Abstract
Human Milk Oligosaccharides (HMO) are one of the major components of human milk. HMO are non-digestible by the human gut, where they are known to play important functions as prebiotics and decoys for binding pathogens. Moreover, it has been proposed that HMO may provide sialic acids to the infant that are important in brain development, however this would require absorption of HMO into the bloodstream. HMO have consistently been found in the urine of humans and other mammals, suggesting systemic absorption. Here we present a procedure for the profiling of milk oligosaccharides (MO) in plasma samples obtained from 13 term infants hospitalized for surgery for congenital heart disease. The method comprises protein denaturation, oligosaccharide reduction and porous graphitized carbon solid phase extraction for purification followed by analysis using nHPLC-PGC-chip-TOF-MS. Approximately 15 free MO were typically observed in the plasma of human infants, including LNT, LDFP, LNFT, 3’SL, 6’SL, 3’SLN and 6’SLN, of which the presence was confirmed using fragmentation studies. A novel third isomer of SLN, not found in human or bovine milk was also consistently detected. Differences in the free MO profiles were observed between infants that were totally formula-fed and infants that received at least some part breast milk. Our results indicate that free MO similar in structure to those found in human milk and urine are present in the blood of infants. The method and results presented here will facilitate further research toward the possible roles of free MO in the development of the infant.
Keywords: HMO, plasma, sialyllactosamine, nHPLC-MS, porous graphitized carbon
INTRODUCTION
Human milk has historically been the sole source of nutrition during the early stages of an infant's life. Evolution has thus crafted nature's most complex food to balance the nutritional and immunoprotective needs of the infant and the health of the mother; indeed human milk is the ‘perfect food’ [1,2]. Milk oligosaccharides (MO) are one of the major components of human milk, and while they are composed of monosaccharides linked by glycosidic bonds, they are generally regarded to be non-digestible by the infant gut due to lack of human production of the necessary glycosidases. Human milk oligosaccharides (HMO) have at least two important functions. First, HMO are important prebiotics [3,4] selectively stimulating the growth of beneficial bacteria, such as bifidobacteria, which express bacterial glycosidases and can use the HMO as a food source. This prebiotic effect of HMO explains in large part the significant differences in the gut microbiome of breastfed infants compared to that of formula-fed infants [5]. Second, HMO may serve as decoys by binding pathogens within the gut lumen, thus preventing bacterial adhesion to the epithelial cells in the small intestine and colon [6]. Increased levels of HMO in mother's milk and infant feces are associated with decreased incidence of both gastro-intestinal infections and upper respiratory infections in infants [7], suggesting that HMO may play a role in both mucosal immunity within the gut and adaptive immunity through systemic effects. An additional beneficial effect of HMO has been proposed: sialic acids are important in brain development and human milk in the presence of bacteria that express sialidases may provide N-acetylneuraminic acid, the key monomeric precursor of neural glycoproteins, gangliosides, glycosaminoglycans and mucins [8].
From an evolutionary standpoint, infant formula is a very recent development. While current formulae differ significantly from early versions, most are derived from bovine milk and none are enriched with HMO. Term infants that receive human milk have lower rates of otitis media, gastroenteritis, allergies, asthma, urinary tract infections, Crohn's disease and some forms of cancer, and improved cognitive and motor skills [9]. Premature infants that receive human milk have a significantly lower risk of developing necrotizing enterocolitis, a devastating inflammatory disease that appears to be triggered by alterations in the intestinal microbiome [10,11]. It has been hypothesized that these protective effects are in part due to the presence of HMO.
HMOs have complicated structures, but are built up of mainly five monosaccharide residues that may be linked linearly or branched to each other using glycosidic linkages. Typically, HMO have a lactose core, which is further elongated with galactoses (Gal), glucoses (Glc), N-acetylglucosamines (GlcNAc), Fucoses (Fuc) or the sialic acid N-acetylneuraminic acid (Neu5Ac), to a final degree of polymerization of 3-14 [12-14]. The structures of HMO are highly diverse and unique, and differ significantly from the MO profiles of other mammals, such as cows [15] and primates [12]. Bovine milk contains a limited repertoire of MO that are likely present in many current infant formulae in small quantities [15]. Due to their complex structures, it has thus far not been possible to produce HMO on a large scale for addition to infant formula.
