis and applied activation likelihood estimation to our search set. Our results demonstrate consistent working memory networks across genders, but also provide evidence for gender-specific networks whereby females consistently activate more limbic (e.g., amygdala and hippocampus) and prefrontal structures (e.g., right inferior frontal gyrus), and males activate a distributed network inclusive of more parietal regions. These data provide a framework for future investigation using functional or effective connectivity methods to elucidate the underpinnings of gender differences in neural network recruitment during working memory tasks.
Keywords: gender differences, fMRI, brainmap, working memory, sex differences
Introduction
For over a century, unequal abilities between men and women, particularly within the intellectual domain, have been both intriguing and elusive. While evidence for gender differences in psychological processes have been noted across a diverse range of cognitive domains (Bradley et al., 2001; Gur et al., 2000; Koch et al., 2007; Lynn and Irwing, 2002; Ragland et al., 2000; Shaywitz et al., 1995; Volf and Razumnikova, 1999), mixed results (Stevens, 2011) have stunted progression toward an understanding of the potential basis for these differences from a strictly neurological perspective. While the majority of research in this area has focused on specific behavioral performance differences in relation to test performance, this study sought to determine the neurofunctional differences observed during working memory, a pivotal cognitive process shown to be predictive of academic achievement and intelligence (Conway et al., 2003).
Examining working memory as a whole, the observed neural activation patterns observed in functional neuroimaging studies consistently demonstrate prefrontal, temporal, and parietal involvement (Haier et al., 2005) (Baddeley, 1981; Baddeley, 1997, 2000; Baddeley and Logie, 1999; D'Esposito et al., 1998a; D'Esposito et al., 1998b; D'Esposito et al., 2000; Na et al., 2000; Prabhakaran et al., 2000; Repovs and Baddeley, 2006), posited to reflect the components of Baddeley and colleagues (2011) revised model of working memory. However, it is widely accepted that working memory operates differently when presented with verbal compared to spatial information (Reuter-Lorenz et al., 2000; Smith et al., 1996). Verbal working memory preferentially engages the left hemisphere, specifically the inferior parietal lobe, lateral frontal lobe, the supramarginal gyrus (BA 10), premotor areas, and Broca's area (Jonides et al., 1998; Schumacher et al., 1996; Smith et al., 1996; Smith et al., 1998). Spatial working memory has been associated with a more dispersed activation pattern across the hemispheres, consisting of the inferior frontal lobe, posterior parietal lobe, right occipital gyrus, right premotor area, right dorsolateral prefrontal cortex, and the extrastriate cortex in the occipital lobe (D'Esposito et al., 1998a; Jonides et al., 1993; van Asselen et al., 2006). It has long been acknowledged that working memory plays a key role in manipulating incoming information entering the cognitive system, whether the information is verbal or spatial in nature, interacting dynamically with attention and long-term memory. For this reason, working memory is an integral part of general cognitive processing with significant trickle-down effects on other critical processes. Therefore, observing gender differences among working memory networks could have robust effects in other areas of cognitive functioning.
Interestingly, when working memory is deconstructed into spatial and verbal components, evidence suggests that behavioral disparities emerge between genders (Halpern et al., 2007). Research has shown that from a behavioral performance perspective, males demonstrate greater mathematical (Lynn and Irwing, 2008), spatial (Kaufman, 2007; Lejbak et al., 2011; Masters and Sanders, 1993; Nordvik and Amponsah, 1998), and object working memory (Lejbak et al., 2011) compared to females, and females display greater verbal (including episodic memory (Lewin et al., 2001)) and writing skills than males (Bae et al., 2000; Hedges and Nowell, 1995). The discrepancy in male and female spatial ability appears to begin as early as preschool and then becomes even more significant as males and females enter adulthood (Levine et al., 1999), whereas the female superiority in verbal facets tends to appear slightly later, peaking in early adulthood (Willingham and Cole, 1997). Some researchers suggest that the male advantage in spatial ability helps set them above their female counterparts in mathematics, especially in areas like geometry, which involve the visualization of items in space (Casey et al., 1995).
Despite evidence that gender differences exist in working memory, there is an equally strong case for a lack of performance differences. In recent years, as functional neuroimaging has become more commonplace, studies that do not find explicit behavioral differences have the opportunity to view more intrinsic neurofunctional patterns. Multiple studies have found that there are no significant performance differences between the genders during verbal working memory tasks, but there is evidence for neurofunctional differences (Kaufman, 2007; Lejbak et al., 2011; Speck et al., 2000), suggesting that the behavioral differences may still exist, but the studies could be underpowered, or males and females could be using different psychological strategies. Specifically, Speck and colleagues (Speck et al., 2000) observed differences in the functional networks utilized to complete a verbal working memory task, with males accessing more right hemispheric regions such as the lateral prefrontal cortex, posterior cingulate and caudate, while females utilized the left hemisphere more prominently. Females have also shown greater activation in the middle, inferior, and orbital prefrontal regions, despite similar performance to male subjects in other studies (Goldstein et al., 2005). Taken collectively, neuroimaging data support the notion that certain brain regions can function differently in males and females to produce the same behavioral responses, which appears to be the case with working memory (Goldstein et al., 2005). These results suggest that using functional neuroimaging may allow researchers to develop more accurate models of gender differences within specific cognitive domains that would allow for theories of neuroanatomical and neurofunctional differences to be tested empirically (for review, please see Halpern, et al. 2007).
From a neuroimaging perspective, recent research has shown that there are gender differences in functional connectivity during resting state (Filippi et al., 2013). Specifically, Filippi and colleagues (2013) found that women had greater intrinsic functional connectivity inclusive of the cingulate, dorsolateral prefrontal cortex, and the inferior frontal gyrus, while men demonstrated increased functional connectivity in parietal regions, characteristics that the authors attribute to potential strategy differentiation. These observed differences could help explain the disparity in performance between the genders on various cognitive tasks, as well as bringing into question the possibility of inherent neural network differences. The present study focuses on the later implication of the resting state data with regard to working memory, to see if such differences exist during working memory performance. Furthermore, because of the diversity of paradigms used to examine working memory, we chose to pursue a meta-analysis that overcomes task-dependent activation differences, allowing for a more accurate depiction of gender differences within the construct of working memory. Therefore, the present study investigated the neural underpinnings of gender differences in working memory by capitalizing on the structure of the BrainMap database (Fox et al., 2005; Fox and Lancaster, 2002; Laird et al., 2005b), a functional neuroimaging database that archives functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies with a meticulous coding scheme (Laird et al., 2009). Using meta-analysis to develop models of functional connectivity and subsequently probing differences in connectivity networks has been demonstrated to be both robust and effective (Robinson et al., 2010).
Methods
In order to ascertain the neural underpinning of working memory for males and females, the BrainMap database was queried using Sleuth version 2.2 (Fox et al., 2005; Laird et al., 2009; Laird et al., 2005b). In short, Sleuth is a free, publicly available search tool that allows users to search the BrainMap database among any of the meta-data categories contained within the database. We entered the following search criteria: 1) studies coded within the behavioral domain of cognition and paradigm class of working memory (e.g., Experiments → Behavioral Domain → Cognition → Memory – Working), 2) studies reporting activations only (e.g., Experiments → Activation → Activations Only), 3) studies using normal, healthy subjects (e.g., Experiments → Context → Normal Mapping), and 4) studies using only males or only females (e.g., two separate searches, one for each gender, were performed, Subjects → Gender → Females (or Males) Only). Resultant whole-brain coordinates of activation during working memory tasks were then downloaded (males: 44 papers, 2316 locations, 141 experiments, 127 conditions, 701 subjects; females: 15 papers, 402 locations, 36 experiments, 49 conditions, 200 subjects; to download the complete workspace files for the male and female searches, please visit http://aucanlab.com/?page_id=128). Coordinates that were not reported in Talairach space in their original publication were transformed into Talairach space by the GingerALE analysis program using the icbm2tal transform (Laird et al., 2010; Lancaster et al., 2005).
