Abstract
Life-threatening infections caused by Staphylococcus aureus, particularly the community-acquired methicillin-resistant strains of S. aureus (CA-MRSA), continue to pose serious problems. Greater virulence and increased pathogenicity of certain S. aureus strains are attributed to higher prevalence of exotoxins. Of these exotoxins, the superantigens (SAg) are likely most pathogenic because of their ability to rapidly and robustly activate the T cells even in extremely small quantities. Therefore, countering SAg-mediated T cell activation using T regulatory cells (Tregs) might be beneficial in diseases such as toxic shock syndrome (TSS). As the normal numbers of endogenous Tregs in a typical host are insufficient, we hypothesized that increasing the Treg numbers by administration of IL2-anti-IL2 antibody complexes (IL2C) or by adoptive transfer of ex vivo expanded Tregs might be more effective in countering SAg-mediated immune activation. HLA-DR3 transgenic mice that closely recapitulate human TSS, were treated with IL2C to increase endogenous Tregs or received ex vivo expanded Tregs. Subsequently, they were challenged with SAg to induce TSS. Analyses of various parameters reflective of TSS (serum cytokine/chemokine levels, multiple organ pathology and SAg-induced peripheral T cell expansion) indicated that increasing the Tregs failed to mitigate TSS. On the contrary, serum IFN-γ levels were increased in IL2C treated mice. Exploration into the reasons behind the lack of protective effect of Tregs revealed IL-17 and IFN-γ-dependent loss of Tregs during TSS. In addition, significant upregulation of GITR on conventional T cells during TSS could render them resistant to Treg mediated suppression, contributing to failure of Treg-mediated immune regulation.
Keywords: HLA class II transgenic mice, Superantigen, T regulatory cells, Cytokines
Introduction
Life-threatening infections caused by Staphylococcus aureus, particularly the community-acquired methicillin-resistant strains of S. aureus (CA-MRSA), continue to pose serious problems (1–3). It is becoming increasingly evident that higher prevalence of exotoxins might contribute to greater virulence, increased pathogenicity and rapid spread of CA-MRSA strains around the world (4–8). Among the staphylococcal exotoxins, the superantigens (SAg) need a special mention because of their extreme potency and unique biological activities (9). Recent studies from our own group and others have clearly shown that SAg play an important role in the pathogenesis of serious infections caused by S. aureus such as pneumonia, infective endocarditis, sepsis and toxic shock syndrome (TSS) (10–14).
Superantigens are the most potent biological activators of the immune system (15). Unlike conventional antigens, SAg bind directly to MHC class II molecules outside of the peptide-binding groove. Subsequently, they interact with certain TCR Vβ families expressed on both CD4+ as well as CD8+ T cells and crosslink the TCR. This results in rapid activation of 30–50% of the total T cell pool. Activated T cells carryout their respective effector functions, including production of large quantities of several proinflamamtory cytokines and chemokines. This results in a robust systemic inflammatory response syndrome, hypotension, multiple organ failure and ultimately, death. Overall, excessive activation of the immune system by SAg appears to be the primary cause for immunopathology and mortality in diseases caused by toxigenic S. aureus (16). Therefore, countering the SAg-mediated immune activation could be beneficial in such diseases. The immune system is endowed with several natural regulatory pathways to control such heightened immune responses and to limit the collateral immune damage to the host. The CD4+CD25+FoxP3+ T regulatory cells (Tregs) are one such extensively characterized system (17).
Tregs, either natural or induced, have been shown to suppress almost any type of adaptive immune response, whether elicited in a physiological state or in a pathological state (18, 19). For example, Tregs have been shown to be protective in several acute systemic inflammatory conditions such as LPS-induced shock (20), zymosan-induced shock (21), graft-versus-host disease (22–24) sepsis caused by Gram-negative bacteria (25) and CD28 superagonist-induced inflammatory response syndrome (26), which are all analogous to SAg-induced TSS. Given these findings, Tregs are attractive candidates for the prevention and/or treatment of acute inflammatory diseases caused by SAg. However, the high morbidity and mortality associated with TSS and other staphylococcal SAg-mediated diseases indicate that the normal numbers of endogenous Tregs are ineffective. Therefore, increasing the Treg numbers is a possible solution. In the current study, we therefore investigated whether increasing the numbers of endogenous Tregs directly in vivo using IL-2-anti-IL2 immune complexes (27, 28) or by adoptive transfer of ex vivo expanded Tregs (29, 30), could be protective in TSS using HLA-DR3 transgenic mouse model. Unlike conventional laboratory mice expressing endogenous mouse MHC class II molecules, HLA class II transgenic mice respond robustly to staphylococcal enterotoxin B (SEB) and suffer from an acute systemic inflammatory disease mimicking human TSS, without the use of any sensitizing or potentiating agents (31, 32). Hence, the HLA-DR3 transgenic mouse model was chosen.
MATERIALS AND METHODS
Mice
The following mice were used. HLA-DR3 transgenic mice expressing HLA-DRA*0101 and HLA-DRB*0301 and IFN-γ deficient HLA-DR3 transgenic mice have already been described (31–33). These mice do not express any endogenous mouse MHC class II molecules. Mice were bred within the barrier facility of Mayo Clinic Immunogenetics Mouse Colony (Rochester, MN) and moved to a conventional facility after weaning. All the experiments were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Reagents, antibodies and Flow cytometry
Endotoxin-reduced, highly purified staphylococcal enterotoxin B (SEB, Toxin Laboratories, Sarasota, FL) was dissolved in PBS at 1 mg/ml and stored frozen at −80°C in aliquots. The purity of SEB was verified by SDS-PAGE followed by Coomassie blue staining and the absence of certain other staphylococcal SAg was verified using staphylococcal enterotoxin identification visual immunoassay (SET VIA™, 3M, St. Paul, MN, USA). The following antibodies were used for flow cytometry (BD Biosciences, San Jose, CA, USA). CD4 – GK1.5, CD8 – 53–6.7, TCR Vβ6 – RR4–7, TCR Vβ8 – F23.1, B220 – RA3–6B2, Mac-1 – M 1/70, glucocorticoid-induced TNFR family-related receptor (GITR) – DTA-1, CD25 – 7D4, FoxP3 – FJK-16s. Intracellular staining for FoxP3 was done using a kit from eBiosciences (San Diego, CA, USA). In some experiments, in vivo neutralization of IL-17 was achieved by intra-peritoneal administration of 100 µg of purified rat anti-mouse anti-IL-17 antibodies (clone 50104, R&D systems, Minneapolis, MN, USA). Control mice received equivalent amount of purified rat IgG, (R&D systems, Minneapolis, MN, USA). Antibodies were administered 10 min prior to challenge with SEB (50 µg/mouse). Mice were killed at indicated time points; spleens and thymii were collected in PBS, crushed and the distribution of indicated cell types was determined using flurochrome labeled antibodies using a flow cytometer. Flow data was analyzed using FlowJo software (Version 10, TreeStar Inc., Ashland, OR).
In vivo expansion of T regulatory cells with IL-2, anti-IL-2 immune complexes
In vivo expansion of Tregs was achieved by administering IL-2-anti-IL2 complexes (IL2C) as described by Boyman et al. (27). Briefly, 1 µl of murine IL-2 (1 µg/µl, Peprotech, Rocky Hill, NJ, USA) was added to 5 µl of anti-murine IL-2 antibody (0.5 mg/ml, JES6–1A12, eBiosciences), mixed well, incubated for 30 min at 37°C. Subsequently, 194 µl of 1X PBS was added to this mix and 200 µl of this mix was injected into each mouse on days 0, 1 and 2. The amount of IL-2 and anti-IL2 antibodies were scaled up depending on the number of animals. Mice were challenged with SEB on day 5 or left untreated. Mice were bled 3 hours after SEB challenge and all animals were killed 3 days later. Injection of IL2C on days 0, 1 and 2 is hereafter referred to as 3xIL2C. In one set of experiments, mice received 3 times the dose of IL2C described above on days 0, 1 and 2 to study whether increasing the concentration of IL2C would confer better Treg mediated protection.
In some studies, instead of repeated administration of IL2C as described above, IL2C were delivered continuously using a mini-osmotic pumps. For this, 4 µl of murine IL-2 (1 µg/µl) was added to 20 µl of anti-IL-2 antibody, mixed well and incubated for 30min at 37°C. Subsequently, 76 µl of 1X PBS was added to this mix to make the volume to 100 µl, the entire content was then loaded into a 7-day release Alzet mini-osmotic pump as per manufacturer’s protocol (Durect Corporation, Cupertino, CA, USA). The pumps were then surgically implanted sub-cutaneously into the back of mice as per standard procedure as described previously (34). The surgical wounds were closed with surgical staples. Some mice received pumps filled with IL-2 alone or anti-IL2 alone. Mice were challenged with SEB on day 7 or left untreated. Some mice were bled 3 hours after SEB challenge and all animals were killed 3 days after SEB challenge or day 10 after implantation of pump. Delivering IL2C using mini-osmotic pumps is hereafter referred to as pump-IL2C.
Purification, expansion and adoptive transfer of in vitro expanded T regulatory cells
CD4+CD25+ T cells were isolated from naïve HLA-DR3 transgenic mice using magnetic mouse Treg purification kit following the manufacturer’s protocol (Miltenyi Biotec Inc, Auburn, CA, USA). The final positive selection step was repeated twice to ensure purity, which was verified by testing a small aliquot by flow cytometry by intracellular staining for FoxP3. Purified Tregs were expanded in vitro by culturing them with anti-CD3, anti-CD28 beads (Dynabeads® Mouse T-Activator CD3/CD28, Life Technologies, Grand Island, NY, USA) as per manufacturer’s protocol and published literature (29, 30). Culture medium was supplemented with different amounts of IL-2 during each cycle of expansion as recommended in the protocol. Tregs were expanded by 2 rounds of stimulation cycles, each round lasting 6–7 days. The expanded cells were tested by flow cytometry for expression of CD4, CD25 and FoxP3 and more than 95% of the cells were found to be bona fide Tregs phenotypically. Five million ex vivo expanded Tregs were injected intravenously via the tail vein in to unmanipulated recipients. Twenty-four hours later, recipients were challenged with SEB (50 µg/mouse) or left untreated.