There is ample evidence for the presence of HMO in the urine of infants [16-19]. This implies that the HMO are absorbed from the gastro-intestinal tract into the bloodstream and then filtered by the kidney, and therefore it is likely that HMO are present in the blood and could thus have systemic functions. However, thus far, there have been no reports of detection of HMO in blood of human infants. Recently, the presence of HMO was determined in rat serum of animals that were fed isolated HMO [20], thus indicating that HMO can be absorbed from the rat intestinal tract.
Porous graphitic carbon (PGC) is known to be an excellent medium for the enrichment and separation of oligosaccharides [21]. Our group has been the first to apply PGC separation in combination with time of flight (TOF) mass spectrometry for the analysis of HMO from milk, feces and urine [22-24,13,14,25]. Here we employ nanoflow liquid chromatography (nLC) PGC-TOF-MS for the detection of milk oligosaccharide in the plasma of infants. We assessed the repeatability of the method, and performed a pilot study to assess differences in the plasma HMO profile between formula-fed infants and those receiving human milk and formula and differences in oligosaccharide profiles of plasma samples over time.
METHODS AND MATERIALS
Plasma samples
Samples were obtained from term infants with congenital heart disease enrolled in a placebo-controlled pilot study of probiotics. Patient characteristics were summarized in the original publication [26]. Briefly, the original study included 16 term infants who required surgery for congenital heart disease in the neonatal period. Seven were born by C-section, all received antibiotics at some time during their hospitalization, and all had periods of time with no enteral feedings and acid suppression (all factors known to influence the intestinal microbiota). Samples of plasma were available from 13 of the infants (four of whom received only formula and nine of whom received both formula and human milk or human milk fortified with powdered human milk fortifier made from bovine milk) at various time points in the first 30 days of life; these were analyzed in a blinded manner. The blood samples were centrifuged immediately after the blood was obtained and the plasma was frozen at −80°C. The original samples were thawed for analysis of plasma cytokines [26] and left-over plasma was re-frozen and again stored at −80°C until MO analysis. None of these infants were exclusively human milk-fed, in part due to the challenges related to prolonged hospitalization. The study was approved by the Institutional Review Board and registered at Clinicaltrials.gov (NCT01018472).
Preparation of Oligosaccharide samples from plasma
To isolate free oligosaccharides from plasma samples, a 50 μL aliquot of a 50 mM ammonium bicarbonate buffer was added to 50 μL of plasma in an Eppendorf vial and mixed thoroughly. A 400 μL cold ethanol was then added to the samples, mixed and stored at −80°C for one hour to precipitate proteins. Samples were centrifuged at 21.000 rcf in an Eppendorf centrifuge, and 400 μL of the supernatant was transferred to a new vial. The samples were dried in vacuo using a speedvac (EZ-2 Plus from Genevac, Stony Ridge, NY). Since the MO samples were to be analyzed using PGC and PGC is known to separate the anomers of the oligosaccharides, thus complicating structural identification, the oligosaccharides were reduced prior to SPE and analysis. 100 μL of 1 M sodium borohydride in water was added to each of the samples, which were then incubated at 65 °C to reduce the free oligosaccharides. For the absolute quantification of HMO in plasma, 50 μL of a serum standard (Sigma Aldrich, St. Louis, MO) were spiked with different concentrations of lacto-N-tetraose (LNT) and 6'sialyllactose (6’SL) resulting in final concentrations of 1.5 μg/mL, 0.15 μg/mL, 0.015 μg/mL, 0.0015 μg/mL, 0.00015 μg/mL and 0 μg/mL. 400 μl cold ethanol was added to the samples, which were then further prepared as described above.