Activation likelihood estimation (ALE) meta-analysis (Eickhoff et al., 2009; Laird et al., 2005a; Turkeltaub et al., 2002) was performed on the sets of coordinates identified as activated during working memory tasks to identify regions of convergence within each search (i.e., males and females were run separately). ALE capitalizes on the nature of voxel-wise studies that are commonly reported in a standard stereotaxic space (x,y,z) by pooling 3D coordinates from like studies, and providing the probability of an event occurring at each brain voxel. The algorithm treats each coordinate of activation as a spatial probability, and ALE maps are subsequently calculated by computing the convergence of activation probabilities for every voxel. Permutation testing is then applied. Specifically, an ALE null-distribution is created by randomly assigning the same number of foci from the original analysis throughout the brain, and calculating ALE maps reiteratively after every reassignment. The original ALE scores are then compared to the random null distribution to assign p-values (Laird et al., 2005a; Turkeltaub et al., 2002). A revised ALE algorithm was proposed and subsequently implemented in the statistical toolbox GingerALE version 2.3 (Eickhoff et al., 2009). The new algorithm is statistically more robust as it treats the data using a random-effects approach, and models the uncertainty associated with a given coordinate. Furthermore, the analysis is anatomically constrained to exclude deep white matter, with the reasoning that ‘true’ activations originate in the gray matter, thus if we do not constrain the analyses, there is a potential bias in the permutation testing that creates the null-distribution by which p-values are determined (Eickhoff et al., 2009). Our analysis used the revised algorithm proposed by Eickhoff and colleagues (2009). False discovery rate (FDR) is defined as having no more than 5% false positives (i.e., if you are using an FDR corrected p-value of 0.05). In an ALE meta-analysis, FDR is dependent on the number of permutations implemented (Laird et al., 2005a). ALE maps from the present study were thresholded conservatively at an FDR-corrected p-value of 0.05 with a cluster threshold of 100mm3.
Results
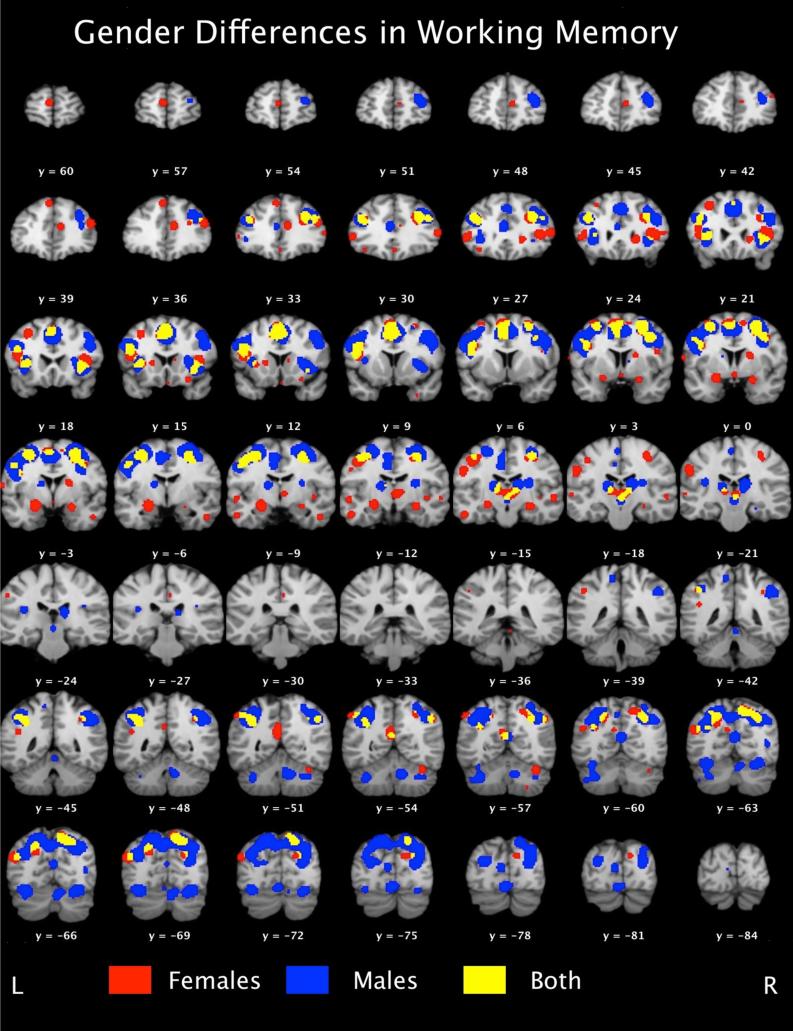
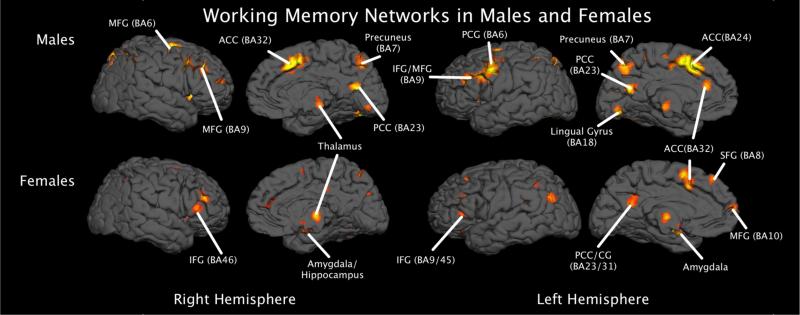
ALE results provide evidence for both common and gender-specific memory network utilization (please see Table 1). Common to both genders, bilateral middle frontal gyri (BA6/9), left cingulate gyrus (BA32), right precuneus (BA7/19), left inferior and superior parietal lobes (BA40,BA7, respectively), right claustrum, and left middle temporal gyrus (BA39) were found to be consistently activated during working memory performance. Gender specific networks also emerged. For females, we found that working memory tasks elicited consistent activity in regions of the limbic system such as the anterior cingulate (BA32), bilateral amygdala, and right hippocampus, in addition to an extensive prefrontal network inclusive of bilateral middle frontal gyri (BA46) and the right medial frontal gyrus (BA9). Males demonstrated a distributed gender-specific working memory network inclusive of the cerebellum, portions of the superior parietal lobe (BA7), the left insula (BA13), and bilateral thalamus (please see Figures 1 and 2).
Table 1.