SEB-induced in vitro splenocyte proliferation assay
For T cell proliferation, single-cell suspensions of splenocytes from HLA-DR3 transgenic mice were depleted of red blood cells by buffered ammonium chloride lysis. Cells were cultured in HEPES-buffered RPMI 1640 containing 5% fetal calf serum, serum supplement, streptomycin and penicillin, at a concentration of 1 × 105 cells/well in 100 µl volumes in 96-well round-bottomed tissue culture plates. SEB was added at indicated concentrations. After 24 hours, the cells were pulsed with tritiated thymidine (1 µg/well). Cells were harvested 18 hours later and the extent of cell proliferation was determined by a standard thymidine incorporation assay.
Serum cytokine quantification and histopathology
For serum cytokine analyses, HLA-DR3 mice were challenged with SEB (50 µg/mouse in 200 µl, i.p) or with PBS. Three hours later, animals were bled and sera separated. The cytokine concentrations in the sera were determined in duplicates using a multiplex bead assay, per the manufacturer's protocol and using their software and hardware (Bio-Plex, Bio-Rad). Tissues collected in buffered formalin were paraffin embedded, cut, and stained with H&E per standard procedure for histopathologic analysis.
Statistical Analyses
Unpaired Student’s t-test was applied to determine the statistical significance when comparing two groups. The difference was considered statistically significant when p<0.05. Statistical analyses and bar charts were generated using GraphPad Prism, version 6.
RESULTS
Administration of IL-2-anti-IL-2 complexes increases T regulatory cell numbers in HLA-DR3 transgenic mice
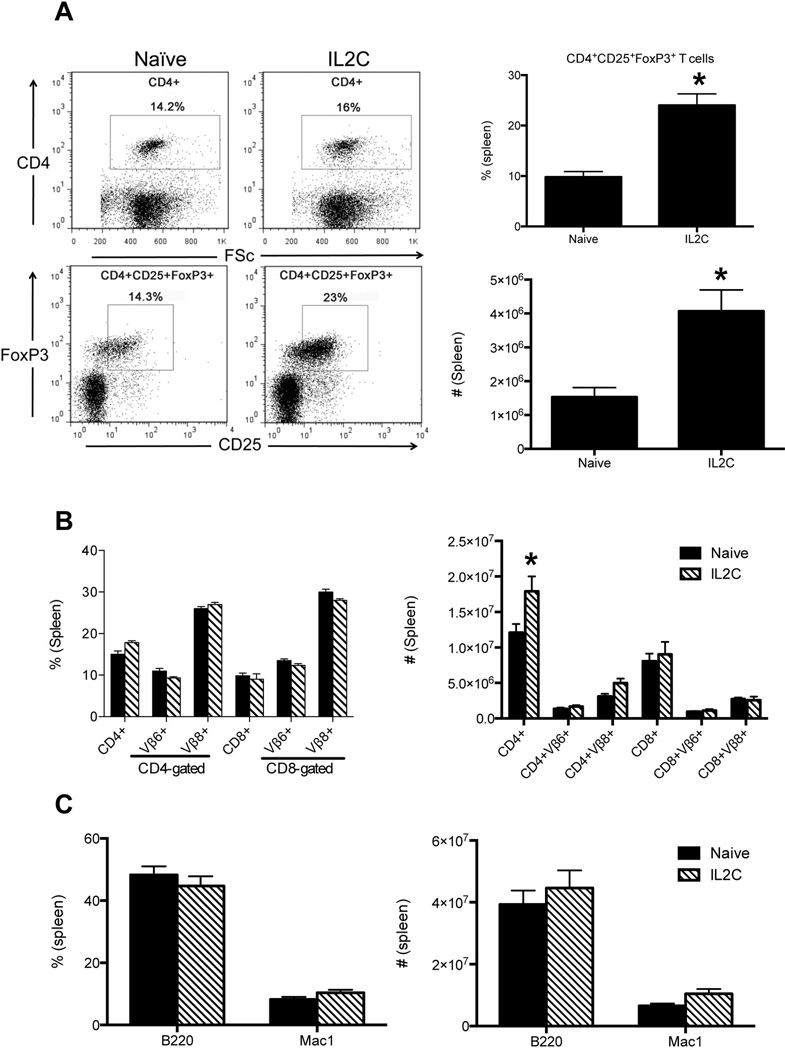
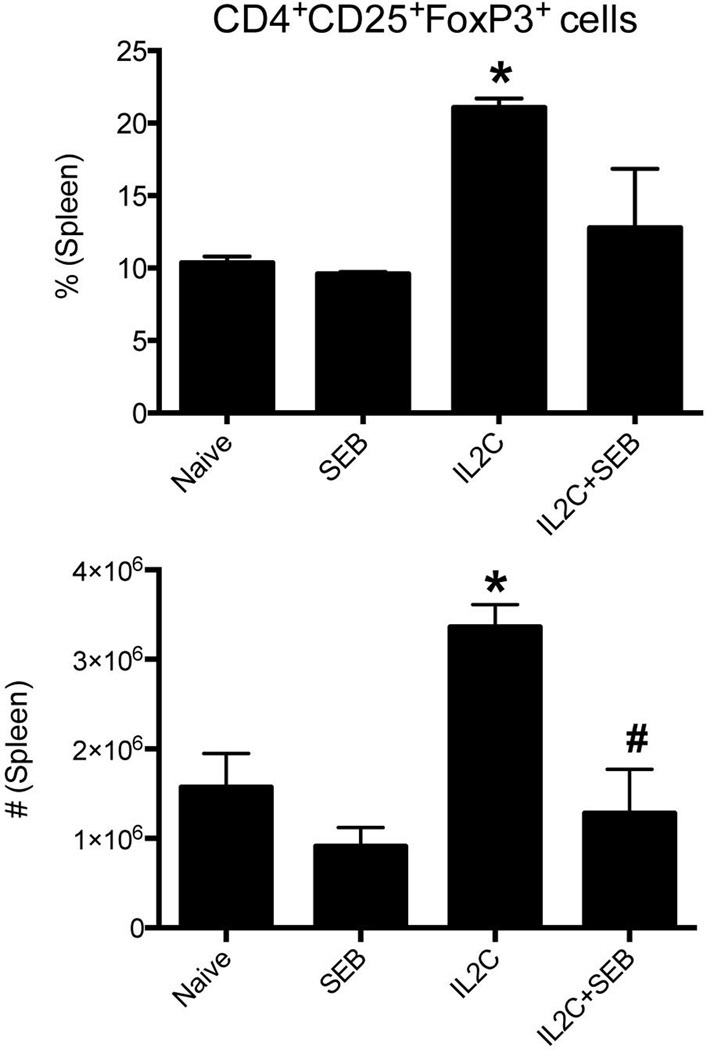
Administration of IL2C on days 0, 1 and 2 resulted in a significant (3–4 folds) increase in the absolute numbers of CD4+CD25+FoxP3+ Tregs in the spleens by day 5 (Fig 1A). In vivo administration of 3xIL2C also resulted in an increase in the total numbers of CD4+ T cells, possibly due to an increase in the Tregs cells. However, there were no significant changes in the numbers of CD8+ T cells, B cells and macrophages in 3xIL2C–treated mice compared to untreated mice (Fig 1B and C). These findings were consistent with previous studies that used IL2C to expand Tregs in vivo (27).
Figure 1. Repeated administration of IL-2-anti-IL-2 complexes increases endogenous Tregs in HLA-DR3 transgenic mice.
HLA-DR3 transgenic mice were injected with immune complexes comprising of IL-2 and anti-IL-2 antibodies on days 0, 1 and 2 (3xIL2C). On day 5, mice were killed and the distribution of various cell types in the spleens was analyzed by flow cytometry. (A) Representative dot plots and bar charts depicting distribution of CD4+CD25+FoxP3+ Tregs in naïve and 3xIL2C treated mice. (B) Bar chart depicting the distribution of CD4+ and CD8+ T cells expressing indicated TCR Vβ families in naïve and 3xIL2C treated mice (C) B cells and macrophages in naïve and 3xIL2C treated mice. Each bar represents mean±SE of data obtained from 4–6 mice in each group. * p<0.05 compared to naïve mice.
Splenocytes from HLA-DR3 transgenic mice treated with IL-2-anti-IL-2 complexes respond robustly to SEB in vitro
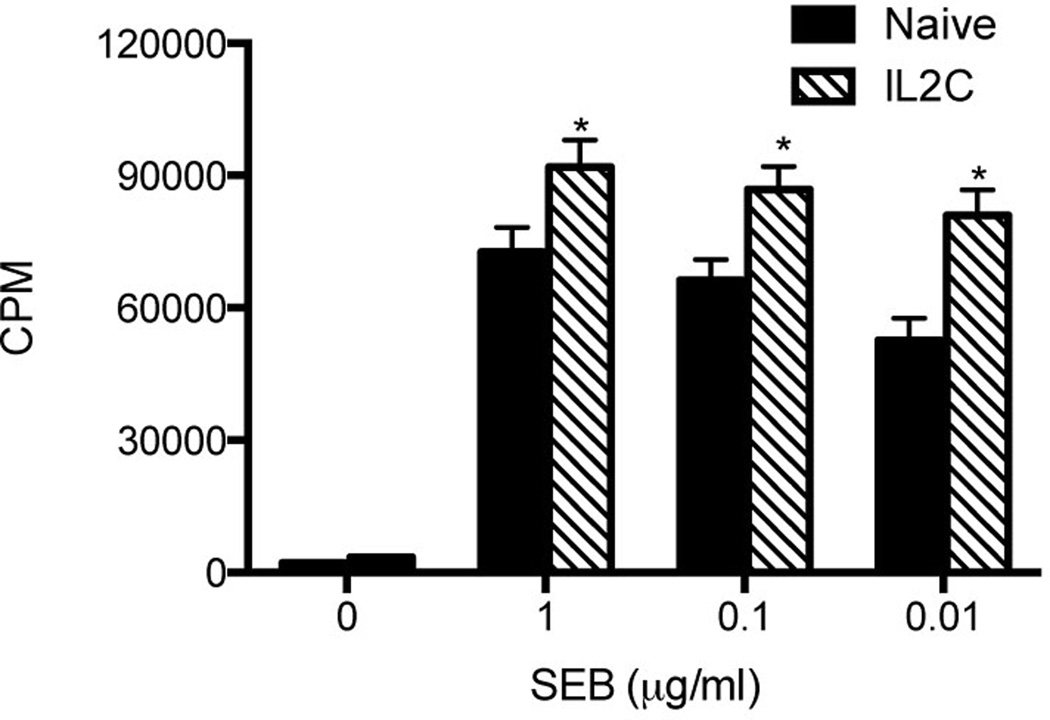
As a measure of Treg suppressor activity relevant to our model, we next determined the extent of SEB-induced proliferation of unfractionated splenocytes isolated from 3xIL2C–treated mice in vitro. This was compared with the responses of splenocytes isolated from naïve mice. Splenocytes from 3xIL2C–treated mice consistently had higher thymidine uptake following stimulation with SEB than the splenocytes from untreated mice (Fig 2). This indicated that even though more Tregs are present in the spleens of 3xIL2C–treated mice, they are unable to suppress the proliferative response elicited by SEB in vitro.