Oligosaccharide purification using solid phase extraction with graphitized carbon
Immediately after reduction, the oligosaccharides were purified using graphitized carbon SPE (Grace, Deerfield, IL). Briefly, cartridges were conditioned using 4 mL of 80% ACN containing 0.05% TFA. (EMD chemicals, Gibbstown, NJ), followed by 4 mL of water containing 0.05% TFA. Oligosaccharide samples were diluted using 400 μL of water and subsequently loaded onto the cartridges. Cartridges were washed using 6 × 4 mL of water, and the oligosaccharides were eluted using 4 mL of 40% ACN containing 0.05% TFA. Samples were dried in vacuo in the speedvac prior to analysis.
nHPLC-chip-TOF-MS analysis
Plasma derived MO were analyzed using an Agilent (Santa Clara, CA) 6200 series nanoHPLC-chip-TOF-MS, consisting of an autosampler, which was maintained at 8 °C, a capillary loading pump, a nanopump, HPLC-chip-MS interface and an Agilent 6210 TOF mass spectrometer. The chip (Glycan Chip II, Agilent) contained a 9 × 0.075 mm i.d. enrichment column coupled to a 43 × 0.075 mm i.d. analytical column; both packed with 5 μm porous graphitized carbon (PGC). Plasma oligosaccharide samples were reconstituted in 50 μL of water prior to analysis; 5 μL of sample was used for injection. Upon injection, the sample was loaded onto the enrichment column using 3% ACN containing 0.1% formic acid (FA, Fluka, St. Louis, MO). The analytical column was then switched on-line so that the nano-pump delivered a gradient of 3% ACN with 0.1% FA (solvent A) to 90% ACN with 0.1% FA (solvent B). The gradient increased from 0% B to 33% B over 13 minutes, followed by an increase to 36% B at 16.5 minutes and 100% B at 17 minutes. The column was then washed at 100% B for 5 minutes, followed by 10 minutes reequilibration at 0% B. Positive ions were generated and mass spectra were acquired over a mass window of 400 m/z to 3000 m/z.
nHPLC-chip-Q-TOF-MS/MS analysis
To allow unambiguous identification of oligosaccharides, samples were analyzed using an Agilent 6200 series nanoHPLC-chip-Q-TOF-MS/MS, consisting of an autosampler, which was maintained at 8 °C, a capillary loading pump, a nanopump, HPLC-chip-MS interface and an Agilent 6520 Quadrupole-Time Of Flight mass spectrometer. The chip and gradient were the same as for the nHPLC-chip-TOF-MS analysis. N-glycans were subjected to collision-induced dissociation (CID) fragmentation with nitrogen as the collision gas using a series of collision energies that were dependent on the m/z values of the oligosaccharides. The collision energies correspond to voltages (Vcollision) that were based on the equation: Vcollision = m/z (1.8/100 Da) Volts - 2.4 V, where the slope and offset of the voltages were set at 1.8/100 Da and -2.4, respectively. All data was acquired in the positive ionization mode.
Data analysis
Data analysis was performed using Masshunter® Qualitative Analysis (version B.03.01, Agilent) and Microsoft® Excel® for Mac 2011 (version 14.1.3, Microsoft). Data was loaded into Masshunter, and glycan features were integrated using the Molecular Feature Extractor algorithm. First, signals above a signal to noise threshold of 5.0 were considered. Then, signals were deconvoluted using a tolerance of 0.0025 m/z ± 10 ppm. To identify the oligosaccharide structures, their mass and retention times were compared to a library previously developed for milk in our group [13,14], where a 15 ppm mass error was allowed. Moreover, comparisons to previous analyses of urine samples [23], which should have more similar oligosaccharide compositions to plasma than milk, were performed. Oligosaccharide compositions, retention times and volume were exported to csv-format. Integrals of the oligosaccharides observed in at least 50% of the samples were considered for statistical analysis. To compare the different feeding groups and time patterns, student's t-tests were used on the integrals relative to the total oligosaccharide content.
RESULTS AND DISCUSSION
Human milk oligosaccharides have consistently been found in the urine of infants [16,23] and therefore it has been assumed that HMO are absorbed from the gut and transported through the bloodstream to the urine. However, thus far MO have not been detected in blood from human infants. Here, MO were purified from plasma of infants, followed by analysis using nLC-PGC-chip-TOF-MS.