Gender Differences in Working Memory Across All Working Memory Tasks
| Convergent Brain Regions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Region | BA | Females | Males | ALE | |||||
| x | y | z | x | y | z | |||||
| Frontal | Right Middle Frontal Gyrus | 6 | 26 | 2 | 52 | 28 | −6 | 52 | 0.032 | 0.095 |
| 34 | 2 | 38 | 32 | 2 | 34 | 0.018 | 0.058 | |||
| 9 | 28 | 28 | 30 | 32 | 30 | 32 | 0.018 | 0.067 | ||
| Left Middle Frontal Gyrus | 6/9 | −28 | −4 | 50 | −26 | −8 | 54 | 0.030 | 0.083 | |
| −36 | 28 | 26 | −40 | 26 | 26 | 0.026 | 0.043 | |||
| Limbic | Left Cingulate Gyrus | 32 | −4 | 10 | 42 | −2 | 16 | 40 | 0.029 | 0.054 |
| Parietal | Right Precuneus | 7 | 12 | −64 | 48 | 16 | −72 | 44 | 0.023 | 0.107 |
| 19 | 30 | −60 | 40 | 30 | −70 | 38 | 0.022 | 0.051 | ||
| Left Superior Parietal Lobule | 7 | −28 | −62 | 48 | −30 | −54 | 48 | 0.014 | 0.079 | |
| Left Inferior Parietal Lobe | 40 | −34 | −50 | 36 | −38 | −52 | 38 | 0.028 | 0.072 | |
| Sub-lobar | Right Claustrum | 32 | 14 | 0 | 30 | 14 | 6 | 0.016 | 0.054 | |
| Temporal | Left Middle Temporal Gyrus | 39 | 32 | −60 | 30 | −34 | −68 | 30 | 0.017 | 0.047 |
|
Female-Specific Network | ||||||||||
| Anterior | Right Culmen | 34 | −56 | −22 | 0.023 | |||||
| 4 | −36 | −8 | 0.014 | |||||||
| Frontal | Left Precentral Gryus | 4 | −44 | −8 | 40 | 0.019 | ||||
| Left Frontal Gyrus | −6 | 6 | 54 | 0.030 | ||||||
| Right Medial Frontal Gyrus | 6 | 10 | 0 | 56 | 0.016 | |||||
| Left Precentral Gryus | −40 | 2 | 28 | 0.015 | ||||||
| Right Medial Frontal Gyrus | 9 | 8 | 46 | 16 | 0.015 | |||||
| Right Inferior Frontal Gyrus | 13 | 38 | 22 | 10 | 0.029 | |||||
| Left Inferior Frontal Gyrus | 4 | −50 | 28 | 6 | 0.020 | |||||
| Right Inferior Frontal Gyrus | 52 | 28 | 12 | 0.024 | ||||||
| Left Middle Frontal Gyrus | 46 | −42 | 14 | 20 | 0.023 | |||||
| Right Middle Frontal Gyrus | 46 | 38 | 22 | 0.026 | ||||||
| Right Inferior Frontal Gyrus | 47 | 26 | 14 | −12 | 0.017 | |||||
| Limbic | Right Anterior Cingulate | 32 | 8 | 36 | 20 | 0.020 | ||||
| Left Amygdala | −22 | −6 | −10 | 0.031 | ||||||
| Right Amygdala | 22 | −2 | −12 | 0.023 | ||||||
| Right Hippocampus | 28 | −14 | −10 | 0.024 | ||||||
| Occipital | Right Cuneus | 18 | 12 | −78 | 28 | 0.018 | ||||
| Right Precuneus | 31 | 20 | −72 | 28 | 0.017 | |||||
| Parietal | Left Postcentral Gyrus | 2 | −54 | −18 | 28 | 0.018 | ||||
| Left Precuneus | 7 | −22 | −66 | 36 | 0.022 | |||||
| 31 | −2 | −50 | 30 | 0.017 | ||||||
| Right Inferior Parietal Lobule | 40 | 46 | −54 | 40 | 0.023 | |||||
| 34 | −46 | 40 | 0.017 | |||||||
| Sub-lobar | Right Thalamus (Medial Dorsal Nucleus) | 4 | −16 | 4 | 0.031 | |||||
| Left Thalamus | −12 | −18 | 6 | 0.018 | ||||||
| Right Caudate Head | 18 | 24 | 4 | 0.024 | ||||||
| Left Claustrum | −30 | 14 | 8 | 0.016 | ||||||
| Left Putamen (Lenitform Nucleus) | −18 | 12 | 8 | 0.016 | ||||||
| Temporal | Left Superior Temporal Gyrus | 13 | −42 | −46 | 24 | 0.020 | ||||
| Left Middle Temporal Gyrus | 39 | −46 | −68 | 26 | 0.037 | |||||
|
Male Specific Network | ||||||||||
| Anterior | Right Cerebllum Nodule | 10 | −52 | −28 | 0.060 | |||||
| Left Middle Frontal Gyrus | 6 | −48 | 0 | 38 | 0.072 | |||||
| Left Superior Frontal Gyrus | 6 | 0 | 8 | 48 | 0.120 | |||||
| Left Medial Frontal Gyrus | −4 | −20 | 56 | 0.031 | ||||||
| −8 | −10 | 48 | 0.042 | |||||||
| Right Middle Frontal Gyrus | 9 | 42 | 12 | 40 | 0.048 | |||||
| Left Inferior Frontal Gyrus | 9 | −50 | 10 | 30 | 0.063 | |||||
| Left Middle Frontal Gyrus | −44 | 14 | 26 | 0.043 | ||||||
| Right Middle Frontal Gyrus | 10 | 34 | 48 | 16 | 0.046 | |||||
| Midbrain | Left Brainstem (Red Nucleus) | 0 | −20 | −4 | 0.040 | |||||
| Occipital | Right Cuneus | 18 | 26 | −76 | 20 | 0.065 | ||||
| Left Cuneus | −18 | −74 | 20 | 0.059 | ||||||
| Left Middle Occipital Gyrus | 19 | −28 | −78 | 20 | 0.042 | |||||
| Right Middle Occipital Gyrus | 37 | 40 | −64 | 10 | 0.031 | |||||
| Right Precuneus | 28 | −56 | 52 | 0.045 | ||||||
| Left Precuneus | 7 | −14 | −70 | 48 | 0.066 | |||||
| −6 | −68 | 40 | 0.049 | |||||||
| Right Supramarginal Gyrus | 40 | 40 | −46 | 36 | 0.030 | |||||
| Posterior | Left Declive | −32 | −66 | −14 | 0.071 | |||||
| Right Declive | 26 | −68 | −16 | 0.065 | ||||||
| Left Cerebellar Tonsil | −32 | −56 | −32 | 0.041 | ||||||
| −40 | −58 | −34 | 0.031 | |||||||
| Left Declive | −2 | −76 | −10 | 0.054 | ||||||
| Right Declive | 10 | −68 | −16 | 0.040 | ||||||
| Sub-lobar | Left Insula | 13 | −34 | 16 | 10 | 0.050 | ||||
| Right Thalamus | 14 | −20 | 16 | 0.067 | ||||||
| Left Thalamus (Ventral Lateral Nucleus) | −16 | −16 | 14 | 0.059 | ||||||
Figure 1.
Mosaic view of working memory networks in males (blue) and females (red). Brain regions recruited by both genders during working memory tasks are depicted by yellow. Maps were thresholded at p < 0.05, FDR-corrected.
Figure 2.
3D rendering of the working memory networks in males and females.
Post-hoc Decomposition of Working Memory
Our initial findings revealed neural network recruitment differences in working memory, such that females demonstrated more limbic activation. Because of the disparate search set sizes, and to ensure our data were driven by cognitively coded papers, we did post-hoc analyses examining the two most prevalent working memory tasks: the n-back and the delayed match to sample (DMTS) task. For these searches, we followed the above procedure, but in addition to the search criteria of ‘Experiments → Behavioral Domain → Cognition → Memory – Working’, we also included Experiments → Paradigm Class → Delayed Match to Sample (or n-back)’. This allowed us to narrow our search to only those studies implementing n-back or DMTS tasks within the behavioral domain of ‘Cognition’. The DMTS and n-back search specific to females yielded 15 papers, 195 subjects, 45 experiments, 53 conditions, and 484 locations. The male workspace consisted of 30 papers, 397 subjects, 76 experiments, 89 conditions, and 757 locations. ALE was implemented as described above. Maps were thresholded at an FDR-corrected p-value of 0.05, with a cluster threshold of 100mm3. We also performed a quantitative contrast of the resultant ALE maps to objectively determine the differences between male and female networks in a statistically sound manner using the GingerALE program within the BrainMap environment. To do this, GingerALE performs a subtraction of one ALE image from the other. Similar to a traditional ALE analysis, GingerALE creates simulated data by pooling the coordinates from the original datasets and randomly dividing them into two new groupings of the same size as the original datasets, then subtracting these new pairings (i.e., permutations are used to create a null distribution of which the real-data is then compared). The resultant images are converted to z-score maps.