Figure 2. Modulatory effect of unfractionated Tregs from HLA-DR3 transgenic mice expanded with IL-2-anti-IL-2 complexes on SEB-induced splenocyte proliferative responses in vitro.
Unfractionated splenocytes isolated from naïve and 3xIL2C–treated mice were stimulated with indicated concentrations of SEB and the extent of proliferation was determined by tritiated thymidine incorporation assay. Each bar represents mean±SE of data obtained from 4–6 mice in each group. * p<0.05 compared to naïve mice.
Elevated systemic IFN-γ levels in HLA-DR3 transgenic mice treated with IL-2-anti-IL-2 complexes following SEB challenge
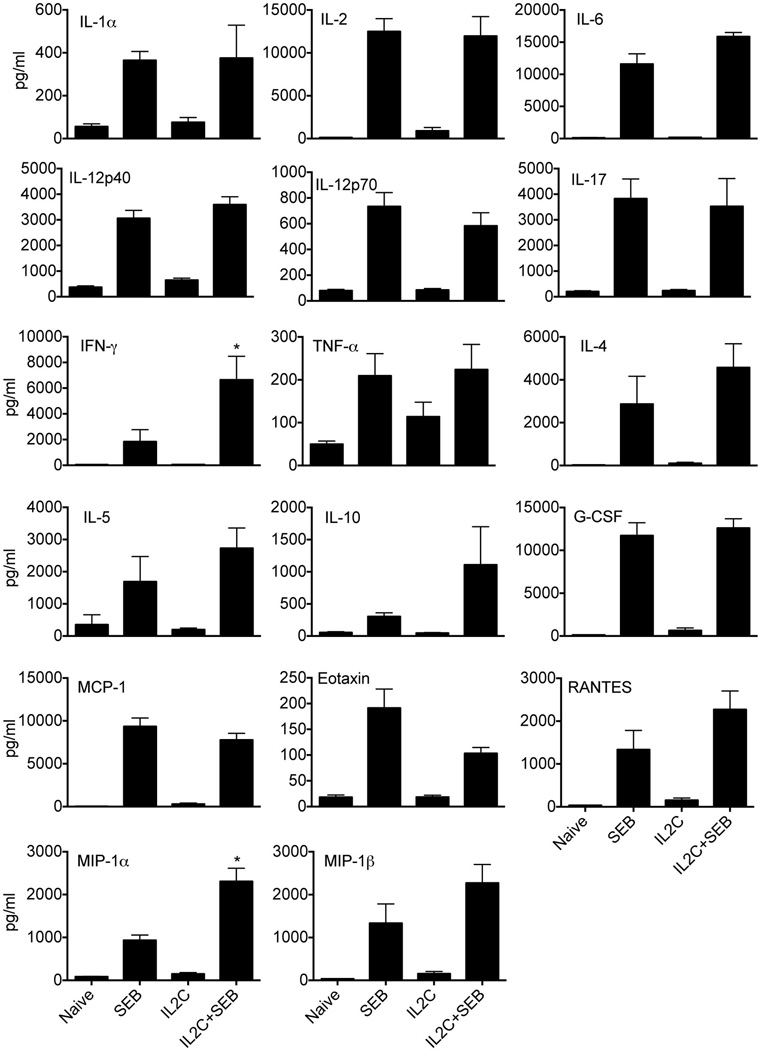
TSS in humans is characterized by a systemic cytokine and chemokine storm, which culminates in multiple organ dysfunction and often death (35). Challenging HLA-DR3 transgenic mice with SEB elicits a similar acute response (31). Therefore, in the next set of experiments, the ability of in vivo expanded Tregs to dampen SEB-induced systemic cytokine and chemokine surge was investigated. For this, naïve and 3xIL2C–treated HLA-DR3 transgenic mice were challenged with SEB, bled 3 hours later and levels of several cytokines and chemokines in the sera were determined. While naïve HLA-DR3 mice had low levels of cytokines and chemokines in their sera as expected, challenging with SEB resulted in a dramatic elevation in serum levels of all cytokines (Th1, Th2 and Th17) and chemokines tested (Fig 3), as shown by us earlier (31–33). Sera from control 3xIL2C–treated mice also had low levels of cytokines and chemokines similar to naïve HLA-DR3 mice suggesting that administration of 3xIL2C does not elicit an inflammatory response nor does it result in elevated basal levels of anti-inflammatory cytokines such as IL-10. However, contrary to the expectation, the serum cytokine/chemokine levels were still highly elevated in 3xIL2C–treated mice challenged with SEB similar to SEB-challenged HLA-DR3 mice that did not receive 3xIL2C.
Figure 3. SEB-induced systemic cytokine/chemokine surge in naïve and IL-2-anti-IL-2 complex-treated HLA-DR3 transgenic.
HLA-DR3 transgenic mice were left untreated or injected with immune complexes comprising of IL-2 and anti-IL-2 antibodies on days 0, 1 and 2 (3xIL2C). On day 5, mice were challenged with SEB (50 µg/mouse) or PBS and bled 3 hours later. Concentrations of indicated cytokines/chemokines were determined using multiplex assay. Each bar represents mean±SE of data obtained from 6–8 mice in each group. * p<0.05 compared to naïve mice.
Interestingly, SEB-challenged, 3xIL2C–treated mice had significantly higher levels of IFN-γ (6642 ± 1840 pg/ml, N=6) compared to 3xIL2C–untreated SEB-challenged HLA-DR3 mice (1836 ± 932.4 pg/ml N=8, p = 0.02) (Fig 3). Even though 3xIL2C–treated mice challenged with SEB had higher mean serum levels of IL-4, IL-5 and IL-10 than 3xIL2C–untreated SEB-challenged HLA-DR3 mice, these differences were not statistically significant (Fig 3). With respect to chemokines, 3xIL2C–treated mice challenged with SEB had significantly higher serum levels MIP-1α compared to SEB-challenged HLA-DR3 mice not treated with 3xIL2C (2306 ± 307.2 pg/ml versus 934.3 ± 120.9 pg/ml, p=0.0006) (Fig 3).
We next tested using a small cohort of HLA-DR3 mice whether increasing the concentration of IL2C by threefold would suppress SEB-induced systemic cytokine/chemokine storm. HLA-DR3 transgenic mice received thrice the normal dose of IL2C on days 0, 1 and 2. On day 3, mice were challenged with SEB, sera collected 3 hours later and assayed. While we did not enumerate the Treg numbers in these mice, the serum levels of most the cytokines/ chemokines were much higher than in SEB-challenged HLA-DR3 mice treated with regular dose of IL2C. For example, the serum level of IFN-γ was 13,720 ± 1149 pg/ml, IL-17 was 8093 ± 643 pg/ml and IL-6 was 16693 ± 520 pg/ml (n=4, mean ± SE) in this group (additional data not shown). Overall, these results showed that even though IL2C treatment increased the number of Tregs in vivo, the SEB-induced systemic inflammatory response was largely unaffected. On the contrary some cytokines, such as IFN-γ, was significantly elevated in IL2C treated group.
Expansion of peripheral T cells bearing SEB-reactive TCR is unaffected in mice treated with IL-2-anti-IL-2 complexes
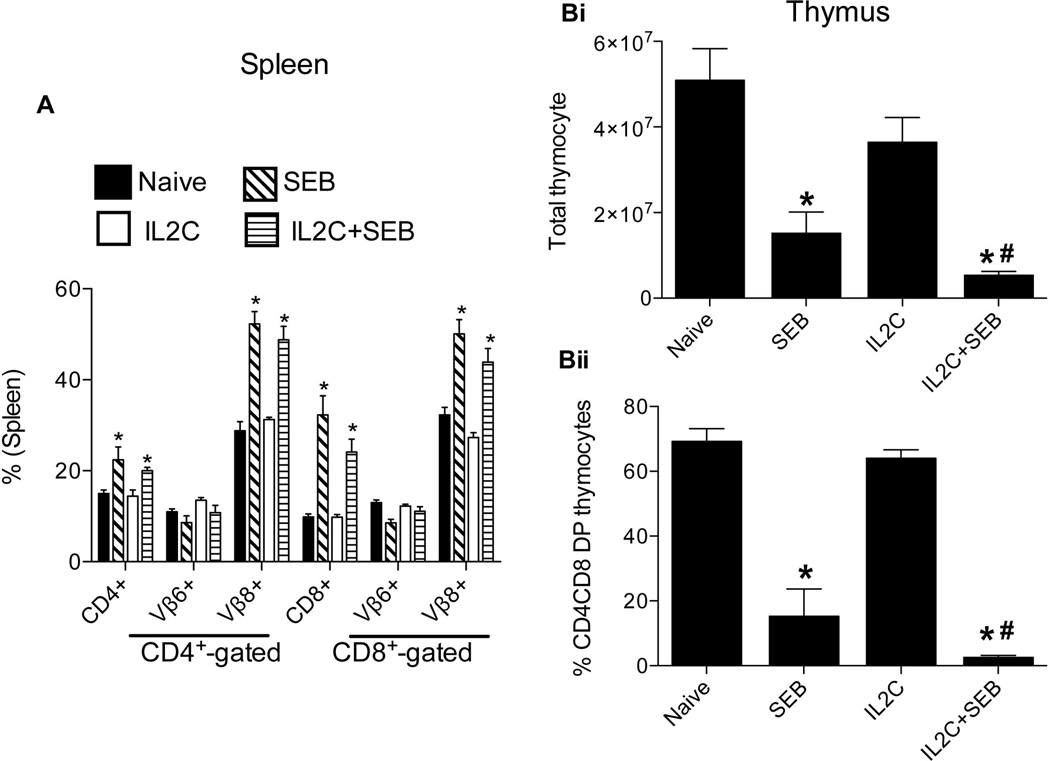
In addition to the systemic cytokine/chemokine storm, expansion of mature, peripheral CD4+ and CD8+ T cells bearing certain TCR Vβ families (which peaks by day 3) is the hallmark of SAg-mediated T cell activation in vivo. Since Tregs are known to suppress T cell activation and expansion either directly or indirectly (36), we next determined the numbers of splenic CD4+ and CD8+ T cells bearing SEB-reactive, TCR Vβ8 and the SEB-non-reactive, TCR Vβ6 as controls. As shown in Fig 4A, administration of SEB resulted in a significant increase in CD4+ and CD8+ T cells bearing TCR Vβ8, but not TCR Vβ6, compared to naïve mice. However, CD4+ and CD8+ T cells bearing TCR Vβ8 were also significantly elevated even in 3xIL2C–treated HLA-DR3 mice challenged with SEB consistent with the serum cytokine/chemokine data.