Detection of free oligosaccharides in plasma of infants
A typical profile of oligosaccharides obtained from infant plasma is depicted in Figure 1A with the major oligosaccharide peaks annotated. Approximately 15 milk oligosaccharides are observed in a typical chromatogram, ranging between 7 and 19. The number is significantly smaller than that detected in human milk, which typically contains a hundred compounds per individual, but is more comparable to the number of HMO identified in urine [23]. Neutral HMO commonly observed in infant plasma include LNT, LNnT, LNFP III and 2’FL. LNT, which is often the most abundant HMO in breast milk, is observed at low abundances in the plasma of infants (and was found in small quantities in exclusively formula-fed infants). The detection of 2’fucosyllactose (2’FL) has potential clinical relevance as it is a marker of the mother's secretor status. As expected, 2’FL was not found in exclusively formula-fed infants [24,27,28].
Figure 1. Representative extracted compound chromatogram of the oligosaccharides found in infant plasma.
(A) Chromatogram showing oligosaccharide profile at time point 1. Major peaks are annotated with their respective structures according to the following glycan symbol key: blue square: N-acetylglucosamine, green ball: mannose, yellow ball: galactose, red triangle: fucose and purple diamond: sialic acid. (B) Overlay of three extracted compound chromatograms from the repeated analysis of one infant at time point 1.
Interestingly, several sialylated oligosaccharides are highly abundant in infant plasma. These include two isomers of sialyllactose (3’SL and 6’SL), an LST, and two sialyllactosamine isomers (3’SLN and 6’SLN). In addition a new isomer of SLN (unknown SLN or U-SLN) is found that is neither 6’SLN nor 3’SLN. The large amount of SLN identified in infant plasma is somewhat unexpected. Typically, SLN is found in low abundances in human milk and at moderate abundances in bovine milk [15,29]. It may be that the observed SLN is mostly derived from bovine milk, however the relative levels of SLN observed in this study are higher than what would typically be expected from bovine milk. Similarly, SLN have also been reported to be present at relatively higher concentrations in urine of breast-fed infants [23], suggesting their specific absorption or production after intake by the infant. The presence of U-SLN is intriguing. This compound is abundant in plasma but not observed in either bovine or human milk. We have previously shown that there are glycosidases produced by commensal bacteria in the infant gut [30], therefore we may speculate that U-SLN and other sialylated compounds can be produced by the interaction of milk, milk products and bacterial enzymes, i.e. an in vivo example of a new oligosaccharide structure produced by the gut microbiota.
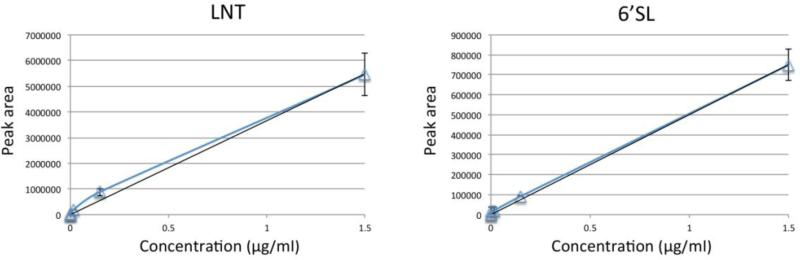
While excellent stability of nLC-chip-TOF-MS for the analysis of oligosaccharides has been reported [31], the repeatability of the sample preparation method for the isolation of HMO from blood has not been shown. To assess the stability of the method, two infant plasma samples, of which sufficient volumes were available, were prepared in triplicates and analyzed. An overlay of the extracted compound chromatograms of one of the samples is depicted in Figure 1B. Using relative abundances, an average RSD of 15% could be calculated for the oligosaccharides observed, indicating good method repeatability. To assess the absolute abundance of HMO in the infants’ plasma, a standard serum sample was used. Different concentrations of LNT and 6’SL (1500 ng/ml, 150 ng/ml, 15 ng/ml, 1.5 ng/ml, 0.15 ng/ml and 0 ng/ml) were spiked into the standard serum, which was then further processed and analyzed similarly to the infant plasma samples. Notably, no HMO were observed in the standard sample in which no LNT and 6’SL were added, indicating that HMO are typically not found in blood of healthy adults. Figure 2 reports the resulting calibration curves. A linear function was fitted with an R2 of 0.994 for LNT and 0.998 for 6’SL and the HMO concentrations were determined for the two triplicate samples. Based on the calibration curve, the average absolute abundances of LNT were calculated to be 59 and 168 ng/ml (corresponding to approximately 8.4 × 10−8 M and 2.4 × 10−7 M, respectively) with RSD's of 3% and 7%, respectively. No 6’SL was observed in one of the samples, while the average concentration in the other sample was 626 ng/ml (corresponding to 9.8 × 10−7 M) with an RSD of 11%.