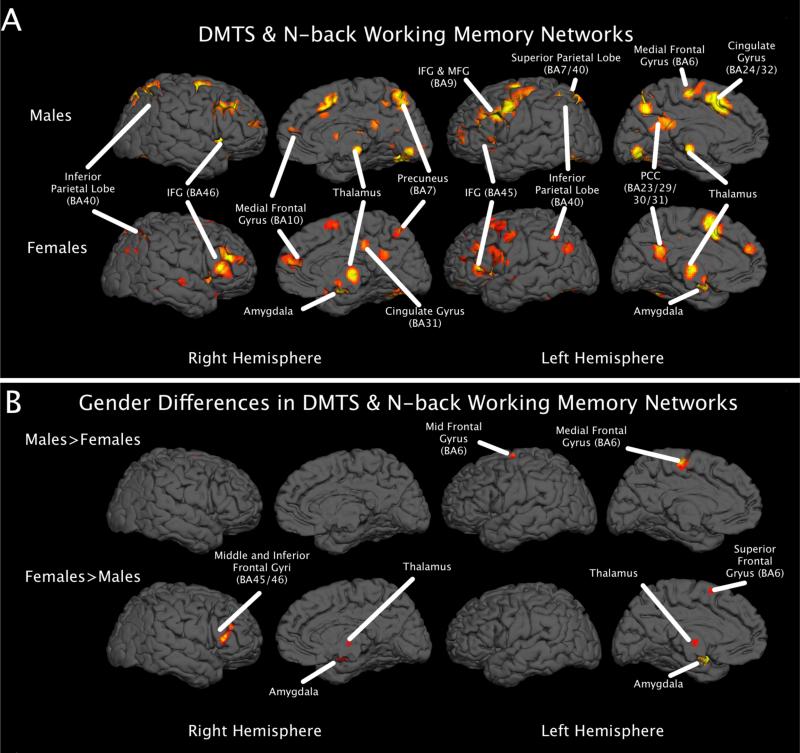
Our results largely mirror the results obtained from including all working memory studies, with females demonstrating more activation throughout the limbic and prefrontal regions, including bilateral amygdalae and cingulate regions, and males activating more parietal areas, such as the inferior and superior parietal lobe and the precuneus (please see Tables, 2, 3, and 4). The quantitative assessment of gender differences on the resultant ALE maps from the post-hoc analysis corroborated with evidence from visual assessment. Specifically, the females showed greater activation of limbic structures inclusive of the amygdalae, in addition to frontal regions such as the left medial and superior frontal gyri and the right middle and inferior gyri. Males demonstrated greater activation consistently in the left precuneus and superior parietal lobule, as well as the right insula (please see Table 5 and Figure 3, Panel B).
Table 2.
Gender Differences in DMTS and N-back Working Memory Tasks
| Convergent Brain Regions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lobe | Region | BA | Females | Males | ALE Female | ALE Male | ||||
| x | y | z | x | y | z | |||||
| Anterior | Right Culmen | 2 | −50 | −20 | 6 | −42 | −20 | 0.009 | 0.012 | |
| Frontal | Left Middle Frontal Gyrus | −26 | −4 | 50 | −26 | −8 | 56 | 0.022 | 0.051 | |
| Left Precentral Gyrus | 6 | −40 | 0 | 28 | −44 | 0 | 30 | 0.035 | 0.051 | |
| Right Precentral Gyrus | 42 | 2 | 28 | 32 | 0 | 34 | 0.010 | 0.049 | ||
| Right Sub-Gyral | 26 | 2 | 52 | 20 | −6 | 56 | 0.029 | 0.047 | ||
| Left Inferior Frontal Gyrus | −56 | 12 | 24 | −52 | 10 | 30 | 0.011 | 0.034 | ||
| Right Medial Frontal Gyrus | 9 | 8 | 48 | 16 | 8 | 50 | 16 | 0.016 | 0.014 | |
| Right Middle Frontal Gyrus | 28 | 34 | 24 | 32 | 30 | 32 | 0.013 | 0.039 | ||
| 48 | 16 | 34 | 48 | 16 | 36 | 0.009 | 0.021 | |||
| Left Middle Frontal Gyrus | −38 | 44 | 16 | −42 | 50 | 4 | 0.012 | 0.013 | ||
| Right Middle/Superior Frontal Gyrus | 10 | 38 | 48 | 20 | 36 | 46 | 16 | 0.014 | 0.026 | |
| Left Inferior Frontal Gyrus | 45 | −50 | 28 | 6 | −52 | 18 | 4 | 0.021 | 0.013 | |
| Left Extra-Nuclear/Inferior Frontal Gyrus | 47 | −30 | 18 | −10 | −32 | 20 | −8 | 0.014 | 0.017 | |
| Limbic | Left Cingulate Gyrus | 31 | 0 | −50 | 26 | −2 | −50 | 28 | 0.020 | 0.016 |
| 32 | −4 | 10 | 42 | −12 | 6 | 40 | 0.032 | 0.024 | ||
| Occipital | Left Lingual Gyrus | 18 | −20 | −78 | −8 | −14 | −82 | −10 | 0.008 | 0.012 |
| Right Cuneus | 26 | −68 | 18 | 26 | −76 | 20 | 0.011 | 0.057 | ||
| Parietal | Left Postcentral Gyrus | 3 | −54 | −18 | 26 | −50 | −18 | 38 | 0.015 | 0.011 |
| Left Precuneus | 7 | −20 | −64 | 38 | −14 | −70 | 48 | 0.020 | 0.041 | |
| Right Precuneus | 12 | −64 | 48 | 18 | −70 | 46 | 0.019 | 0.064 | ||
| 19 | 30 | −60 | 40 | 32 | −66 | 38 | 0.022 | 0.025 | ||
| Right Superior Parietal Lobule | 7 | 38 | −58 | 52 | 28 | −58 | 54 | 0.009 | 0.032 | |
| Right Inferior Parietal Lobule | 40 | 46 | −54 | 40 | 44 | −50 | 40 | 0.023 | 0.019 | |
| Posterior | Left Cerebellar Tonsil | −36 | −52 | −44 | −36 | −56 | −44 | 0.010 | 0.011 | |
| Right Cerebellar Tonsil | 24 | −58 | −44 | 28 | −58 | −36 | 0.014 | 0.012 | ||
| Right Declive | 26 | −70 | −16 | 26 | −68 | −16 | 0.015 | 0.058 | ||
| Sub-lobar | Left Insula | 13 | −36 | 18 | 8 | −34 | 16 | 10 | 0.024 | 0.025 |
| −32 | 20 | 2 | 0.023 | |||||||
| Right Claustrum | 32 | 14 | 0 | 32 | 12 | 4 | 0.019 | 0.029 | ||
| Left Caudate Body | −6 | 0 | 10 | −6 | 2 | 18 | 0.011 | 0.011 | ||
| Temporal | Left Superior Temporal Gyrus | 22 | −44 | −34 | −2 | −46 | −36 | 0 | 0.019 | 0.011 |
| Left Fusiform Gyrus | 37 | −40 | −54 | −18 | −42 | −44 | −12 | 0.027 | 0.010 | |
| Right Superior Temporal Gyrus | 38 | 44 | 20 | −18 | 42 | 20 | −18 | 0.009 | 0.011 | |
| Left Middle Temporal Gyrus | 39 | −32 | −60 | 30 | −34 | −68 | 30 | 0.014 | 0.018 | |
Table 3.