Figure 4. Modulatory effects of IL-2-anti-IL-2 complex treatment on SEB-induced expansion of peripheral T cells and deletion of thymocytes in HLA-DR3 transgenic mice.
HLA-DR3 transgenic mice were left untreated or injected with immune complexes comprising of IL-2 and anti-IL-2 on days 0, 1 and 2 (3xIL2C). On day 5, mice were challenged with SEB (50 µg/mouse) or PBS and killed 3 days later for flow cytometric analysis. (A) Distribution of CD4+ and CD8+ T cells expressing TCR Vβ8 (SEB-responsive) and TCR Vβ6 (SEB non-responsive) in the spleens and (B) Thymus. Each bar represents mean±SE of data obtained from 6–8 mice in each group. * p<0.05 compared to naïve mice. # p<0.05 compared to SEB group.
Superantigen-induced deletion of double of positive thymocytes is more pronounced in IL2C treated mice
While the mature peripheral T cells expressing certain TCR Vβ families undergo robust expansion following administration of SEB, the immature CD4CD8 double positive thymocytes undergo massive apoptosis in a TCR and cytokine-dependent manner (37, 38). Therefore, we next examined whether Tregs can protect CD4CD8 DP thymocytes from SEB-induced apoptosis. Challenging HLA-DR3 transgenic mice with SEB resulted in a massive reduction in the CD4CD8 DP thymocytes, causing a profound reduction in the total thymocyte numbers as well (Fig 4B). However, in vivo expansion of Tregs with 3xIL2C conferred little protection from SEB-induced CD4CD8 DP thymocyte deletion (Fig 4B).
Course of SEB-induced TSS in IL2C–treated HLA-DR3 transgenic mice
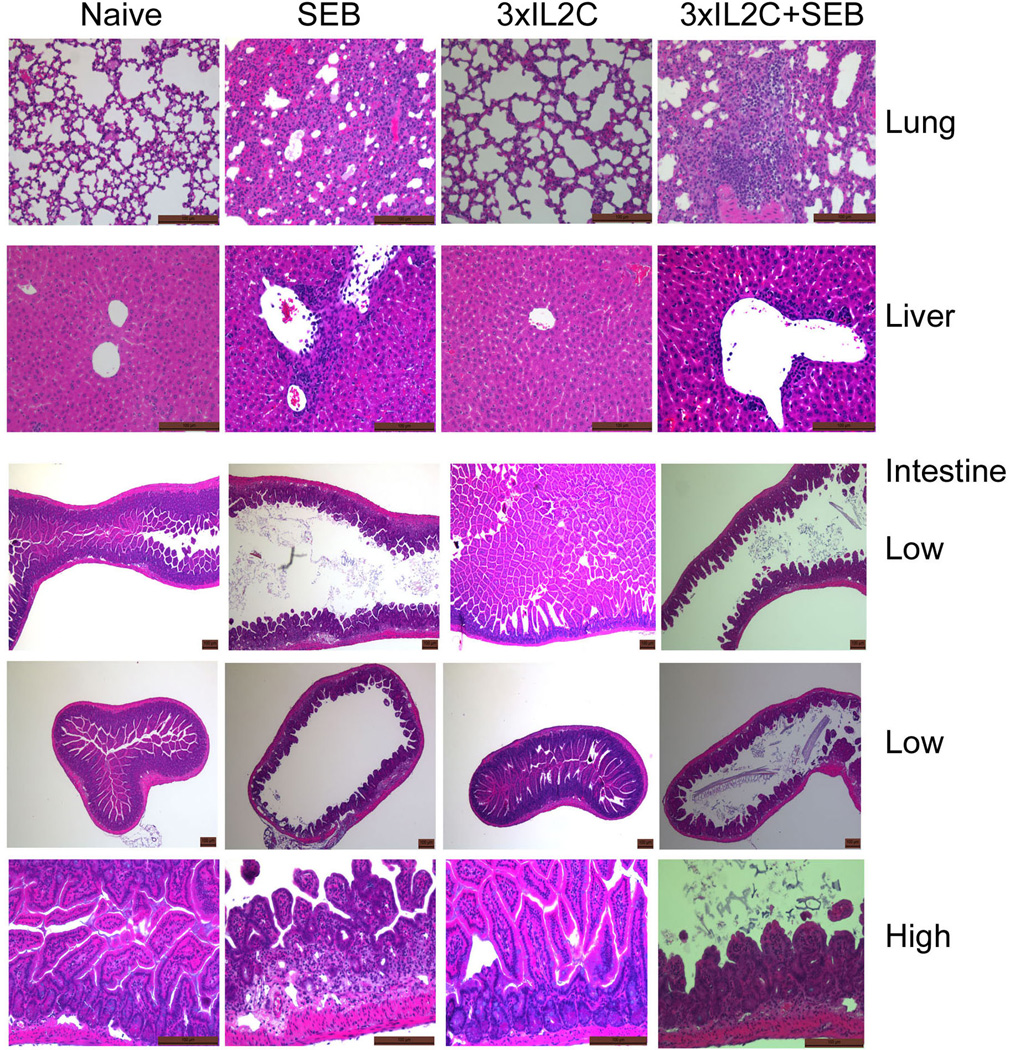
We have previously shown that SEB-induced TSS in HLA-DR3 transgenic mice is characterized by extensive inflammatory changes in various vital organs, which culminates in death, similar to human TSS (31). Therefore, we next studied the extent of inflammation in lung, liver and the small intestines, the organs that are primarily affected during TSS. As shown in Fig 5, lung, liver and small intestines from naïve as well as HLA-DR3 mice treated with 3xIL2C showed no signs of inflammation. However, mice challenged with SEB had extensive inflammation in the lungs, liver and the small intestines irrespective of whether they received 3xIL2C or not, indicating that in vivo administration of 3xIL2C had little protective effect from organ pathology.
Figure 5. Organ pathology during SEB-induced toxic shock syndrome in HLA-DR3 transgenic mice and its modulation by IL-2-anti-IL-2 complexes.
HLA-DR3 transgenic mice were left untreated or injected with IL-2, anti-IL-2 immune complexes on days 0, 1 and 2 (3xIL2C) to expand endogenous Tregs. On day 5, mice were challenged with SEB (50 µg/mouse) or PBS and killed 3 days later. Organs were collected in buffered formalin, paraffin-embedded and processed. Hematoxylin and Eosin stained sections were evaluated by light microscopy. Representative low and high magnification images are shown.
We also studied the effect of 3xIL2C treatment on overall protection from TSS in HLA-DR3 transgenic mice. Throughout the study, 40% (4/10 mice) of the 3xIL2C–untreated HLA-DR3 transgenic mice succumbed to SEB-induced TSS. However, 50% (6/12 mice) of 3xIL2C–treated HLA-DR3 mice died of SEB-induced TSS. There was no statistically significant difference in the survival between these 2 groups (Additional data not shown). Taken together, these results suggested that even though administration of 3xIL2C resulted in an increase in the total number of Tregs in vivo, neither SEB-induced systemic inflammatory response, multiple organ pathology, nor mortality were reduced. Rather, some of the key inflammatory mediators, such as IFN-γ and MIP-1α, were increased in 3xIL2C–treated mice.
Tregs expanded in vivo with IL2C delivered via mini-osmotic pump also fail to attenuate SEB-induced inflammatory responses
Considering the mechanisms by which IL2C cause expansion of Tregs (39, 40), we hypothesized that continuous delivery of IL2C using mini-osmotic pumps would cause a more robust expansion of Tregs, which could be more effective in mitigating the systemic inflammatory response seen during TSS. As we hypothesized, pump-IL2C mice harbored more Tregs than all other groups (Mean±SE of CD25+FoxP3+ T cells within CD4+ gated cells in the spleens were as follows. Naïve = 0.95±0.13; IL2 = 2.43±0.19; αIL2 = 1.59±0.14 and pump-IL2C = 4.3±0.35 millions; naïve versus IL-2, naïve versus pump-IL2C and IL-2 versus pump-IL2C p<0.05). However, chronic delivery of IL2C using mini-osmotic pump did not significantly increase the Treg numbers beyond to that seen mice treated with 3xIL2C. This might suggest the presence of homeostatic threshold for total number of Tregs. We next tested the efficacy of Tregs expanded with pump-IL2C in attenuating SEB-induced systemic cytokine/chemokine surge. SEB unchallenged naïve and pump-IL2C treated mice had comparable low levels of cytokines and chemokines tested (Supp. Figure 1). Interestingly, serum levels of IFN-γ in pump-IL2C mice challenged with SEB were significantly higher than control SEB-challenged mice, a phenomenon, which was observed even with 3xIL2C, treated mice (Supp. Figure 1). Serum IL-5 was also elevated in SEB-challenged pump-IL2C treated mice. While the serum levels of IL12p40, IL-17 and KC were statistically lower in pump-IL2C mice challenged with SEB compared to control SEB-challenged mice, these mediators were still very high compared to naïve SEB-unchallenged pump-IL2C mice (Supp. Figure 1). Neither the expansion of mature peripheral T cells expressing certain TCR Vβ family nor deletion of CD4CD8DP thymocytes were blocked in SEB-challenged pump-IL2C treated mice (Data not shown). As would be expected from the above findings, lungs, livers, kidneys and intestines from SEB-challenged pump-IL2C treated mice displayed similar pathology to that of SEB-challenged HLA-DR3 mice without pump-IL2C treatment (Not shown).