Figure 2. Calibration curves of a standard serum sample spiked in with different concentrations of LNT (left) and 6’SL (right).
Error bars represent the standard deviation (n=3).
These results strongly support the hypothesis that MO are absorbed in the infants’ gut and circulate through their bloodstream. Furthermore the results are in accordance with the recent report, where it was observed that sialylated HMO peaks are more abundant in the feces and urine, but not the milk of mother-infant dyads [23].
Identification and structural analysis of milk oligosaccharides in plasma
The milk oligosaccharides were identified using a library of structures as described in earlier publications [13,14], and tandem MS was used to confirm the structures. Tandem MS spectra of two of the oligosaccharides studied, namely LNFP III at m/z 865.33 and 3’SL at m/z 636.23 are depicted in Figure 3. The spectra clearly show typical glycan fragmentation patterns with b- and y- type ions, thus confirming their identification as oligosaccharides. Characteristic fragment ions for fucosylated oligosaccharides were observed for LNFP III at m/z 350.15 and 512.21, corresponding to HexNAc1Fuc1 and Hex1HexNAc1Fuc1, respectively, indicating a glycosidic linkage between the fucose and the glucosamine, thus confirming the LNFP III isomer. For the sialylated compound 3’SL, distinct sialylated fragments were observed at 292.11 and 454.16, corresponding to NeuAc and Hex1NeuAc1, respectively.
Figure 3. Fragmentation mass spectra of two oligosaccharides found in infant plasma.
CID fragmentation spectra are depicted for LNFP III at m/z 865.33 and 3’SL at m/z 636.23. Major peaks are annotated with their respective fragments. For glycan symbol key, see Figure 1.
The tandem MS of the three isomers of sialyllactosamine are shown in Figure 4. The 6’SLN and 3’SLN were identified based on their retention times. U-SLN did not match either structure, however the fragmentation pattern is very similar to the other two isomers under identical CID conditions. Because we do not have sufficient amounts of U-SLN to purify and perform a comprehensive structural analysis of the oligosaccharide, we may only speculate on the structure of the U-SLN. Currently, there are only three known linkages of sialic acids. They can be bound to an oligosaccharide chain via α(2,3) and α(2,6). While sialic acids may also be linked to another sialic acid residue via α(2,8), such a linkage has not been reported with any other monosaccharide, and is thus very unlikely for U-SLN. Because sialic acids are typically found to be the terminal residue on the non-reducing end and the sialic acids can only be linked to the 3- and 6-position of galactose, we may conclude that the disaccharide core is not a standard lactosamine, but an isomer with a different combination of a hexose and a hexosamine residue. Possibly, the disaccharide core is of bacterial origin rather than human. While the fragmentation patterns of the three SLN are similar, there are variations in the relative abundances of the products. The fragmentation patterns of U-SLN and 3’SLN are more similar compared to 6’SLN, which is more distinct, maybe hinting toward a 3’ linkage of the sialic acid to the disaccharide core.
Figure 4. Determination of three isomers of sialyllactosamine (SLN) in the plasma of infants.