Female-Specific Network in DMTS and N-back Working Memory Tasks
| Lobe | Region | BA | x | y | z | ALE |
|---|---|---|---|---|---|---|
| Anterior | Right Pyramis | 2 | −64 | −26 | 0.017 | |
| 4 | −42 | −22 | 0.009 | |||
| Right Culmen | 10 | −36 | −20 | 0.009 | ||
| 34 | −56 | −22 | 0.027 | |||
| Frontal | Right Precentral Gyrus | 4 | 32 | −18 | 48 | 0.009 |
| 6 | 24 | −14 | 46 | 0.008 | ||
| Left Precentral Gyrus | 6 | −62 | 0 | 14 | 0.016 | |
| 6 | −44 | −8 | 40 | 0.021 | ||
| Left Middle Frontal Gyrus | 6 | −22 | 14 | 56 | 0.011 | |
| Left Superior Frontal Gyrus | 6 | −6 | 6 | 54 | 0.039 | |
| Right Medial Frontal Gyrus | 6 | 10 | 0 | 56 | 0.010 | |
| Right Middle Frontal Gyrus | 6 | 16 | 14 | 58 | 0.011 | |
| 6 | 38 | 0 | 40 | 0.016 | ||
| Left Middle Frontal Gyrus | 8 | −34 | 16 | 42 | 0.013 | |
| Left Medial Frontal Gyrus | 8 | −10 | 40 | 40 | 0.008 | |
| Left Inferior Frontal Gyrus | 9 | −54 | 4 | 22 | 0.014 | |
| Left Middle Frontal Gyrus | 9 | −52 | 14 | 32 | 0.010 | |
| 9 | −36 | 28 | 26 | 0.026 | ||
| Left Medial Frontal Gyrus | 9 | −4 | 48 | 26 | 0.016 | |
| 10 | −16 | 48 | 6 | 0.009 | ||
| Left Middle Frontal Gyrus | 11 | −20 | 48 | −8 | 0.010 | |
| Right Middle Frontal Gyrus | 11 | 24 | 48 | −10 | 0.009 | |
| Right Inferior Frontal Gyrus | 13 | 34 | 10 | −12 | 0.015 | |
| Right Medial Frontal Gyrus | 25 | 2 | 14 | −16 | 0.015 | |
| Right Inferior Frontal Gyrus | 44 | 42 | 16 | 10 | 0.013 | |
| Left Inferior Frontal Gyrus | 45 | −42 | 16 | 16 | 0.021 | |
| Right Middle Frontal Gyrus | 46 | 46 | 38 | 22 | 0.026 | |
| Right Inferior Frontal Gyrus | 46 | 52 | 28 | 12 | 0.024 | |
| 47 | 26 | 14 | −10 | 0.012 | ||
| Left Inferior Frontal Gyrus | 47 | −40 | 28 | 0 | 0.012 | |
| Limbic | Left Anterior Cingulate | 25 | 0 | 0 | −6 | 0.015 |
| Left Posterior Cingulate | 31 | −10 | −54 | 18 | 0.012 | |
| Right Cingulate Gyrus | 31 | 4 | −30 | 36 | 0.014 | |
| Left Amygdala | −22 | −6 | −12 | 0.030 | ||
| Right Amygdala | 22 | −2 | −12 | 0.025 | ||
| Right Hippocampus | 28 | −14 | −12 | 0.025 | ||
| Midbrain | Left Substania Nigra | −8 | −20 | −8 | 0.016 | |
| Occipital | Left Cuneus | 18 | −8 | −80 | 20 | 0.012 |
| Left Middle Temporal Gyrus | 19 | −40 | −60 | 16 | 0.009 | |
| Right Middle Occipital Gyrus | 19 | 30 | −80 | 22 | 0.012 | |
| Left Precuneus | 31 | −8 | −60 | 26 | 0.008 | |
| Parietal | Right Superior Parietal Lobule | 7 | 36 | −66 | 48 | 0.008 |
| Left Angular Gyrus | 39 | −46 | −66 | 28 | 0.015 | |
| Right Angular Gyrus | 39 | 54 | −64 | 32 | 0.010 | |
| Left Inferior Parietal Lobule | 40 | −52 | −54 | 44 | 0.017 | |
| Left Inferior Parietal Lobule | 40 | −34 | −50 | 36 | 0.026 | |
| Right Inferior Parietal Lobule | 40 | 34 | −48 | 40 | 0.019 | |
| Right Inferior Parietal Lobule | 40 | 60 | −32 | 30 | 0.009 | |
| Posterior | Right Declive | 32 | −64 | −12 | 0.015 | |
| Sub-lobar | Left Insula | 13 | −42 | −28 | 24 | 0.011 |
| Right Insula | 13 | 36 | 20 | 18 | 0.009 | |
| 13 | 40 | −12 | −2 | 0.015 | ||
| Left Amygdala | −24 | −10 | −10 | 0.029 | ||
| Left Thalamus | −12 | −18 | 6 | 0.024 | ||
| Right Thalamus (Medial Dorsal Nucleus) | 4 | −16 | 4 | 0.031 | ||
| Right Lateral Globus Pallidus | 12 | 2 | 4 | 0.009 | ||
| Right Caudate Head | 18 | 24 | 4 | 0.024 | ||
| Right Caudate Body | 20 | −2 | 20 | 0.008 | ||
| Right Lateral Globus Pallidus | 22 | −12 | 2 | 0.019 | ||
| Right Thalamus (Pulvinar) | 26 | −30 | 6 | 0.016 | ||
| Temporal | Right Fusiform Gyrus | 20 | 46 | −6 | −20 | 0.009 |
| Right Middle Temporal Gyrus | 20 | 58 | −42 | −10 | 0.009 | |
| 21 | 56 | −14 | −6 | 0.013 | ||
| Left Middle Temporal Gyrus | 21 | −54 | −12 | −6 | 0.017 | |
| 22 | −48 | −46 | 2 | 0.014 | ||
| 38 | −42 | 4 | −8 | 0.008 | ||
| Left Superior Temporal Gyrus | 38 | −38 | 8 | −14 | 0.009 | |
| 38 | −36 | 4 | −14 | 0.009 | ||
| Right Angular Gyrus | 39 | 46 | −74 | 30 | 0.010 | |
Table 4.