Adoptive transfer of ex vivo expanded Tregs
Since in vivo expansion of endogenous Tregs using IL2C did not present any appreciable benefits, we next investigated whether adoptive transfer of ex vivo expanded Tregs would be protective in TSS. Naïve Tregs isolated from HLA-DR3 transgenic mice were expanded using anti-CD3-anti-CD28 beads. Phenotypic analysis of the expanded cells prior to adoptive transfer confirmed that more than 95% of the cells were CD4+CD25+FoxP3+. One day after adoptive transfer of 5 million Tregs, the recipients were challenged with SEB; serum cytokine and chemokine levels were quantified 3 hours later. As shown in Supp. Figure 2, HLA-DR3 mice challenged with SEB had significantly elevated levels of all the cytokines and chemokines tested compared to naïve mice concordant with previous results. While, adoptive transfer of Tregs per se did not cause any significant elevation in cytokines or chemokines (Supp. Figure 2), SEB-challenged HLA-DR3 mice that had received Tregs still had significantly elevated levels of these mediators. However, certain chemokines (such as eotaxin, MCP-1 and KC) as well as IL-6 were significantly lower (p<0.05) in SEB-challenged Tregs-treated HLA-DR3 mice compared to SEB-challenged HLA-DR3 mice that did not receive any Tregs. Interestingly, IL-5 was significantly elevated in the sera of SEB-challenged HLA-DR3 mice that received Tregs compared to those that did not receive Tregs (4236±229 pg/ml versus 1410±147 pg/ml, p<0.05, respectively, Supp. Figure 2). This indicated that while adoptive transfer of Tregs suppressed some chemokines, most of the potentially pathogenic cytokines/chemokines such as IL-1, IL-12, IL-17, IFN-γ, MIP, MCP and RANTES were still elevated. Moreover, SEB-induced expansion of peripheral T cells and deletion of CD4CD8 DP thymocytes were not attenuated in Treg-treated, SEB challenged HLA-DR3 mice (Not shown). Notably, organ pathology in these mice was also similar to SEB-challenged HLA-DR3 mice that did not receive any Tregs (Data not shown). Overall, these results indicated that increasing the Treg numbers, either by expansion of endogenous Tregs or by adoptive transfer, does not confer protection from SEB-induced TSS.
Homeostasis of T regulatory cells in IL-2-anti-IL-2 complexes treated mice challenged with SEB
As most of the SAg-driven immunopathological processes were not attenuated by Tregs either expanded in vivo or transferred adoptively after 2 rounds of ex vivo expansion, we next investigated the fate of Tregs in mice undergoing TSS. Surprisingly, the numbers of Tregs still remained elevated in mice treated with IL2C even on day 8 (i.e., 6 days after cessation of treatment of IL2C) when not challenged with SEB. However, the numbers of Tregs in these mice fell drastically when they were challenged with SEB (Fig 6). Similar results were observed with pump-IL2C treated mice and mice with adoptively transferred Tregs. In both these groups, the Tregs remained elevated in recipients that were not challenged with SEB. However, in recipients that were challenged with SEB, the Treg numbers were significantly reduced. In pump-IL2C mice, the Tregs numbers fell to the level seen in naïve mice and in mice that received ex vivo expanded Tregs, the Treg numbers were significantly below that seen in naïve mice (Not shown). Overall, a reduction in the Treg numbers in mice challenged with SEB could partly explain why SAg-induced inflammatory responses were unabated (quantitative defect).
Figure 6. Homeostasis of Tregs during SEB-induced toxic shock syndrome in HLA-DR3 transgenic mice.
HLA-DR3 transgenic mice were left untreated or injected with IL-2, anti-IL-2 immune complexes on days 0, 1 and 2 to expand Tregs (3xIL2C). On day 5, mice were challenged with SEB (50 µg/mouse) or PBS and killed 3 days later. The percentage of CD25+FoxP3+ T cells in the CD4+ gated splenocytes were determined by flow cytometry and their absolute numbers were deduced. Each bar represents mean±SE of data obtained from 6–8 mice in each group. * p<0.05 compared to naïve mice. # p<0.05 compared to IL2C group.
Another possibility is that the SAg-induced inflammatory milieu somehow compromised the immunoregulatory capabilities of Tregs (qualitative defect), at the same time rendering the effector T cells less amenable to Treg-mediated suppression. GITR is one such pathway that can accomplish both these consequences (41). GITR is a classical cell surface marker that is constitutively expressed on Tregs. On the contrary, naïve conventional T (Tconv) cells express very low levels of GITR. However, GITR expression is upregulated on Tconv cells upon activation and functions as a costimulatory molecule (42). Engagement of GITR on Tregs by GITR-L can negatively affect their suppressor activity, while ligation of GITR expressed on activated Tconv cells could render them less susceptible to Treg-mediated suppression (43). Given its overall anti-immunosuppressive property, we next investigated the expression profile of GITR on Tregs as well as on Tconv cells during TSS in HLA-DR3 mice.
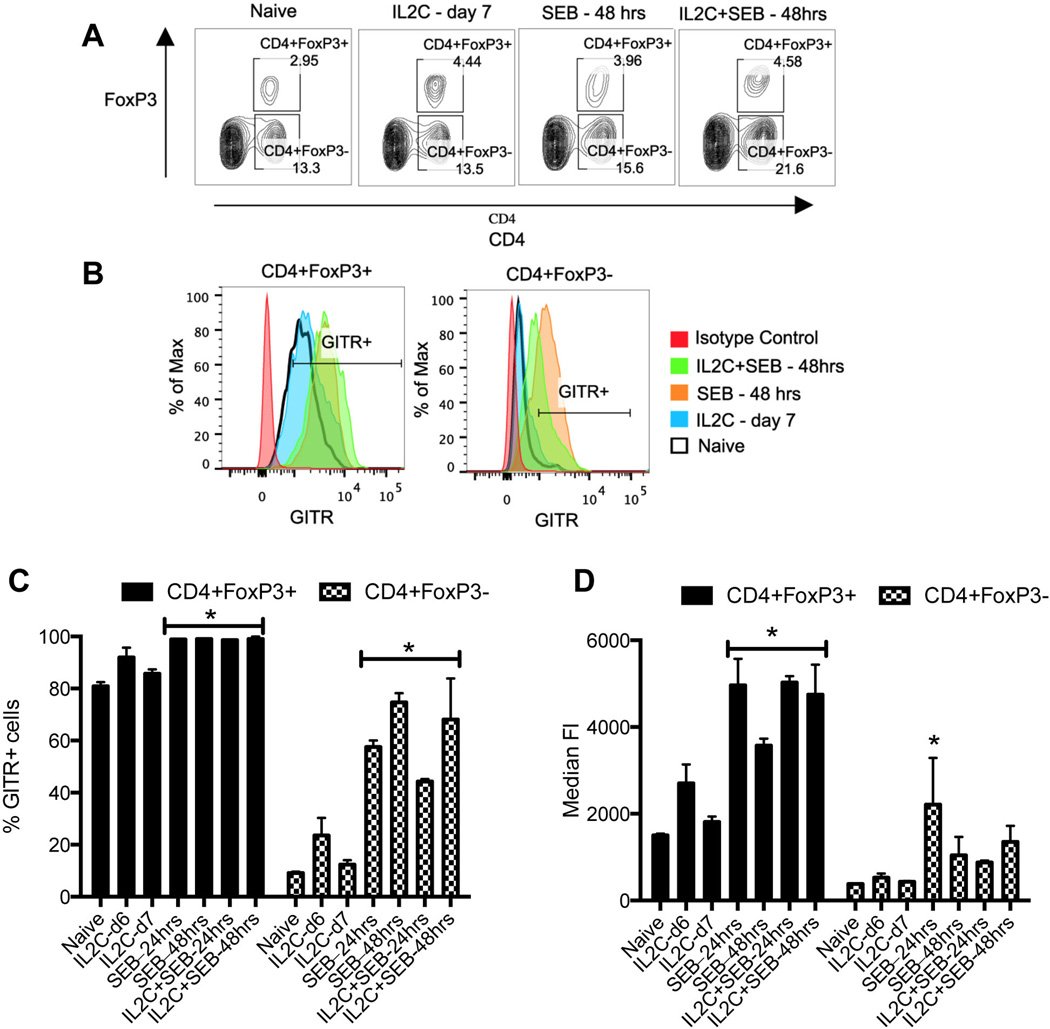
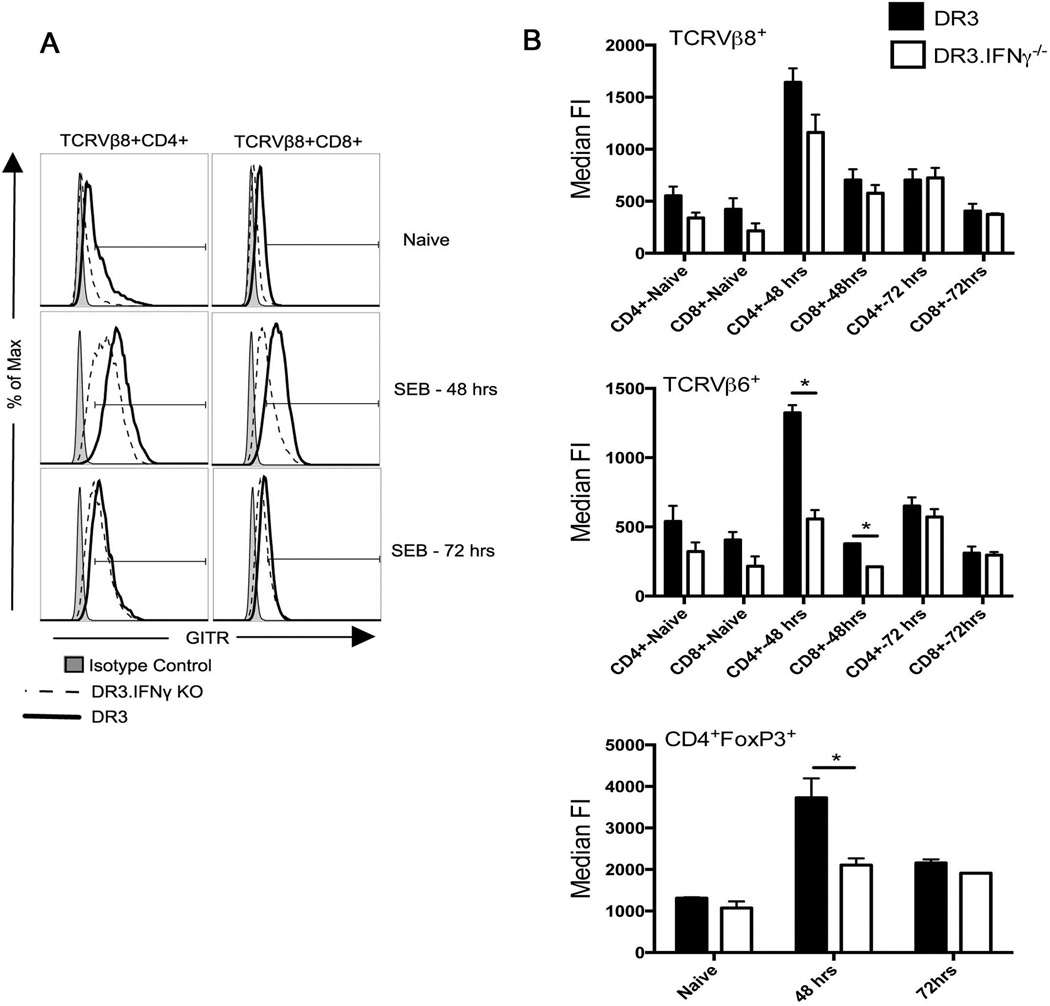
As shown in Fig 7, in naïve HLA-DR3 mice, close to 80% of the FoxP3+CD4+ T cells (Tregs) were GITR+ as expected. Even the median fluorescent intensity (MFI) of GITR was also high on naïve FoxP3+ CD4+ T cells. While IL2C treated mice had slightly higher percentage of GITR+ Tregs as well as higher MFI of GITR at day 6, they reduced to naive levels by day 7. Interestingly, in mice challenged with SEB, almost 100% of the FoxP3+ CD4+ T cells expressed GITR and the MFI of GITR on Tregs also increased. SEB-induced upregulation of GITR expression on Tregs could also be appreciated in 3xIL2C treated mice (Fig 7). These results suggested that in the systemic inflammatory milieu, the expression of GITR on Tregs increases significantly.