(A) Extracted ion chromatogram for sialyllactosamine (m/z 677.25) with annotation of the known SLN structures. (B) Fragmentation spectrum of 6’SLN from infant plasma. (C) Fragmentation spectrum of the unknown SLN from infant plasma. (D) Fragmentation spectrum of 3’SLN from infant plasma. All spectra were obtained using CID in the positive ionization mode, and their major fragments have been annotated, for symbol key, see Figure 1. Monosaccharides for which the exact structure could not be identified, have been indicated with a colorless symbol (square: N-acetylhexosamine and ball: hexose).
Variations in milk oligosaccharides between infants
The absolute concentrations of LNT and 6’SL were compared between solely formula-fed infants and partially breastfed infants. For LNT, an average concentration of 77ng/ml (1.1 × 10−7 M) was observed in the partially breastfed infants, while only 7 ng/ml LNT (9.8 × 10−9 M) was present in the plasma of formula-fed infants (p=0.045 using a t-test). Similarly, lower concentrations of 6’SL were observed in solely formula-fed infants compared to partially breast-fed infants, though this was not significant due to the small sample size: 23 ng/ml compared to 150 ng/ml, respectively, corresponding to 3.6 × 10−8 M and 2.4 × 10−7 M.
To further assess differences in the overall MO profile, the relative abundances of plasma MO in samples from solely formula-fed infants were compared to those of partially breastfed infants and the average relative abundances for the oligosaccharides determined consistently are plotted in Figure 5. In spite of the small sample size, significant p-values (<0.05) were observed for the three neutral oligosaccharides 2’FL, LNFP III and LNT (p-values of 0.022, 0.030 and 0.013, respectively, marked with an asterisk in Figure 5), while no significant differences were observed for the sialylated compounds. The relative levels of all three neutral oligosaccharides were lower in solely formula-fed infants, and as expected, 2’FL and LNFP III were nearly absent in plasma from formula-fed infants.
Figure 5. Variation in abundances of oligosaccharides in the plasma of infants exclusively formula-fed and infants that received some breast milk.
Average abundances relative to the overall oligosaccharide content are depicted, with formula-fed infants represented in light grey, and partially breast-fed infants in dark grey. Error bars represent standard deviations. MO of which significantly different levels were observed between formula-fed infants and infants that received some breast milk are indicated with an asteriks.
Given the low volume of plasma available for analysis in the study, we were able to profile the oligosaccharides in samples of only six infants at two time points (the second sample obtained 3-5 weeks after the first). No significant differences were observed between the time points, indicating that in these infants the plasma oligosaccharide profile did not change much over time. No significant effects of the probiotic treatment on the plasma oligosaccharide profile were observed.
CONCLUSION
This study shows, for the first time, that milk oligosaccharides with structures derived from human and bovine milk oligosaccharides can be detected in the plasma of infants using nLC-PGC-chip-TOF-MS. This study has some drawbacks inherent in the study design primarily due to the difficulty in obtaining blood samples from healthy infants. The infants enrolled in the study were not healthy and may have had altered gut permeability due to chronic illness, antibiotic use, or surgery. The exact volume of human milk and/or formula consumed by a given infant in the days before sample collection would have been valuable information but was not available. Nonetheless, the methods described are suitable for future larger studies to analyze correlations between plasma HMO composition and gut permeability, intestinal dysbiosis, allergies, and even neurodevelopment.
The structural properties of the oligosaccharides strongly indicate that most of them originate from human and bovine milk. However, the high abundance of sialylated oligosaccharides, together with their presence in infants not receiving human milk, suggests that at least part of the structures are likely specifically absorbed, produced in the infant's gut or added to the infant formula; this is further indicated by the presence of a novel, unknown sialyllactose structure in the infants’ plasma. Access to the source mothers’ milk was not possible in this study. However, we have examined thousands of human and bovine milk samples in other studies [Reference], and we have no reason to believe that the composition of HMO in the milk samples here would differ significantly from other human and bovine milk specimens, respectively.