Male-Specific Network in DMTS and N-back Working Memory Tasks
| Lobe | Region | BA | x | y | z | ALE |
|---|---|---|---|---|---|---|
| Anterior | Right Cerebellar Lingual | 2 | −42 | −8 | 0.022 | |
| Right Nodule | 10 | −52 | −28 | 0.051 | ||
| Right Culmen | 12 | −60 | −2 | 0.013 | ||
| Frontal | Left Middle Frontal Gyrus | 6 | −46 | 0 | 38 | 0.053 |
| Left Medial Frontal Gyrus | 6 | −8 | −10 | 48 | 0.015 | |
| 6 | −4 | −20 | 56 | 0.027 | ||
| Left Superior Frontal Gyrus | 6 | 0 | 8 | 48 | 0.065 | |
| Right Middle Frontal Gyrus | 6 | 28 | −6 | 54 | 0.042 | |
| Left Superior Frontal Gyrus | 10 | −38 | 50 | 18 | 0.012 | |
| Left Precentral Gyrus | 44 | −52 | 6 | 10 | 0.010 | |
| Left Inferior Frontal Gyrus | 46 | −42 | 30 | 10 | 0.014 | |
| Left Middle Frontal Gyrus | 46 | −42 | 18 | 26 | 0.027 | |
| Left Inferior Frontal Gyrus | 47 | −48 | 18 | −6 | 0.013 | |
| Limbic | Left Posterior Cingulate | 23 | −4 | −56 | 20 | 0.014 |
| 29 | 0 | −42 | 22 | 0.018 | ||
| Midbrain | Left Red Nucleus | 0 | −20 | −6 | 0.029 | |
| Occipital | Left Cuneus | 17 | −6 | −78 | 14 | 0.013 |
| Right Lingual Gyrus | 17 | 10 | −88 | −4 | 0.016 | |
| Left Cuneus | 18 | −18 | −82 | 28 | 0.011 | |
| Left Middle Occipital Gyrus | 19 | −28 | −78 | 18 | 0.023 | |
| Left Lingual Gyrus | 19 | −18 | −60 | −4 | 0.012 | |
| Right Middle Occipital Gyrus | 19 | 38 | −64 | 10 | 0.023 | |
| Left Inferior Temporal Gyrus | 37 | −44 | −64 | −2 | 0.011 | |
| Parietal | Left Postcentral Gyrus | 3 | −40 | −26 | 56 | 0.015 |
| Left Superior Parietal Lobule | 7 | −30 | −54 | 46 | 0.052 | |
| 7 | 4 | −52 | 60 | 0.011 | ||
| Right Precuneus | 7 | 6 | −70 | 42 | 0.027 | |
| 7 | 8 | −50 | 44 | 0.015 | ||
| 7 | 28 | −44 | 42 | 0.011 | ||
| Left Precuneus | 7 | −4 | −68 | 36 | 0.027 | |
| 19 | −10 | −84 | 44 | 0.010 | ||
| Left Inferior Parietal Lobule | 40 | −36 | −52 | 36 | 0.034 | |
| Posterior | Left Cerebellar Tonsil | −42 | −58 | −32 | 0.019 | |
| −34 | −68 | −14 | 0.052 | |||
| Left Declive | −26 | −84 | −16 | 0.013 | ||
| −12 | −68 | −18 | 0.025 | |||
| −2 | −76 | −10 | 0.042 | |||
| Right Uvula | 6 | −66 | −34 | 0.015 | ||
| Right Declive | 10 | −68 | −16 | 0.040 | ||
| Sub-lobar | Left Insula | 13 | −40 | 0 | 14 | 0.010 |
| Right Insula | 13 | 36 | −24 | 22 | 0.024 | |
| Left Caudate Body | −16 | −2 | 16 | 0.014 | ||
| Left Thalamus (Ventral Lateral Nucleus) | −16 | −16 | 12 | 0.048 | ||
| Right Caudate Body | 8 | 4 | 10 | 0.020 | ||
| Right Thalamus (Lateral Dorsal Nucleus) | 12 | −20 | 16 | 0.052 | ||
| Left Cerebellum | −2 | −82 | −24 | 0.013 | ||
Table 5.
Gender Differences in DMTS and N-back Working Memory Tasks
| Females > Males | ||||||
|---|---|---|---|---|---|---|
| Lobe | Region | BA | x | y | z | Z-Score |
| Anterior | Right Culmen | 30 | −56 | −24 | 3.01 | |
| Frontal | Left Medial Frontal Gyrus | 6 | −13 | 10 | 53 | 3.35 |
| −8 | 6 | 56 | 3.09 | |||
| Left Superior Frontal Gyrus | −10 | 12 | 58 | 3.29 | ||
| Right Inferior Frontal Gyrus | 45 | 50 | 22 | 11.14 | 3.72 | |
| 54 | 26 | 14 | 3.43 | |||
| Right Middle Frontal Gyrus | 46 | 46 | 32 | 24 | 3.29 | |
| 50 | 32 | 18 | 3.09 | |||
| Limbic | 28 | 26 | −20 | −10 | 3.43 | |
| Right Parahippocampal Gyrus | 20 | −3.6 | −9.2 | 3.12 | ||
| 34 | 21 | −12 | −16 | 2.66 | ||
| Left Uncus | −22.6 | −0.53 | −13.57 | 3.89 | ||
| Left Amygdala | −16.67 | −4 | −18 | 3.43 | ||
| −16 | −8 | −10 | 3.35 | |||
| 25 | −3 | −11.5 | 2.85 | |||
| Right Amygdala | 19.5 | −9.5 | −12 | 2.83 | ||
| 18 | −4 | −16 | 2.82 | |||
| Right Hippocampus | 32 | −10 | −14 | 2.70 | ||
| Sub-lobar | Left Insula | 13 | −42 | −6 | −6 | 3.09 |
| Left Thalamus | −2 | −11 | 2 | 2.97 | ||
| Right Claustrum | 36.86 | −12.86 | −0.29 | 3.72 | ||
| Right Lateral Globus Pallidus | 25.6 | −14 | −4.8 | 3.24 | ||
| Right Medial Globus Pallidus | 18.67 | −4.67 | −8 | 2.79 | ||
| 30 | −18 | −8 | 3.54 | |||
| Right Putamen | 29 | −15 | −6 | 3.35 | ||
| 28 | −8 | −8 | 3.19 | |||
| Right Thalamus | 6 | −8 | 2 | 2.82 | ||
| Temporal | Left Sub-Gyral | 21 | −44 | −6 | −10 | 3.24 |
| Left Superior Temporal Gyrus | 22 | −50.5 | −8.75 | −4.25 | 3.72 | |
| −46 | −11 | −4 | 3.35 | |||
|
Males > Females | ||||||
| Frontal | −12.8 | −17.4 | 55.6 | 3.89 | ||
| Left Medial Frontal Gyrus | −4 | −24 | 59 | 3.72 | ||
| −4.8 | −17.2 | 58.4 | 3.29 | |||
| 6 | 0 | −14 | 56 | 2.85 | ||
| Left Middle Frontal Gyrus | −19 | −7 | 60 | 3.16 | ||
| Left Precentral Gyrus | −28 | −14 | 62 | 2.99 | ||
| Right Sub-Gyral | 24 | −10 | 54 | 3.29 | ||
| Parietal | Left Precuneus | −26 | −56 | 54 | 3.04 | |
| Left Superior Parietal Lobule | 7 | −30 | −61 | 45 | 2.95 | |
| −26 | −62 | 54 | 2.93 | |||
| Sub-lobar | Right Insula | 13 | 36 | −22 | 25 | 3.04 |
Figure 3.
A) 3D rendering of networks involved in n-back and DMTS tasks, thresholded at p < 0.05, FDR-corrected. B) 3D rendering from the contrast analysis of the resultant ALE maps from panel A, thresholded at z > 2.3.
Discussion
Despite over a century of scientific inquiry, little progress has been made in addressing the substrates of gender differences, specifically as they relate to working memory. Using a novel approach, we used the BrainMap database to probe neurofunctional differences in working memory. Our results provide evidence for differential network recruitment by males and females undergoing working memory tasks. The results are consistent with previous literature suggesting that males utilize more spatial processing related networks (i.e., parietal regions) than females, and females tend to recruit more prefrontal regions (Haier et al., 2005), suggesting that men and women may use different strategies to solve complex problems (Haier et al., 2005).