Figure 7. Expression profile of GITR on Tregs and Tconv cells in HLA-DR3 transgenic mice.
HLA-DR3 transgenic mice were left untreated or injected with IL-2, anti-IL-2 immune complexes on days 0, 1 and 2 to expand Tregs. On day 5, mice were challenged with SEB (50 µg/mouse) or PBS. Mice were killed 24 (day 6) or 48 hours (day 7) later, splenocytes isolated and stained with indicated antibodies. (A) Representative dot plots depicting distribution of CD4+FoxP3+ and CD4+FoxP3 T cells in different groups of mice (B) Histogram overlays depicting expression of GITR on the cells gated as above (C) Bar chart depicting percentages of GITR+ cells within the indicated gates and (D) Bar chart depicting median fluorescent intensity of GITR expression on cells within the indicated gates. Each bar represents mean±SE of data obtained from 3 mice in each group. * p<0.05 compared to naïve mice.
In the Tconv cell (FoxP3-) compartment, only 10% of the CD4+ T cells expressed GITR in naïve mice and even the MFI was also very low (Fig 7B and C), consistent with previous results (42). However, challenging with SEB resulted in a time-dependent increase in percentage of GITR+ CD4+ Tconv cells. At 24 hrs, close to 60% of the CD4+ Tconv cells were GITR+, while by 48 hrs, nearly 80% of them were GITR+. Even the MFI of expression of GITR was also high on Tconv cells (Fig 7 and Supp. Figure 3). A similar pattern was seen in IL2C–treated HLA-DR3 mice challenged with SEB (Fig 7 and Supp. Figure 3). We also analyzed the expression of GITR on CD4+ and CD8+ T cells expressing SEB-reactive TCR Vβ8 and SEB-non-reactive TCR Vβ6 during TSS. As shown in Supp. Figure 4, a low percentage of TCR Vβ6+ as well as Vβ8+ CD4+ and CD8+ T cells from naïve HLA-DR3 mice expressed GITR. Upon challenge with SEB, almost 100% of SEB-reactive Vβ8+ CD4+ and CD8+ T cells expressed GITR as expected. Interestingly, even though TCR Vβ6 does not bind to SEB (and hence TCR Vβ6-bearing CD4+ and CD8+ T cells are not directly activated by SEB), more than 75% of TCR Vβ6+ CD4+ and CD8+ T cells from SEB challenged mice expressed GITR. Thus, challenging with SEB resulted in upregulation of GITR, an important anti-immunoregulatory molecule, on the Tregs and to a much higher degree in the Tconv cells.
IFN-γ and IL-17 destabilize T regulatory cells during SEB-induced SIRS
Recent studies have suggested that the phenotype of Tregs is not stable and that under certain inflammatory conditions, particularly in the presence of IFN-γ and IL-17, the Tregs may lose their FoxP3 expression, convert to pro-inflammatory type and may actively produce proinflammatory cytokines such as IFN-γ and IL-17 (44–46). Since TSS is characterized by a profound elevation in systemic levels of several inflammatory mediators, particularly IFN-γ and IL-17, we hypothesized that this inflammatory milieu may not be conducive for the maintenance of Tregs. Therefore, we next investigated the roles of IFN-γ and IL-17 in Treg homeostasis during TSS using IFN-γ-deficient HLA-DR3 transgenic mice and neutralizing anti-IL-17 antibodies.
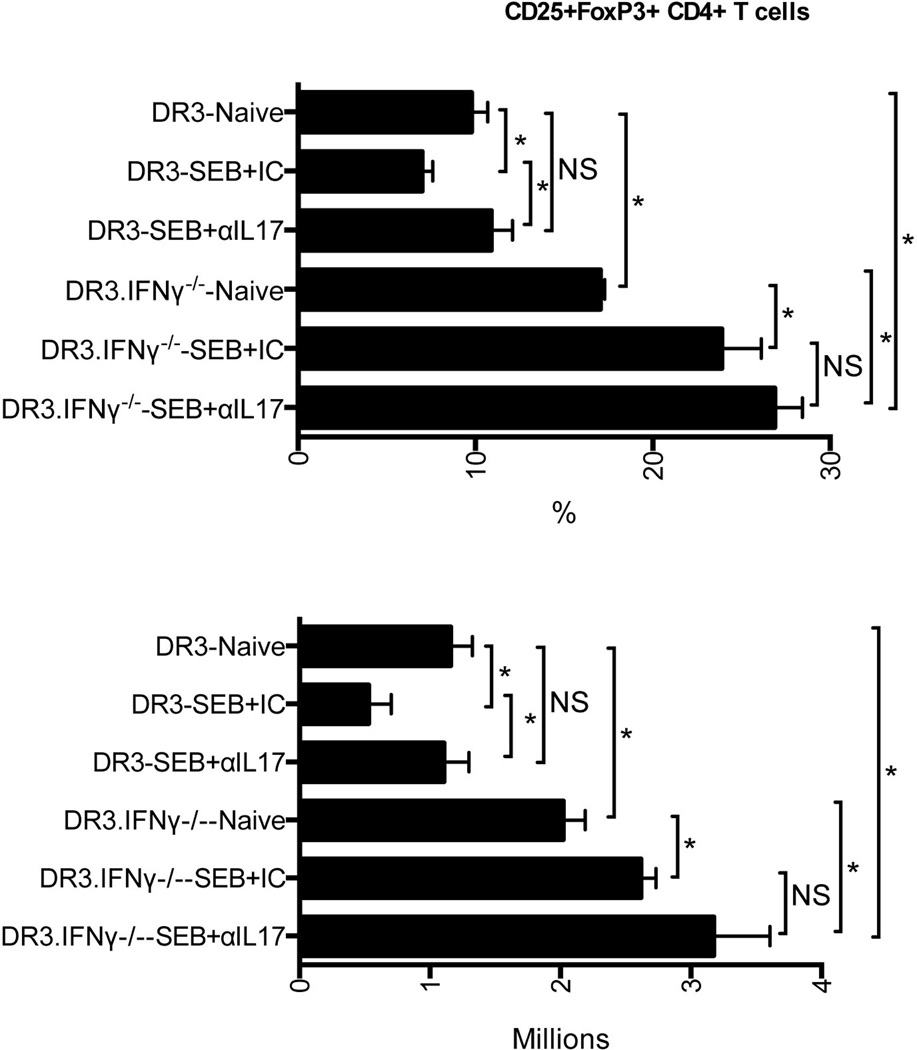
First of all, the number of Tregs in the spleens of HLA-DR3 mice challenged with SEB and treated with isotype control antibodies was significantly reduced compared to naive animals, consistent with the previous results (Fig 8). Interestingly, neutralization of IL-17 in vivo with antibodies restored the numbers of Tregs recovered from HLA-DR3 transgenic mice undergoing TSS to that seen in naïve mice suggesting that IL-17 plays some role in Treg homeostasis. With respect to IFNγ, interestingly, even the naïve HLA-DR3.IFNγ−/− mice had significantly higher numbers of Tregs compared to HLA-DR3.IFNγ+/+ mice. Surprisingly, HLA-DR3.IFNγ−/− mice challenged with SEB and treated with isotype control antibodies yielded more Tregs, while maximum Tregs were present in HLA-DR3.IFNγ−/− mice treated with anti-IL-17 antibodies. These results suggested that IFN-γ and IL-17 act together to reduce the number of Tregs while blocking IFN-γ and IL-17 led to an increase in Tregs during TSS.
Figure 8. Roles of IFN-γ and IL-17 in the homeostasis of T regulatory cells during SEB-induced toxic shock syndrome in HLA-DR3 transgenic mice.