The presence of large amounts of sialylated species in infant plasma and the abundance of sialic acid in the developing brain support the hypothesis that sialic acids or sialic acid containing oligosaccharides are transported from the milk to the lumen of the small intestine or colon and then absorbed into the plasma and across the blood-brain barrier into the immature brain. Similarly, the rich lymphoid component of the gut could facilitate systemic absorption of HMO from the gut influencing development of both the innate and adaptive immune systems.
ACKNOWLEDGEMENTS
Funding was provided by the NIH (R01 HD059127 and UL1 TR000002)
ABBREVIATIONS
- FA
Formic acid
- FL
Fucosyllactose
- Fuc
Fucose
- Gal
Galactose
- Glc
Glucose
- GlcNAc
N-acetylglucosamine
- HMO
Human milk oligosaccharide
- LDFP
Lacto-difucosyl-pentaose
- LNFP
Lacto-N-fucopentaose
- LNnT
Lacto-N-neotetraose
- LNT
Lacto-N-tetraose
- MO
Milk oligosaccharide
- Neu5Ac
N-acetylneuraminic acid
- PGC
Porous graphitized carbon
- RSD
Relative standard deviation
- SL
Sialyllactose
- SLN
Sialyllactosamine
- TFA
Trifluoroacetic acid
Footnotes
the authors declare no conflict of interest.
REFERENCES
- 1.German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:205–218. doi: 10.1159/000146322. discussion 218-222. doi:10.1159/000146322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A 108 Suppl. 2011;1:4653–4658. doi: 10.1073/pnas.1000083107. doi:1000083107 [pii]10.1073/pnas.1000083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O. The first prebiotics in humans: human milk oligosaccharides. J Clin Gastroenterol. 2004;38(6 Suppl):S80–83. doi: 10.1097/01.mcg.0000128926.14285.25. [DOI] [PubMed] [Google Scholar]
- 4.Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 18 Suppl. 2012;4:12–15. doi: 10.1111/j.1469-0691.2012.03863.x. doi:10.1111/j.1469-0691.2012.03863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094. doi:10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newburg DS. Innate immunity and human milk. J Nutr. 2005;135(5):1308–1312. doi: 10.1093/jn/135.5.1308. [DOI] [PubMed] [Google Scholar]
- 7.Stepans MB, Wilhelm SL, Hertzog M, Rodehorst TK, Blaney S, Clemens B, Polak JJ, Newburg DS. Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med. 2006;1(4):207–215. doi: 10.1089/bfm.2006.1.207. doi:10.1089/bfm.2006.1.207. [DOI] [PubMed] [Google Scholar]
- 8.Wang B. Molecular Mechanism Underlying Sialic Acid as an Essential Nutrient for Brain Development and Cognition. Adv Nutr. 2012;3(3):465S–472S. doi: 10.3945/an.112.001875. doi:Doi 10.3945/An.112.001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Section on B. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–841. doi: 10.1542/peds.2011-3552. doi:10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 10.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. 2009;29(1):57–62. doi: 10.1038/jp.2008.117. doi:10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. doi:10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao N, Wu S, Kim J, An HJ, Hinde K, Power ML, Gagneux P, German JB, Lebrilla CB. Evolutionary glycomics: characterization of milk oligosaccharides in primates. J Proteome Res. 2011;10(4):1548–1557. doi: 10.1021/pr1009367. doi:10.1021/pr1009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10(2):856–868. doi: 10.1021/pr101006u. doi:10.1021/pr101006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9(8):4138–4151. doi: 10.1021/pr100362f. doi:10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB, Barile D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology. 2013;23(6):664–676. doi: 10.1093/glycob/cwt007. doi:10.1093/glycob/cwt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. Urinary excretion of in vivo (1)(3)C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 2012;107(7):957–963. doi: 10.1017/S0007114511004016. doi:10.1017/S0007114511004016. [DOI] [PubMed] [Google Scholar]