The congruent areas of activation are not surprising as they are the anatomical structures most associated with working memory processes. Across studies, there has been consistent activation patterns seen in the frontal, temporal, and parietal regions (Baddeley, 1981; Baddeley, 1997, 2000; Baddeley and Logie, 1999; D'Esposito et al., 1998a; D'Esposito et al., 1998b; D'Esposito et al., 2000; Na et al., 2000; Prabhakaran et al., 2000; Repovs and Baddeley, 2006). Baddeley and Hitch's revised theory of working memory (2000) can be used to explain the observed activation patterns. In their theory, working memory was composed of four interconnecting systems: 1) the phonological loop, responsible for the storage and maintenance of speech-based information, 2) the visuospatial sketchpad, which stores and maintains visual and spatial information, 3) the central executive, responsible for controlling and integrating the information from the prior systems while also manipulating the information within working memory, and lastly, the most recently added component, 4) the episodic buffer, which assists with the binding of information to create episodes (Baddeley, 2000; Repovs and Baddeley, 2006). These systems are not mutually exclusive, but rather are thought to have overlapping neural components inclusive of the regions we identified as convergent in our dataset. The prefrontal cortex has been found to reliably activate during working memory tasks, which can be related back to the role of the central executive as well as the episodic buffer. Research has shown that the prefrontal cortex is critical in the maintenance and integration of verbal and spatial information (Prabhakaran et al., 2000), one of the primary roles of the central executive and a feature of the episodic buffer. Solidifying this, research has demonstrated that tasks employing the episodic buffer reliably activate the right prefrontal cortex (Repovs and Baddeley, 2006). The activation seen in areas associated with language can be interpreted as a function of the phonological loop due to their importance in linguistic processing. Furthermore, activation observed in both the inferior and superior parietal cortices may be related to the visuospatial sketchpad due to their known pertinence in the integration of visual information and spatial cognition (please see Na et al., 2000 for a review).
Our data demonstrates consistency with the working memory literature, but also highlights differences that should be examined more thoroughly in future research. Differences in neurophysiology (i.e., cerebral glucose metabolism, cerebral blood flow) during rest have been observed between genders (Davidson et al., 1976; Gur et al., 1995; Ray et al., 1976). Given that our results are based on functional neuroimaging results, which are tightly correlated with these physiological measurements, it is not surprising that differences in neural network recruitment exist during an active state as well. It is possible that the differences observed during rest ‘prime’ the brain to utilize certain networks preferentially. Given the strong limbic activation in the female dataset, it is also possible that females have more limbic contributions to working memory processing than males, a theory that should be investigated further using more advanced analysis techniques such as effective and functional connectivity.
Data from this study and previous research supports the notion that males and females rely on different brain networks to perform the same function, with the implications must notable in the academic realm. Halpern and colleagues (2007) suggest that we can use this knowledge to teach female and male students ways to solve problems that correspond to their most efficient cognitive process (i.e. verbal versus visuospatial solution strategies) to allow more flexibility in their problem solving and positively impact performance overall. Furthermore, a trickle down effect of understanding the neural differences underlying working memory processes between genders may lead to advancements in unbiased test design, particularly with regard to popular standardized tests such as the GRE and SAT, which have been criticized for having gender-biased questions. Such considerations may alleviate the gender discrepancy observed in academics.
Working memory is utilized during many complex cognitive functions, and the knowledge of gender differences could bring into question preferential strategy use, and unlock methods that would eliminate the gender gap. Due to working memory's pivotal role across a diverse set of cognitive functions, there is a possibility of neurofunctional differences during processing, and if this is the case, research addressing these differences will yield greater insight into gender specific cognitive function and expand the literature on gender differences in these constructs. Furthermore, with the robust and sensitive cognitive neuroscience tools, we may delineate the neurophysiological basis of the differences.
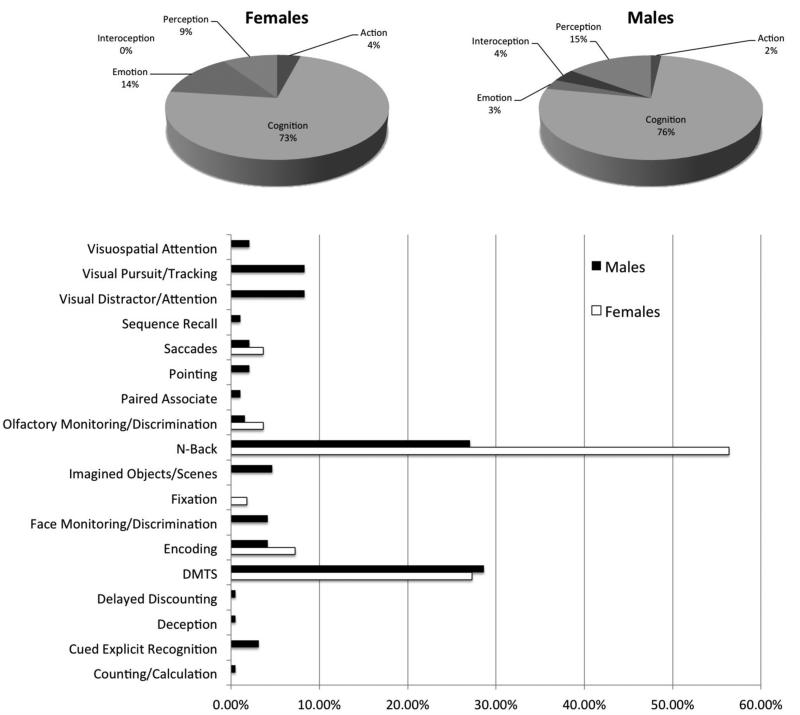
Possible limitations on the present study are those that are shared among meta-analysis based methods. We were unable to control for specific attributes of the participants that could add possible confounds to the overall data such as handedness and where the female participants were in their menstrual cycle, both of which have been shown to impact imaging data. There were also more males than females in the studies included in our meta-analysis. In this study, we did not select working memory tasks based on their content either (i.e., verbal versus spatial). Research has shown that different working memory tasks utilize different brain networks, so depending on the tasks used in the experiments some differences could be related to proportions of specific tasks used (Na et al., 2000) in each workspace. We examined the behavioral domains and paradigms within each of our search sets (Figure 3). As noted in the figure, only a very small percentage of data were coded as emotion, perception, interoception, or action (73% of the female dataset and 76% of the male dataset were coded as cognition). The majority of both data sets were drawn from classic working memory paradigms (84% of paradigms in the female dataset and 56% in the male dataset were either delayed match to sample or n-back paradigms). In the deconstruction analysis that we carried out post-hoc, we limited our search to only those tasks that were coded as n-back or DMTS, and coded under the behavioral domain of ‘Cognition’. These additional analyses did not change our initial findings, thus, we believe our sample is robust and likely offsets the possibility of the above confounds.
Future studies should attempt to have an even gender distribution to control for any effects caused by the greater depth of the male workspace. As shown in Figure 4, the male dataset also had a more diverse profile of working memory paradigms compared to the female workspace. However, we do note that our post-hoc analysis that just examined n-back and DMTS cognitive tasks still demonstrated gender differences. Therefore, future studies should focus on increasing the number of verbal and spatial working memory papers to further deconstruct the observed differences. Additionally, future neuroimaging studies should use the models presented in this paper to look at functional and effective connectivity differences during working memory tasks. Using this strategy, we may be able to probe the strategic differences and their effects on the neurofunctional networks subservient to working memory. These differences may exist even when activation patterns don't demonstrate differences between genders.
Figure 4.
Behavioral domain (top panels, shown in pie graph form) and paradigm breakdown (bottom panel) of the male and female workspaces. Because of the disparate workspace sizes, all values are shown as percentages within each gender-specific workspace, respectively.