IFN-γ-sufficient and IFN-γ-deficient HLA-DR3 transgenic mice were injected with 100 µg of rat anti-mouse IL-17 antibodies or isotype controls. Ten minutes later, mice were challenged with SEB (50 µg/mouse) or PBS and killed 3 days later. Distribution of CD4+CD25+FoxP3+ Tregs was determined by flow cytometry. Each bar represents mean±SE of data obtained from 3–6 mice in each group. * p<0.05.
Modulation of GITR expression by IFN-γ during TSS
In the next set of experiments, we investigated the effect of IFN-γ on expression of GITR on Tconv and Tregs during TSS. For this, HLA-DR3.IFNγ+/+ and HLA-DR3.IFNγ−/− mice were challenged with 10 microgram of SEB, killed 48 and 72 hours later and the expression of profile of GITR was analyzed by flow cytometry. As shown in Fig 9A, both CD4+ as well as CD8+ TCR Vβ8+ T cells from naive HLA-DR3.IFNγ−/− mice expressed reduced levels of GITR compared to naïve HLA-DR3.IFNγ+/+ mice. Even though the CD4+ as well as CD8+ TCR Vβ8+ T cells from HLA-DR3.IFNγ−/− mice upregulated GITR expression following SEB challenge at 48 hours, their GITR levels were lower when compared to cells from HLA-DR3.IFNγ+/+ mice. The overall expression of GITR fell by 72 hours and was comparable between HLA-DR3.IFNγ+/+ and HLA-DR3.IFNγ−/− groups. Similar pattern was seen with CD4+ as well as CD8+ TCR Vβ6+ T cells and CD4+FoxP3+ T cells (summarized in Fig 9, Panel B).
Figure 9. Role of IFN-γ on upregulation of GITR on Tconv and Tregs cells during TSS.
IFN-γ-sufficient and IFN-γ-deficient HLA-DR3 transgenic mice were challenged with SEB (10 µg/mouse). Mice were killed 48 or 72 hours later, splenocytes isolated and stained with indicated antibodies. (A) Representative histogram overlays depicting the expression profile of GITR on indicated gated population (B) Bar charts depicting median fluorescent intensity of GITR expression (mean±SE) on cells within the indicated gates. * p<0.05 between IFN-γ-sufficient and IFN-γ-deficient group.
Discussion
Staphylococcus aureus is still one of the leading causes of lethal infections in humans (47). Recent reports have shown that at least in the US, S. aureus causes more deaths than HIV/AIDS (48, 49). Higher prevalence of various exotoxins, including SAg, could be one of the contributing factors for the greater virulence of certain S. aureus strains (4–8). As SAg are the most potent biological activators of the immune system, it is believed that SAg that are produced in vivo following an infection with toxigenic S. aureus causes a rapid immune activation, a robust systemic inflammatory response syndrome, multiple organ failure and ultimately, death (10–13). Therefore, attenuating the magnitude of immune activation using Tregs could prove to be beneficial in diseases involving SAg, such as TSS. Observations that such an approach is effective in certain infectious disease models (50) and in other models of acute systemic inflammation that are analogous to TSS (20–26), lend support to this hypothesis. Nonetheless, high morbidity and mortality associated with TSS suggests that normal numbers of endogenous Tregs are ineffective in countering SAg-mediated immune activation. Therefore, we investigated whether increasing the Tregs above the endogenous levels could be beneficial in TSS. Our study revealed that increasing the Treg numbers, either by in vivo expansion of endogenous Tregs using IL2C or adoptive transfer of Tregs expanded ex vivo using anti-CD3-anti-CD28 beads, were ineffective. While the precise reasons as to why Tregs were ineffective in our model could not be identified, we put forth the following hypotheses.
Instability of the Tregs in an inflammatory milieu could be one of the mechanisms. Even though higher numbers of Tregs persisted for a long period (even at day 8) in mice that were treated with IL2C or adoptively transferred with Tregs, challenging with SEB led to a rapid decline in the numbers of Tregs. This could be due to deletion of Tregs and/or conversion of Tregs to effector T cells. Recent studies have demonstrated that an inflammatory environment, such as in TSS, not only leads to loss of Treg suppressor functions, this can also promote conversion of Tregs to pro-inflammatory T effector cells, thereby accentuating the inflammation, rather than suppressing inflammation (44–46, 51–56). Elevated serum INF-γ levels in SEB-challenged IL-2C treated mice (Fig 3, Supp. Figure 1), supports this Treg to T effector cell conversion theory. Notably, IFN-γ producing Tregs have also been demonstrated in humans under inflammatory conditions (57, 58). Increased recovery of Tregs from SEB-challenged mice in which IL-17 and IFN-γ were blocked (Fig 8), further supports the emerging theme that excessive amounts of proinflammatory cytokines, such as IL-17 and IFN-γ, destabilize Tregs rendering them ineffective as suppressor cells and covert them to inflammatory cells (59–61). Given the pathogenic role of IFN-γ in TSS, conversion of Treg to IFN-γ-producing type may be detrimental during TSS (33). It is also possible that administration of IL2C led to a small but appreciable increase in Tconv in vivo, which resulted in elevated cytokine levels.
In addition to the quantitative defect in Tregs as discussed above, there could be qualitative defects in Tregs in mice undergoing TSS. Increased resistance of effector T cells to Treg-mediated immune regulation could be another reason for the ineffectiveness of Tregs in our model. Multiple factors could compromise Treg function at the same time helping the effector T cells overcome Treg-mediated immunosuppression. These include the toll-like receptor (TLR) pathway (62, 63) and the GITR pathway (41, 64). TLR pathway might disrupt Treg functions during infection with toxigenic S. aureus where staphylococcal TLR agonists (such as lipoteichoic acid, CpG DNA motifs, cell wall peptidoglycan etc.) could contribute to overriding Treg-mediated suppression (65). However, in the SAg-induced TSS model, we believe that the GITR pathway plays a major role.
GITR is a member of the TNFR superfamily. GITR is constitutively expressed on Tregs, while it is rapidly upregulated on activated T cells where it functions as a costimulatory molecule (42). Several studies have demonstrated that ligation of GITR on Tregs can detrimentally affect their suppressor activity, while ligation of GITR on effector T cells makes them more resistant to Treg-mediated suppression (41, 43, 66). In our study, the SAg-induced systemic inflammatory milieu not only increased the expression of GITR on Tregs, it caused a rapid upregulation of GITR Tconv cells such that 80–90% of the CD4+ as well as CD8+ Tconv cells were GITR+. As GITRL is constitutively expressed on several cell types including DC, B cells and macrophages (43, 67), ligation of GITR on Tregs could abrogate their suppressor activity while in T effector cells, GITR could protect them from Treg-mediated immune regulation.
Further studies using HLA-DR3.IFNγ+/+ and HLA-DR3.IFNγ−/− mice suggested that upregulation of GITR on Tconv and Tregs during TSS could be partly mediated through IFNγ. Nonetheless, GITR was still upregulated on T cells from HLA-DR3.IFNγ−/− mice, suggesting that other pathways are also involved. Overall, contradictory to the widespread notion that Tregs could be beneficial in several inflammatory conditions, our study showed that at least in SAg-induced TSS, Tregs are ineffective due to several reasons. On the contrary, Tregs may even promote immunopathology by converting into an inflammatory phenotype.
Supplementary Material
ACKOWLEDGEMENTS
We are thankful to Julie A. Hanson for meticulous mouse husbandry and Michele K Smart for mouse genotyping.
Footnotes
This study was supported by NIH grants AI68741 to CSD and GR and AI101172 to GR.