- 17.Rudloff S, Pohlentz G, Diekmann L, Egge H, Kunz C. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta paediatr. 1996;85(5):598–603. doi: 10.1111/j.1651-2227.1996.tb14095.x. [DOI] [PubMed] [Google Scholar]
- 18.Obermeier S, Rudloff S, Pohlentz G, Lentze MJ, Kunz C. Secretion of 13C-labelled oligosaccharides into human milk and infant's urine after an oral [13C]galactose load. Isotopes Environ Health Stud. 1999;35(1-2):119–125. doi: 10.1080/10256019908234084. doi:10.1080/10256019908234084. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS. Survival of human milk oligosaccharides in the intestine of infants. Adv Exp Med Biol. 2001;501:315–323. doi: 10.1007/978-1-4615-1371-1_39. [DOI] [PubMed] [Google Scholar]
- 20.Santos-Fandila A, Zafra-Gómez A, Vazquez E, Navalón A, Rueda R, Ramírez M. Ultra high performance liquid chromatography–tandem mass spectrometry method for the determination of soluble milk glycans in rat serum. Talanta. 2013 doi: 10.1016/j.talanta.2013.10.013. doi: http://dx.doi.org/10.1016/j.talanta.2013.10.013. [DOI] [PubMed]
- 21.Ruhaak LR, Deelder AM, Wuhrer M. Oligosaccharide analysis by graphitized carbon liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2009;394(1):163–174. doi: 10.1007/s00216-009-2664-5. doi:10.1007/s00216-009-2664-5. [DOI] [PubMed] [Google Scholar]
- 22.Ninonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B, Lebrilla C. Nanoliquid chromatography-mass spectrometry of oligosaccharides employing graphitized carbon chromatography on microchip with a high-accuracy mass analyzer. Electrophoresis. 2005;26(19):3641–3649. doi: 10.1002/elps.200500246. [DOI] [PubMed] [Google Scholar]
- 23.De Leoz ML, Wu S, Strum JS, Ninonuevo MR, Gaerlan SC, Mirmiran M, German JB, Mills DA, Lebrilla CB, Underwood MA. A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal Bioanal Chem. 2013;405(12):4089–4105. doi: 10.1007/s00216-013-6817-1. doi:10.1007/s00216-013-6817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res. 2012;11(12):6124–6133. doi: 10.1021/pr300769g. doi:10.1021/pr300769g. [DOI] [PubMed] [Google Scholar]
- 25.Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, Lebrilla CB. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54(20):7471–7480. doi: 10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- 26.Ellis CL, Bokulich NA, Kalanetra KM, Mirmiran M, Elumalai J, Haapanen L, Schegg T, Rutledge JC, Raff G, Mills DA, Underwood MA. Probiotic administration in congenital heart disease: a pilot study. J Perinatol. 2013;33(9):691–697. doi: 10.1038/jp.2013.41. doi:10.1038/jp.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blank D, Gebhardt S, Maass K, Lochnit G, Dotz V, Blank J, Geyer R, Kunz C. High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal Bioanal Chem. 2011;401(8):2495–2510. doi: 10.1007/s00216-011-5349-9. doi:10.1007/s00216-011-5349-9. [DOI] [PubMed] [Google Scholar]
- 28.Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104(9):1261–1271. doi: 10.1017/S0007114510002072. doi:10.1017/S0007114510002072. [DOI] [PubMed] [Google Scholar]
- 29.Tao N, DePeters EJ, Freeman S, German JB, Grimm R, Lebrilla CB. Bovine milk glycome. J dairy sci. 2008;91(10):3768–3778. doi: 10.3168/jds.2008-1305. doi:10.3168/jds.2008-1305. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, An HJ, Garrido D, German JB, Lebrilla CB, Mills DA. Proteomic analysis of Bifidobacterium longum subsp. infantis reveals the metabolic insight on consumption of prebiotics and host glycans. PLoS One. 2013;8(2):e57535. doi: 10.1371/journal.pone.0057535. doi:10.1371/journal.pone.0057535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruhaak LR, Taylor SL, Miyamoto S, Kelly K, Leiserowitz GS, Gandara D, Lebrilla CB, Kim K. Chip-based nLC-TOF-MS is a highly stable technology for large-scale high-throughput analyses. Anal Bioanal Chem. 2013;405(14):4953–4958. doi: 10.1007/s00216-013-6908-z. doi:10.1007/s00216-013-6908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]