Although gender differences are socially and scientifically important to understand, few studies have addressed their potential neurophysiological basis. Addressing these issues could lead to advances in our understanding of the underlying neural networks that may be responsible for gender differences in working memory, potentially leading to tailored developmental cognitive programs or novel strategy development that could reduce the gender gap that is thought to exist in some areas of cognition (Irwing and Lynn, 2005, 2006; Lynn and Irwing, 2002). It also provides a foundation to further investigate brain based gender differences and the implications they have for all areas of cognition (Davidson et al., 1976; Gur et al., 1995). To our knowledge, this is the first study addressing neural network differences in working memory using meta-analytic modeling, a powerful and robust technique that capitalizes on the advantages of archived functional neuroimaging studies (Laird et al., 2005c; Minzenberg et al., 2009). Here, we have provided a preliminary model of neurofunctional gender-specific working memory networks. Further research directions could use this model to ascertain why and how males and females use different neural networks during working memory tasks, or could attempt to assess when these neurofunctional differences first appear in development as well as the possible stimuli influencing the emergence of these observed difference.
Highlights.
- Our results provide evidence for gender-specific working memory networks.
- Females activate more limbic structures such as the amygdala and hippocampus.
- Males activate a distributed network inclusive of more parietal regions.
- Our data provide a foundation for future network analyses.
Acknowledgements
This work was supported by NIMH R01-MH074457 (PI: PTF AND ARL). A Collaborative Use Agreement exists between JLR and the BrainMap® Database.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baddeley A. The concept of working memory: A view of its current state and probable future development. Cognition. 1981;10:17–23. doi: 10.1016/0010-0277(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. In: Gazzaniga MS, editor. The Cognitive Neurosciences. The MIT Press; Cambridge, MA: 1997. pp. 755–764. [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Logie RH. Working memory: The multiple-component model. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge University Press; New York, NY, US: 1999. pp. 28–61. [Google Scholar]
- Bae Y, Choy S, Geddes C, Sable J, Snyder T. Trends in education equity for girls and women. U.S. Government Printing Office; Washington, D.C.: 2000. [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- Casey MB, Nuttall RL, Pezaris E, Benbow CP. The influence of spatial ability on gender differences in mathematics college entrance test scores across diverse samples. Developmental Psychology. 1995;31:697–705. [Google Scholar]
- Conway ARA, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences. 2003;7:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Research. Cognitive Brain Research. 1998a;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Ballard D, Aguirre GK, Zarahn E. Human prefrontal cortex is not specific for working memory: a functional MRI study. Neuroimage. 1998b;8:274–282. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Schwartz GE, Pugash E, Bromfield E. Sex differences in patterns of EEG asymmetry. Biological Psychology. 1976;4:119–138. doi: 10.1016/0301-0511(76)90012-0. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Valsasina P, Misci P, Falini A, Comi G, Rocca MA. The organization of intrinsic brain activity differs between genders: A resting-state fMRI study in a large cohort of young healthy subjects. Human Brain Mapping. 2013;34:1330–1343. doi: 10.1002/hbm.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: Description and evaluation. Human Brain Mapping. 2005;25 doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Opinion: Mapping context and content: the BrainMap model. Nature Reviews Neuroscience. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19:509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Gur RC, Alsop D, Glahn D, Petty R, Swanson CL, Maldjian JA, Turetsky BI, Detre JA, Gee J, Gur RE. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain and Language. 2000;74:157–170. doi: 10.1006/brln.2000.2325. [DOI] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267(5197):528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: Sex matters. NeuroImage. 2005;25:320–327. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA. The science of sex differences in science and mathematics. Psychological Science. 2007;8:1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Nowell A. Sex differences in mental scores, variability, and numbers of high-scoring individuals. Science. 1995;269:41–45. doi: 10.1126/science.7604277. [DOI] [PubMed] [Google Scholar]
- Irwing P, Lynn R. Sex differences in means and variability on the progressive matrices in university students: A meta-analysis. British Journal of Psychology. 2005;96:505–524. doi: 10.1348/000712605X53542. [DOI] [PubMed] [Google Scholar]
- Irwing P, Lynn R. Intelligence: Is there a sex difference in IQ scores? Nature. 2006;442:E1–E2. doi: 10.1038/nature04966. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The Role of Parietal Cortex in Verbal Working Memory. The Journal of Neuroscience. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kaufman SB. Sex differences in mental rotation and spatial visualization ability: Can they be accounted for by differences in working memory capacity? Intelligence. 2007;35:211–223. [Google Scholar]
- Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, Stocker T, Shah NJ, Amunts K, Kircher T, Schneider F, Habel U. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the BrainMap database: Progress towards a probabilistic functional brain atlas. Frontiers in Neuroinformatics. 2009;3:1–11. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005a;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 2005b;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and activation likelihood estimation in the Stroop task. Human Brain Mapping. 2005c;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutierrez D, Moran ST, Gonzales SM, Ray KL, Franklin C, Glahn DC, Fox PT, Lancaster JL. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. NeuroImage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Laird AR, Fox PM, Glahn DE, Fox PT. Automated analysis of meta-analysis networks. Human Brain Mapping. 2005;25:174–184. doi: 10.1002/hbm.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejbak L, Crossley M, Vrbancic M. A male advantage for spatial and object but not verbal working memory using the n-back task. Brain and Cognition. 2011;76:191–196. doi: 10.1016/j.bandc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Levine SC, Huttenlocher J, Taylor A, Langrock A. Early sex differenes in spatial skill. Developmental Psychology. 1999;35 doi: 10.1037//0012-1649.35.4.940. [DOI] [PubMed] [Google Scholar]
- Lewin C, Wolgers G, Herlitz A. Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology. 2001;15 doi: 10.1037//0894-4105.15.2.165. [DOI] [PubMed] [Google Scholar]
- Lynn R, Irwing P. Sex differences in general knowledge, semantic memory and reasoning ability. British Journal of Psychology. 2002;93:545–556. doi: 10.1348/000712602761381394. [DOI] [PubMed] [Google Scholar]
- Lynn R, Irwing P. Sex differences in mental arithmetic, digit span, and g as defined as working memory capacity. Intelligence. 2008;36:226–235. [Google Scholar]
- Masters MS, Sanders B. Is the gender difference in mental rotation disappearing? Behavior Genetics. 1993;23:337–341. doi: 10.1007/BF01067434. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. MEta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DG, Ryu JW, Byun HS, Choi DS, Lee EJ, Chung WI, Cho JM, Han BK. Functional MR Imaging of Working Memory in the Human Brain. Korean J Radiol. 2000;1:19–24. doi: 10.3348/kjr.2000.1.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordvik H, Amponsah B. Gender differences in spatial abilities and spatial activity among university students in an egalitarian educational system. Sex Roles. 1998;38 [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD. Integration of diverse information in working memory within the frontal lobe. Nature Neuroscience. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Coleman AR, Gur RC, Glahn DC, Gur RE. Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia. 2000;38:451–461. doi: 10.1016/s0028-3932(99)00086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WJ, Morell M, Frediani AW. Sex differences and lateral specialization of hemispheric functioning. Neuropsychologia. 1976;14:391–394. doi: 10.1016/0028-3932(76)90035-x. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA. PET Evidence for an Amodal Verbal Working Memory System. NeuroImage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373(6515):607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating Verbal and Spatial Working Memory Using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: Evidence from neuroimaging. Proceedings of the National Academy of Sciences. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. NeuroReport. 2000;11:2581–2585. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- Stevens C. Integrating community outreach into the undergraduate neuroscience classroom. J Undergrad Neurosci Ed. 2011;10:A44–A49. [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. NeuroImage. 2002;16:765. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- van Asselen M, Kessels RPC, Neggers SFW, Kappelle LJ, Frijns CJM, Postma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44:1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Volf NV, Razumnikova OM. Sex differences in EEG coherence during a verbal memory task in normal adults. International Journal of Psychophysiology. 1999;34:113–122. doi: 10.1016/s0167-8760(99)00067-7. [DOI] [PubMed] [Google Scholar]
- Willingham WW, Cole NS. Gender and fair assessment. Erlbaum; Mahwah, N.J.: 1997. [Google Scholar]