References
- 1.Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Curr. Opin. Microbiol. 2012;15:588–595. doi: 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Iwamoto M, Mu Y, Lynfield R, Bulens SN, Nadle J, Aragon D, Petit S, Ray SM, Harrison LH, Dumyati G, Townes JM, Schaffner W, Gorwitz RJ, Lessa FC. Trends in Invasive Methicillin-Resistant Staphylococcus aureus Infections. Pediatrics. 2013;132:e817–e824. doi: 10.1542/peds.2013-1112. [DOI] [PubMed] [Google Scholar]
- 3.Shilo N, Quach C. Pulmonary Infections and Community Associated Methicillin Resistant Staphylococcus aureus: A Dangerous Mix? Paediatr. Respir. Rev. 2011;12:182–189. doi: 10.1016/j.prrv.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Hu D-L, Omoe K, Inoue F, Kasai T, Yasujima M, Shinagawa K, Nakane A. Comparative prevalence of superantigenic toxin genes in meticillin-resistant and meticillin-susceptible Staphylococcus aureus isolates. J. Med. Microbiol. 2008;57:1106–1112. doi: 10.1099/jmm.0.2008/002790-0. [DOI] [PubMed] [Google Scholar]
- 5.Wilson GJ, Seo KS, Cartwright RA, Connelley T, Chuang-Smith ON, Merriman JA, Guinane CM, Park JY, Bohach GA, Schlievert PM, Morrison WI, Fitzgerald JR. A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia. PLoS Pathog. 2011;7:e1002271. doi: 10.1371/journal.ppat.1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J. Allergy Clin. Immunol. 2010;125:39–49. doi: 10.1016/j.jaci.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu D, Li X, Yang Y, Zheng Y, Wang C, Deng L, Liu L, Li C, Shang Y, Zhao C, Yu S, Shen X. Superantigen gene profiles and presence of exfoliative toxin genes in community-acquired meticillin-resistant Staphylococcus aureus isolated from Chinese children. J. Med. Microbiol. 2011;60:35–45. doi: 10.1099/jmm.0.023465-0. [DOI] [PubMed] [Google Scholar]
- 8.Chini V, Dimitracopoulos G, Spiliopoulou I. Occurrence of the Enterotoxin Gene Cluster and the Toxic Shock Syndrome Toxin 1 Gene among Clinical Isolates of Methicillin-Resistant Staphylococcus aureus Is Related to Clonal Type and agr Group. J. Clin. Microbiol. 2006;44:1881–1883. doi: 10.1128/JCM.44.5.1881-1883.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgado-Pabón W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, Peterson ML, Schlievert PM. Superantigens Are Critical for Staphylococcus aureus Infective Endocarditis, Sepsis, and Acute Kidney Injury. mBio. 2013:4. doi: 10.1128/mBio.00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karau MJ, Tilahun A, Schmidt S, Clark CR, Patel R, Rajagopalan G. Linezolid is Superior to Vancomycin in Experimental Pneumonia Caused by Superantigen-Producing Staphylococcus aureus in HLA class II Transgenic Mice. Antimicrob. Agents Chemother. 2012 doi: 10.1128/AAC.01080-12. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaulding AR, Lin Y-C, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine. 2012;30:5099–5109. doi: 10.1016/j.vaccine.2012.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varshney AK, Wang X, Scharff MD, MacIntyre J, Zollner RS, Kovalenko OV, Martinez LR, Byrne FR, Fries BC. Staphylococcal Enterotoxin B–Specific Monoclonal Antibody 20B1 Successfully Treats Diverse Staphylococcus aureus Infections. J. Infect. Dis. 2013 doi: 10.1093/infdis/jit421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaulding AR, Salgado-Pabón W, Merriman JA, Stach CS, Ji Y, Gillman AN, Peterson ML, Schlievert PM. Vaccination Against Staphylococcus aureus Pneumonia. J. Infect. Dis. 2013 doi: 10.1093/infdis/jit823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser DJ, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 2008;225:226–243. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 16.Spaulding AR, Salgado-Pabón W, Kohler PL, Horswill AR, Leung DYM, Schlievert PM. Staphylococcal and Streptococcal Superantigen Exotoxins. Clin. Microbiol. Rev. 2013;26:422–447. doi: 10.1128/CMR.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S. Regulatory T Cells: Key Controllers of Immunologic Self-Tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 18.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 19.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annual Review of Immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okeke EB, Okwor I, mou Z, Jia P, Uzonna JE. CD4+CD25+ Regulatory T Cells Attenuates LPS-induced Systemic Inflammatory Responses and Promotes Survival in Murine Escherichia coli infection. Shock Publish Ahead of Print: 10.1097/SHK.1090b1013e318296e318265b. 9000 doi: 10.1097/SHK.0b013e318296e65b. [DOI] [PubMed] [Google Scholar]
- 21.Jia W, Cao L, Yang S, Dong H, Zhang Y, Wei H, Jing W, Hou L, Wang C. Regulatory T Cells Are Protective in Systemic Inflammation Response Syndrome Induced by Zymosan in Mice. PLoS ONE. 2013;8:e64397. doi: 10.1371/journal.pone.0064397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitazawa Y, Li XK, Liu Z, Kimura H, Isaka Y, Hunig T, Takahara S. Prevention of graft-versus-host diseases by in vivo supCD28mAb-expanded antigen-specific nTreg cells. Cell Transplant. 2010;19:765–774. doi: 10.3727/096368910X508870. [DOI] [PubMed] [Google Scholar]
- 23.Duramad O, Laysang A, Li J, Ishii Y, Namikawa R. Pharmacologic Expansion of Donor-Derived, Naturally Occurring CD4+Foxp3+ Regulatory T Cells Reduces Acute Graft-versus-Host Disease Lethality Without Abrogating the Graft-versus-Leukemia Effect in Murine Models. Biology of Blood and Marrow Transplantation. 2011;17:1154–1168. doi: 10.1016/j.bbmt.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JL, Trenado Al, Vasey D, Klatzmann D, Salomon BAL. CD4+CD25+ Immunoregulatory T Cells: New Therapeutics for Graft-Versus-Host Disease. The Journal of Experimental Medicine. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, Vonderfecht SL, Na S. Adoptive Transfer of In Vitro-Stimulated CD4+CD25+ Regulatory T Cells Increases Bacterial Clearance and Improves Survival in Polymicrobial Sepsis. The Journal of Immunology. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 26.Gogishvili T, Langenhorst D, Lühder F, Elias F, Elflein K, Dennehy KM, Gold R, Hünig T. Rapid Regulatory T-Cell Response Prevents Cytokine Storm in CD28 Superagonist Treated Mice. PLoS ONE. 2009;4:e4643. doi: 10.1371/journal.pone.0004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective Stimulation of T Cell Subsets with Antibody-Cytokine Immune Complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 28.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of Experimental Medicine. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In Vitro-expanded Antigen-specific Regulatory T Cells Suppress Autoimmune Diabetes. The Journal of Experimental Medicine. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siemasko KF, Gao J, Calder VL, Hanna R, Calonge M, Pflugfelder SC, Niederkorn JY, Stern ME. In Vitro Expanded CD4+CD25+Foxp3+ Regulatory T Cells Maintain a Normal Phenotype and Suppress Immune-Mediated Ocular Surface Inflammation. Investigative Ophthalmology & Visual Science. 2008;49:5434–5440. doi: 10.1167/iovs.08-2075. [DOI] [PubMed] [Google Scholar]
- 31.Tilahun AY, Marietta EV, Wu T-T, Patel R, David CS, Rajagopalan G. Human leukocyte antigen class II transgenic mouse model unmasks the significant extrahepatic pathology in toxic shock syndrome. Am. J. Pathol. 2011;178:2760–2773. doi: 10.1016/j.ajpath.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilahun AY, Theuer JE, Patel R, David CS, Rajagopalan G. Detrimental effect of the proteasome inhibitor, bortezomib in bacterial superantigen- and lipopolysaccharide-induced systemic inflammation. Mol. Ther. 2010;18:1143–1154. doi: 10.1038/mt.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilahun AY, Holz M, Wu T-T, David CS, Rajagopalan G. Interferon gamma-dependent intestinal pathology contributes to the lethality in bacterial superantigen-Induced toxic shock syndrome. PLoS ONE. 2011;6:e16764. doi: 10.1371/journal.pone.0016764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowdhary VR, Tilahun AY, Clark CR, Grande JP, Rajagopalan G. Chronic Exposure to Staphylococcal Superantigen Elicits a Systemic Inflammatory Disease Mimicking Lupus. The Journal of Immunology. 2012;189:2054–2062. doi: 10.4049/jimmunol.1201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi S. Naturally Arising CD4+ Regulatory T Cells for Immunologic Self-Tolerance and Negative Control of Immune Responses. Annual Review of Immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 37.Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J. Immunol. 2002;169:1837–1843. doi: 10.4049/jimmunol.169.4.1837. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes in vivo. J. Exp. Med. 1998;187:1427–1438. doi: 10.1084/jem.187.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Létourneau S, van Leeuwen EMM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor α subunit CD25. Proceedings of the National Academy of Sciences. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Martínez K, León K. Modeling the role of IL2 in the interplay between CD4+ helper and regulatory T cells: studying the impact of IL2 modulation therapies. International Immunology. 2012;24:427–446. doi: 10.1093/intimm/dxr120. [DOI] [PubMed] [Google Scholar]
- 41.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 42.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proceedings of the National Academy of Sciences. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of Glucocorticoid-Induced TNFR Family-Related Receptor on Effector T Cells by its Ligand Mediates Resistance to Suppression by CD4+CD25+ T Cells. The Journal of Immunology. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 44.Bailey-Bucktrout, Samantha L, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling Hans J, Bluestone Jeffrey A. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4+ FoxP3+ T cells. Current Opinion in Immunology. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. M. I. for the Active Bacterial Core surveillance Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 48.Otto M. Understanding the epidemic of community-associated MRSA and finding a cure: are we asking the right questions? Expert Rev. Anti Infect. Ther. 2009;7:141–143. doi: 10.1586/14787210.7.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008;(46 Suppl. 5):S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 50.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 51.Wisnoski N, Chung C-S, Chen Y, Huang X, Ayala A. The Contribution of Cd4+ Cd25+ T-Regulatory-Cells to Immune Suppression in Sepsis. Shock. 2007;27:251–257. doi: 10.1097/01.shk.0000239780.33398.e4. 210.1097/1001.shk.0000239780.0000233398.e0000239784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng X-H, Jetten AM, Dong C. Molecular Antagonism and Plasticity of Regulatory and Inflammatory T Cell Programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yurchenko E, Shio MT, Huang TC, Da Silva Martins M, Szyf M, Levings MK, Olivier M, Piccirillo CA. Inflammation-Driven Reprogramming of CD4+Foxp3+ Regulatory T Cells into Pathogenic Th1/Th17 T Effectors Is Abrogated by mTOR Inhibition in vivo. PLoS ONE. 2012;7:e35572. doi: 10.1371/journal.pone.0035572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos santos L, O'Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg Cell Number and Acquisition of Effector Cell Phenotype during Lethal Infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, O'Shea JJ, Fowler DH. STAT3 Transcription Factor Promotes Instability of nTreg Cells and Limits Generation of iTreg Cells during Acute Murine Graft-versus-Host Disease. Immunity. 2012;37:209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmüller U, Baron U, Olek S, Bluestone JA, Brusko TM. Plasticity of Human Regulatory T Cells in Healthy Subjects and Patients with Type 1 Diabetes. The Journal of Immunology. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang J-H, Kim Y-J, Han S-H, Kang C-Y. IFN-γ-STAT1 signal regulates the differentiation of inducible Treg: Potential role for ROS-mediated apoptosis. European Journal of Immunology. 2009;39:1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 60.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting Edge: The Th1 Response Inhibits the Generation of Peripheral Regulatory T Cells. The Journal of Immunology. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao X, Leonard K, Collins LI, Cai SF, Mayer JC, Payton JE, Walter MJ, Piwnica-Worms D, Schreiber RD, Ley TJ. Interleukin 12 Stimulates IFN-γ-Mediated Inhibition of Tumor-Induced Regulatory T-Cell Proliferation and Enhances Tumor Clearance. Cancer Research. 2009;69:8700–8709. doi: 10.1158/0008-5472.CAN-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasare C, Medzhitov R. Toll Pathway-Dependent Blockade of CD4+CD25+ T Cell-Mediated Suppression by Dendritic Cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 63.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes and Infection. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: More than an effector T cell co-stimulatory system. European Journal of Immunology. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 65.Tilahun AY, Karau MJ, Ballard A, Gunaratna MP, Thapa A, David CS, Patel R, Rajagopalan G. The impact of Staphylococcus aureus-associated molecular patterns on staphylococcal superantigen-induced toxic shock syndrome and pneumonia. Mediators Inflamm. 2014 doi: 10.1155/2014/468285. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji H-b, Liao G, Faubion WA, Abadía-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting Edge: The Natural Ligand for Glucocorticoid-Induced TNF Receptor-Related Protein Abrogates Regulatory T Cell Suppression. The Journal of Immunology. 2004;172:5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 67.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proceedings of the National Academy of Sciences. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.